In Vitro Characterization of an Anodized Surface of a Dental Implant Collar and Dental Abutment on Peri-Implant Cellular Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
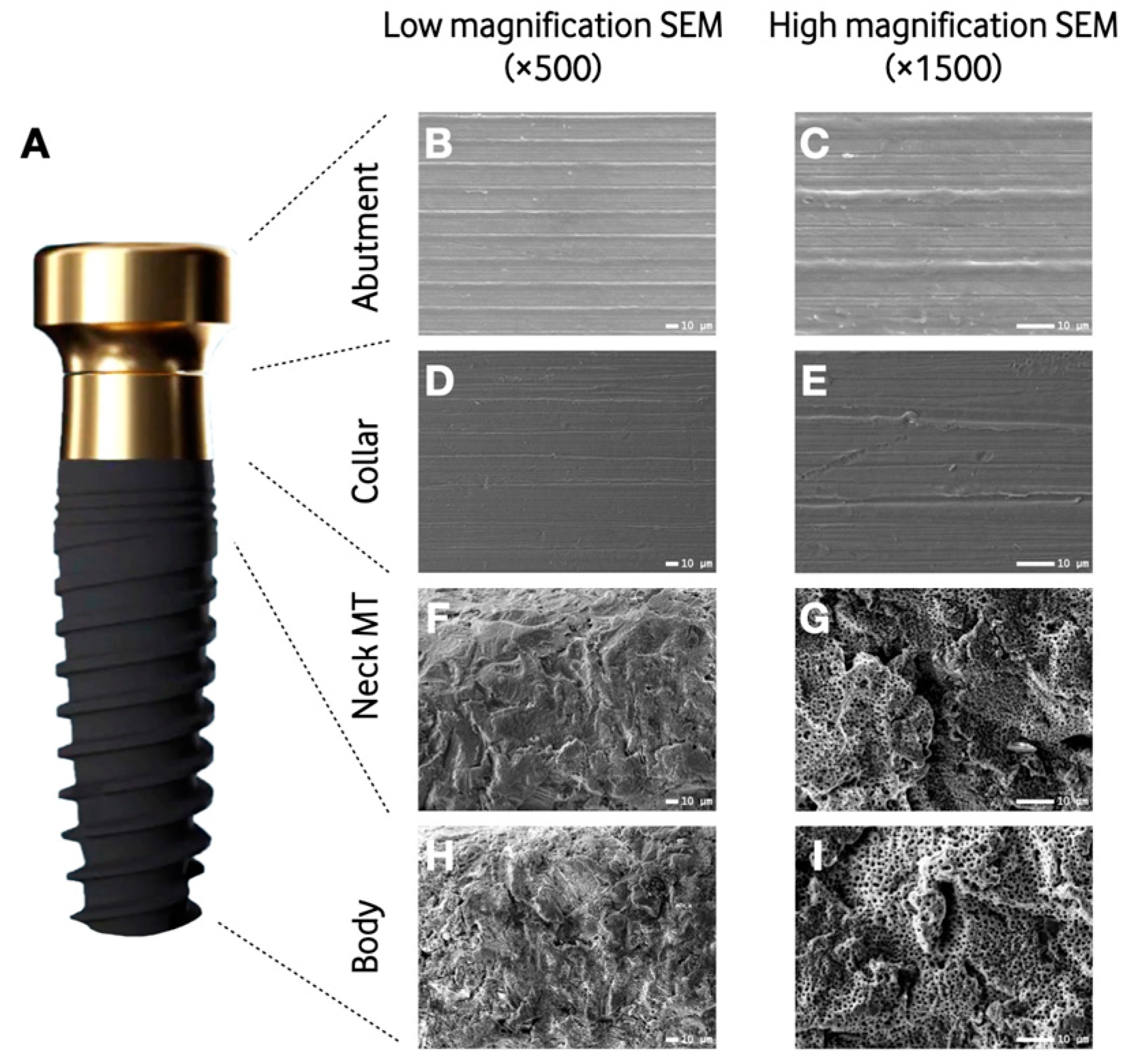
2.2. Scanning Electron Microscopy (SEM)
2.3. Surface Topography Analyses
- -
- Sa (arithmetic mean height) is defined as the difference in height of each point compared to the arithmetical mean of the surface. Expressed as µm.
- -
- Sz (average maximum height) is defined as the sum of the largest peak height value and the largest pit depth value within the defined area. Expressed as µm.
- -
- Ssk (skewness of topography height distribution) is defined as the degree of bias of the rough shape.
- -
- Sdr (developed interfacial area ratio) is defined as the ratio between the area of the “real” developed surface and the area of the “projected” surface. Expressed as percentage.
2.4. Surface Chemistry
2.5. Cellular Assays
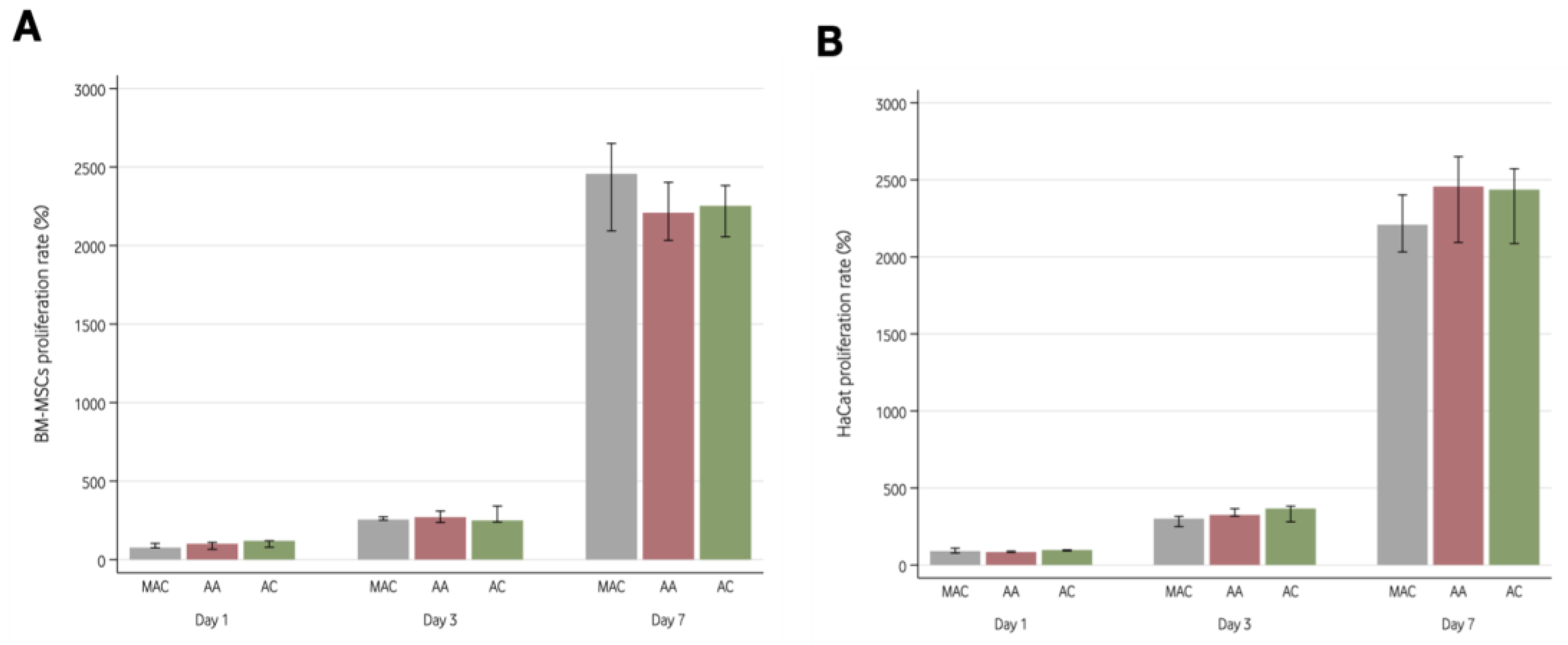
2.6. Cell Proliferation Analysis
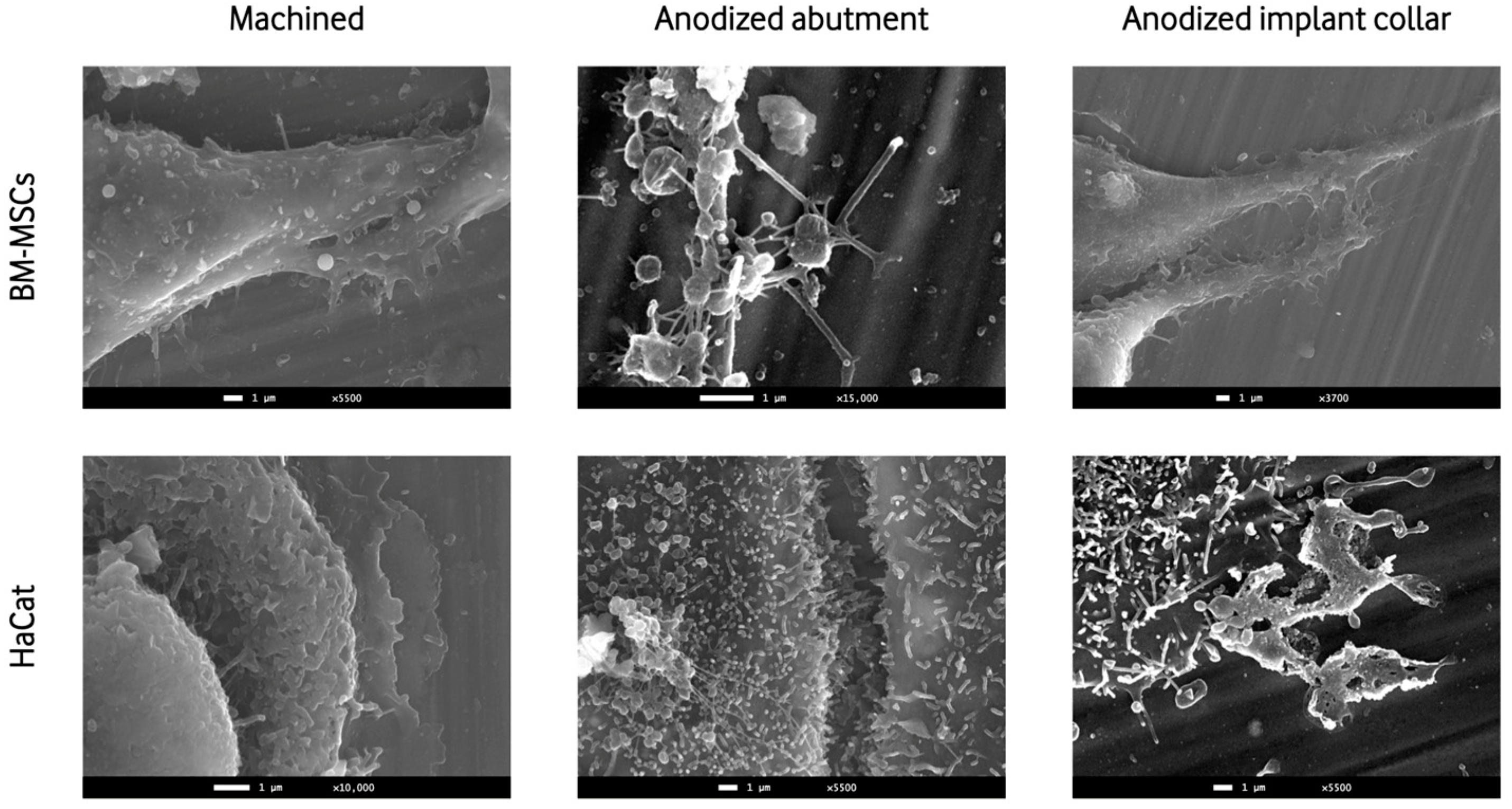
2.7. SEM of Cell Seeding on Implants
2.8. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy
3.2. Surface Topography Analysis
3.3. Surface Composition
3.4. Cellular Assays
3.4.1. Cell Proliferation Analysis
3.4.2. SEM of Cell Seeding on Implants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic Review of the Survival Rate and the Incidence of Biological, Technical, and Aesthetic Complications of Single Crowns on Implants Reported in Longitudinal Studies with a Mean Follow-up of 5 Years. Clin. Oral Implants Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A Systematic Review of the Survival and Complication Rates of Implant-Supported Fixed Dental Prostheses (FDPs) after a Mean Observation Period of at Least 5 Years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 22–38. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on Implants and Osseointegration. Periodontology 2000 2019, 79, 178–189. [Google Scholar] [CrossRef]
- Block, M.S. Dental Implants: The Last 100 Years. J. Oral Maxillofac. Surg. 2018, 76, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Barfeie, A.; Wilson, J.; Rees, J. Implant Surface Characteristics and Their Effect on Osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of Cells. Clin. Oral Implants Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Trujillo, E.G.; Pradies, G.; Petrillo, S.; Muzzi, M.; Carossa, S.; Mussano, F. Fibroblast Interaction with Different Abutment Surfaces: In Vitro Study. Int. J. Mol. Sci. 2020, 21, 1919. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface Characteristics of Dental Implants: A Review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Mosca Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface, Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Röser, K.; Albrektsson, T. Qualitative and Quantitative Observations of Bone Tissue Reactions to Anodised Implants. Biomaterials 2002, 23, 1809–1817. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M. Anodic Oxidation of Titanium: From Technical Aspects to Biomedical Applications. J. Appl. Biomater. Biomech. 2011, 9, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Milleret, V.; Lienemann, P.S.; Gasser, A.; Bauer, S.; Ehrbar, M.; Wennerberg, A. Rational Design and in Vitro Characterization of Novel Dental Implant and Abutment Surfaces for Balancing Clinical and Biological Needs. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. S1), 15–24. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, M.B.; Marques, J.F.; Peñarrieta-Juanito, G.M.; Costa, M.; Souza, J.C.M.; Magini, R.S.; Miranda, G.; Silva, F.S.; Caramês, J.M.M.; da Mata, A.D.S.P. Bioactive-Enhanced Polyetheretherketone Dental Implant Materials: Mechanical Characterization and Cellular Responses. J. Oral Implantol. 2021, 47, 9–17. [Google Scholar] [CrossRef]
- Lavenus, S.; Trichet, V.; Le Chevalier, S.; Hoornaert, A.; Louarn, G.; Layrolle, P. Cell Differentiation and Osseointegration Influenced by Nanoscale Anodized Titanium Surfaces. Nanomedicine 2012, 7, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Zicola, E.; Munaron, L.; Rivolo, P.; Mandracci, P.; Carossa, S. Early Response of Fibroblasts and Epithelial Cells to Pink-Shaded Anodized Dental Implant Abutments: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 571–579. [Google Scholar] [CrossRef]
- Farrag, K.M.; Khamis, M.M. Effect of Anodized Titanium Abutment Collars on Peri-Implant Soft Tissue: A Split-Mouth Clinical Study. J. Prosthet. Dent. 2021, 130, 59–67. [Google Scholar] [CrossRef]
- Martínez-Rus, F.; Prieto, M.; Salido, M.; Madrigal, C.; Özcan, M.; Pradíes, G. A Clinical Study Assessing the Influence of Anodized Titanium and Zirconium Dioxide Abutments and Peri-Implant Soft Tissue Thickness on the Optical Outcome of Implant-Supported Lithium Disilicate Single Crowns. Int. J. Oral Maxillofac. Implant. 2017, 32, 156–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Wang, L.; Lu, Q.; Fan, Z. Influence of Anodized Titanium Abutments on the Esthetics of the Peri-Implant Soft Tissue: A Clinical Study. J. Prosthet. Dent. 2021, 125, 445–452. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft Tissue Sealing around Dental Implants Based on Histological Interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef]
- Osman, M.A.; Alamoush, R.A.; Kushnerev, E.; Seymour, K.G.; Watts, D.C.; Yates, J.M. Biological Response of Epithelial and Connective Tissue Cells to Titanium Surfaces with Different Ranges of Roughness: An In-Vitro Study. Dent. Mater. 2022, 38, 1777–1788. [Google Scholar] [CrossRef]
- Giannasi, C.; Pagni, G.; Polenghi, C.; Niada, S.; Manfredi, B.; Brini, A.; Rasperini, G. Impact of Dental Implant Surface Modifications on Adhesion and Proliferation of Primary Human Gingival Keratinocytes and Progenitor Cells. Int. J. Periodontics Restor. Dent. 2018, 38, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Di Pietro, N.; Pandolfi, A.; D’arcangelo, C.; De Angelis, F.; Mandatori, D.; Schiavone, V.; Piattelli, A.; et al. Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2021, 22, 9871. [Google Scholar] [CrossRef]
- Baharloo, B.; Textor, M.; Brunette, D.M. Substratum Roughness Alters the Growth, Area, and Focal Adhesions of Epithelial Cells, and Their Proximity to Titanium Surfaces. J. Biomed. Mater. Res. A 2005, 74, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential Behavior of Fibroblasts and Epithelial Cells on Structured Implant Abutment Materials: A Comparison of Materials and Surface Topographies. Clin. Implant. Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Ogle, O.E. Implant Surface Material, Design, and Osseointegration. Dent. Clin. N. Am. 2015, 59, 505–520. [Google Scholar] [CrossRef]
- Schwartz, Z.; Martin, J.Y.; Dean, D.D.; Simpson, J.; Cochran, D.L.; Boyan, B.D. Effect of Titanium Surface Roughness on Chondrocyte Proliferation, Matrix Production, and Differentiation Depends on the State of Cell Maturation. J. Biomed. Mater. Res. 1996, 30, 145–155. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral Implant Surfaces: Part 1—Review Focusing on Topographic and Chemical Properties of Different Surfaces and in Vivo Responses to Them. Available online: https://pubmed.ncbi.nlm.nih.gov/15543910/ (accessed on 20 July 2023).
- Messias, A.; Nicolau, P.; Guerra, F. Titanium Dental Implants with Different Collar Design and Surface Modifications: A Systematic Review on Survival Rates and Marginal Bone Levels. Clin. Oral Implant. Res. 2019, 30, 20–48. [Google Scholar] [CrossRef]
- Randall, E.; Abou-Arraj, R.; Geurs, N.; Griffin, R.; Reddy, M.; Geisinger, M. The Effect of Dental Implant Collar Design on Crestal Bone Loss at 1 Year After Implant Placement. Int. J. Periodontics Restor. Dent. 2019, 39, 165–173. [Google Scholar] [CrossRef]
- Lindhe, J.; Berglundh, T. The Interface between the Mucosa and the Implant. Periodontology 2000 1998, 17, 47–54. [Google Scholar] [CrossRef]
- Cai, X.; Yang, F.; Yan, X.; Yang, W.; Yu, N.; Oortgiesen, D.A.W.; Wang, Y.; Jansen, J.A.; Walboomers, X.F. Influence of Bone Marrow-Derived Mesenchymal Stem Cells Pre-Implantation Differentiation Approach on Periodontal Regeneration In Vivo. J. Clin. Periodontol. 2015, 42, 380–389. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thornsen, P. The Soft Tissue Barrier at Implants and Teeth. Clin. Oral Implant. Res. 1991, 2, 81–90. [Google Scholar] [CrossRef]
- Gould, T.R.L.; Brunette, D.M.; Westsury, L. The Attachment Mechanism of Epithelial Cells to Titanium In Vitro. J. Periodontal Res. 1981, 16, 611–616. [Google Scholar] [CrossRef]
- Dorkhan, M.; Yücel-Lindberg, T.; Hall, J.; Svensäter, G.; Davies, J.R. Adherence of Human Oral Keratinocytes and Gingival Fibroblasts to Nano-Structured Titanium Surfaces. BMC Oral Health 2014, 14, 75. [Google Scholar] [CrossRef]
- Pera, F.; Menini, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Asero, S.; Marchetti, C.; Canepa, C.; Merlini, L.; Pesce, P.; et al. Can Abutment with Novel Superlattice CrN/NbN Coatings Influence Peri-Implant Tissue Health and Implant Survival Rate Compared to Machined Abutment? 6-Month Results from a Multi-Center Split-Mouth Randomized Control Trial. Materials 2022, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Koodaryan, R.; Hafezeqoran, A. Evaluation of Implant Collar Surfaces for Marginal Bone Loss: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2016, 2016, 4987526. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.; Senna, P.; Francischone, C.; Francischone Junior, C.; de Souza Picorelli Assis, N.; Sotto-Maior, B. Retrospective Evaluation of the Influence of the Collar Surface Topography on Peri-Implant Bone Preservation. Int. J. Oral Maxillofac. Implant. 2017, 32, 858–863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Wang, L.; Lu, Q.; Fan, Z. Changes in the Esthetic, Physical, and Biological Properties of a Titanium Alloy Abutment Treated by Anodic Oxidation. J. Prosthet. Dent. 2019, 121, 156–165. [Google Scholar] [CrossRef]
- Chai, W.L.; Brook, I.M.; Palmquist, A.; Van Noort, R.; Moharamzadeh, K. The Biological Seal of the Implant-Soft Tissue Interface Evaluated in a Tissue-Engineered Oral Mucosal Model. J. R. Soc. Interface 2012, 9, 3528–3538. [Google Scholar] [CrossRef]
- Marei, M.K.; Saad, M.M.; El-Ashwah, A.M.; Ei-Backly, R.M.; Al-Khodary, M.A. Experimental Formation of Periodontal Structure around Titanium Implants Utilizing Bone Marrow Mesenchymal Stem Cells: A Pilot Study. J. Oral Implantol. 2009, 35, 106–129. [Google Scholar] [CrossRef]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human Gingival Fibroblast Functions Are Stimulated by Oxidized Nano-Structured Titanium Surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The Influence of Titanium Abutment Surface Roughness on Plaque Accumulation and Gingivitis: Short-Term Observations. Available online: https://pubmed.ncbi.nlm.nih.gov/8666447/ (accessed on 13 March 2023).
- Hempel, U.; Hefti, T.; Dieter, P.; Schlottig, F. Response of Human Bone Marrow Stromal Cells, MG-63, and SaOS-2 to Titanium-Based Dental Implant Surfaces with Different Topography and Surface Energy. Clin. Oral Implant. Res. 2013, 24, 174–182. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM Analysis of Recent Dental Implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive Coatings for Orthopaedic Implants-Recent Trends in Development of Implant Coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Murakami, H.; Chehroudi, B.; Textor, M.; Brunette, D.M. Effects of Surface Topography on the Connective Tissue Attachment to Subcutaneous Implants. Int. J. Oral Maxillofac. Implant. 2006, 21, 354–365. [Google Scholar]
- Hamilton, D.W.; Brunette, D.M. “Gap Guidance” of Fibroblasts and Epithelial Cells by Discontinuous Edged Surfaces. Exp. Cell Res. 2005, 309, 429–437. [Google Scholar] [CrossRef]
- Jin, C.; Ren, L.; Ding, H.; Shi, G.; Lin, H.; Zhang, F. Enhanced Attachment, Proliferation, and Differentiation of Human Gingival Fibroblasts on Titanium Surface Modified with Biomolecules. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Chen, H.; Liu, T.; Yang, P.; Zhao, Y.; Huang, N. A Novel Coating of Type IV Collagen and Hyaluronic Acid on Stent Material-Titanium for Promoting Smooth Muscle Cell Contractile Phenotype. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 235–243. [Google Scholar] [CrossRef]
- Riivari, S.; Närvä, E.; Kangasniemi, I.; Willberg, J.; Närhi, T. Epithelial Cell Attachment and Adhesion Protein Expression on Novel in Sol TiO2 Coated Zirconia and Titanium Alloy Surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2533. [Google Scholar] [CrossRef]
| Voltage | Time | Ratio Ca-P | |
|---|---|---|---|
| Implant collar | 58 V | 90 s | 2:1 |
| Implant body | 138 V | 90 s | 2:1 |
| Prosthetic abutment | 58.8 V | 75 s | 0 |
| Sa (µm) | Sz (µm) | Ssk | Sdr (%) | |
|---|---|---|---|---|
| Treated samples (n = 3) | ||||
| Prosthetic abutment | 0.25 (0.06) | 1.19 (0.55) | −0.05 (0.12) | 0.43 (0.72) |
| Implant collar | 0.11 (0.02) | 1.28 (0.57) | −0.02 (0.27) | 0.62 (0.83) |
| Implant neck microthreads | 1.20 (0.11) | 18.37 (1.87) | −0.20 (0.30) | 74.52 (21.73) |
| Implant body thread | 0.95 (0.58) | 18.58 (8.93) | −0.27 (0.31) | 61.38 (20.52) |
| Implant body valley | 1.68 (0.24) | 16.38 (1.67) | −0.12 (0.27) | 56.38 (18.57) |
| Machined samples (n = 3) | ||||
| Prosthetic abutment | 0.13 (0.02) | 1.11 (0.68) | −0.05 (0.26) | 0.28 (0.21) |
| Implant collar | 0.10 (0.03) | 1.08 (0.71) | 0.02 (0.23) | 0.23 (0.24) |
| Implant neck microthreads | 0.13 (0.07) | 1.79 (2.21) | −0.20 (0.79) | 1.12 (0.32) |
| Implant body thread | 0.07 (0.08) | 2.21 (0.76) | 0.79 (4.65) | 0.32 (0.85) |
| Implant body valley | 0.14 (0.03) | 1.44 (0.65) | 0.22 (0.27) | 0.55 (0.04) |
| O | Na | Al | P | Ca | Ti | V | |
|---|---|---|---|---|---|---|---|
| Treated samples (n = 3) | |||||||
| Prosthetic abutment | 30.15 (1.96) | - | 4.17 (0.32) | 0.29 (0.09) | - | 62.71 (2.17) | 2.37 (0.21) |
| Implant collar | 31.52 (0.85) | - | 4.53 (0.20) | - | - | 61.82 (0.55) | 2.22 (0.58) |
| Implant Body | 46.44 (1.04) | 0.44 (0.13) | 9.24 (0.30) | 1.05 (0.31) | 0.63 (0.26) | 41.22 (1.37) | 1.67 (0.13) |
| Machined samples (n = 3) | |||||||
| Prosthetic abutment | - | - | 6.42 (0.31) | - | - | 90.15 (0.28) | 3.23 (0.20) |
| Implant collar | - | - | 6.61 (0.37) | - | - | 90.45 (0.26) | 3.41 (0.44) |
| Implant Body | - | - | 5.95 (0.66) | - | - | 90.17 (0.30) | 3.60 (0.38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traver-Méndez, V.; Camps-Font, O.; Ventura, F.; Nicolau-Sansó, M.A.; Subirà-Pifarré, C.; Figueiredo, R.; Valmaseda-Castellón, E. In Vitro Characterization of an Anodized Surface of a Dental Implant Collar and Dental Abutment on Peri-Implant Cellular Response. Materials 2023, 16, 6012. https://doi.org/10.3390/ma16176012
Traver-Méndez V, Camps-Font O, Ventura F, Nicolau-Sansó MA, Subirà-Pifarré C, Figueiredo R, Valmaseda-Castellón E. In Vitro Characterization of an Anodized Surface of a Dental Implant Collar and Dental Abutment on Peri-Implant Cellular Response. Materials. 2023; 16(17):6012. https://doi.org/10.3390/ma16176012
Chicago/Turabian StyleTraver-Méndez, Valeria, Octavi Camps-Font, Francesc Ventura, Miquel Angel Nicolau-Sansó, Carles Subirà-Pifarré, Rui Figueiredo, and Eduard Valmaseda-Castellón. 2023. "In Vitro Characterization of an Anodized Surface of a Dental Implant Collar and Dental Abutment on Peri-Implant Cellular Response" Materials 16, no. 17: 6012. https://doi.org/10.3390/ma16176012
APA StyleTraver-Méndez, V., Camps-Font, O., Ventura, F., Nicolau-Sansó, M. A., Subirà-Pifarré, C., Figueiredo, R., & Valmaseda-Castellón, E. (2023). In Vitro Characterization of an Anodized Surface of a Dental Implant Collar and Dental Abutment on Peri-Implant Cellular Response. Materials, 16(17), 6012. https://doi.org/10.3390/ma16176012








