Dye-Modified, Sonochemically Obtained Nano-SnS2 as an Efficient Photocatalyst for Metanil Yellow Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sonochemical Syntheses
2.3. Evaluation of Sonochemical Efficiency and Percentage Yield of the Synthesis Process
2.4. Characterization of Products
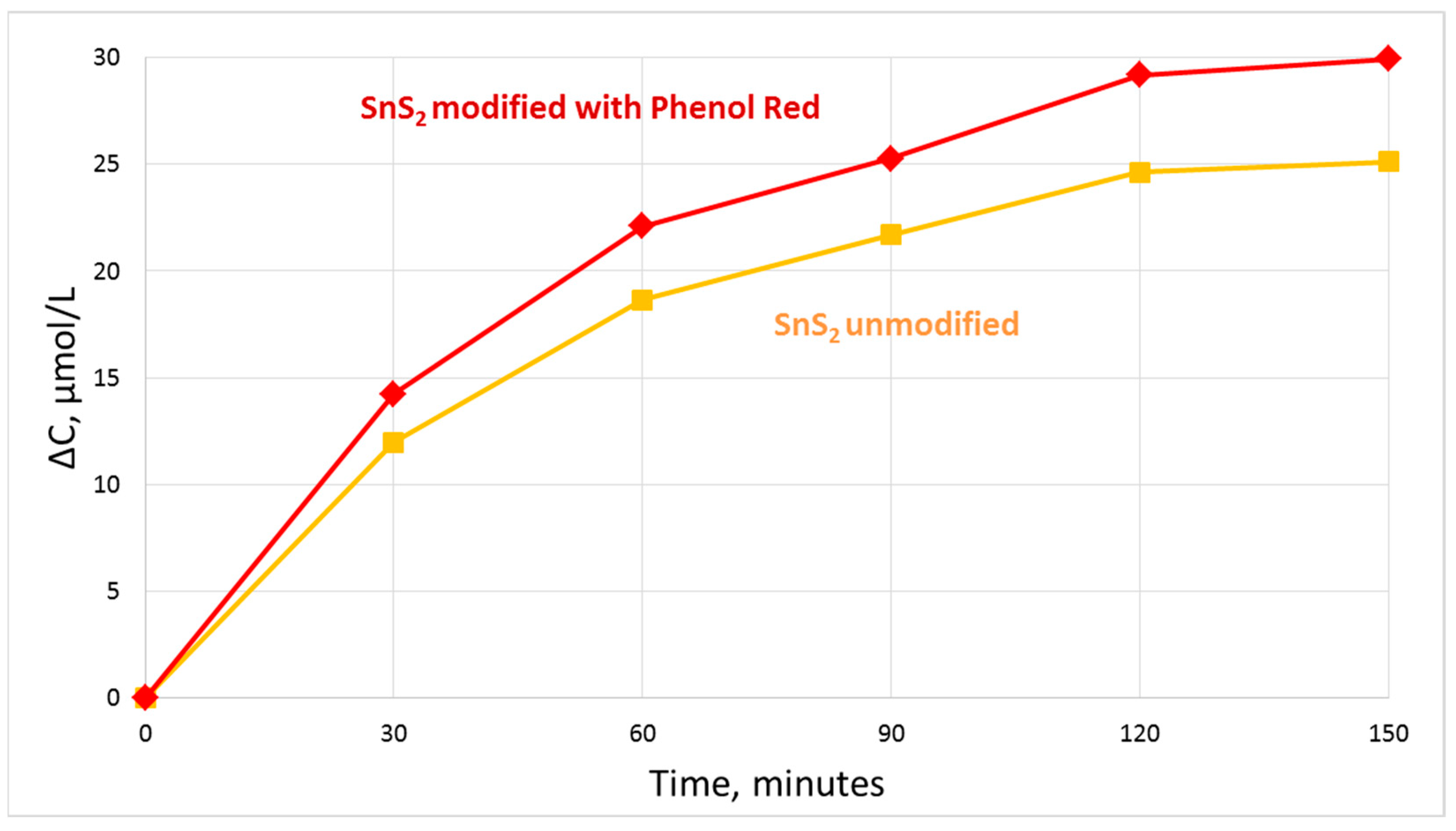
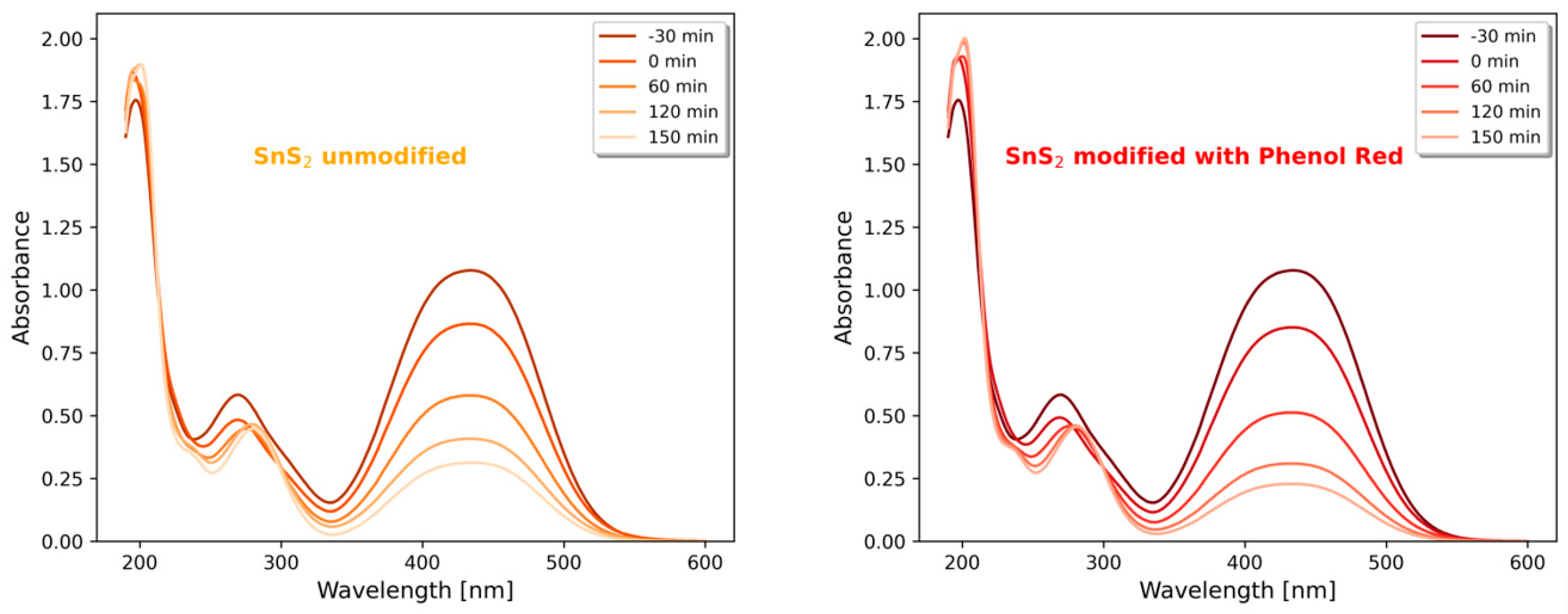
2.5. Photocatalytic Degradation of Metanil Yellow
2.6. N2 Physisorption Measurements
3. Results and Discussion
- –
- the photocatalyst absorbs the photon and is excited, then generates a pair of positive hole and negative electron:SnS2 + hν → SnS2 + e− + h+
- –
- the hole reacts with a molecule of water generating hydroxyl radicals:h+ + H2O → H+ + ·OH
- –
- the hydroxyl radicals attack the dye molecules and degrade (oxidize) them in many consecutive steps:Dye molecule + x ·OH →→→ y CO2 + z H2O
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oftedal, I. Die Kristallstruktur von SnS2. Nor. Geol. Tidsskr. IX 1926, 9, 225–233. [Google Scholar]
- Gao, Y.; Bai, L.; Zhang, X.; Yang, F. Non-Parallel Photo-Assisted Electrocatalysis Mechanism of SnS2/NiO Heterojunction for Efficient Electrocatalytic Oxygen Evolution Reaction. ChemElectroChem 2021, 8, 2087–2093. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Liang, T. Research Progress of SnS2/rGO Material in Gas Sensor. E3S Web Conf. 2021, 267, 02045. [Google Scholar] [CrossRef]
- Liang, A.; Ming, J.; Zhu, W.; Guan, H.; Han, X.; Zhang, S.; Lin, Y.; Dong, J.; Huang, Y.; Qiu, W.; et al. Tin Disulfide-Coated Microfiber for Humidity Sensing with Fast Response and High Sensitivity. Crystals 2021, 11, 648. [Google Scholar] [CrossRef]
- Liu, X.; Najam, T.; Yasin, G.; Kumar, M.; Wang, M. One-Pot Synthesis of High-Performance Tin Chalcogenides/C Anodes for Li-Ion Batteries. ACS Omega 2021, 6, 17391–17399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wan, F.; Ping, H.; Wang, H.; Wang, W.; Fu, Z. Biotemplating synthesis of rod-shaped tin sulfides assembled by interconnected nanosheets for energy storage. J. Power Sources 2021, 506, 230180. [Google Scholar] [CrossRef]
- Qiao, L.; Yu, C.; Sun, R.; Tao, Y.; Li, Y.; Yan, Y. Three-dimensional magnetic stannic disulfide composites for the solid-phase extraction of sulfonamide antibiotics. J. Chromatogr. A 2021, 1652, 462372. [Google Scholar] [CrossRef]
- Gao, F.; Chen, H.; Feng, W.; Hu, Y.; Shang, H.; Xu, B.; Zhang, J.; Xu, C.-Y.; Hu, P. High-Performance van der Waals Metal-Insulator-Semiconductor Photodetector Optimized with Valence Band Matching. Adv. Funct. Mater. 2021, 31, 2104359. [Google Scholar] [CrossRef]
- Arunkumar, M.; Veerakumar, S.; Mohanavel, V.; Vairamuthu, J.; Vijayan, V.; Senthilkumar, N. A Novel Visible Light-Driven p-Type BiFeO3/n-Type SnS2 Heterojunction Photocatalyst for Efficient Charge Separation and Enhanced Photocatalytic Activity. J. Clust. Sci. 2021, 32, 1431–1439. [Google Scholar] [CrossRef]
- Kumar, M.; Rani, S.; Singh, Y.; Gour, K.S.; Singh, V.N. Tin-selenide as a futuristic material: Properties and applications. RSC Adv. 2021, 11, 6477–6503. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Ji, Y.; Zhang, H.; Shen, X. Two-dimensional SnS nanosheets fabricated by a novel hydrothermal method. J. Mater. Sci. 2005, 40, 591–595. [Google Scholar] [CrossRef]
- An, C.; Tang, K.; Shen, G.; Wang, C.; Yang, Q.; Hai, B.; Qian, Y. Growth of belt-like SnS crystals from ethylenediamine solution. J. Cryst. Growth 2002, 244, 333–338. [Google Scholar] [CrossRef]
- Hickey, S.G.; Waurisch, C.; Rellinghaus, B.; Eychmüller, A. Size and Shape Control of Colloidally Synthesized IV-VI Nanoparticulate Tin(II) Sulfide. J. Am. Chem. Soc. 2008, 130, 14978–14980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, D.; Wang, G. Synthesis and characterization of SnS nanowires in cetyltrimethylammoniumbromide (CTAB) aqueous solution. Chem. Phys. Lett. 2003, 379, 67–73. [Google Scholar] [CrossRef]
- Shen, G.; Chen, D.; Tang, K.; Huang, L.; Qian, Y.; Zhou, G. Novel polyol route to nanoscale tin sulfides flaky crystallines. Inorg. Chem. Commun. 2003, 6, 178–180. [Google Scholar] [CrossRef]
- Gajendiran, J.; Rajendran, V. Synthesis of SnS2 nanoparticles by a surfactant-mediated hydrothermal method and their characterization. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015001. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.C. In air synthesis of SnS2 nanoplates from tin, sulfur and ammonium choride powders. Mater. Chem. Phys. 2008, 112, 742–744. [Google Scholar] [CrossRef]
- Giberti, A.; Gaiardo, A.; Fabbri, B.; Gherardi, S.; Guidi, V.; Malagu, C.; Bellutti, P.; Zonta, G.; Casotti, D.; Cruciani, G. Tin(IV) sulfide nanorods as a new gas sensing material. Sens. Actuators B Chem. 2016, 223, 827–833. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Metters, J.P.; Banks, C.E.; Pollet, B.G. Sonoelectrochemical Synthesis of Nanomaterials. In Cavitation: A Novel Energy-Efficient Technique for the Generation of Nanomaterials; Manickam, S., Ashokkumar, M., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 173–201. [Google Scholar]
- Islam, M.H.; Paul, M.T.Y.; Burheim, O.S.; Pollet, B.G. Recent developments in the sonoelectrochemical synthesis of nanomaterials. Ultrason. Sonochem. 2019, 59, 104711. [Google Scholar] [CrossRef]
- Khimani, A.J.; Chaki, S.H.; Chauhan, S.M.; Mangrola, A.V.; Meena, R.R.; Deshpande, M.P. Synthesis, characterization, antimicrobial and antioxidant study of the facile sonochemically synthesized SnS2 nanoparticles. Nano-Struct. Nano-Objects 2019, 18, 100286. [Google Scholar] [CrossRef]
- Matyszczak, G.; Fidler, A.; Polesiak, E.; Sobieska, M.; Morawiec, K.; Zajkowska, W.; Lawniczak-Jablonska, K.; Kuzmiuk, P. Application of sonochemically synthesized SnS and SnS2 in the electro-Fenton proces: Kinetics and enhanced decolorization. Ultrason. Sonochem. 2020, 68, 105186. [Google Scholar] [CrossRef] [PubMed]
- Matyszczak, G.; Jóźwik, P.; Polesiak, E.; Sobieska, M.; Krawczyk, K.; Jastrzębski, C.; Płociński, T. Sonochemical preparation of SnS and SnS2 nano- and micropowders and their characterization. Ultrason. Sonochem. 2021, 75, 105594. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef] [PubMed]
- Elashery, S.E.A.; Ibrahim, I.; Gomaa, H.; El-Bouraie, M.M.; Moneam, I.A. Comparative Study of the Photocatalytic Degradation of Crystal Violet Using Ferromagnetic Magnesium Oxide Nanoparticles and MgO-Bentonite Nanocomposite. Magnetochemistry 2023, 9, 56. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver decorated TiO2/g-C3N4 bifunctional nanocomposites for photocatalytic elimination of water pollutants under UV and artificial solar light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Ibrahim, I.; Kaltzoglou, A.; Athanasekou, C.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Ioannidis, N.; Perraki, M.; Tsakiridis, P.; Sygellou, L.; et al. Magnetically separable TiO2/CoFe2O4/Ag nanocomposited for the photocatalytic reduction of hexavalent chromium pollutant under UV and artificial solar light. Chem. Eng. J. 2020, 381, 122730. [Google Scholar] [CrossRef]
- Fu, C.-F.; Wu, X.; Yang, J. Material Design for Photocatalytic Water Splitting from a Theoretical Perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef]
- Hagiwara, H.; Nagatomo, M.; Seto, C.; Ida, S.; Ishiahara, T. Dye Modification Effects on TaON for Photocatalytic Hydrogen Production from Water. Catalysts 2013, 3, 614–624. [Google Scholar] [CrossRef]
- Hagiwara, H.; Higashi, K.; Watanabe, M.; Kakigi, R.; Ida, S.; Ishihara, T. Effect of Porphyrin Molecular Structure on Water Splitting Activity of a KTaO3 Photocatalyst. Catalysts 2016, 6, 42. [Google Scholar] [CrossRef]
- Hagiwara, H.; Nagatomo, M.; Seto, C.; Ida, S.; Ishihara, T. Dye-modification effects on water splitting activity of GaN:ZnO photocatalyst. J. Photochem. Photobiol. A Chem. 2013, 272, 41–48. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S.; Rao, N.N. Visible light assisted photodegradation of halocarbons on the dye modified TiO2 surface using visible light. Sol. Energy Mater. Sol. Cells 2006, 90, 1013–1020. [Google Scholar] [CrossRef]
- Chatterjee, D. Effect of excited state redox properties of dye sensitizers on hydrogen production through photo-splitting of water over TiO2 photocatalyst. Catal. Commun. 2010, 11, 336–339. [Google Scholar] [CrossRef]
- Hagiwara, H.; Nagatomo, M.; Ida, S.; Ishihara, T. Photocatalytic splitting of water into hydrogen and oxygen on organic dye modified KTa(Zr)O3 catalyst. Energy Procedia 2012, 22, 53–60. [Google Scholar] [CrossRef]
- Yamamoto, A.; Teramura, K.; Hosokawa, S.; Shishido, T.; Tanaka, T. Visible-Light-Assisted Selective Catalytic Reduction of Nitric Oxide with Ammonia over Dye-Modified Titania Photocatalysts. ChemCatChem 2015, 7, 1818–1825. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Wu, D.; Sun, Y. Visible-light responsive dye-modified TiO2 photocatalyst. J. Solid State Chem. 2008, 181, 593–602. [Google Scholar] [CrossRef]
- Yu, Y.-Z.; Zhang, Y.-R.; Geng, C.-H.; Sun, L.; Guo, Y.; Feng, Y.-R.; Wang, Y.-X.; Zhang, X.-M. Precise and Wide-Ranged Band-Gap Tuning of Ti6-Core-Based Titanium Oxo Clusters by the Type and Number of Chromophore Ligands. Inorg. Chem. 2019, 58, 16785–16791. [Google Scholar] [CrossRef]
- Yang, S.; Prendergast, D.; Neaton, J.B. Tuning Semiconductor Band Edge Energies for Solar Photocatalysis via Surface Ligand Passivation. Nano Lett. 2012, 12, 383–388. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.-E. Stabilization and Band-Gap Tuning of the 1T-MoS2 Monolayer by Covalent Functionalization. Chem. Mater. 2015, 27, 3743–3748. [Google Scholar] [CrossRef]
- Zhou, T.; Anderson, R.T.; Li, H.; Bell, J.; Yang, Y.; Gorman, B.P.; Pylypenko, S.; Lusk, M.T.; Sellinger, A. Bandgap Tuning of Silicon Quantum Dots by Surface Functionalization with Conjugated Organic Groups. Nano Lett. 2015, 15, 3657–3663. [Google Scholar] [CrossRef]
- Podsiadło, S.; Białogłowski, M.; Fadaghi, M.; Matyszczak, G.; Kardas, K.; Dłużewski, P.; Data, P.; Łapkowski, M. Synthesis of kesterite nanopowders with bandgap tuning ligands. Cryst. Res. Technol. 2015, 50, 743–746. [Google Scholar] [CrossRef]
- Chowdhury, P.; Viraraghavan, T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes—A review. Sci. Total Environ. 2009, 407, 2474–2492. [Google Scholar] [CrossRef]
- Yanagida, H.; Masubuchi, Y.; Minagawa, K.; Ogata, T.; Takimoto, J.; Koyama, K. A reaction kinetics model of water sonolysis in the presence of a spin-trap. Ultrason. Sonochem. 1999, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Singha, P.S.; Firdaus, S.B.; Ghosh, S. Metanil Yellow: The toxic food colorant. Asian Pac. J. Health Sci. 2017, 4, 65–66. [Google Scholar] [CrossRef]
- Khan, I.S.; Ali, M.N.; Hamid, R.; Ganie, S.A. Genotoxic effect of two commonly used food dyes metanil yellow and carmoisine using Allium cepa L. as indicator. Toxicol. Rep. 2020, 7, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Muliadi, F.N.A.; Halmi, M.I.E.; Wahid, S.B.A.; Gani, S.S.A.; Zaidan, U.H.; Mahmud, K.; Shukor, M.Y.A. Biostimulation of Microbial Communities from Malaysian Agricultural Soil for Detoxification of Metanil Yellow Dye; a Response Surface Methodological Approach. Sustainability 2021, 13, 138. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Schmidt, W.; Qin, J.; Kim, M.; Chan, D. Evaluation of Turmeric Powder Adulterated with Metanil Yellow Using FT-Raman and FT-IR Spectroscopy. Foods 2016, 5, 36. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Alexander, V.N.; James, P.O.; Rodriguez-Reinoso, F.; Rouquerol, J.; Kenneth, S.W.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Burton, L.A.; Whittles, T.J.; Hesp, D.; Linhart, W.M.; Skelton, J.M.; Hou, B.; Webster, R.F.; O’Dowd, G.; Reece, C.; Cherns, D.; et al. Electronic and optical properties of single crystal SnS2: An earth-abundant disulfide photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef]
- El-Rehim, H.A.A.; Hegazy, E.-S.A.; Diaa, D.A. Photo-catalytic degradation of Metanil Yellow dye using TiO2 immobilized into polyvinyl alcohol/acrylic acid microgels prepared by ionizing radiation. React. Funct. Polym. 2012, 72, 823–831. [Google Scholar] [CrossRef]
- Tizaoui, C.; Grima, N.M.; Derdar, M.Z. Effect of the radical scavenger t-butanol on gas-liquid mass transfer. Chem. Eng. Sci. 2009, 64, 4375–4382. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef] [PubMed]
- Falara, P.P.; Ibrahim, I.; Zourou, A.; Sygellou, L.; Sanchez, D.E.; Romanos, G.E.; Givalou, L.; Antoniadou, M.; Arfanis, M.K.; Han, C.; et al. Bi-functional photocatalytic heterostructures combining titania thin films with carbon quantum dots (C-QDs/TiO2) for effective elimination of water pollutants. Environ. Sci. Pollut. Res. 2023, 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]

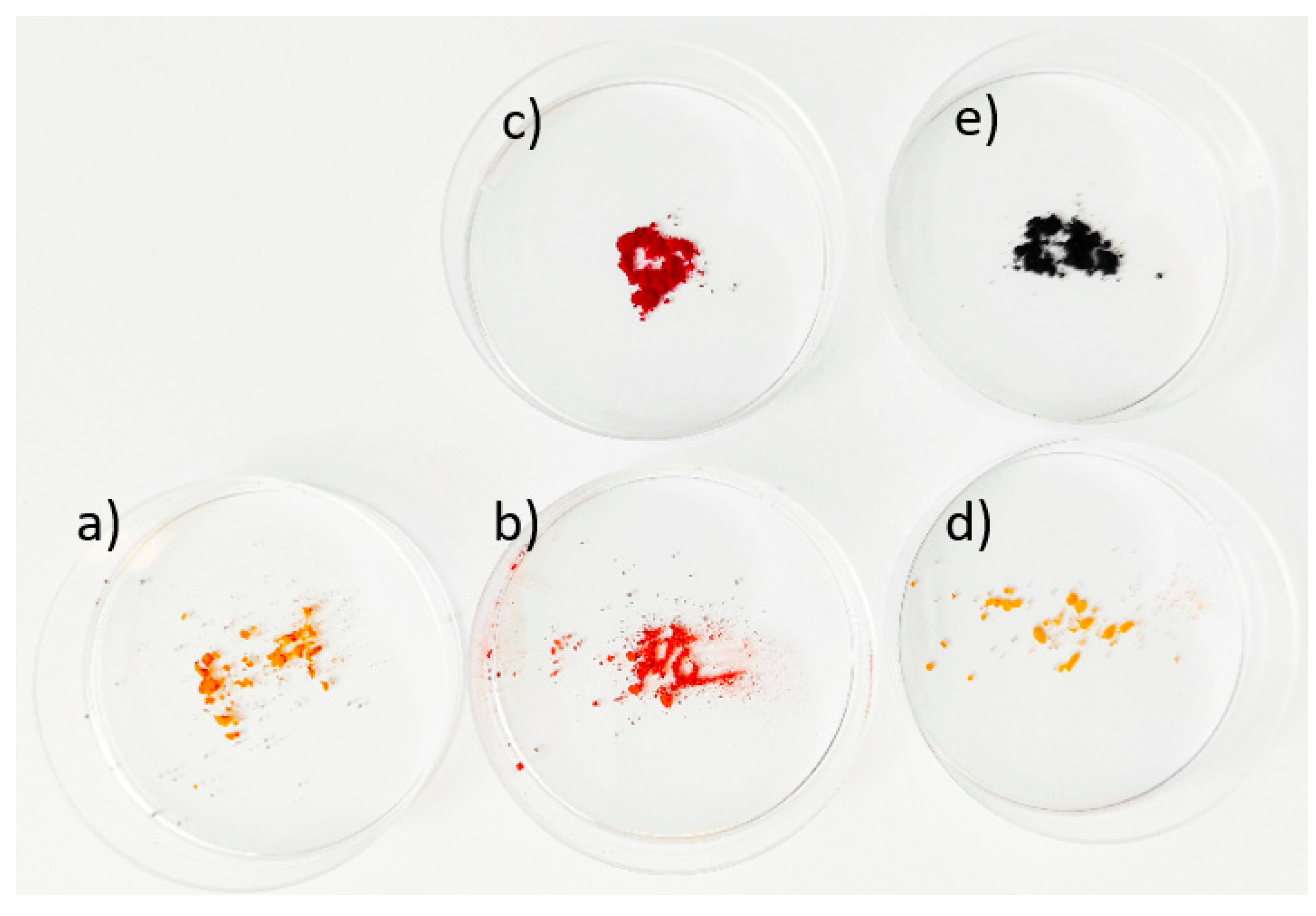
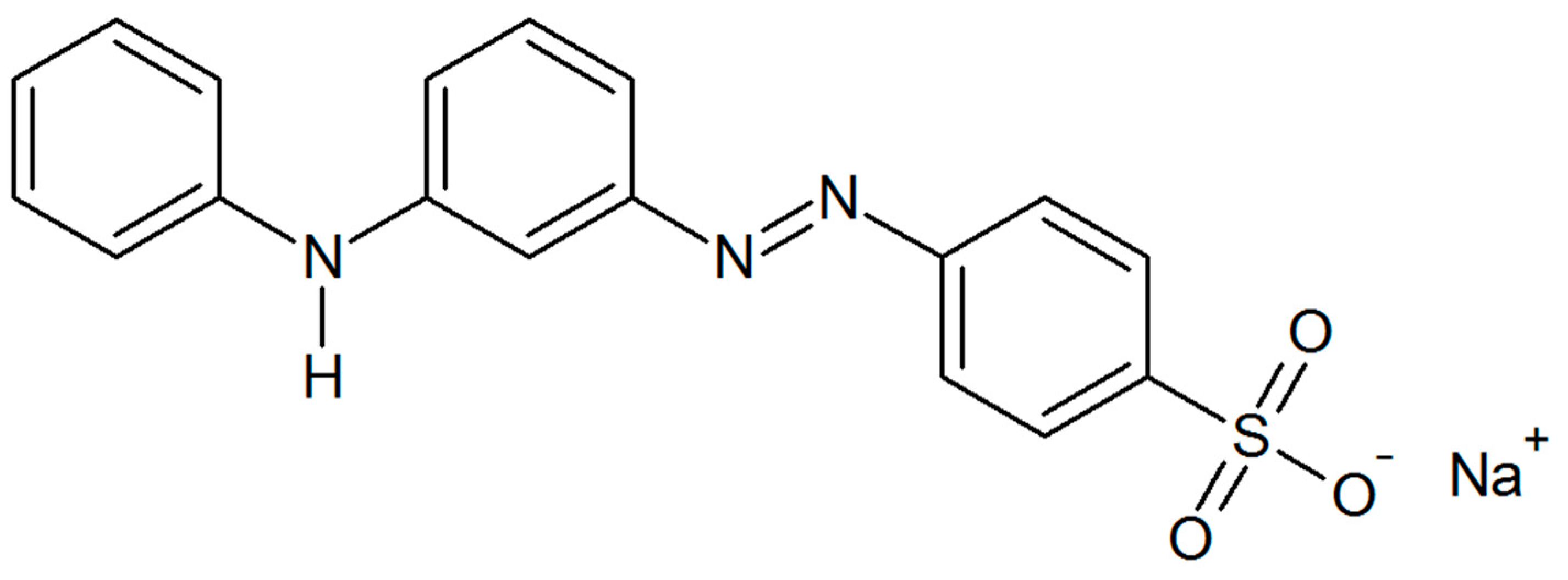




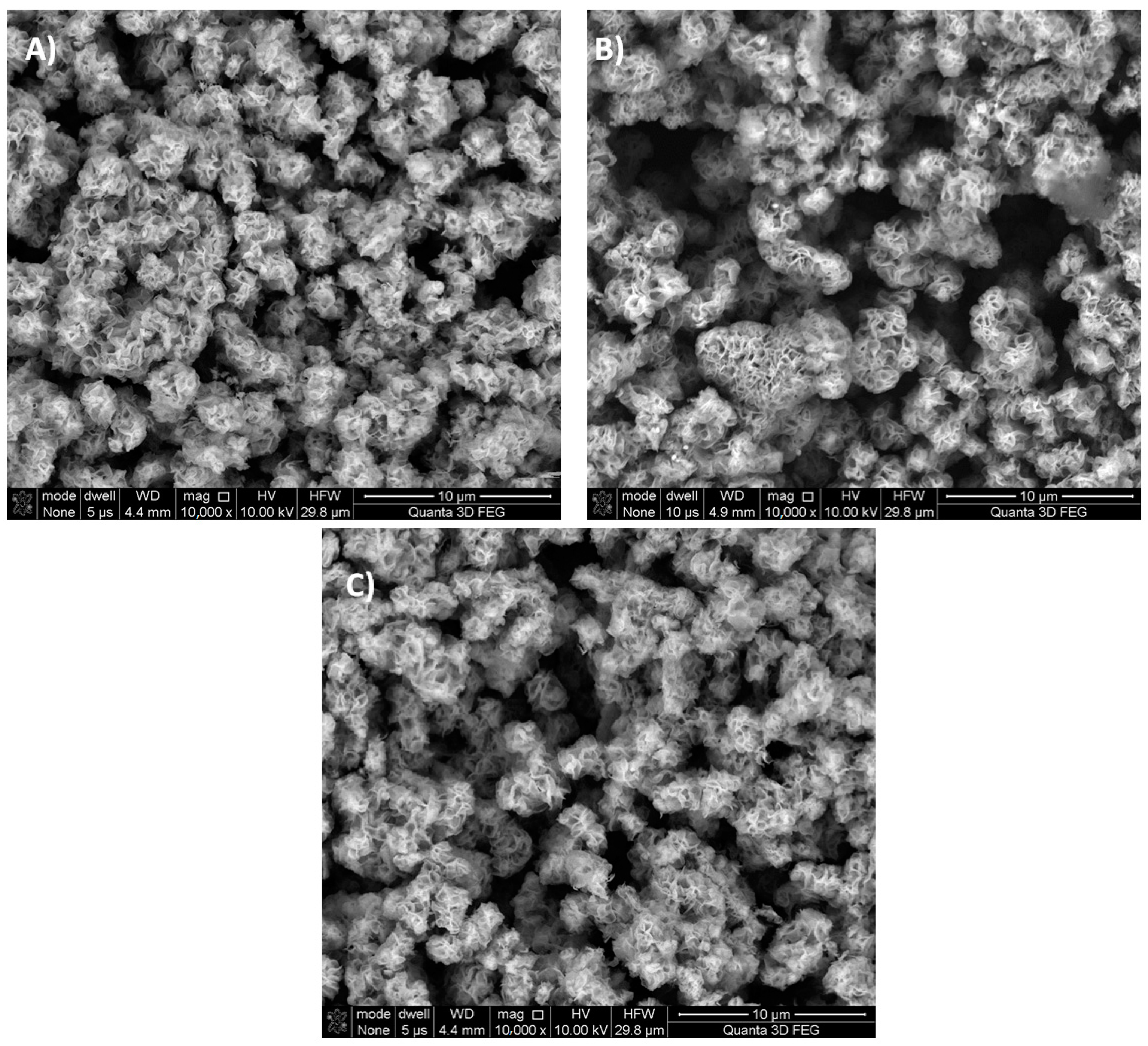


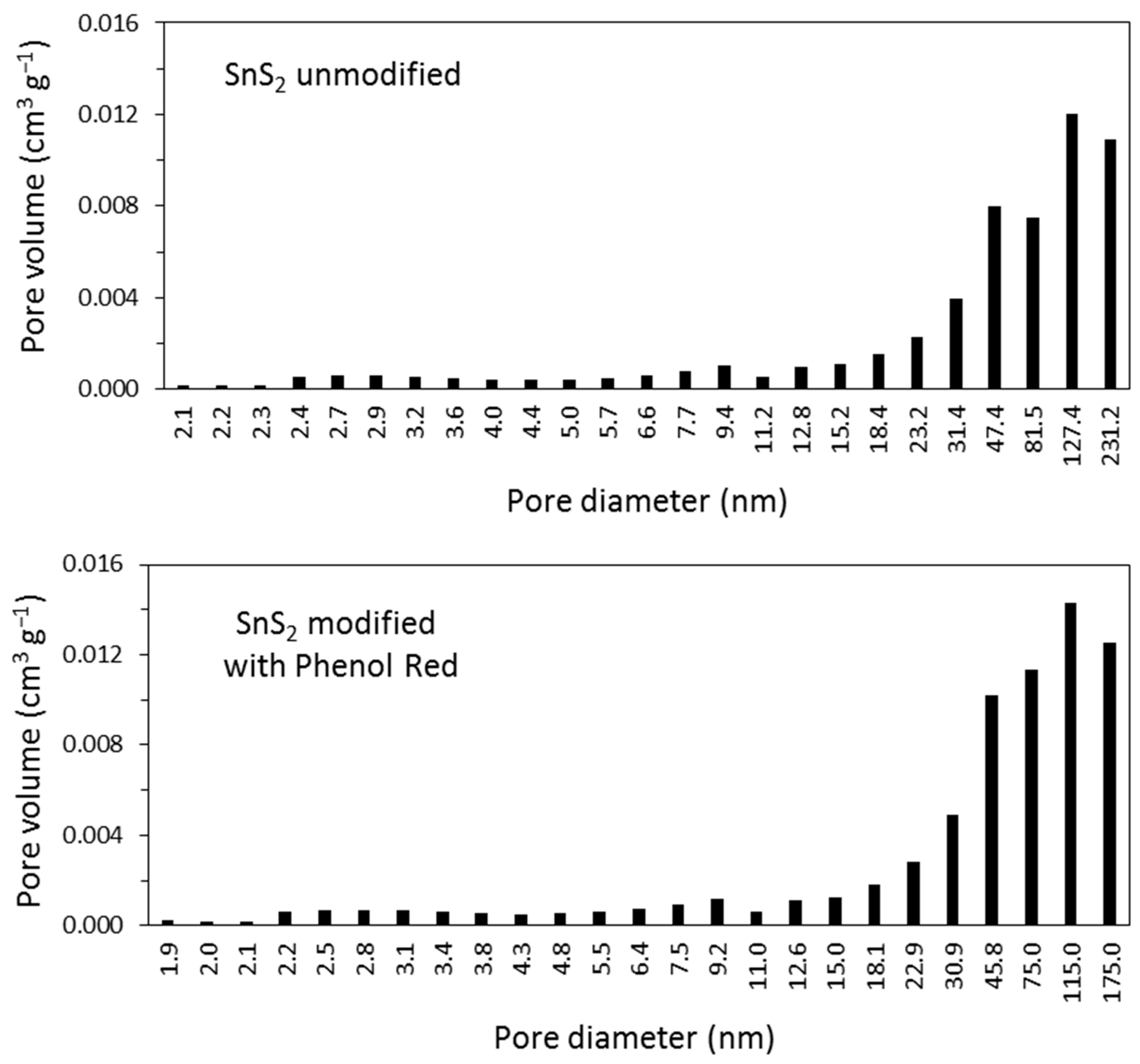
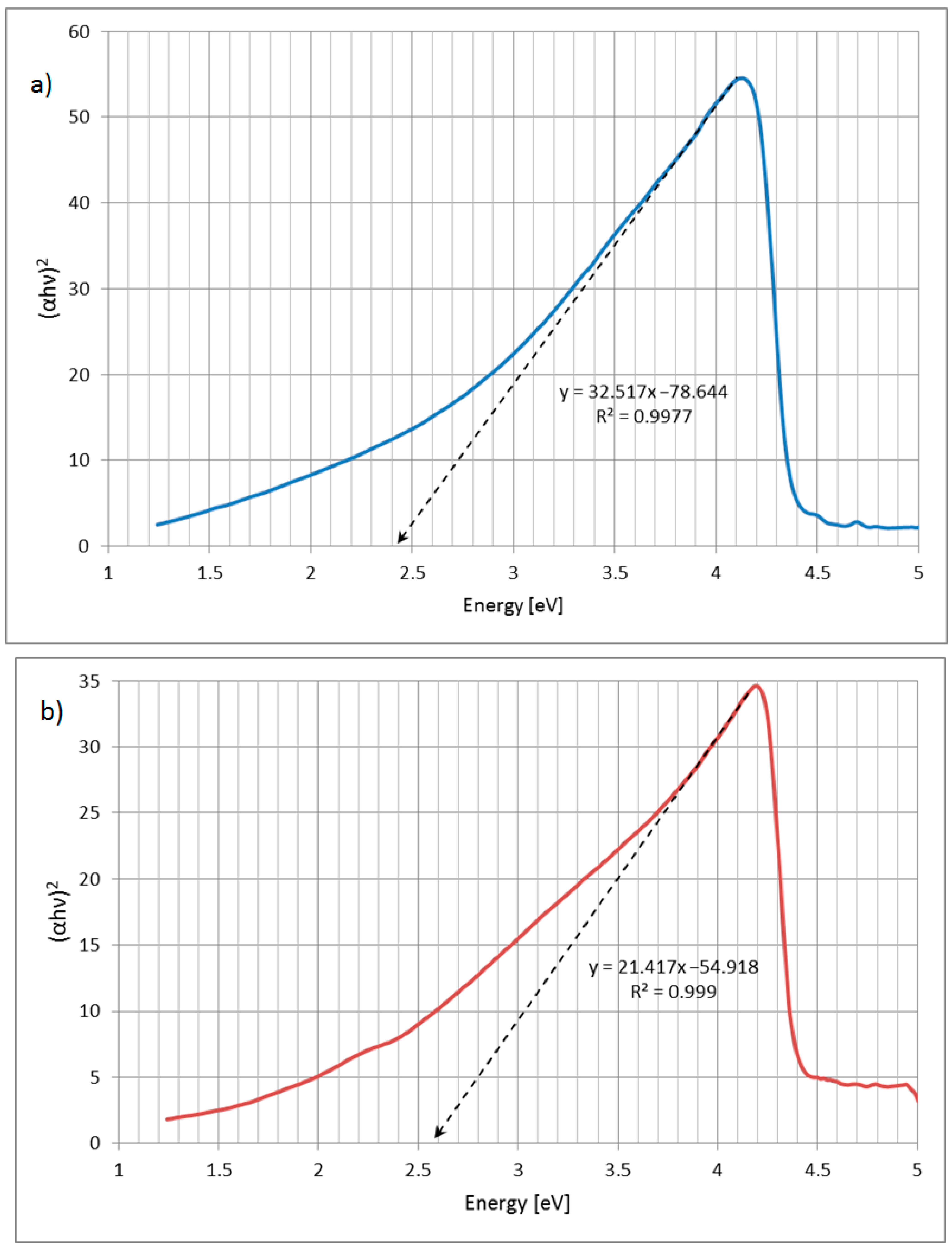
| Sample | Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) |
|---|---|---|
| SnS2 unmodified | 11.4 | 0.056 |
| SnS2 modified with Phenol Red | 13.1 | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matyszczak, G.; Jóźwik, P.; Zybert, M.; Yedzikhanau, A.; Krawczyk, K. Dye-Modified, Sonochemically Obtained Nano-SnS2 as an Efficient Photocatalyst for Metanil Yellow Removal. Materials 2023, 16, 5774. https://doi.org/10.3390/ma16175774
Matyszczak G, Jóźwik P, Zybert M, Yedzikhanau A, Krawczyk K. Dye-Modified, Sonochemically Obtained Nano-SnS2 as an Efficient Photocatalyst for Metanil Yellow Removal. Materials. 2023; 16(17):5774. https://doi.org/10.3390/ma16175774
Chicago/Turabian StyleMatyszczak, Grzegorz, Paweł Jóźwik, Magdalena Zybert, Albert Yedzikhanau, and Krzysztof Krawczyk. 2023. "Dye-Modified, Sonochemically Obtained Nano-SnS2 as an Efficient Photocatalyst for Metanil Yellow Removal" Materials 16, no. 17: 5774. https://doi.org/10.3390/ma16175774
APA StyleMatyszczak, G., Jóźwik, P., Zybert, M., Yedzikhanau, A., & Krawczyk, K. (2023). Dye-Modified, Sonochemically Obtained Nano-SnS2 as an Efficient Photocatalyst for Metanil Yellow Removal. Materials, 16(17), 5774. https://doi.org/10.3390/ma16175774










