Assessment of the Composition Effect of a Bio-Cementation Solution on the Efficiency of Microbially Induced Calcite Precipitation Processes in Loose Sandy Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
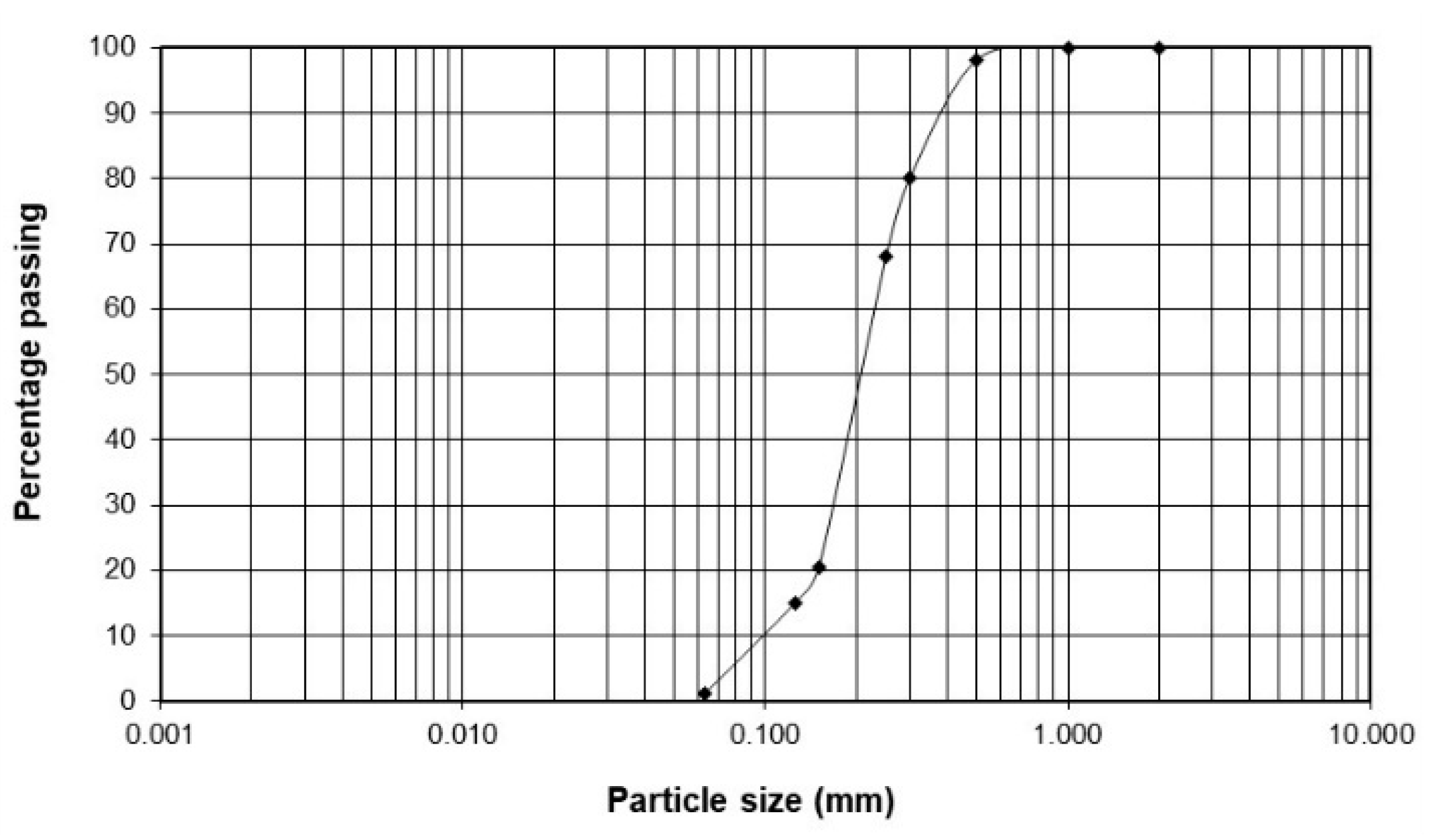
2.1.1. Soil
2.1.2. Bacteria Strains
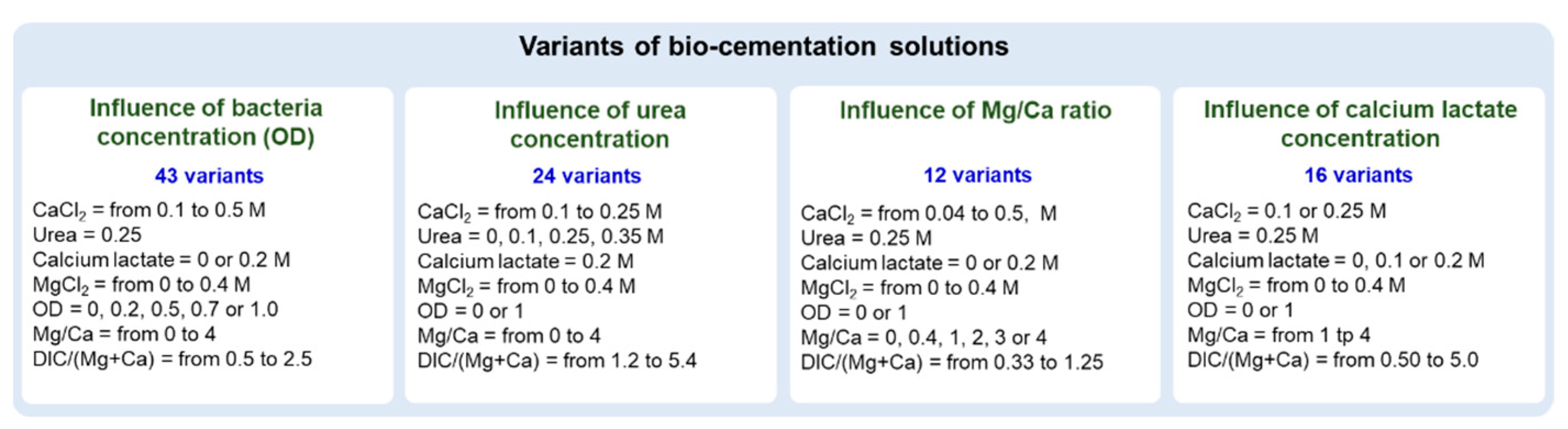
2.1.3. Cementation Solutions
2.2. Sample Preparation and Treatment
2.3. Methods for Verifying the Bio-Cementation Process
2.3.1. Methods for Verifying the Bio-Cementation Process
2.3.2. Phase Composition Analysis
2.3.3. Pocket Penetrometer Test
3. Results
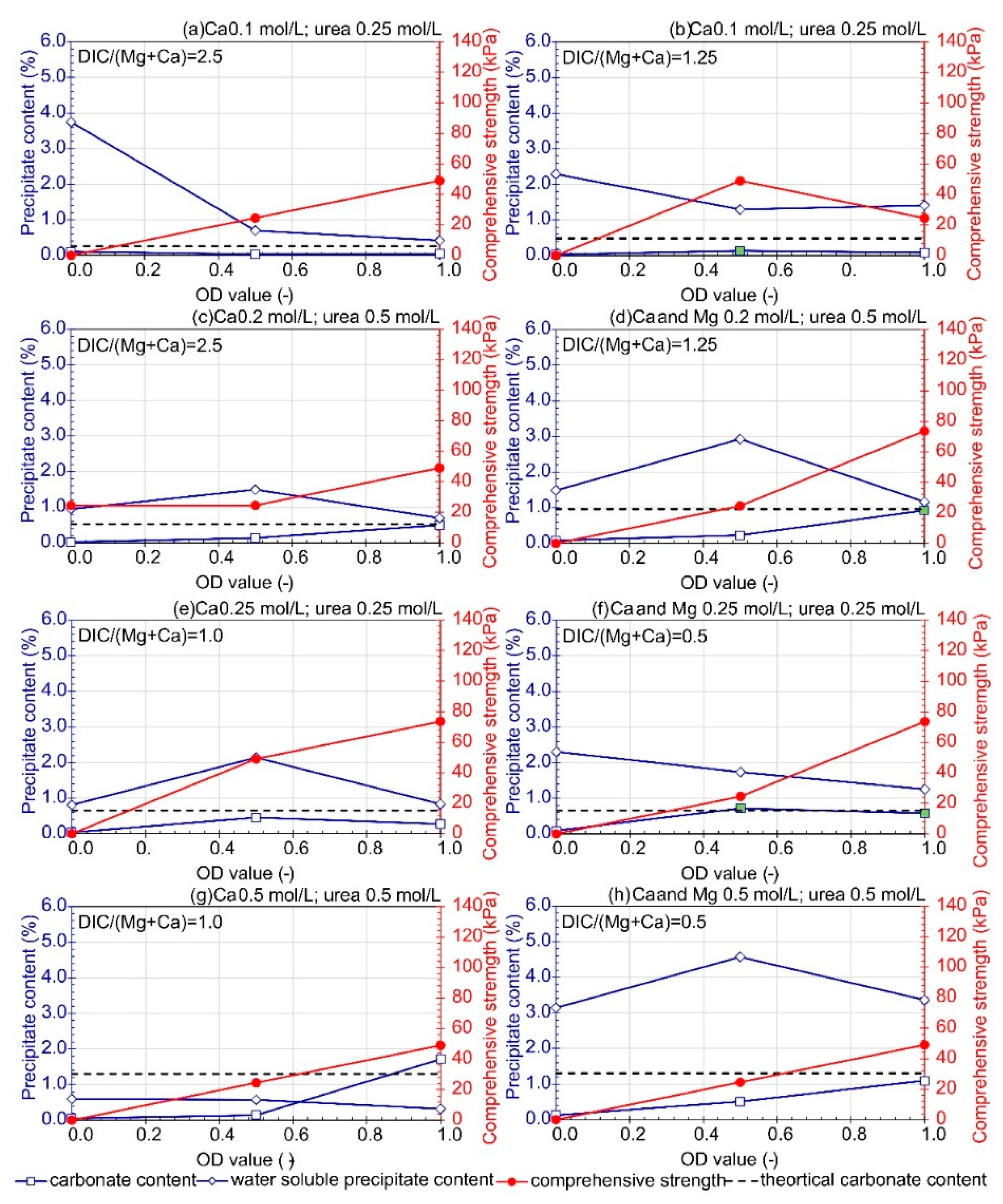
3.1. Influence of Bacteria Concentration
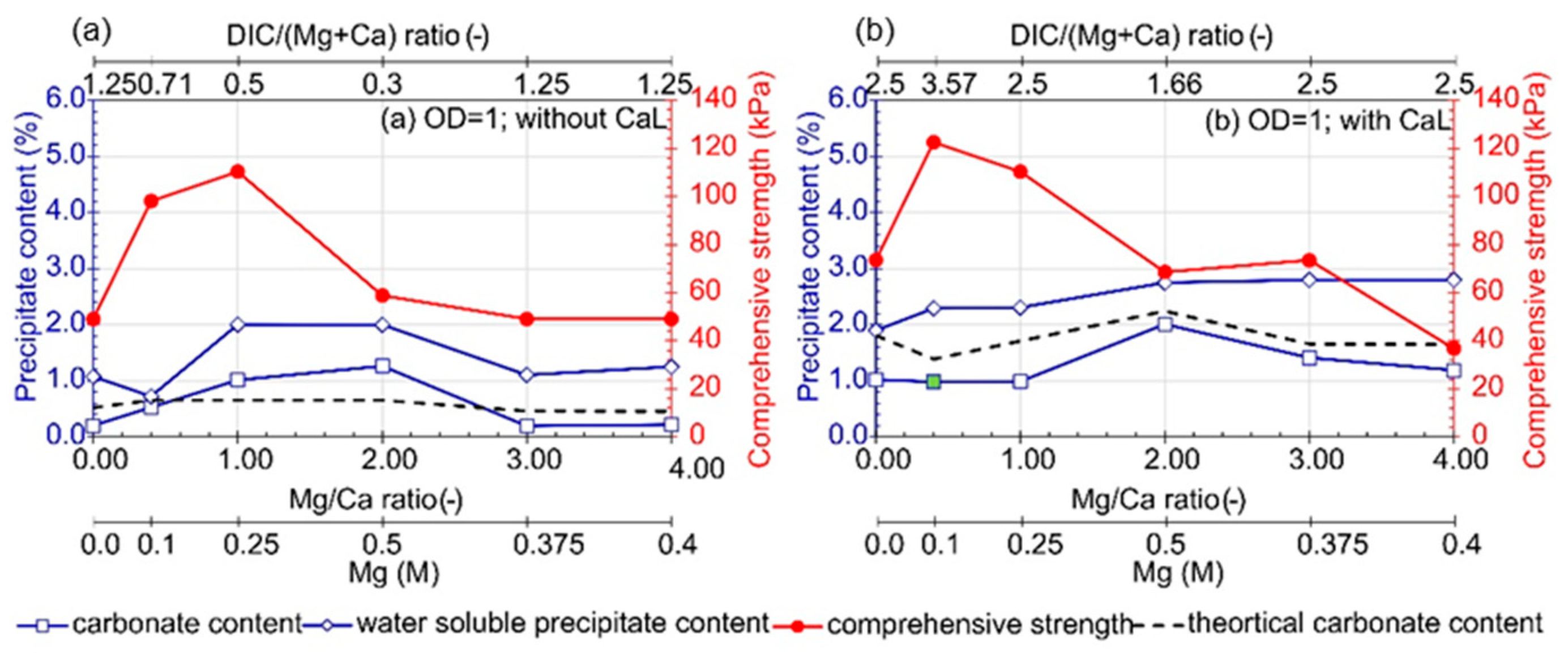
3.2. Influence of Mg/Ca Molar Ratio
3.3. Influence of Urea Concentration
 —0.2 M CaL), (
—0.2 M CaL), ( —0.15 M CaL), (
—0.15 M CaL), ( —0.1 M CaL). The green marker indicates a well-cemented sample based on macroscopic evaluation.
—0.1 M CaL). The green marker indicates a well-cemented sample based on macroscopic evaluation.
 —0.2 M CaL), (
—0.2 M CaL), ( —0.15 M CaL), (
—0.15 M CaL), ( —0.1 M CaL). The green marker indicates a well-cemented sample based on macroscopic evaluation.
—0.1 M CaL). The green marker indicates a well-cemented sample based on macroscopic evaluation.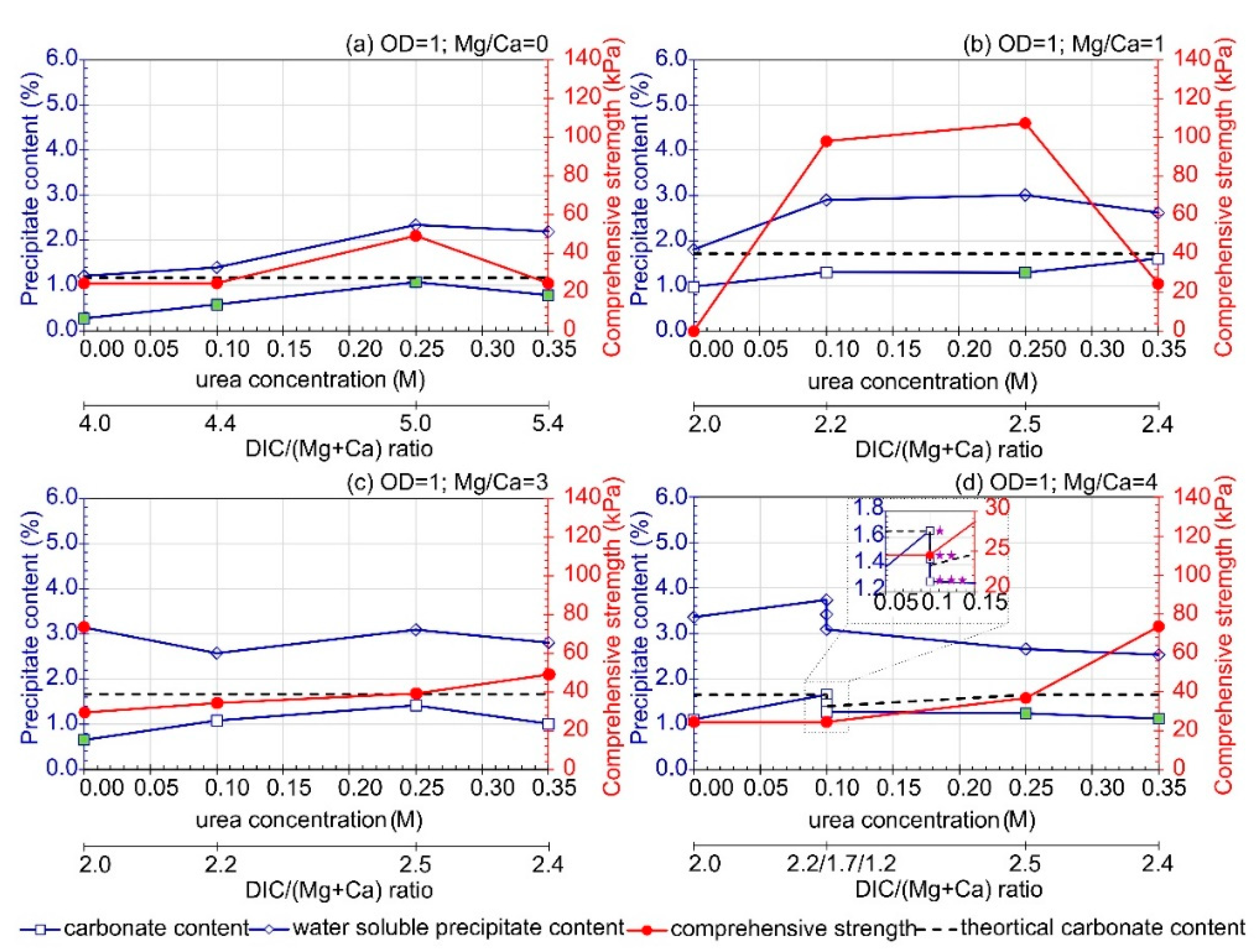
3.4. Influence of Calcium Lactate Concentration

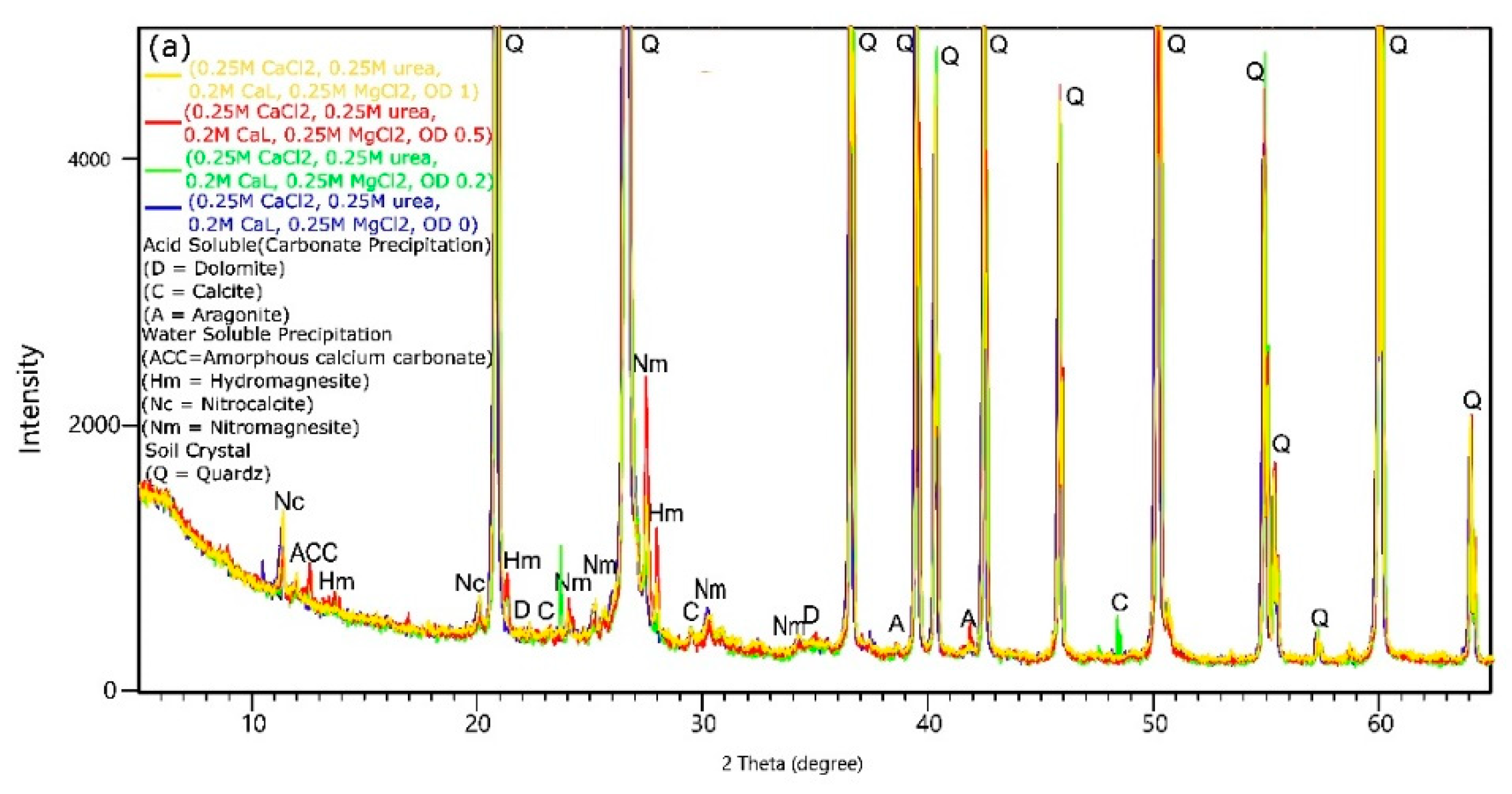
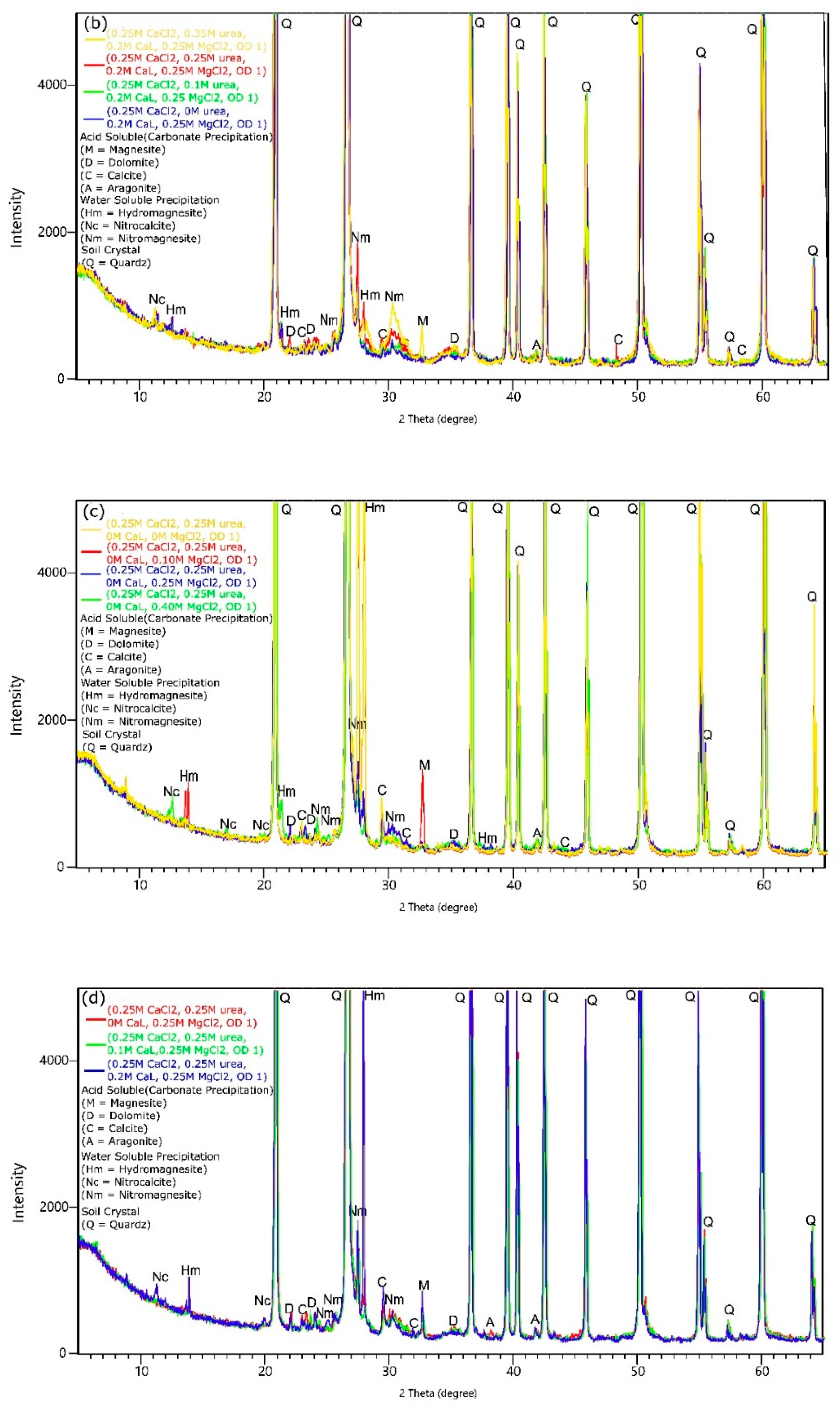
3.5. XRD Analysis
4. Discussion
5. Conclusions
- Under the adopted test conditions, increasing CaL concentration resulted in the formation of undesirable water-soluble forms that reduced the effectiveness of the method. Additionally, in the presence of CaL, aragonite, calcite, magnesite, dolomite, and hydromagnesite were formed. However, the question of how CaL affects the amount of precipitated carbonates and the improvement of soil strength parameters has not been answered. Water-soluble content also increased with Mg ions concentrations and Mg/Ca molar ratios, and it decreased with bacteria concentration.
- An increase in the Mg/Ca molar ratio decreased the efficiency of soil bio-stabilization. However, the presence of Mg ions in lower concentrations stimulated the formation of more stable crystals of dolomite and the improvement of soil. The optimum combination of bio-cementation solution containing B. subtilis bacteria strain, assuming a single application treatment, based on the comprehensive strength of cemented sand, is as follows: OD equal to 1, urea concentration of 0.25 M, CaL concentration of 0.1–0.2 M, and the Mg/Ca molar ratio between 0.4 and 1. The highest comprehensive strength was achieved with Mg2+ and Ca2+ concentrations equal to 0.1 M and 0.25 M, respectively. This composition of bio-cementation solution can be recommended for in situ application; however, the efficiency of the MICP processes may depend on the type of treated soil.
- The comprehensive strength and carbonate content depended on bacteria cell concentrations. Moreover, the correlation between carbonate content and bacteria concentration, available dissolved inorganic carbon, calcium lactate and magnesium chloride concentration, as well as the sum and molar ratio of Mg and Ca ions, was revealed. Additionally, an increase in comprehensive strength was related to an increase in carbonate content.
- The results presented in the article, however, do not answer several important questions. Therefore, it would be reasonable to conduct numerical analysis of the precipitation processes to determine the relationships between parameter concentrations and WSC, CC, and comprehensive strength. Additionally, for environmental reasons, further studies are needed to eliminate urea or at least significantly increase the contribution of the non-ureolytic carbonate precipitation pathway in the application of the MICP method under in situ conditions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanov, V.; Chu, J. Applications of Microbiology to Geotechnical Engineering for Bioclogging and Biocementation of Soil In Situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Imran, M.A.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Durability Improvement of Biocemented Sand by Fiber-Reinforced MICP for Coastal Erosion Protection. Materials 2022, 15, 2389. [Google Scholar] [CrossRef]
- Tian, X.; Xiao, H.; Li, Z.; Li, Z.; Su, H.; Ouyang, Q. Experimental Study on the Strength Characteristics of Expansive Soils Improved by the MICP Method. Geofluids 2022, 2022, 3089820. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Li, X. Review article Geotechnical Engineering Properties of Soils Solidified by Microbially Induced CaCO3 Precipitation (MICP). Adv. Civ. Eng. 2021, 6683930. [Google Scholar]
- Zhao, Y.; Wang, Q.; Yuan, M.; Chen, X.; Xiao, Z.; Hao, X.; Zhang, J.; Tang, Q. The Effect of MICP on Physical and Mechanical Properties of Silt with Different Fine Particle Content and Pore Ratio. J. Appl. Sci. 2021, 12, 139. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially Induced Carbonate Precipitation for Wind Erosion Control of Desert Soil: Field-Scale Test. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
- Tsai, C.P.; Ye, J.H.; Ko, C.H.; Lin, Y.R. An Experimental Investigation of Microbial-induced Carbonate Precipitation on Mitigating Beach Erosion. Sustainability 2022, 14, 2513. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R.; Wang, H.; Xia, J. Surface Rainfall Erosion Resistance and Freeze-Thaw Durability of Bio-Cemented and Polymer-Modified Loess Slopes. J. Environ. Manag. 2022, 301, 113883. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A.D. Review on Microbial Induced Calcite Precipitation Mechanisms Leading to Bacterial Selection for Microbial Concrete. Constr. Build. Mater. 2019, 225, 67–75. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Liu, H.; Zhao, Y.; Yang, Y.; Xu, X.; Cheng, X.; Wit, B.D. Examining Energy Consumption and Carbon Emissions of Microbial Induced Carbonate Precipitation Using the Life Cycle Assessment Method. Sustainability 2021, 13, 4856. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281. [Google Scholar] [CrossRef]
- Tang, C.S.; Yin, L.Y.; Jiang, N.J.; Zhu, C.; Zeng, H.; Shi, B. Factors affecting the performance of microbial induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 79–94. [Google Scholar] [CrossRef]
- Yu, T. Review on engineering properties of MICP-treated soils. Geomech. Eng. 2021, 27, 13–30. [Google Scholar]
- Fukue, M.; Lechowicz, Z.; Fujimri, Y.; Emori, K.; Mulligan, C.N. Incorporation of Optical Density into Blending Design for a Biocement Solution. Materials 2022, 15, 1951. [Google Scholar] [CrossRef]
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 2023, 1, 100002. [Google Scholar] [CrossRef]
- Lv, C.; Tsang, C.S.; Zhang, J.Z.; Pan, X.H.; Liu, H. Effects of Calcium Sources and Magnesium Ions on the Mechanical Behavior of MICP-treated calcareous sand: Experimental Evidence and Precipitated Crystal Insights. Acta Geotech. 2023, 18, 2703–2717. [Google Scholar] [CrossRef]
- Hemayati, M.; Nikooee, E.; Habibagahi, G.; Niazi, A.; Afzali, S.F. New Non-Ureolytic Heterotrophic Microbial Induced Carbonate Precipitation for Suppression of Sand Dune Wind Erosion. Sci. Rep. 2023, 13, 5845. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of Bacteria as Self-Healing Agent for the Development of Sustainable Concrete. Ecol. Eng. 2010, 35, 230–235. [Google Scholar]
- Al Qabany, A.; Soga, K. Effect of Chemical Treatment Used in MICP on Engineering Properties of Cemented Soils. Geotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Ogbonnaya, U.; Wen, K.; Tian, A.; Amini, F. Influence of Fiber Addition on Mechanical Properties of MICP-Treated Sand. J. Mater. Civ. Eng. 2016, 28, 04015166. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Mu, B.; Gui, Z.; Lu, F.; Petropoulos, E.; Yu, Y. Microbial-Induced Carbonate Precipitation Improves Physical and Structural Properties of Nanjing Ancient City Walls. Materials 2021, 14, 5665. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nusslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. 2006, 131, 1381–1392. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Passen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Paassen, L.A.V.; Ghose, R.; Linden, T.J.M.; Star, W.R.L.; Loosderch, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Harkes, M.P.; Van Passen, L.A.; Booster, J.L. Fixation and Distribution of Bacterial Activity in Sand to Induce Carbonate Precipitation for Ground Reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.H.; Kim, S.G.; Park, J.Y.; Kang, C.H.; Jeong, J.H.; So, J.S. Effects of Different Calcium Salts on Calcium Carbonate Crystal Formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Choi, S.G.; Wu, S.; Chu, J. Biocementation for Sand Using an Eggshell as Calcium Source. J. Geotech. Geoenviron. Eng. 2016, 142, 06016010. [Google Scholar] [CrossRef]
- Jiang, N.J.; Soga, K. The Applicability of Microbially Induced Calcite Precipitation (MICP) for Internal Erosion Control in Gravel–Sand Mixtures. Géotechnique 2016, 67, 42–55. [Google Scholar] [CrossRef]
- Terzis, D.; Latman, R.B.; Laloui, L. Fabric Characteristics and Mechanical Response of Bio-Improved Sand to Various Treatment Conditions. Géotechnique 2016, 6, 52–57. [Google Scholar] [CrossRef]
- Gomez, M.G.; DeJong, J.T. Engineering Properties of Bio-Cementation Improved Sandy Soils. In Proceedings of the Grouting 2017, Honolulu, HI, USA, 9–12 July 2017; pp. 23–33. [Google Scholar]
- Nafisi, A.; Montoya, B.M. A New Framework for Identifying Cementation Level of MICP-Treated Sands. In Proceedings of the IFCEE 2018, Orlando, FL, USA, 5–10 March 2018; Volume 296, pp. 37–47. [Google Scholar]
- Choi, S.G.; Chu, J.; Kwon, T.H. Effect of Chemical Concentrations on Strength and Crystal Size of Biocemented Sand. Geomech. Eng. 2019, 17, 465–475. [Google Scholar]
- Marin, S.; Cabestrero, O.; Demergasso, C.; Olivares, S.; Zetola, V.; Vera, M. An Indigenous Bacterium with Enhanced Performance of Microbially-Induced Ca-Carbonate Biomineralization Under Extreme Alkaline Conditions for Concrete and Soil-Improvement Industries. Acta Biomater. 2021, 120, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Satyam, N.; Reddy, K.R. Liqufaction Resistance of Biotreated Sand Before and After Exposing to Weathering Conditions. Indian Geotech. J. 2022, 52, 328–340. [Google Scholar] [CrossRef]
- Hadi, Z.S.; Saeed, K.A. Effect of Microbial-Induced Calcite Precipitation (MICP) on The Strength of Soil Contaminated with Lead Nitrate. J. Mech. Behav. Biomed. Mater. 2022, 31, 143–149. [Google Scholar] [CrossRef]
- Al-Thawadi, S.M. Consolidation of Sand Particles by Aggregates of Calcite Nanoparticles Synthesized by Ureolytic Bacteria under Non-Sterile Conditions. J. Chem. Technol. 2013, 2, 141–146. [Google Scholar]
- Yureo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Fukue, M.; Fujimori, Y.; Sato, Y.; Nakagawa, T.; Mulligan, C.N. Evidence of The Production and Dissolution of Carbonate Phases in Bentonite Formations. Appl. Clay Sci. 2010, 47, 133–138. [Google Scholar] [CrossRef]
- Fukue, M.; Ono, S.; Sato, Y. Cementation of Sands Due to Microbiologically-Induced Carbonate Precipitation. Soils Found. 2011, 51, 83–93. [Google Scholar] [CrossRef]
- Lin, W.; Gao, Y.; Lin, W.; Zhuo, Z.; Wu, W.; Cheng, Z. Seawater-based bio-cementation of natural sea sand via microbially induced carbonate precipitation. Environ. Technol. Innov. 2023, 29, 103010. [Google Scholar] [CrossRef]
- Nayanthara, P.G.N.; Dassanayake, A.B.N.; Nakashima, K.; Kawasaki, S. Microbial Induced Carbonate Precipitation Using a Native Inland Bacterium for Beach Sand Stabilization in Nearshore Areas. Appl. Sci. 2019, 9, 3201. [Google Scholar] [CrossRef]
- Murugan, R.; Suraishkumar, G.K.; Mukherjee, A.; Dhami, N.K. Insights into the Influence of Cell Concentration in Design And Development of Microbially Induced Calcium Carbonate Precipitation (MICP) Process. PLoS ONE 2021, 16, e0254536. [Google Scholar] [CrossRef] [PubMed]
- Poazytek, K.; Ciezkowska, M.; Sklodowska, A.; Drewniak, L. Microbial Consortium with High Cellulolytic Activity (MCHCA) for Enhanced Biogas Production. Front. Microbiol. 2016, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Kardogan, B.; Sekercioglu, K.; Ersan, Y.C. Compatibility and Biomineralization Oriented Optimization of Nutrient Content in Nitrate-Reducing-Biogranules-Based Microbial Self-Healing Concrete. Sustainability 2021, 13, 8990. [Google Scholar] [CrossRef]
- Zeitouny, J.; Lieske, W.; Lavasan, A.A.; Heinz, E.; Wichern, M.; Wichtmann, T. Impact of New Combined Treatment Method on the Mechanical Properties and Microstructure of MICP-Improved Sand. Geotechnics 2023, 3, 661–685. [Google Scholar] [CrossRef]
- Choi, S.G.; Park, S.S.; Wu, S.; Chu, J. Methods for Calcium Carbonate Content Measurement of Biocemented Soils. J. Mater. Civ. Eng. 2017, 29, 06017015. [Google Scholar] [CrossRef]
- Vinjay, K.; Murmu, M. Effect of Calcium Lactate on Compressive Strength and Self-Healing of Cracks in Microbial Concrete. Front. Struct. Civ. Eng. 2019, 13, 515–525. [Google Scholar]
- Rock Identifier. Available online: https://rockidentifier.com/wiki/Nitrocalcite.html (accessed on 13 June 2023).
- Zhuang, C.; Liu, C.; Cui, Z.; Yang, Z.; Chen, Y.; Dou, Z. Microbially-Induced Calcium Carbonate Precipitation Test on Yellow Sandstone Based on LF-NMR Monitoring. Int. J. Environ. Res. Public Health 2022, 19, 16860. [Google Scholar] [CrossRef]
- Cuthbert, M.O.; Riley, M.S.; Sidhu, S.H.; Renshaw, J.C.; Tobler, D.J.; Phoenix, V.R.; Mackay, R. Controls on the Rate of Ureolysis and the Morphology of Carbonate Precipitated by S. pasteurii Biofilms and Limits due to Bacterial Encapsulation. Ecol. Eng. 2012, 41, 32–40. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, M.L.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Konstantinou, C.; Wang, Y.; Biscontin, G.; Soga, K. The Role of Bacterial Urease Activity on The Uniformity of Carbonate Precipitation Profiles of Bio-Treated Coarse Sand Specimens. Sci. Rep. 2021, 11, 6161. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, H.; Cheng, X.; Li, M. The Promotion of Magnesium Ions on Aragonite Precipitation in MICP Process. Constr. Build. Mater. 2020, 263, 120057. [Google Scholar] [CrossRef]
- Reddy, M.M.; Nancollas, G.H. The Crystallization of Calcium Carbonate: IV. The Effect of Magnesium, Strontium and Sulfate Ions. J. Cryst. Growth 1976, 35, 33–38. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Shi, T.; Yang, B.; Li, C.; Xu, H.; Yin, W. Preparation, Properties and Phase Transition of Mesoporous Hydromagnesite with Various Morphologies from Natural Magnesite. J. Powder Technol. 2020, 364, 822–830. [Google Scholar] [CrossRef]
- Gowthaman, S.; Iki, T.; Nakashima, K.; Ebina, K.; Kawasaki, S. Feasibility Study for Slope Soil Stabilization by Microbial Induced Carbonate Precipitation (MICP) using Indigenous Bacteria Isolated from Cold Subarctic Region. Appl. Sci. 2019, 1, 1480. [Google Scholar] [CrossRef]
- Phang, I.R.K.; Wong, K.S.; Chan, Y.S.; Lau, S.Y. Effect of Microbial-Induced Calcite Precipitation Towards Strength and Permeability of Peat. Bull. Eng. Geol. Environ. 2022, 81, 314. [Google Scholar] [CrossRef]
- Sharma, A.; Ramkrishnan, R. Study on Effect of Microbial Induced Calcite Precipitates on Strength of Fine Grained Soils. Prospect. Sci. 2016, 8, 198–202. [Google Scholar] [CrossRef]
- Shannoon, L.K.; Ibrahim, M.A. Bio-Cementation of Sandy Soil through Bacterial Processing to Precipitate Carbonate. Al-Nahrain J. Eng. Sci. 2020, 23, 225–231. [Google Scholar] [CrossRef]
- Fukue, M.; Lechowicz, Z.; Fujimori, Y.; Emori, K.; Mulligan, C.N. Inhibited and Retarded Behaviour by Ca2+ and Ca2+/OD Loading Rate on Ureolytic Bacteria in MICP Process. Materials 2023, 16, 3357. [Google Scholar] [CrossRef]
- Erdmann, N.; Strieth, D. Influencing Factors on Microbiologically Induced Calcium Carbonate Precipitation for Biocementation. World J. Microbiol. Biotechnol. 2023, 38, 61. [Google Scholar] [CrossRef]
- Erdmann, N.; Payrebrune, K.M.D.; Ulber, R.; Strieth, D. Optimizing Compressive Strength of Sand Treated with MICP Using Response Surface Methodology. SN Appl. Sci. 2022, 4, 282. [Google Scholar] [CrossRef]
- Molnár, Z.; Pekker, P.; Dódony, I.; Pósfai, M. Clay minerals affect calcium (magnesium) carbonate precipitation and aging. Earth Planet. Sci. Lett. 2021, 567, 116971. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L. The Comparison of Microbiologically-Induced Calcium Carbonate Precipitation and Magnesium Carbonate Precipitation. In Proceedings of the 8th International Congress on Environmental Geotechnics Volume 3: Towards a Sustainable Geoenvironment, Hangzhou, China, 28 October–1 November 2018; Springer: Singapore, 2019; pp. 302–308. [Google Scholar]
- Naveed, M.; Duan, J.; Uddin, S.; Suleman, M.; Hui, Y. Application of Microbially Induced Calcium Carbonate Precipitation with Urea Hydrolysis to Improve the Mechanical Properties of Soil. Ecol. Eng. 2020, 153, 105885. [Google Scholar] [CrossRef]
- Shahraki, R.S.; Zomorodian, S.M.A.; Niazi, A.; O’Kelly, B.C. Improving Sand with Microbial-Induced Carbonate Precipitation. Ground Improv. 2014, 168, 217–230. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of Calcium Carbonates and Their Engineered Applications: A Review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Ruwisch, R.C. Surface Percolation for Soil Improvement by Biocementation Utilizing In-Situ Enriched Indigenous Aerobic and Anaerobic Ureolytic Soil Microorganisms. Geomicrobiol. J. 2016, 34, 546–556. [Google Scholar] [CrossRef]
- Fernández, A.R.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Román, M.S. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals 2022, 12, 1562. [Google Scholar] [CrossRef]
- Yang, F.S.Y.; Zhang, Y.Q.H. Combining Microbially Induced Calcite Precipitation (MICP) with Zeolite: A New Technique to Reduce Ammonia Emission and Enhanced Soil Treatment Ability of MICP Technology. J. Environ. Chem. Eng. 2022, 3, 107770. [Google Scholar]
- Keykha, H.A.; Mohamadzadeh, H.; Asadi, A.; Kawasaki, S. Ammonium-Free Carbonate-Producing Bacteria as an Ecofriendly Soil Biostabilizer. Geotech. Test. J. 2018, 42. [Google Scholar] [CrossRef]
- Gowthaman, S.; Mohsenzadeh, A.; Najashima, K. Removal of Ammonium by-products from the Effluent of Bio-Cementation System through Struvite Precipitation. Mater. Today Proc. 2022, 61, 243–249. [Google Scholar] [CrossRef]


| Bacteria Strain | Growth Medium | DIC Source (Concentration) | Metal (Ca2+) Source (Concentration) | Additional Components (Concentration) | Refs. |
|---|---|---|---|---|---|
| B. pasteurii (ATCC 6453) | urea growth medium (3 g/L bacteria nutrient broth, 20 g/L urea, 10 g/L NH4Cl, 2.12 g/L NaHCO3, 25.5 mM CaCl2), pH 7.5 | urea (20 g/L or 0.33 M) | CaCl2 (140 g/L or 1.26 M) | - | [24] |
| S. pasteurii (DSMZ 33) | growth medium (20 g/L YE, 10 g/L NH4Cl, pH 9.0) | urea (1.1 M) | CaCl2 (1.1 M) | fixation solution: CaCl2 (0.05 M) | [25] |
| S. pasteurii (DSM 33) | nutrient broth containing (20 g/L), yeast extract, NH4Cl (10 g/L), NiCl2 (10µM) | urea (1 mol/L) | CaCl2 (1.1 mol/L) | - | [26] |
| S. pasteurii (DSMZ 33) | - | urea (1.0 M) | CaCl2 (1.0 M) | fixation solution: CaCl2 (0.05 M); deionized water, fresh surface water, saline water (9 g/L NaCl) | [27] |
| S. pasteurii (NO-A10) | growth medium in EDC solution (10 g/L), culture medium, 5% NaCl solution | urea (1.0 M) | MgCl2 (0.5 M), CaCl2 (0.5 M or 1.0 M) | buffer solution (0.01 M NH4OH+NH4Cl) | [28] |
| S. pasteurii (ATCC 11859) | NH4-YE medium (20 g YE, 10 g (NH4)2SO4, 20 g of agar in 0.13 M Tris buffer, pH = 9.0), OD = 0.8–1.2 | - | equimolar urea- CaCl2 (0.1, 0.25, 0.5, 1.0 M) | nutrient broth (3 g/L), NH4Cl 10 g/L, NaHCO3 (2.12 g/L) | [20] |
| Bacillus sp. | - | urea (1.0 M) | CaCl2 (0.45 M) | - | [29] |
| S. pasteurii (ATCC 6452) | NH4-YE medium (20 g/L YE, 10 g/L (NH4)2SO4, 20 g/L agar in 0.13 M Tris buffer, pH = 9.0) | urea (1.0 M) | CaCl2 (1.0 M) | nutrient broth (3 g/L) | [30] |
| S. pasteurii (ATCC 11859) | (1) 0.5 M urea, 0.187 M NH4Cl, 3 g/L nutrient broth, (2) 0.1 M CaCl2, 0.187 M NH4Cl, 3 g/L nutrient broth (increased salinity to enhance the adsorption of microorganisms on sand grains) | urea (0.25 M) | CaCl2 (0.25 M) | - | [31] |
| S. pasteurii | NH4Cl (0.006 M and 0.012 M, C2H3NaO2(0.0425 M and 0.085 M, and YE (0.1 and 0.2 g/L) | urea (0.25 M and 0.5 M) | CaCl2 (0.125 M and 0.25 M) | C2H3NaO2 (-) | [32] |
| S. pasteurii | growth medium (in 0.13 mol/L tris buffer, 10 g/L (NH4)2SO4, 20 g/L YE) | urea (0.333 M) | CaCl2 (0.1 M) | NH4Cl (0.374 M) | [33] |
| S. pasteurii (ATCC 1859) | YE (20 g), (NH4)2SO4 (10 g), 0.13 M tris buffer (pH = 9.0) solution | urea (0.3 M) | CaCl2 (0.3 M) | - | [34] |
| S. pasteurii (DSMZ 33) + native urease-producing bacteria | ureolytic broth (1 M urea, without (NH4)2SO4) | urea (1.0 M) | CaCl2 (1.0 M) | C2H3NaO2 (13.8 g/L) | [35] |
| B. sphaericus | 25 g/L nutrient broth | urea (0.5 M) | CaCl2 (0.5 M) | NH4Cl (10 g/L) and NaHCO3 were (2.12 g/L) | [36] |
| B. subtilis | peptone (5 g), NaCL (5 g), YE (4 g), beef extract (1 g), urea (50 mL) | urea (30.03 g/L or 0.5 M) | CaCl2 (27.75 g/L or 0.25 M) | NH4Cl (10 g), NaHCO3 (2.12 g) | [37] |
| S. pasteurii (ATCC 11859) | NH4-YE medium (20 g/L YE, 10 g/L (NH4)2SO4, 15.73 g/L Tris base) | urea (0.5 M) | C4H6CaO4, Ca(NO3)2, and CaCl2 (0.5 M). | MgCl2 (0, 0.05, 0.1, 0.25, 0.5 M). | [17] |
| Classification | Results | |
|---|---|---|
| Specific density | t/m3 | 2.593 |
| Dry density min | t/m3 | 1.326 |
| Dry density max | t/m3 | 1.714 |
| pH | - | 8.18 |
| D10 | mm | 0.09 |
| D30 | mm | 0.17 |
| D60 | mm | 0.23 |
| Cu | - | 2.6 |
| Cc | - | 2.4 |
| Variables | Comprehensive Strength (kPa) | WSC (%) | CC (%) |
|---|---|---|---|
| CaCl2 (M) | 0.078 | −0.063 | −0.044 |
| Urea (M) | −0.032 | −0.199 | −0.210 |
| CaL (M) | 0.038 | 0.592 | 0.411 |
| MgCl2 (M) | −0.113 | 0.589 | 0.442 |
| OD (-) | 0.606 | −0.223 | 0.678 |
| Mg/Ca | −0.144 | 0.431 | 0.355 |
| Σ(Ca, Mg) (M) | −0.051 | 0.519 | 0.391 |
| DIC (M) | 0.033 | 0.598 | 0.395 |
| DIC/(Ca+Mg) | 0.044 | 0.194 | 0.115 |
| Comprehensive strength (kPa) | 1 | −0.180 | 0.363 |
| WSC (%) | −0.180 | 1 | 0.154 |
| CC (%) | 0.363 | 0.154 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronczyk, J.; Marchelina, N.; Pyzik, A.; Franus, M. Assessment of the Composition Effect of a Bio-Cementation Solution on the Efficiency of Microbially Induced Calcite Precipitation Processes in Loose Sandy Soil. Materials 2023, 16, 5767. https://doi.org/10.3390/ma16175767
Fronczyk J, Marchelina N, Pyzik A, Franus M. Assessment of the Composition Effect of a Bio-Cementation Solution on the Efficiency of Microbially Induced Calcite Precipitation Processes in Loose Sandy Soil. Materials. 2023; 16(17):5767. https://doi.org/10.3390/ma16175767
Chicago/Turabian StyleFronczyk, Joanna, Nadella Marchelina, Adam Pyzik, and Małgorzata Franus. 2023. "Assessment of the Composition Effect of a Bio-Cementation Solution on the Efficiency of Microbially Induced Calcite Precipitation Processes in Loose Sandy Soil" Materials 16, no. 17: 5767. https://doi.org/10.3390/ma16175767
APA StyleFronczyk, J., Marchelina, N., Pyzik, A., & Franus, M. (2023). Assessment of the Composition Effect of a Bio-Cementation Solution on the Efficiency of Microbially Induced Calcite Precipitation Processes in Loose Sandy Soil. Materials, 16(17), 5767. https://doi.org/10.3390/ma16175767








