Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Agarose Hydrogels
2.3. Characterization of the Physicochemical Properties of the Hydrogels
2.4. Rheological Measurements
2.5. Release Tests of Diclofenac Sodium
3. Results
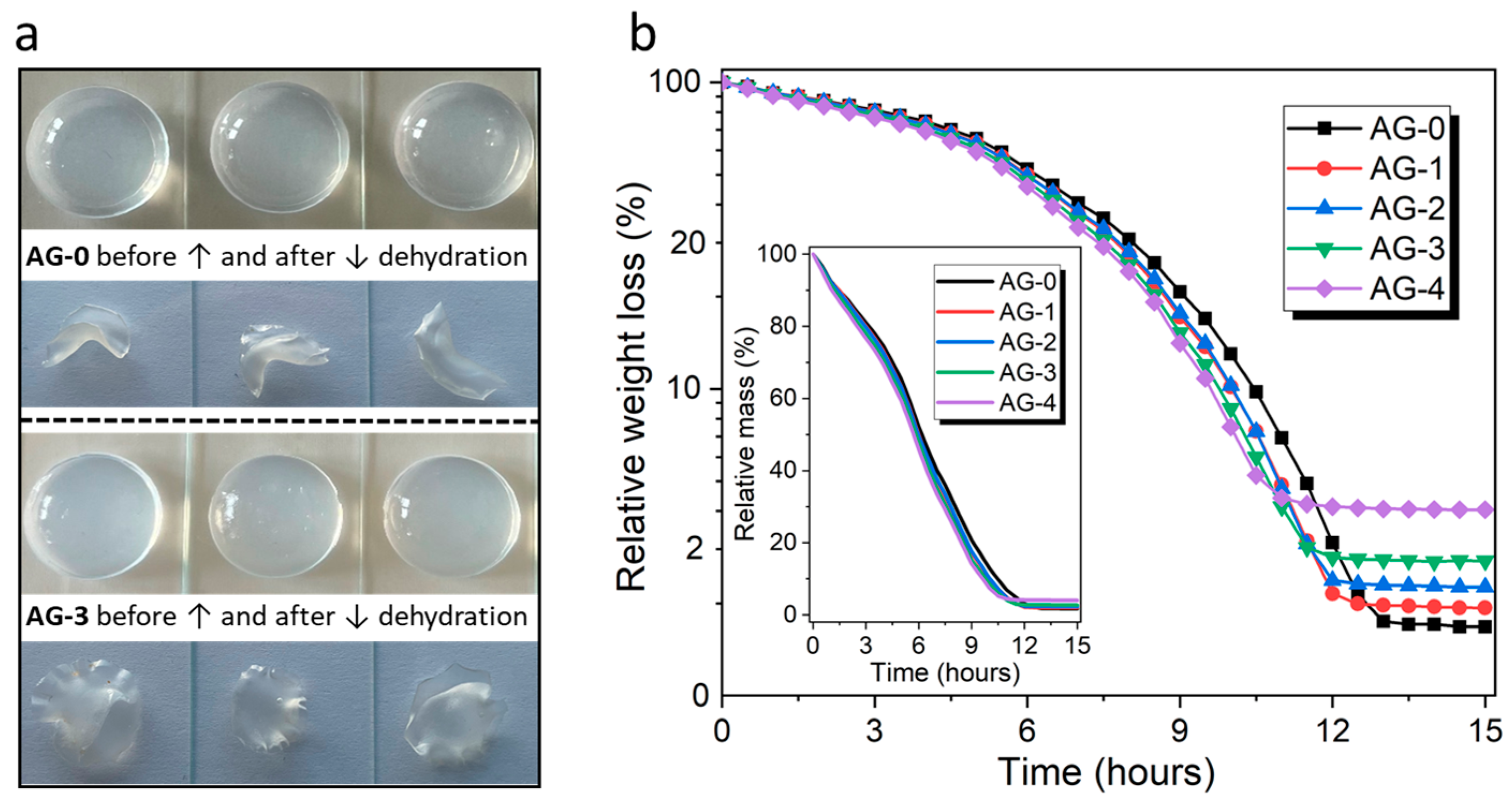
3.1. Dehydration Studies
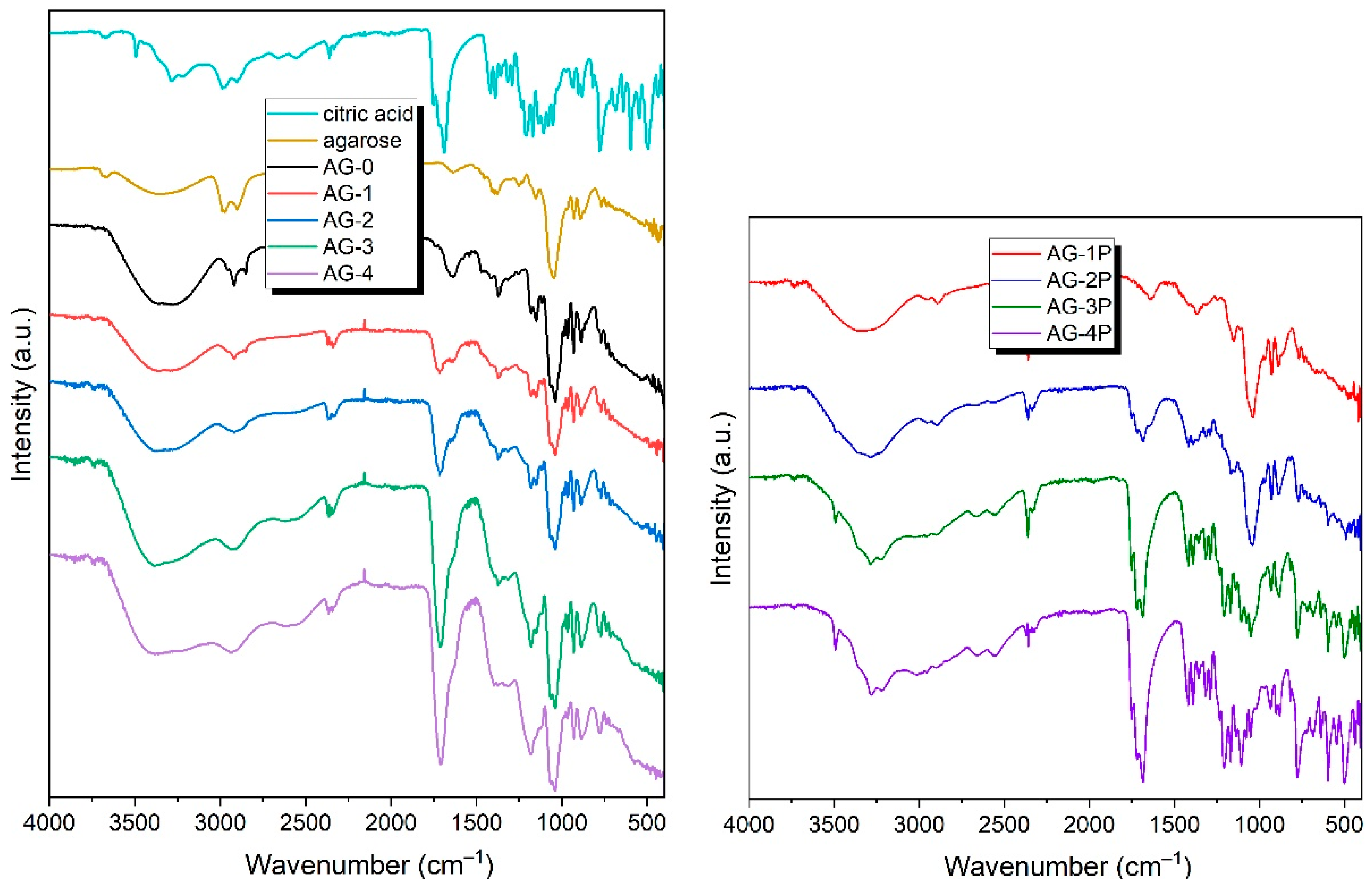
3.2. FTIR
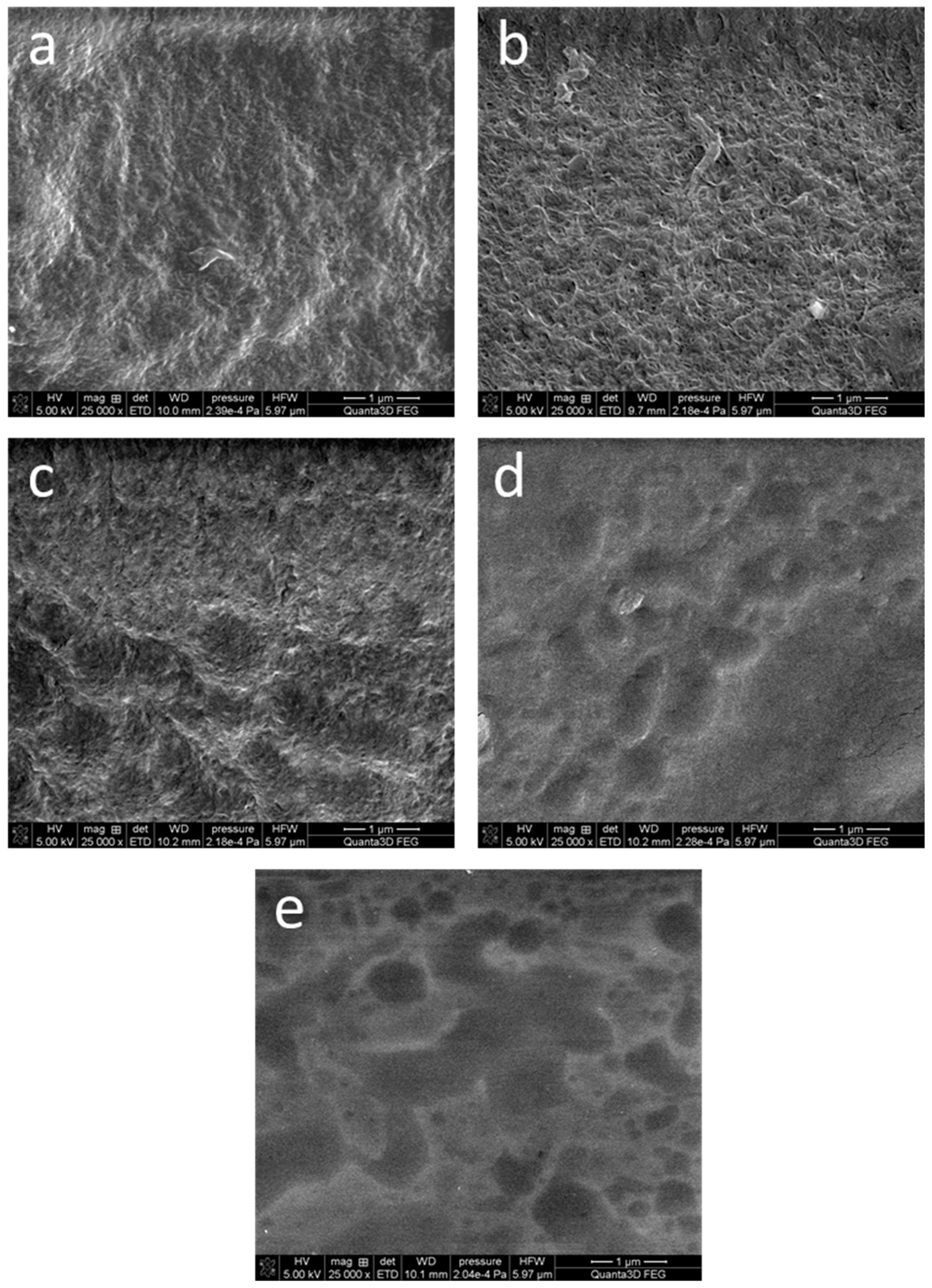
3.3. SEM Imaging
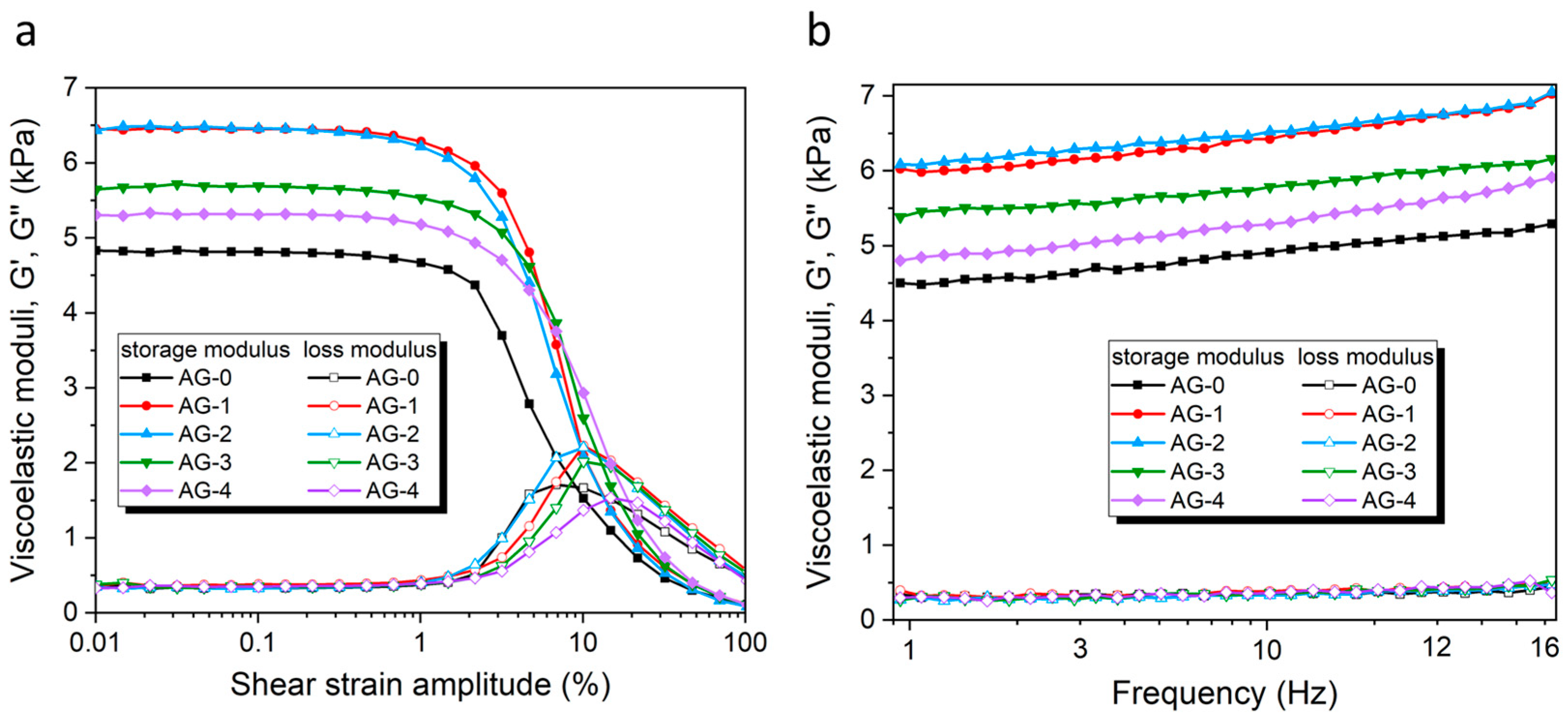
3.4. Rheological Measurements
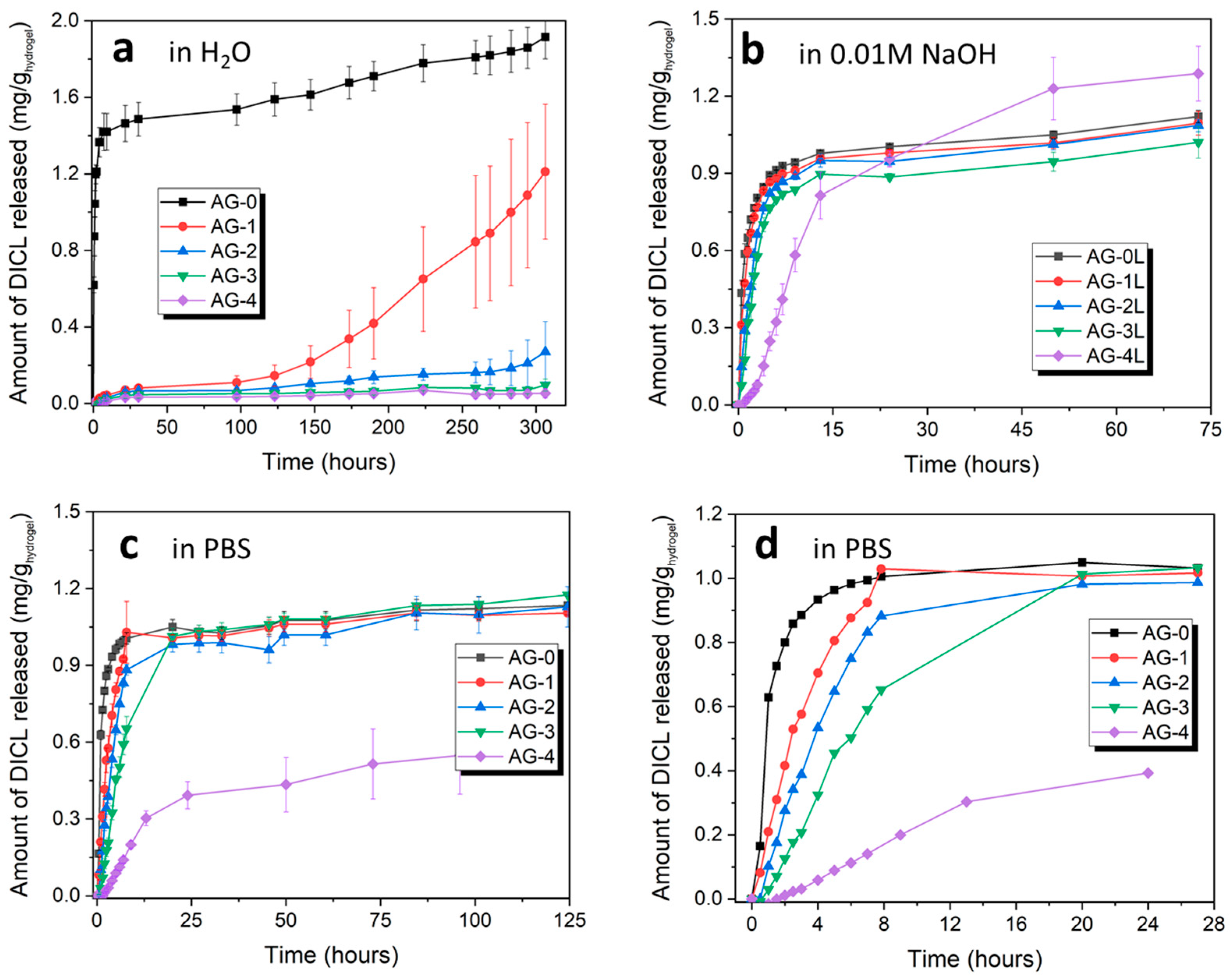
3.5. Release of Diclofenac Sodium (DICL)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Fan, D.Y.; Tian, Y.; Liu, Z.J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 18, pp. 1345–1360. [Google Scholar]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Zakir Hossain, K.M.; Roberts, G.A.F.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohydr. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef]
- Ribba, L.; Garcia, N.L.; D’Accorso, N.; Goyanes, S. Disadvantages of Starch-Based Materials, Feasible Alternatives in Order to Overcome These Limitations. Starch-Based Mater. Food Packag. Process. Charact. Appl. 2017, 37–76. [Google Scholar] [CrossRef]
- Devi, L.S.; Das, A.J.; Das, A.B. Characterization of high amylose starch-microcrystalline cellulose based floatable gel for enhanced gastrointestinal retention and drug delivery. Carbohydr. Polym. Technol. Appl. 2022, 3, 100185. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Fernández-Cossío, S.; León-Mateos, A.; Sampedro, F.G.; Oreja, M.T.C. Biocompatibility of agarose gel as a dermal filler: Histologic evaluation of subcutaneous implants. Plast. Reconstr. Surg. 2007, 120, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Bonhome-Espinosa, A.B.; Chato-Astrain, J.; Sánchez-Porras, D.; García-García, Ó.D.; Carmona, R.; López-López, M.T.; Alaminos, M.; Carriel, V.; Rodriguez, I.A. Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 527560. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Physics of agarose fluid gels: Rheological properties and microstructure. Curr. Res. Food Sci. 2021, 4, 436. [Google Scholar] [CrossRef]
- Nordqvist, D.; Vilgis, T.A. Rheological Study of the Gelation Process of Agarose-Based Solutions. Food Biophys. 2011, 6, 450–460. [Google Scholar] [CrossRef]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-Containing Agarose Hydrogel for Wound and Burn Care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef]
- Soylu, H.M.; Chevallier, P.; Copes, F.; Ponti, F.; Candiani, G.; Yurt, F.; Mantovani, D. A Novel Strategy to Coat Dopamine-Functionalized Titanium Surfaces with Agarose-Based Hydrogels for the Controlled Release of Gentamicin. Front. Cell. Infect. Microbiol. 2021, 11, 678081. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of flexible ph-responsive agarose/succinoglycan hydrogels for controlled drug release. Polymer 2021, 13, 2049. [Google Scholar] [CrossRef]
- Aslam, M.; Barkat, K.; Malik, N.S.; Alqahtani, M.S.; Anjum, I.; Khalid, I.; Tulain, U.R.; Gohar, N.; Zafar, H.; Paiva-Santos, A.C.; et al. pH Sensitive Pluronic Acid/Agarose-Hydrogels as Controlled Drug Delivery Carriers: Design, Characterization and Toxicity Evaluation. Pharmaceutics 2022, 14, 1218. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Javanbakht, S.; Namazi, H.; Shaabani, A. Application or function of citric acid in drug delivery platforms. Med. Res. Rev. 2022, 42, 800–849. [Google Scholar] [CrossRef]
- Barczak, M.; Borowski, P.; Gila-Vilchez, C.; Alaminos, M.; Gonzalez-Caballero, F.; López-López, M.T. Revealing importance of particles’ surface functionalization on the properties of magnetic alginate hydrogels. Carbohydr. Polym. 2020, 247, 116747. [Google Scholar] [CrossRef] [PubMed]
- Barczak, M. Amine-modified mesoporous silicas: Morphology-controlled synthesis toward efficient removal of pharmaceuticals. Microporous Mesoporous Mater. 2019, 278, 354–365. [Google Scholar] [CrossRef]
- Barczak, M.; Borowski, P. Silica xerogels modified with amine groups: Influence of synthesis parameters on porous structure and sorption properties. Microporous Mesoporous Mater. 2019, 281, 32–43. [Google Scholar] [CrossRef]
- Barczak, M.; Pietras-Ożga, D.; Seliem, M.K.; Falco, G.D.; Giannakoudakis, D.A.; Triantafyllidis, K. Mesoporous Silicas Obtained by Time-Controlled Co-Condensation: A Strategy for Tuning Structure and Sorption Properties. Nanomaterials 2023, 13, 2065. [Google Scholar] [CrossRef]
- Awadhiya, A.; Tyeb, S.; Rathore, K.; Verma, V. Agarose bioplastic-based drug delivery system for surgical and wound dressings. Eng. Life Sci. 2017, 17, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Nita, L.E.; Croitoriu, A.; Serban, A.M.; Bercea, M.; Rusu, A.G.; Ghilan, A.; Butnaru, M.; Mititelu-Tartau, L.; Chiriac, A.P. New Hydrogels Based on Agarose/Phytagel and Peptides. Macromol. Biosci. 2023, 23, 2200451. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Srivastava, D.N.; Rogers, R.D.; Kumar, A. Agarose processing in protic and mixed protic–aprotic ionic liquids: Dissolution, regeneration and high conductivity, high strength ionogels. Green Chem. 2012, 14, 2831–2839. [Google Scholar] [CrossRef]
- Uranga, J.; Leceta, I.; Etxabide, A.; Guerrero, P.; De La Caba, K. Cross-linking of fish gelatins to develop sustainable films with enhanced properties. Eur. Polym. J. 2016, 78, 82–90. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; De la Caba, K. The Effect of Cross-Linking with Citric Acid on the Properties of Agar/Fish Gelatin Films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef]
- Trudicova, M.; Smilek, J.; Kalina, M.; Smilkova, M.; Adamkova, K.; Hrubanova, K.; Krzyzanek, V.; Sedlacek, P. Multiscale Experimental Evaluation of Agarose-Based Semi-Interpenetrating Polymer Network Hydrogels as Materials with Tunable Rheological and Transport Performance. Polymers 2020, 12, 2561. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Bonhome-Espinosa, A.B.; Kuzhir, P.; Zubarev, A.; Duran, J.D.G.; Lopez-Lopez, M.T. Rheology of magnetic alginate hydrogels. J. Rheol. 2018, 62, 1083–1096. [Google Scholar] [CrossRef]
- Cvek, M.; Zahoranova, A.; Mrlik, M.; Sramkova, P.; Minarik, A.; Sedlacik, M. Poly(2-oxazoline)-based magnetic hydrogels: Synthesis, performance and cytotoxicity. Colloids Surf. B Biointerfaces 2020, 190, 110912. [Google Scholar] [CrossRef] [PubMed]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications about the Author; Wiley-VCH: Weinheim, Germany, 1994; ISBN 978-0-471-18575-8. [Google Scholar]
- Callejas, A.; Gomez, A.; Melchor, J.; Riveiro, M.; Massó, P.; Torres, J.; López-López, M.T.; Rus, G. Performance study of a torsional wave sensor and cervical tissue characterization. Sensors 2017, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Barczak, M.; Wierzbicka, M.; Borowski, P. Sorption of diclofenac onto functionalized mesoporous silicas: Experimental and theoretical investigations. Microporous Mesoporous Mater. 2018, 264, 254–264. [Google Scholar] [CrossRef]
- Barczak, M.; Dobrowolski, R.; Borowski, P.; Giannakoudakis, D.A. Pyridine-, thiol- and amine-functionalized mesoporous silicas for adsorptive removal of pharmaceuticals. Microporous Mesoporous Mater. 2020, 299, 110132. [Google Scholar] [CrossRef]
| Sample Name | Volume of 2% Agarose | Volume of Water | Volume of Citric Acid | Conc. of Citric Acid | AG and CA Content in Dry Mas * |
|---|---|---|---|---|---|
| AG-0 | 4 mL | 1 mL | 0 mL | --- | 0.08 g/0.0 g |
| AG-1 | 4 mL | 0 mL | 1 mL | 1.0% | 0.08 g/0.01 g |
| AG-2 | 4 mL | 0 mL | 1 mL | 2.5% | 0.08 g/0.025 g |
| AG-3 | 4 mL | 0 mL | 1 mL | 5.0% | 0.08 g/0.05 g |
| AG-4 | 4 mL | 0 mL | 1 mL | 10.0% | 0.08 g/0.1 g |
| Sample Name | Storage Modulus, G′ (Pa) | Loss Modulus, G″ (Pa) | Yielding Point, γL (%) |
|---|---|---|---|
| AG-0 | 4815 ± 14 | 338 ± 8 | ~9 |
| AG-1 | 6450 ± 18 | 373 ± 17 | ~10 |
| AG-2 | 6463 ± 11 | 333 ± 5 | ~10 |
| AG-3 | 5682 ± 20 | 355 ± 26 | ~14 |
| AG-4 | 5313 ± 30 | 349 ± 16 | ~20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, A.; Kapusta, O.; Gugała-Fekner, D.; Barczak, M. Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium. Materials 2023, 16, 6042. https://doi.org/10.3390/ma16176042
Jarosz A, Kapusta O, Gugała-Fekner D, Barczak M. Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium. Materials. 2023; 16(17):6042. https://doi.org/10.3390/ma16176042
Chicago/Turabian StyleJarosz, Anna, Oliwia Kapusta, Dorota Gugała-Fekner, and Mariusz Barczak. 2023. "Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium" Materials 16, no. 17: 6042. https://doi.org/10.3390/ma16176042
APA StyleJarosz, A., Kapusta, O., Gugała-Fekner, D., & Barczak, M. (2023). Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium. Materials, 16(17), 6042. https://doi.org/10.3390/ma16176042







