In Situ SEM, TEM, EBSD Characterization of Nucleation and Early Growth of Pure Fe/Pure Al Intermetallic Compounds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
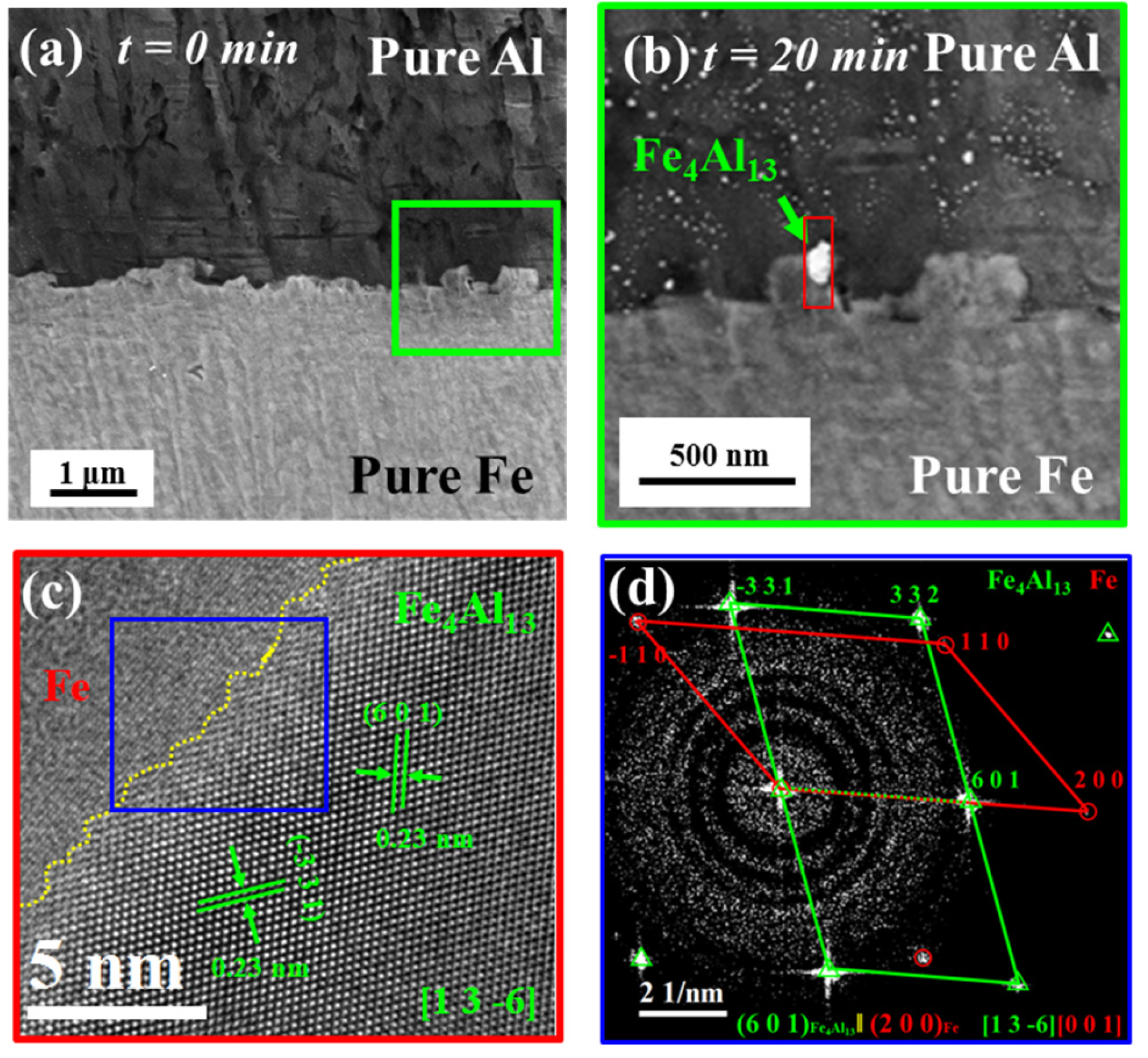
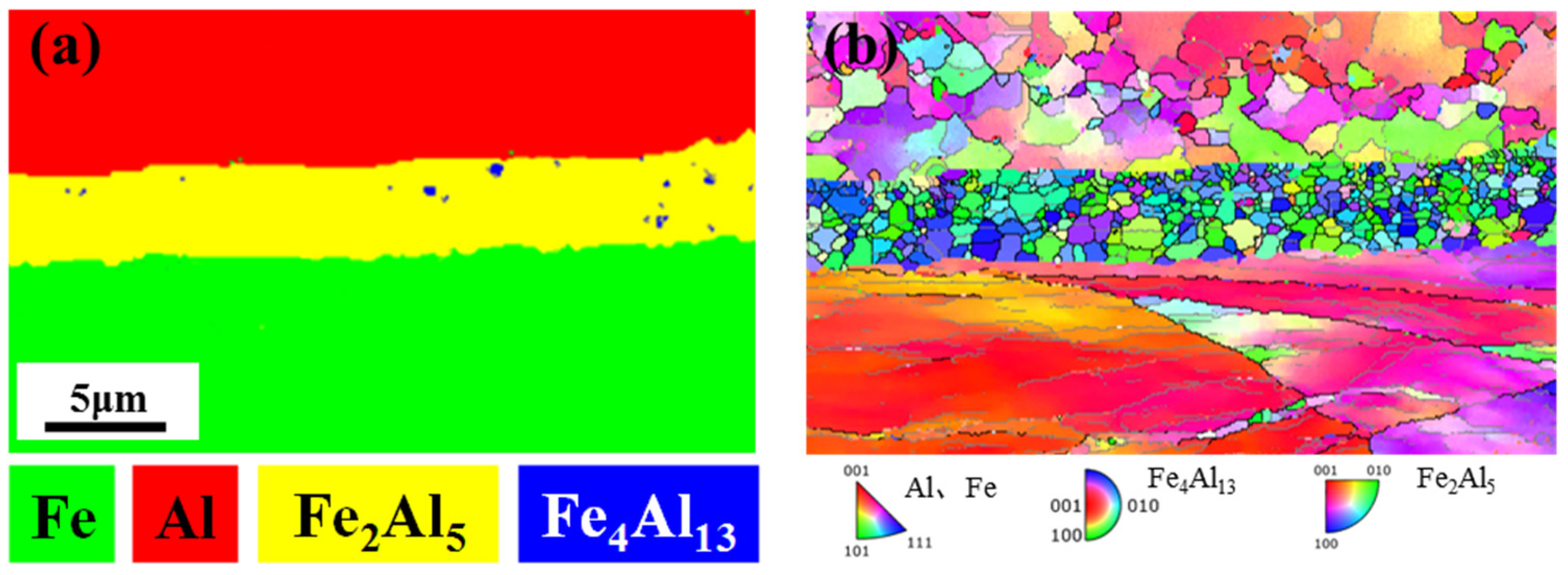
3.1. Nucleation and Characterization of Fe/Al Intermetallic Compounds
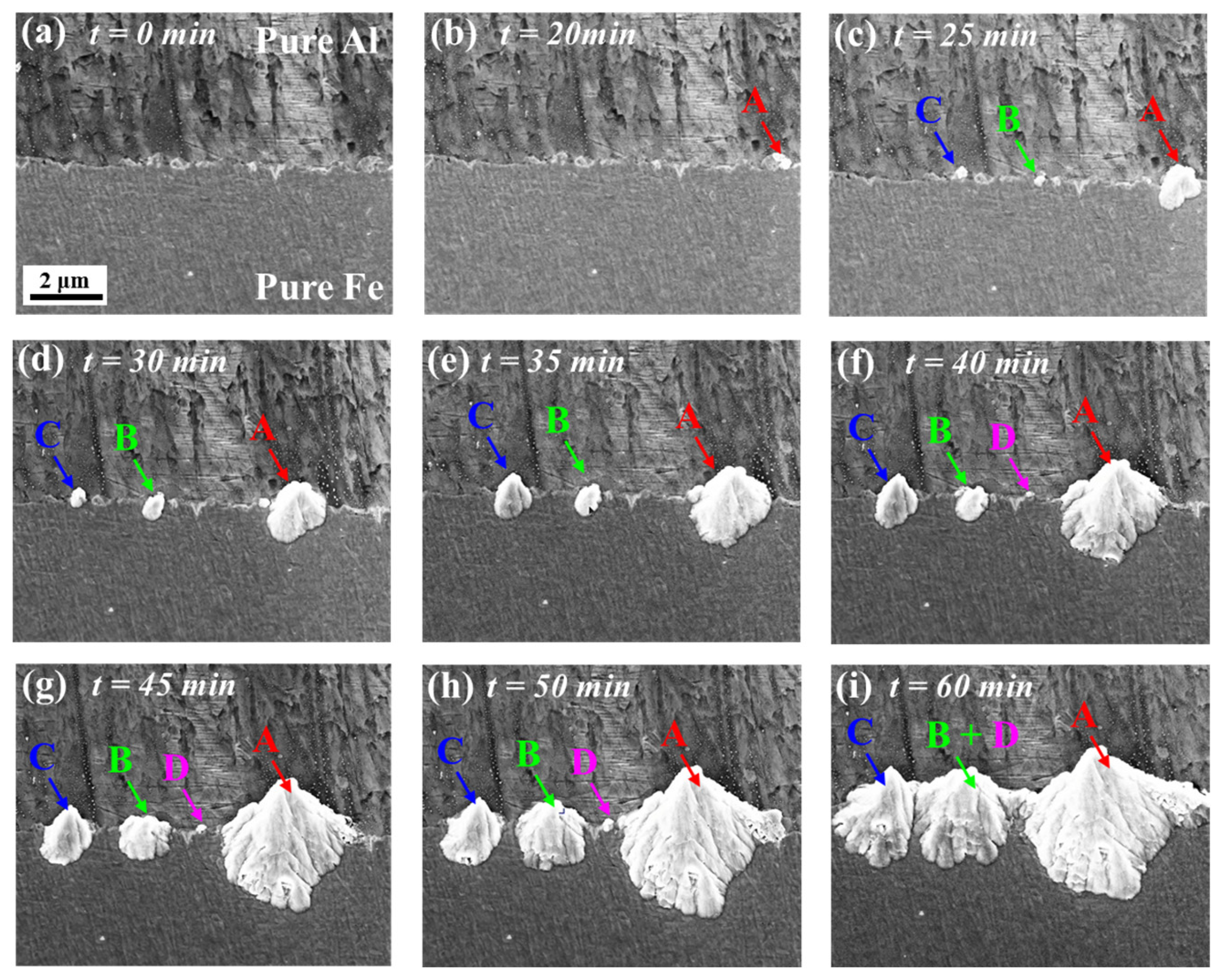
3.2. In Situ Observation and Characterization of Early Growth of IMCs at 380 °C
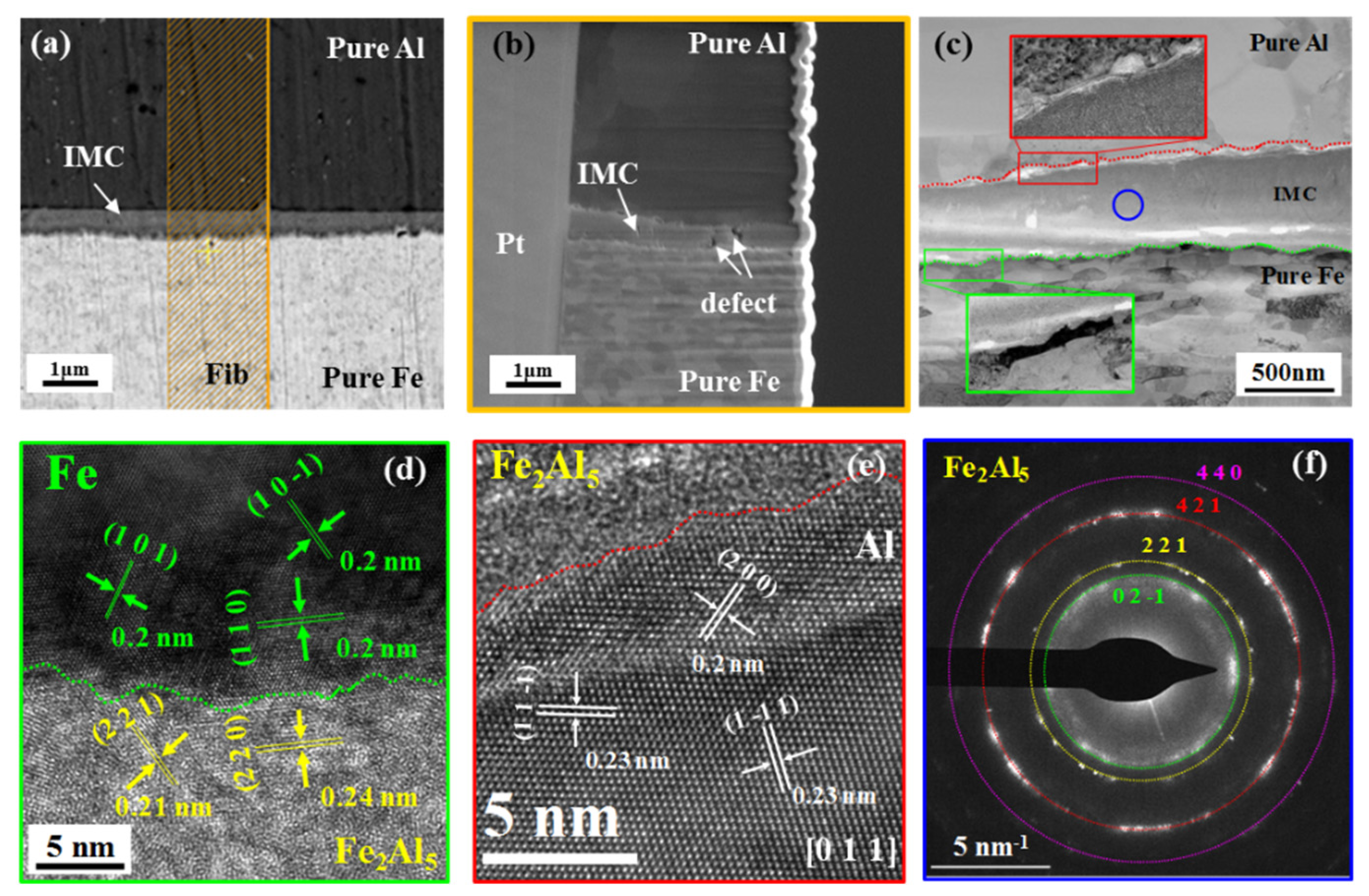
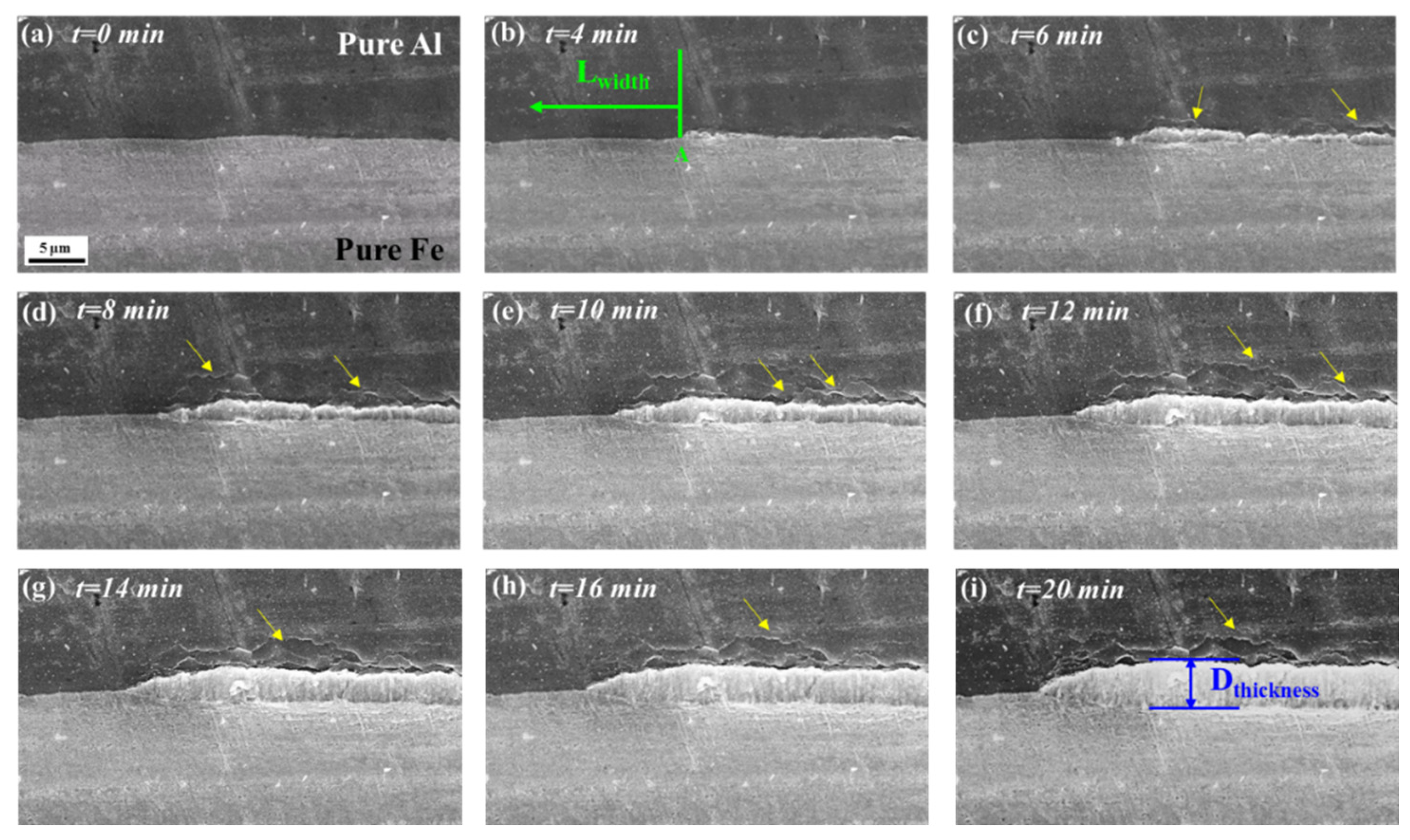
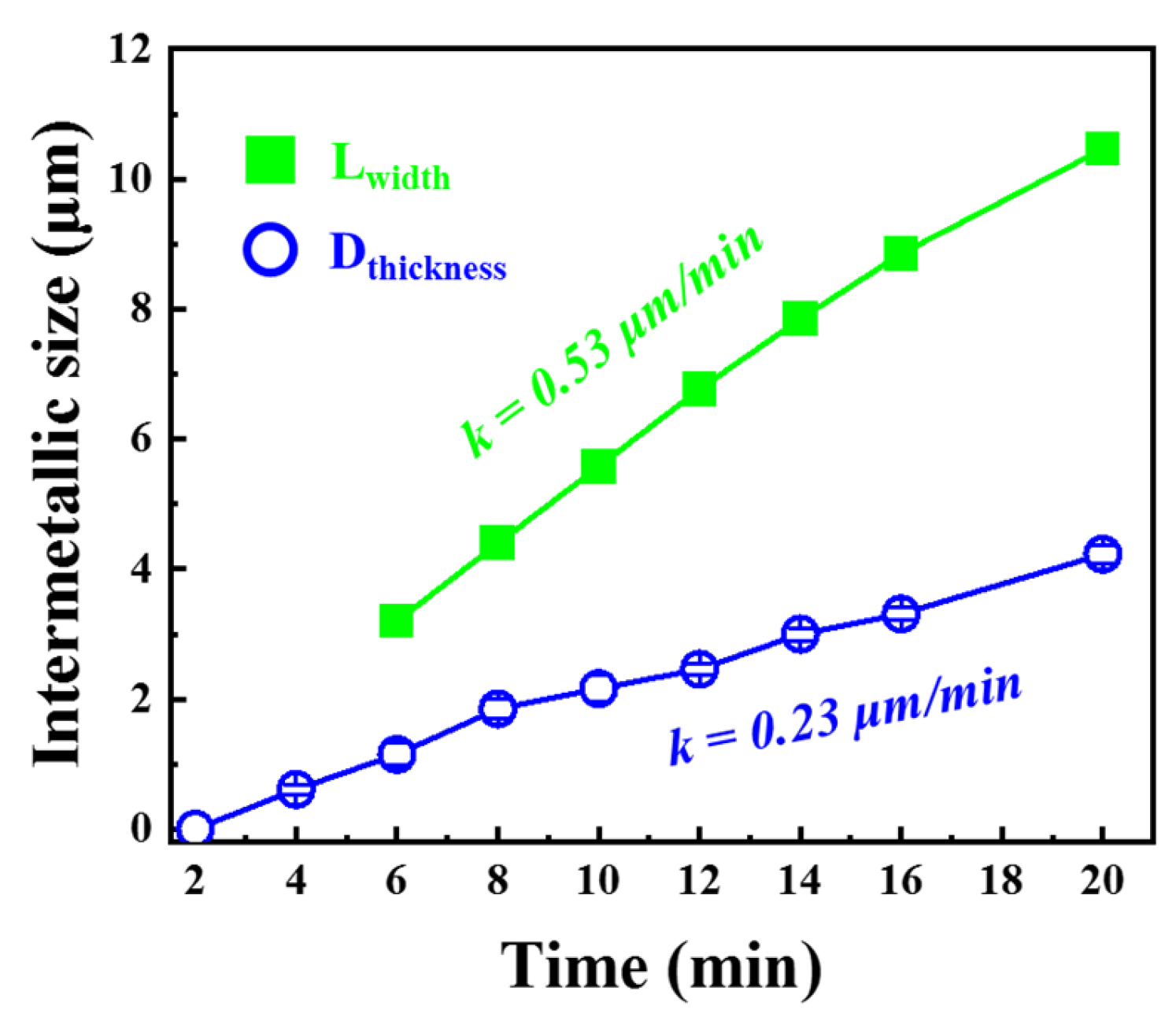
3.3. In Situ Observation and Characterization of IMCs at 520 °C
4. Conclusions
- (1)
- At 380 °C, the primary phase of Fe/Al composite intermetallic compounds was Fe4Al13 formed on the Fe side and habituated with Fe. The IMCs changed from the initial Fe4Al13 to the most stable Fe2Al5 when the heat treatment was extended from 15 min to 60 min, and the Fe2Al5 phase was habitus with Fe.
- (2)
- During heat treatment at 380 °C, the initial growth rate of Fe/Al composite intermetallic compounds was controlled by reaction. The initial growth rate of thickness and horizontal dimensions of IMCs was basically the same, ranging from 0.02–0.17 μm/min. When the thickness reached 4.5 μm, the growth rate of the thickness changed from reaction control to diffusion control, the critical thickness of the transformation was 4.5 μm, and the growth rate decreased to 0.007 μm/min.
- (3)
- After heat treatment at 520 °C (≤20 min), the growth of IMCs was still controlled by the reaction, the horizontal growth rate was 0.53 μm/min, the thickness growth rate was 0.23 μm/min, and the main component of IMCs was Fe2Al5 at 520 °C/20 min.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, G.; Chen, S.; Li, S.; Huang, J.; Yang, J.; Zhao, Z.; Huang, W.; Chen, S. Microstructures and mechanical property of 5052 aluminum alloy/Q235 steel butt joint achieved by laser beam joining with Sn-Zn filler wire. Opt. Laser Technol. 2021, 139, 106996. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Zhou, D.J. Formation and bonding properties of Al(4A60)-steel(08Al) clad strip intermetallic compound. Zhongguo Youse Jinshu Xuebao/Chin. J. Nonferrous Met. 2015, 25, 1176–1184. [Google Scholar]
- Sasaki, T.; Yakou, T. Features of intermetallic compounds in aluminized steels formed using aluminum foil. Surf. Coat. Technol. 2006, 201, 2131–2139. [Google Scholar] [CrossRef]
- Matysik, P.; Jozwiak, S.; Czujko, T. Characterization of Low-Symmetry Structures from Phase Equilibrium of Fe-Al System-Microstructures and Mechanical Properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kajihara, M. Morphology of Compounds Formed by Isothermal Reactive Diffusion between Solid Fe and Liquid Al. Mater. Trans. 2009, 50, 2212–2220. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, K.; Wang, Z.; Hu, X.; Liu, H.; Nie, Z. Effect of intermetallic compounds on interfacial bonding of Al/Fe composites. Mater. Lett. 2023, 333, 133597. [Google Scholar] [CrossRef]
- Shin, D.; Lee, J.-Y.; Heo, H.; Kang, C.-Y. Formation Procedure of Reaction Phases in Al Hot Dipping Process of Steel. Metals 2018, 8, 820. [Google Scholar] [CrossRef]
- Zou, T.P.; Gao-Yang, Y.U.; Chen, S.H.; Huang, J.H.; Yang, J.; Zhao, Z.Y.; Rong, J.P.; Yang, J. Effect of Si content on interfacial reaction and properties between solid steel and liquid aluminum. Trans. Nonferrous Met. Soc. China 2021, 31, 2570–2584. [Google Scholar] [CrossRef]
- Krivska, B.; Slapakova, M.; Vesely, J.; Kihoulou, M.; Fekete, K.; Minarik, P.; Kralik, R.; Grydin, O.; Stolbchenko, M.; Schaper, M. Intermetallic Phases Identification and Diffusion Simulation in Twin-Roll Cast Al-Fe Clad Sheet. Materials 2021, 14, 7771. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, H.; Tian, Y.; Yu, Z.; Wu, D. Effect of zinc on the fracture behavior of galvanized steel/6061 aluminum alloy by laser brazing. Weld. World 2021, 65, 13–22. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, X.; Tan, C.; Chen, B.; Song, X.; Li, L. Effect of Si content on the interfacial reactions in laser welded-brazed Al/steel dissimilar butted joint. J. Mater. Process. Technol. 2018, 258, 9–21. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S.P. Intermetallic compound formation in Fe–Al–Si ternary system: Part II. Mater. Charact. 2002, 49, 293–311. [Google Scholar] [CrossRef]
- Dangi, B.; Brown, T.W.; Kulkarni, K.N. Effect of silicon, manganese and nickel present in iron on the intermetallic growth at iron—Aluminum alloy interface. J. Alloys Compd. 2018, 769, 777–787. [Google Scholar] [CrossRef]
- Bouayad, A.; Gerometta, C.; Belkebir, A.; Ambari, A. Kinetic interactions between solid iron and molten aluminium. Mater. Sci. Eng. A 2003, 363, 53–61. [Google Scholar] [CrossRef]
- Hirose, A.; Imaeda, H.; Kondo, M.; Kobayashi, K.F. Influence of Alloying Elements on Interfacial Reaction and Strength of Aluminum/Steel Dissimilar Joints for Light Weight Car Body. Mater. Sci. Forum 2007, 539–543, 3888–3893. [Google Scholar] [CrossRef]
- Yahiro, A.; Masui, T.; Yoshida, T.; Doi, D. Development of Nonferrous Clad Plate and Sheet by Warm Rolling with Different Temperature of Materials. Trans. Iron Steel Inst. Jpn. 2007, 31, 647–654. [Google Scholar] [CrossRef]
- Furuya, H.S.; Sato, Y.T.; Sato, Y.S.; Kokawa, H.; Tatsumi, Y. Strength Improvement Through Grain Refinement of Intermetallic Compound at Al/Fe Dissimilar Joint Interface by the Addition of Alloying Elements. Metall. Mater. Trans. A 2017, 49, 527–536. [Google Scholar] [CrossRef]
- Szczepaniak, A. Investigation of Intermetallic Layer Formation in Dependence of Process Parameters during the Thermal Joining of Aluminium with Steel. Ph.D. Thesis, Rheinisch-Westfälischen Technischen Hochschule Aachen, Aachen, Germany, 2016; pp. 36–40. [Google Scholar]
- Sapanathan, T.; Sabirov, I.; Xia, P.; Monclús, M.A.; Molina-Aldareguía, J.M.; Jacques, P.J.; Simar, A. High temperature in situ SEM assessment followed by ex situ AFM and EBSD investigation of the nucleation and early growth stages of Fe-Al intermetallics. Scr. Mater. 2021, 200, 113910. [Google Scholar] [CrossRef]
- Křivská, B.; Šlapáková, M.; Minárik, P.; Fekete, K.; Králík, R.; Stolbchenko, M.; Schaper, M.; Grydin, O. In-Situ TEM observation of intermetallic phase growth in Al-steel clad sheet. AIP Conf. Proc. 2021, 2411, 030005. [Google Scholar] [CrossRef]
- Zhang, K.; Cui, C.; Deng, L.; Wang, Y.; Wu, C.; Su, H. In-situ observations of solidification process of Fe–Al–Ta eutectic alloy. J. Phys. Chem. Solids 2023, 172, 111067. [Google Scholar] [CrossRef]
- Wang, J.; Lee, P.; Hamilton, R.; Li, M.; Allison, J. The kinetics of Fe-rich intermetallic formation in aluminium alloys: In situ observation. Scr. Mater. 2009, 60, 516–519. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformation in Metals and Alloys; Chapman and Hall: London, UK, 1992; p. 514. [Google Scholar]
- Perez, M. Gibbs-Thomson effects in phase transformations. Scr. Mater. 2005, 52, 709–712. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; dos Santos, J.F.; Raabe, D. Influence of intermetallic phases and Kirkendall-porosity on the mechanical properties of joints between steel and aluminium alloys. Mater. Sci. Eng. A 2011, 528, 4630–4642. [Google Scholar] [CrossRef]
- Eggersmann, M.; Mehrer, H. Diffusion in intermetallic phases of the Fe-Al system. Philos. Mag. A 2000, 80, 1219–1244. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kang, S.-B.; Sato, T.; Tezuka, H.; Kamio, A. Evolution of iron aluminide in Al/Fe in situ composites fabricated by plasma synthesis method. Mater. Sci. Eng. A 2003, 362, 257–263. [Google Scholar] [CrossRef]
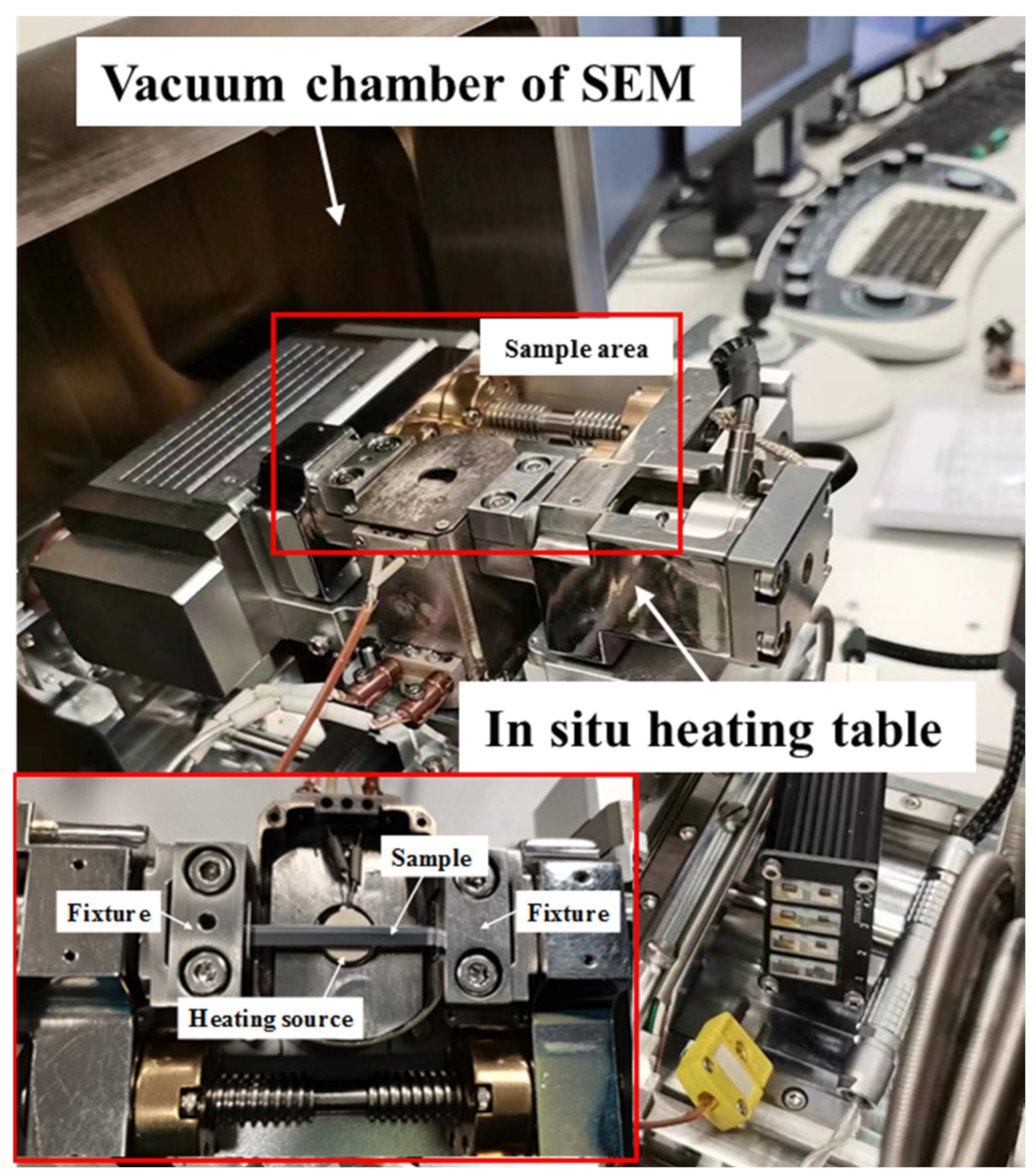

| Sample | Materials | Temperature/°C | Holding Time/min | Characterization Methods |
|---|---|---|---|---|
| 1# | pure Fe/pure Al | 380 | 20 | FIB + TEM |
| 2# | 380 | 60 | FIB + TEM, Size statistics | |
| 3# | 520 | 20 | EBSD, Size statistics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gao, K.; Wang, Z.; Hu, X.; Wang, J.; Nie, Z. In Situ SEM, TEM, EBSD Characterization of Nucleation and Early Growth of Pure Fe/Pure Al Intermetallic Compounds. Materials 2023, 16, 6022. https://doi.org/10.3390/ma16176022
Zhang X, Gao K, Wang Z, Hu X, Wang J, Nie Z. In Situ SEM, TEM, EBSD Characterization of Nucleation and Early Growth of Pure Fe/Pure Al Intermetallic Compounds. Materials. 2023; 16(17):6022. https://doi.org/10.3390/ma16176022
Chicago/Turabian StyleZhang, Xiaojun, Kunyuan Gao, Zhen Wang, Xiuhua Hu, Jianzhu Wang, and Zuoren Nie. 2023. "In Situ SEM, TEM, EBSD Characterization of Nucleation and Early Growth of Pure Fe/Pure Al Intermetallic Compounds" Materials 16, no. 17: 6022. https://doi.org/10.3390/ma16176022
APA StyleZhang, X., Gao, K., Wang, Z., Hu, X., Wang, J., & Nie, Z. (2023). In Situ SEM, TEM, EBSD Characterization of Nucleation and Early Growth of Pure Fe/Pure Al Intermetallic Compounds. Materials, 16(17), 6022. https://doi.org/10.3390/ma16176022






