Effect of Sodium Hydroxide and Tripolyphosphate on Curcumin Release from Chitosan-Based Macroparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
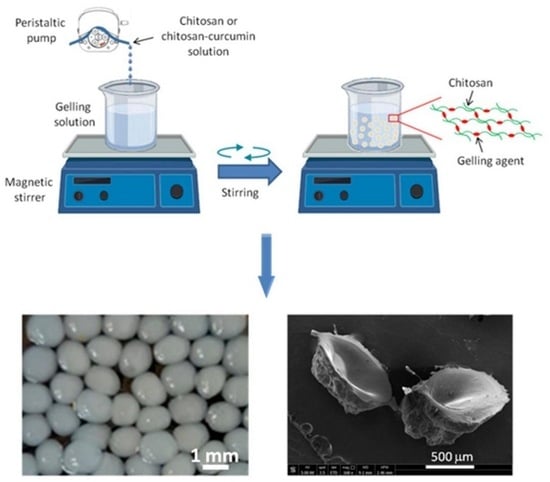
2.2. Preparation of the CS- and CS- CUR- Based Macrobeads
2.3. Characterization of the CS- and CS-CUR Macrobeads
3. Results
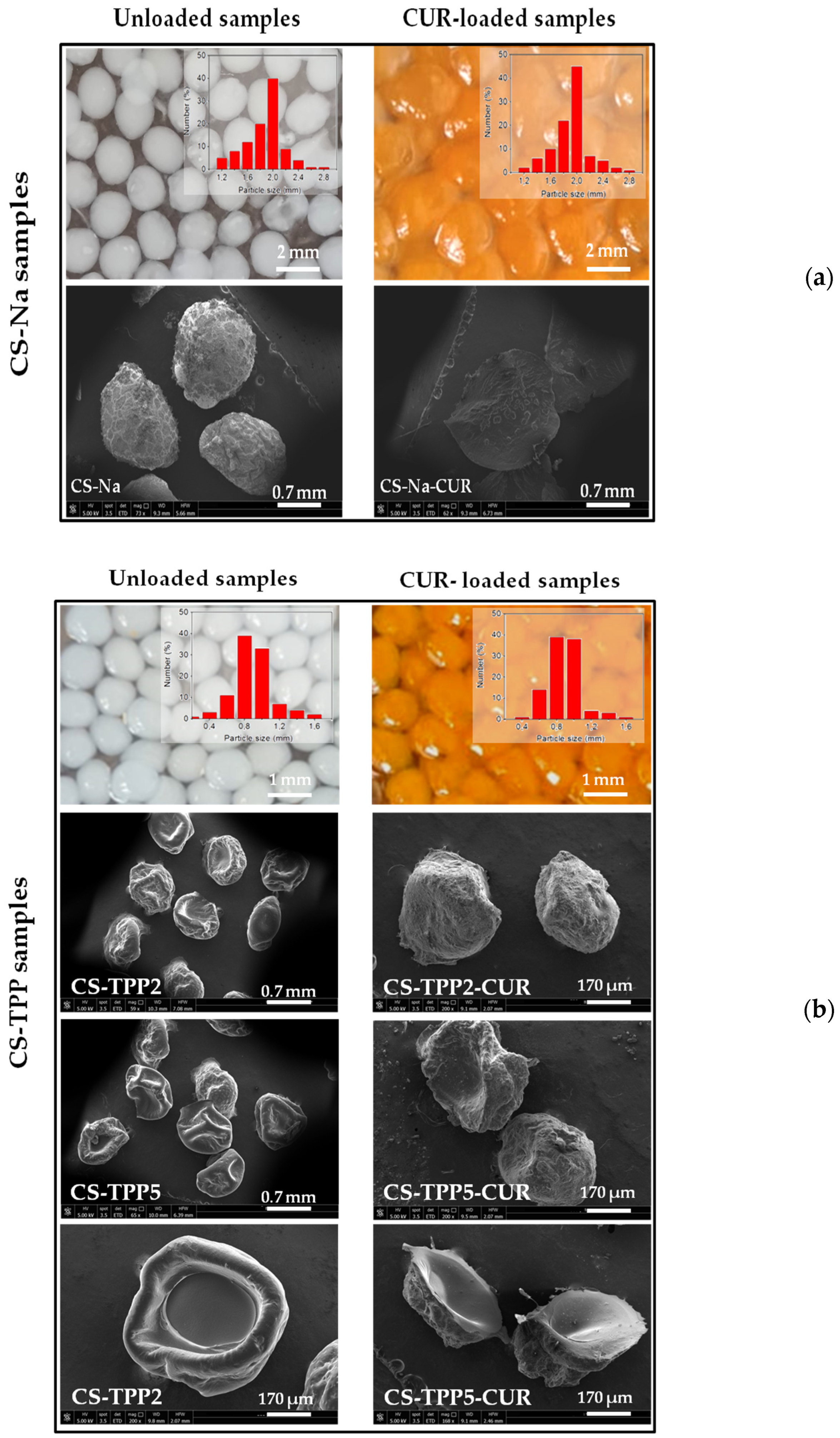
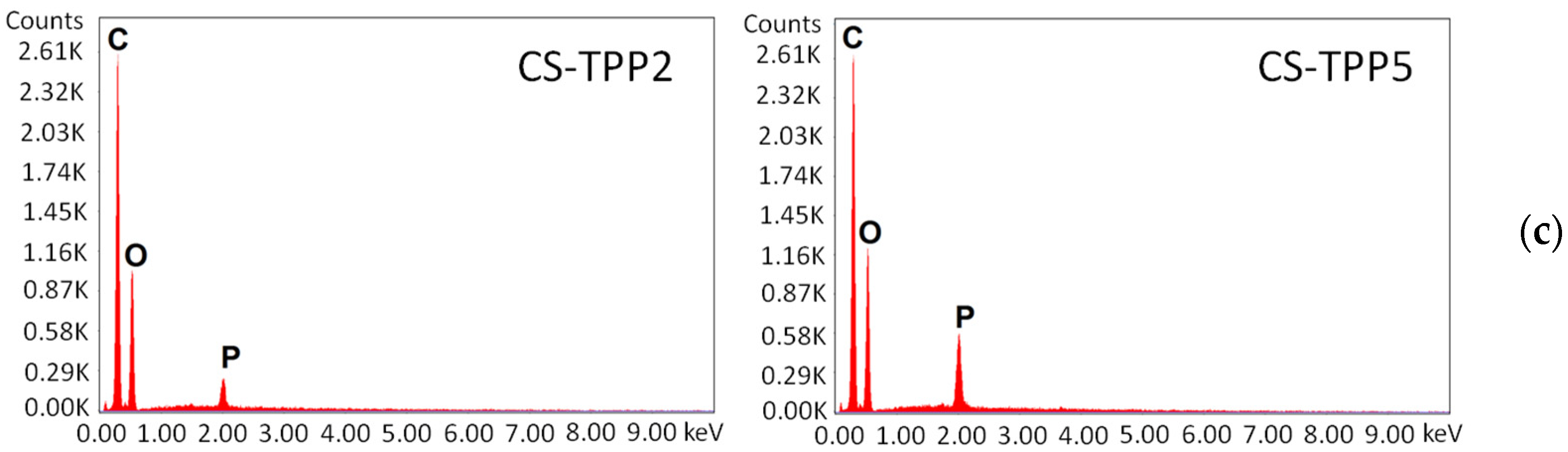
3.1. Morphological Characterization of the Macrobeads
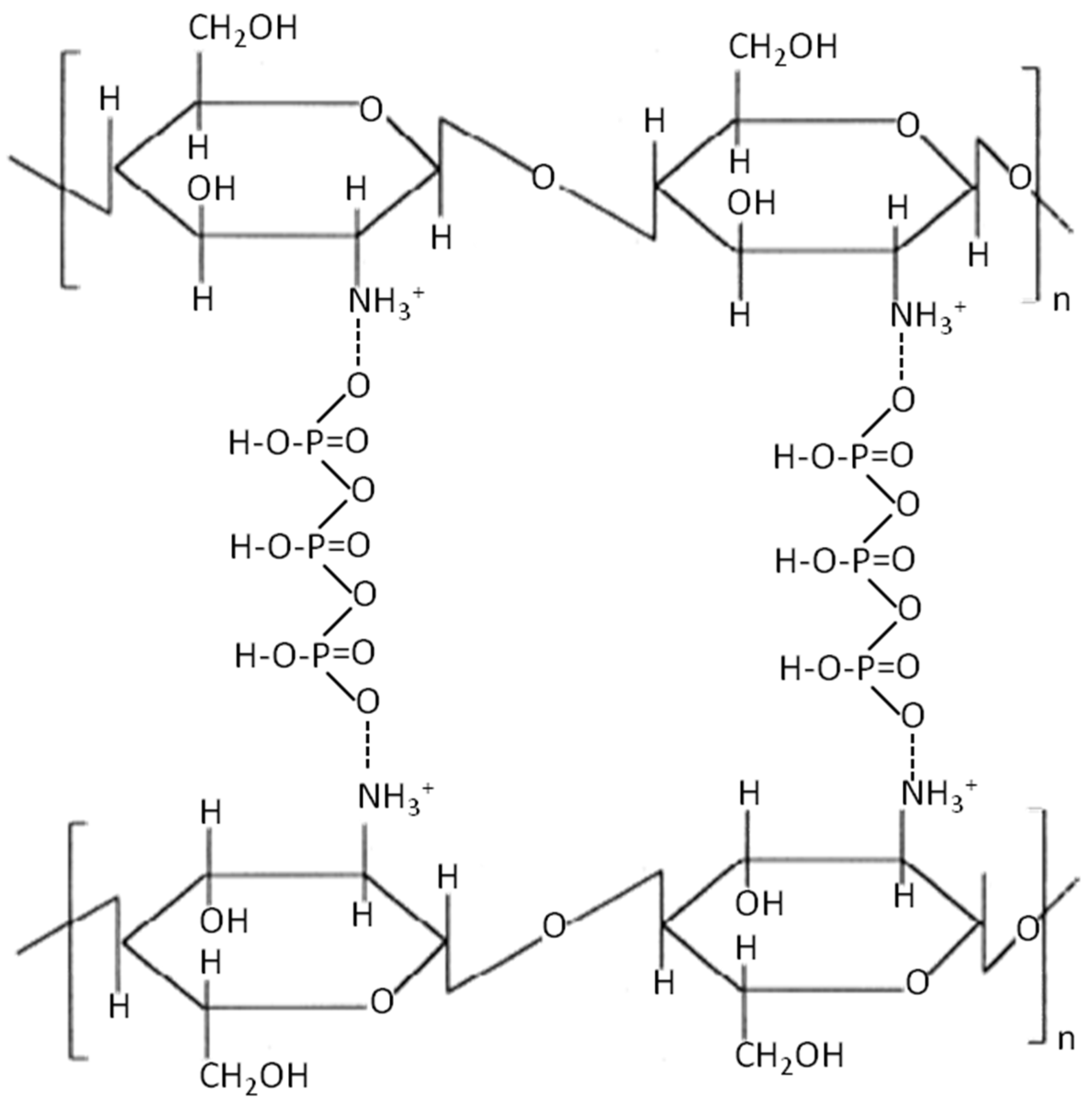
3.2. FTIR Analyses
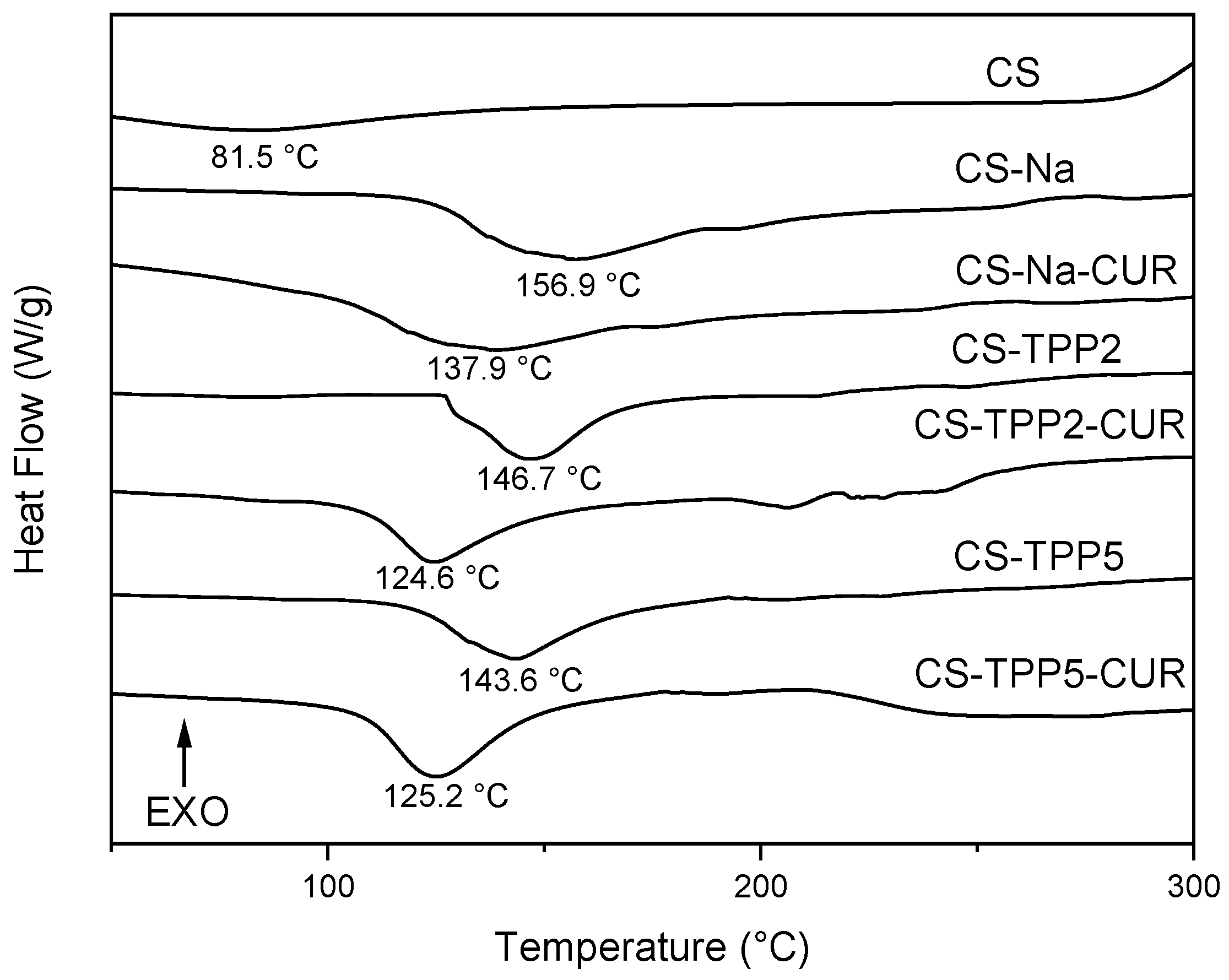
3.3. TGA and DSC Analyses
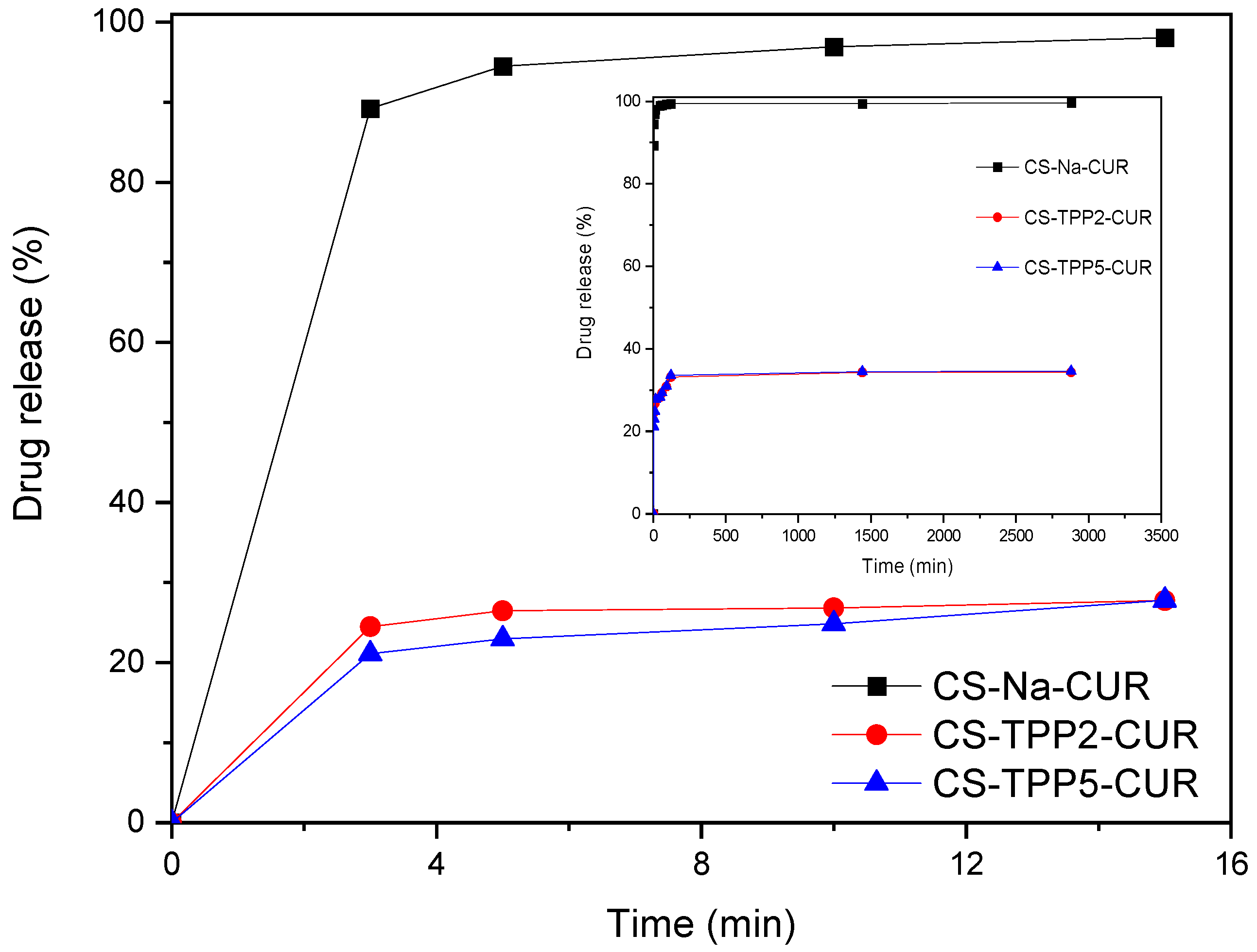
3.4. Evaluation of Encapsulation and Swelling Degree of CUR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, I.; Kertmen, A. Methods of Chitosan Identification: History and Trends. Lett. Appl. NanoBioSci. 2023, 12, 94. [Google Scholar]
- Madian, N.G.; El-Ashmanty, B.A.; Abdel-Rahim, H.K. Improvement of Chitosan Films Properties by Blending with Cellulose, Honey and Curcumin. Polymers 2023, 15, 2587. [Google Scholar] [CrossRef] [PubMed]
- Dhanavel, S.; Nivethaa, E.A.K.; Narayanan, V.; Stephen, A. In vitro cytotoxicity study of dual drug loaded chitosan/palladium nanocomposite towards HT-29 cancer cells. Mater. Sci. Eng. C 2017, 75, 1399–1410. [Google Scholar] [CrossRef]
- Lal, J.; Gupta, S.K.; Agarwal, D.D. Chitosan: An efficient biodegradable and recyclable green catalyst for one-pot synthesis of 3,4-dihydropyrimidinones of curcumin in aqueous media. Catal. Commun. 2012, 27, 38–43. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J.; Maroniche, G.A.; Borrajo, M.P.; Pereyra, M.A.; Creus, C.M. A novel, green, low-cost chitosan-starch hydrogel as potential delivery system for plant growth-promoting bacteria. Carbohydr. Polym. 2018, 202, 409–417. [Google Scholar] [CrossRef]
- Dhanavel, S.; Praveena, P.; Narayanan, V.; Stephen, A. Chitosan/reduced graphene oxide/Pd nanocomposites for co-delivery of 5-fluorouracil and curcumin towards HT-29 colon cancer cells. Polym. Bull. 2020, 77, 5681–5696. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Qian, L.-H.; Zhang, Y.-H.; Gong, P.-X.; Liu, W.; Li, H.-J. Fabrication of foxtail millet prolamin/caseinate/chitosan hydrochloride composite nanoparticles using antisolvent and pH-driven methods for curcumin delivery. Food Chem. 2023, 404, 134604. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Li, M.; Wu, H.; Zhen, T.; Xiong, L.; Sun, Q. pH-Sensitive Chitosan-Sodium Phytate Core-Shell Hollow Beads and Nanocapsules for the Encapsulation of Active Ingredients. J. Agric. Food Chem. 2019, 67, 2894–2905. [Google Scholar] [CrossRef]
- Soliman, G.M.; Zhang, Y.L.; Merle, G.; Cerruti, M.; Barralet, J. Hydrocaffeic acid-chitosan nanoparticles with enhanced stability, mucoadhesion and permeation properties. Eur. J. Pharm. Biopharm. 2014, 88, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y.; Dong, L.; Chen, P.; Liu, W.; Yang, L. Cartilage-targeting and inflammatory-responsive nanocarriers for effective osteoarthritis treatment via reactive oxygen species scavenging and anti-angiogenesis. J. Mater. Sci. Technol. 2023, 143, 30–42. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T.; Gopinath, S.C.B. Laboratory to industrial scale synthesis of chitosan-based nanomaterials: A review. Process Biochem. 2023, 130, 147–155. [Google Scholar] [CrossRef]
- Jagtap, S.; Thakre, D.; Wanjari, S.; Kamble, S.; Labhsetwar, N.; Rayalu, S. New modified chitosan-based adsorbent for defluoridation of water. J. Colloid Interface Sci. 2009, 332, 280–290. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, P.; Zeng, H.; Rui, Z. Construction of porous chitosan macrospheres via dual pore-forming strategy as host for alkaline protease immobilization with high activity and stability. Carbohydr. Polym. 2023, 305, 120476. [Google Scholar] [CrossRef]
- Fajardo, H.V.; Martins, A.O.; de Almeida, R.M.; Noda, L.K.; Probst, L.F.D.; Carreño, N.L.V.; Valentini, A. Synthesis of mesoporous Al2O3 macrospheres using the biopolymer chitosan as a template: A novel active catalyst system for CO2 reforming of methane. Mater. Lett. 2005, 59, 3963–3967. [Google Scholar] [CrossRef]
- Muresan, E.I.; Drobota, M.; Bargan, A.; Dumitriu, C.A.M. Hard porous chromium containing macrospheres as new catalysts for the esterification reaction of acetic acid with epichlorohydrin. Cent. Eur. J. Chem. 2014, 12, 528–536. [Google Scholar] [CrossRef]
- Luan, Q.; Zhang, H.; Wang, J.; Li, Y.; Gan, M.; Deng, Q.; Cai, L.; Tang, H.; Huang, F. Electrostatically reinforced and sealed nanocellulose-based macrosphere by alginate/chitosan multi-layer coatings for delivery of probiotics. Food Hydrocoll. 2023, 142, 108804. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Ma, M.; Sheng, L.; Huang, X. Effect of eggshell membrane as porogen on the physicochemical structure and protease immobilization of chitosan-based macroparticles. Carbohydr. Polym. 2020, 242, 116387. [Google Scholar] [CrossRef]
- Behbahani, E.; Ghaedi, M.; Abbaspour, M.; Rostamizadeh, K.; Dashtian, K. Curcumin loaded nanostructured lipid carriers: In vitro digestion and release studies. Polyhedron 2019, 164, 113–122. [Google Scholar] [CrossRef]
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar] [CrossRef] [PubMed]
- Fitriani, L.; Azizah, H.; Hasanah, U.; Zaini, E. Enhancement of curcumin solubility and dissolution by adsorption in mesoporous SBA-15. Int. J. Appl. Pharm. 2023, 15, 61–67. [Google Scholar] [CrossRef]
- Krishnan, V.; Venkatasubbu, G.D.; Kalaivani, T. Investigation of hemolysis and antibacterial analysis of curcumin-loaded mesoporous SiO2 nanoparticles. Appl. Nanosci. 2023, 13, 811–818. [Google Scholar] [CrossRef]
- Liang, F.; Wang, M.; Hu, Y.; Guo, Z.; Yang, W. Cetyltrimethylammonium bromide promoted dispersing and incorporation of curcumin into silica particles in alkaline ethanol/water mixture. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126789. [Google Scholar] [CrossRef]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W. High storage capacity of curcumin loaded onto hollow mesoporous silica nanoparticles prepared via improved hard-templating method optimized by Taguchi DoE. Eng. Sci. Technol. Int. J. 2022, 33, 101070. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Chitosan-based Pickering emulsion: A comprehensive review on their stabilizers, bioavailability, applications and regulations. Carbohydr. Polym. 2023, 304, 120491. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Elekhtiar, R.; El-Hefnawy, M.; Mahrous, H.; Alhayyani, S.; Al-Goul, S.; Orif, M.; Tayel, A. Fabrication and assessment of potent anticancer nanoconjugates from chitosan nanoparticles, curcumin, and eugenol. Front. Bioeng. Biotechnol. 2022, 10, 1030936. [Google Scholar] [CrossRef]
- Asif, H.M.; Zafar, F.; Ahmad, K.; Iqbal, A.; Shaheen, G.; Ansari, K.A.; Rana, S.; Zahid, R.; Ghaffar, S. Synthesis, characterization and evaluation of anti-arthritic and anti-inflammatory potential of curcumin loaded chitosan nanoparticles. Sci. Rep. 2023, 13, 10274. [Google Scholar] [CrossRef]
- Duse, L.; Baghdan, E.; Pinnapireddy, S.R.; Engelhardt, K.H.; Jedelská, J.; Schaefer, J.; Quendt, P.; Bakowsky, U. Preparation and Characterization of Curcumin Loaded Chitosan Nanoparticles for Photodynamic Therapy. Phys. Status Solidi (A) 2018, 215, 1700709. [Google Scholar] [CrossRef]
- Martins, A.F.; de Oliveira, D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Chitosan/TPP microparticles obtained by microemulsion method applied in controlled release of heparin. Int. J. Biol. Macromol. 2012, 51, 1127–1133. [Google Scholar] [CrossRef]
- Ishak, N.A.; Hamidon, T.S.; Zi-Hui, T.; Hussin, M.H. Extracts of curcumin-incorporated hybrid sol–gel coatings for the corrosion mitigation of mild steel in 0.5 M HCl. J. Coat. Technol. Res. 2020, 17, 1515–1535. [Google Scholar] [CrossRef]
- Biró, E.; Németh, A.S.; Sisak, C.; Feczkó, T.; Gyenis, J. Preparation of chitosan particles suitable for enzyme immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X.-Y. Molecular interactions, characterization and antimicrobial activity of curcumin–chitosan blend films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. AAPS PharmSciTech 2006, 7, 50. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Inamdar, M. Aqueous Behaviour of Chitosan. Int. J. Polym. Sci. 2010, 2010, 939536. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Development of chitosan–tripolyphosphate fibers through pH dependent ionotropic gelation. Carbohydr. Res. 2011, 346, 2582–2588. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur. J. Pharm. Sci. 2008, 35, 404–416. [Google Scholar] [CrossRef]
- Parize, A.; Stulzer, H.; Laranjeira, M.; Brighente, I.I.; Souza, T. Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray drying. Química Nova 2012, 35, 1127–1132. [Google Scholar] [CrossRef]
- Desai, K.G.; Park, H. Preparation and characterization of drug-loaded chitosan-tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Desai, K.G.; Park, H.J. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J. Microencapsul. 2005, 22, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Stevens, W.F.; Remuñán-López, C. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 2006, 312, 166–173. [Google Scholar] [CrossRef]
- Liu, C.; Desai, K.G.; Tang, X.; Chen, X. Drug Release Kinetics of Spray-Dried Chitosan Microspheres. Dry. Technol. 2006, 24, 769–776. [Google Scholar] [CrossRef]
- RemunanLopez, C.; Bodmeier, R. Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J. Control. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Katas, H.; Hussain, Z.; Ling, T.C. Chitosan Nanoparticles as a Percutaneous Drug Delivery System for Hydrocortisone. J. Nanomater. 2012, 2012, 372725. [Google Scholar] [CrossRef]
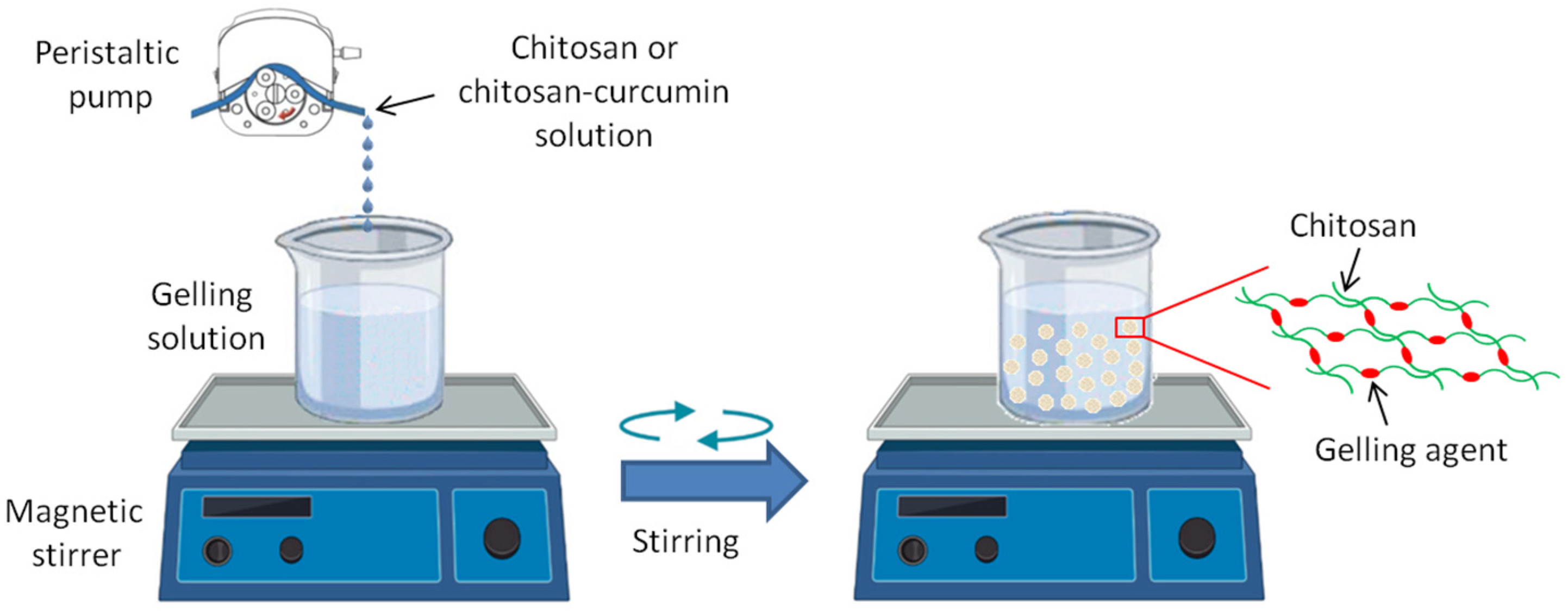


| Sample Code | CS (g) | Gelling Agent | CUR (g) |
|---|---|---|---|
| CS-Na | 0.5 | NaOH 4 wt% | - |
| CS-Na-CUR | 0.5 | NaOH 4 wt% | 0.1 |
| CS-TPP2 | 0.5 | TPP 2 wt% | - |
| CS-TPP2-CUR | 0.5 | TPP 2 wt% | 0.1 |
| CS-TPP5 | 0.5 | TPP 5 wt% | - |
| CS-TPP5-CUR | 0.5 | TPP 5 wt% | 0.1 |
| Sample Code | Encapsulation Efficiency (wt%) | Swelling Degree (wt%) |
|---|---|---|
| CS-Na-CUR | 99.8 | n.d. |
| CS-TPP2-CUR | 95.9 | 120 |
| CS-TPP5-CUR | 91.4 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistone, A.; de Gaetano, A.; Piperopoulos, E.; Abate, C. Effect of Sodium Hydroxide and Tripolyphosphate on Curcumin Release from Chitosan-Based Macroparticles. Materials 2023, 16, 5850. https://doi.org/10.3390/ma16175850
Pistone A, de Gaetano A, Piperopoulos E, Abate C. Effect of Sodium Hydroxide and Tripolyphosphate on Curcumin Release from Chitosan-Based Macroparticles. Materials. 2023; 16(17):5850. https://doi.org/10.3390/ma16175850
Chicago/Turabian StylePistone, Alessandro, Annamaria de Gaetano, Elpida Piperopoulos, and Chiara Abate. 2023. "Effect of Sodium Hydroxide and Tripolyphosphate on Curcumin Release from Chitosan-Based Macroparticles" Materials 16, no. 17: 5850. https://doi.org/10.3390/ma16175850
APA StylePistone, A., de Gaetano, A., Piperopoulos, E., & Abate, C. (2023). Effect of Sodium Hydroxide and Tripolyphosphate on Curcumin Release from Chitosan-Based Macroparticles. Materials, 16(17), 5850. https://doi.org/10.3390/ma16175850