Charge Carrier Dynamics in Non-Fullerene Acceptor-Based Organic Solar Cells: Investigating the Influence of Processing Additives Using Transient Absorption Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication and J–V Characterization
2.2. UV-Vis Absorption and Photoluminescence Spectroscopy
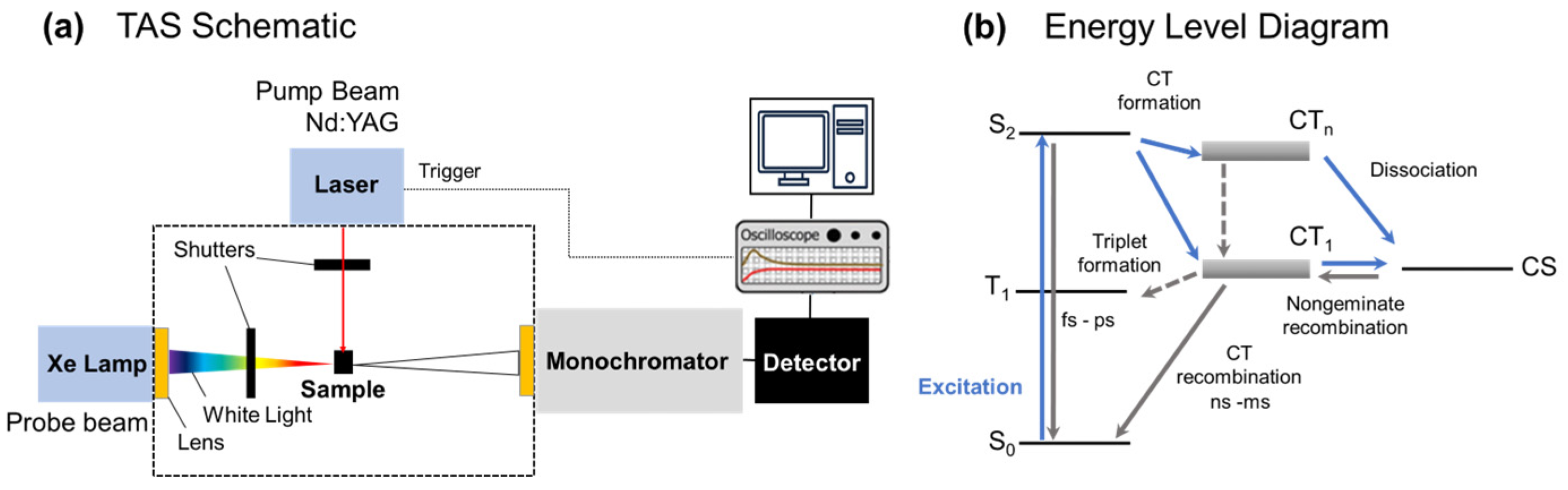
2.3. Transient Absorption Spectroscopy
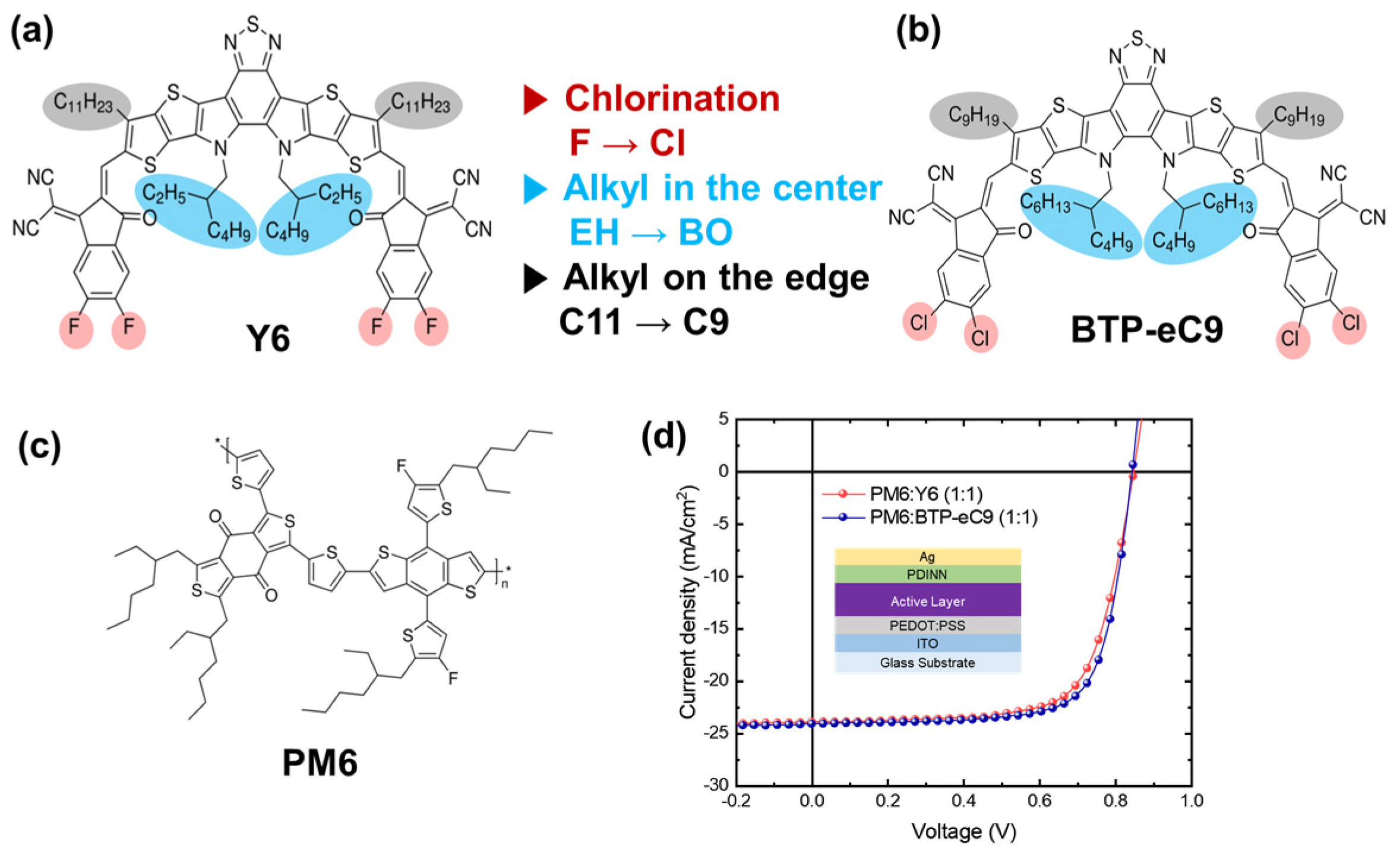
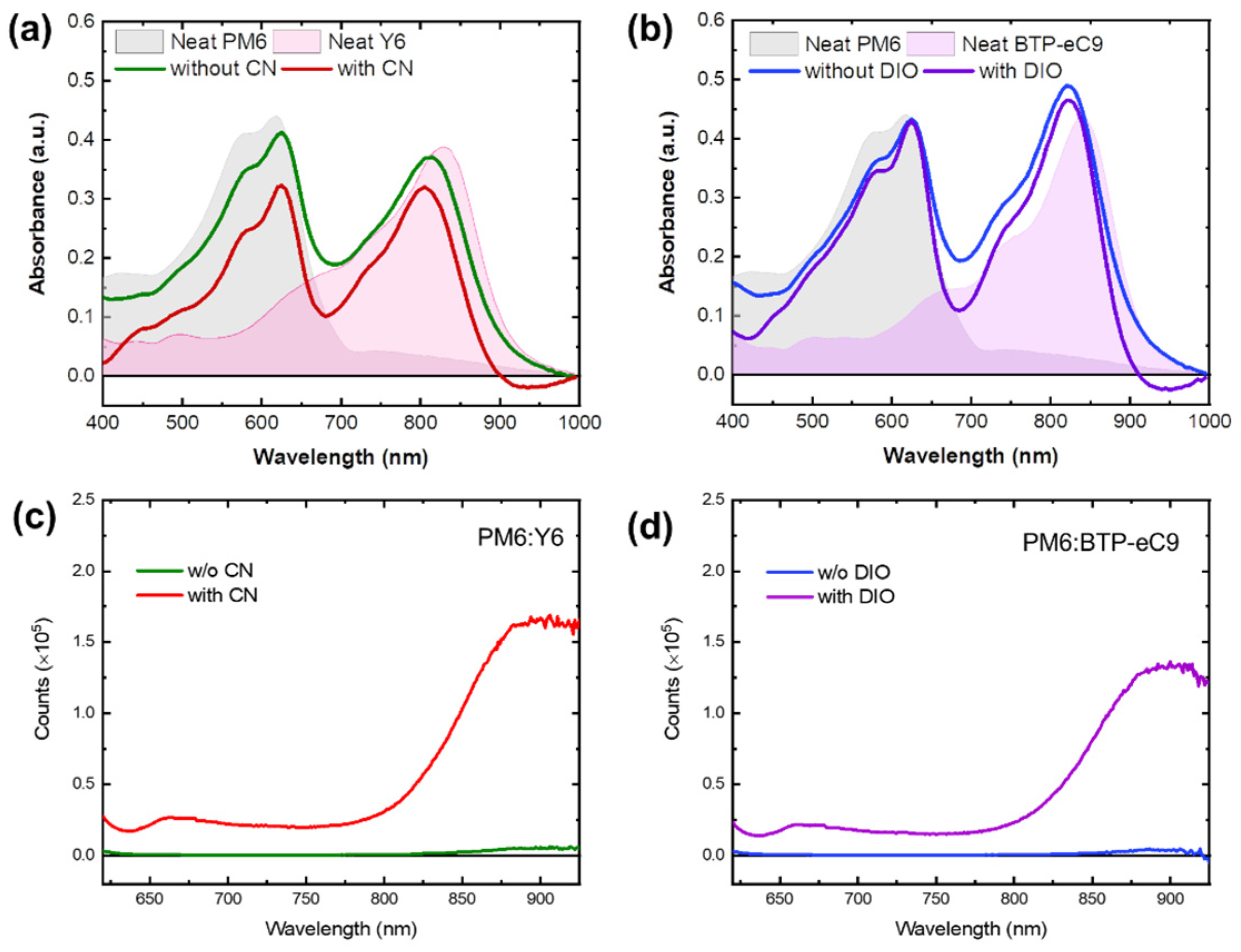
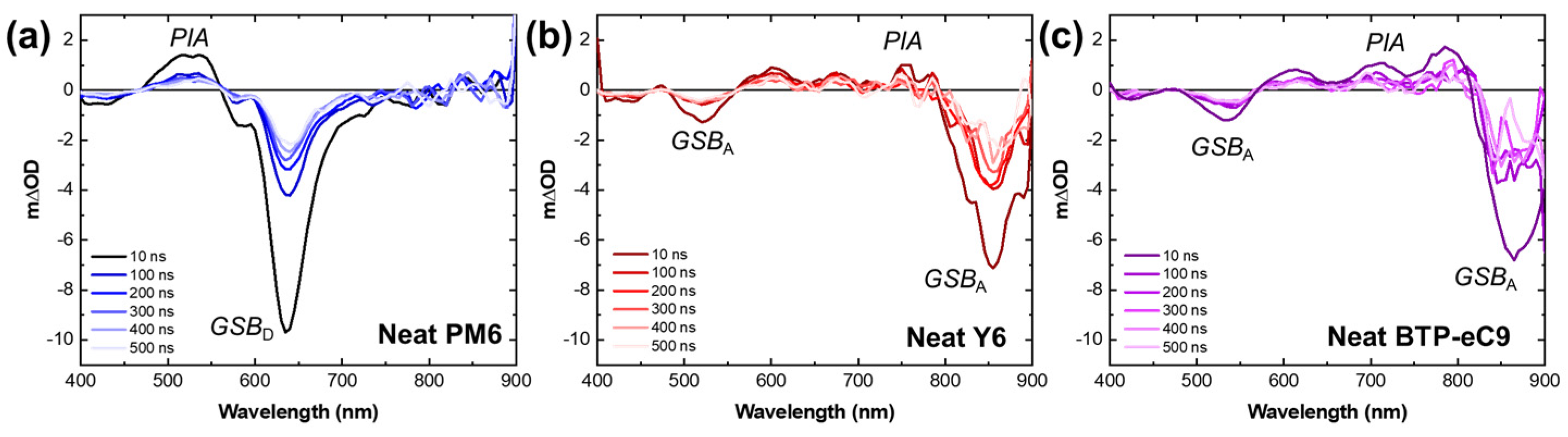
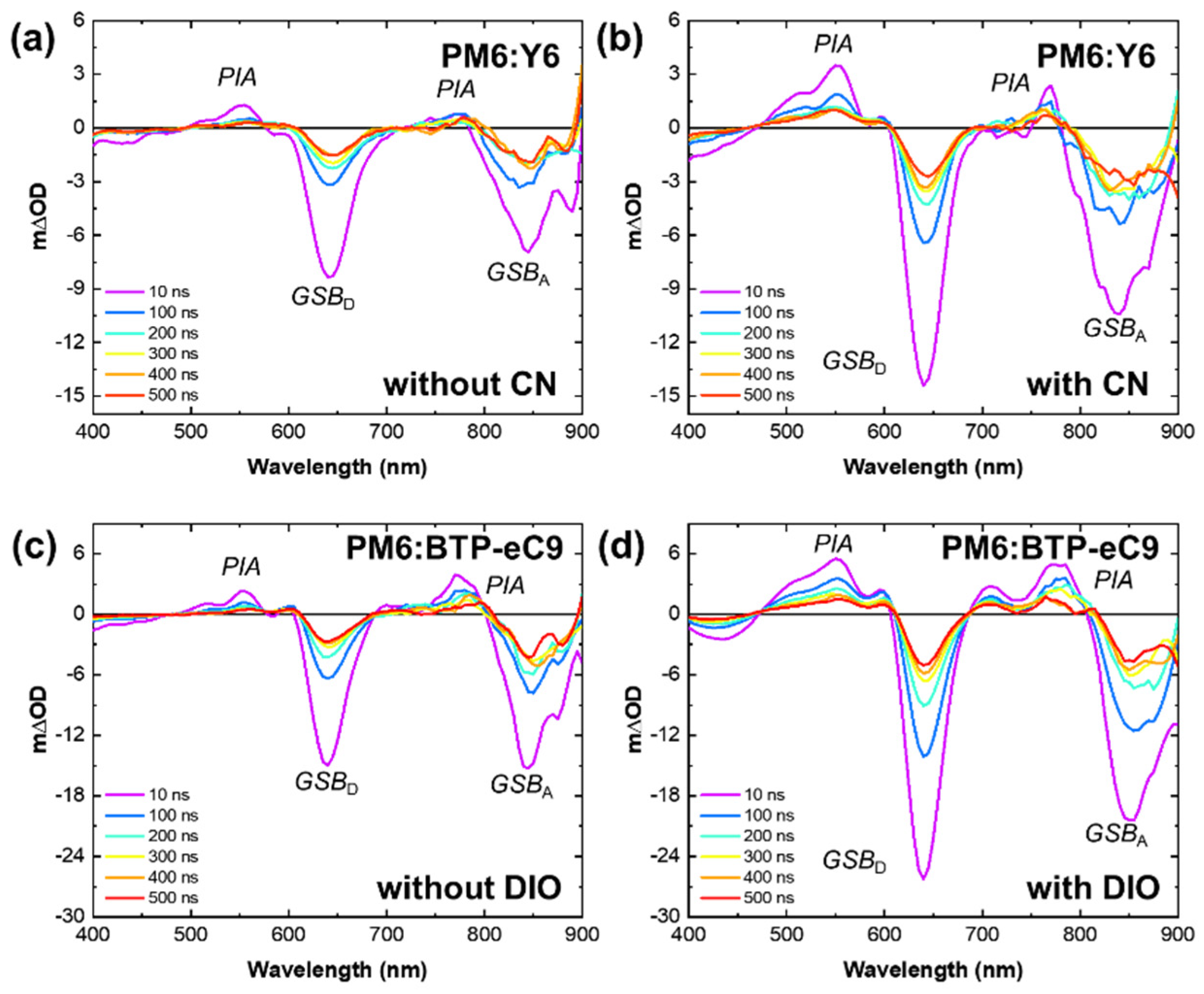
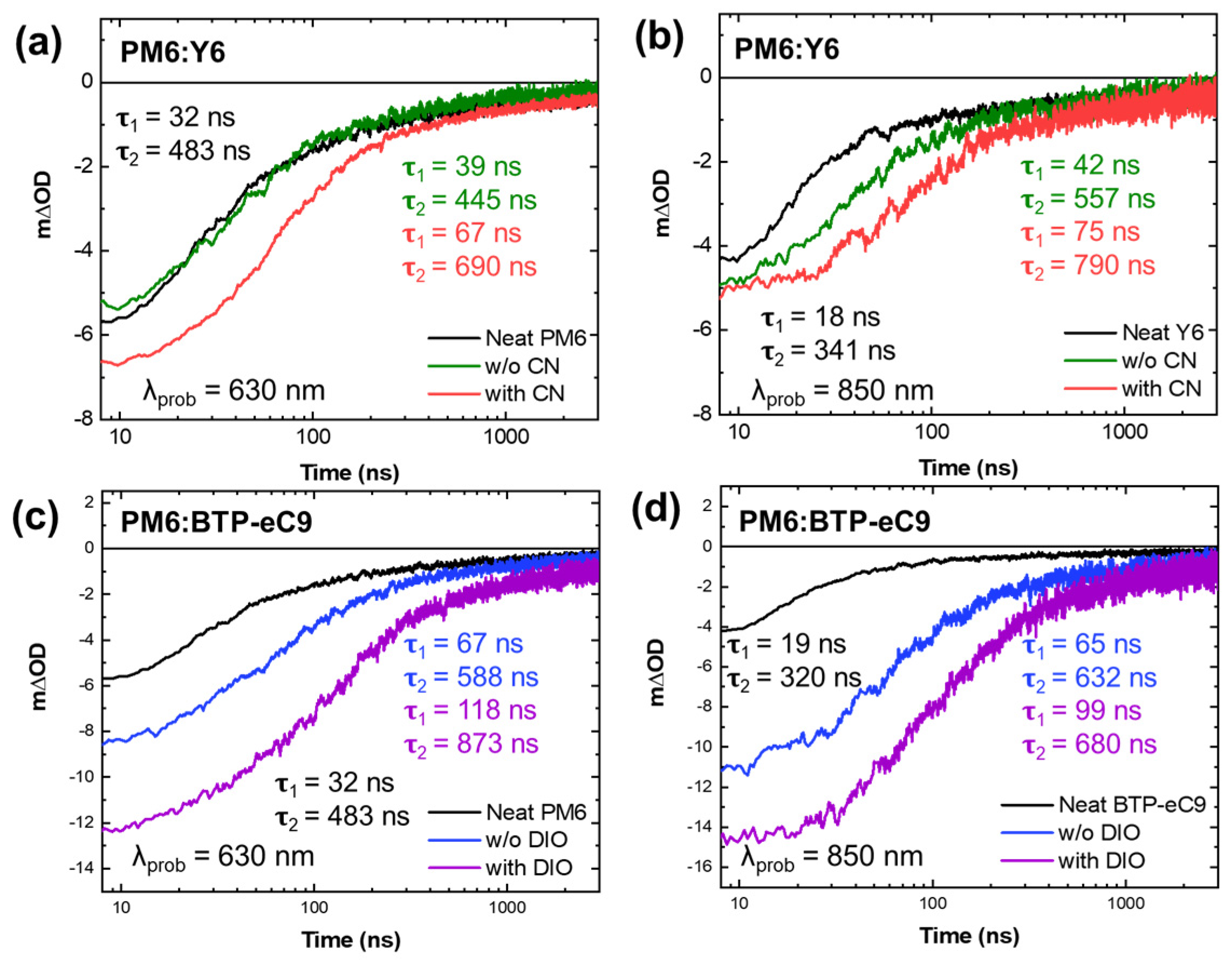
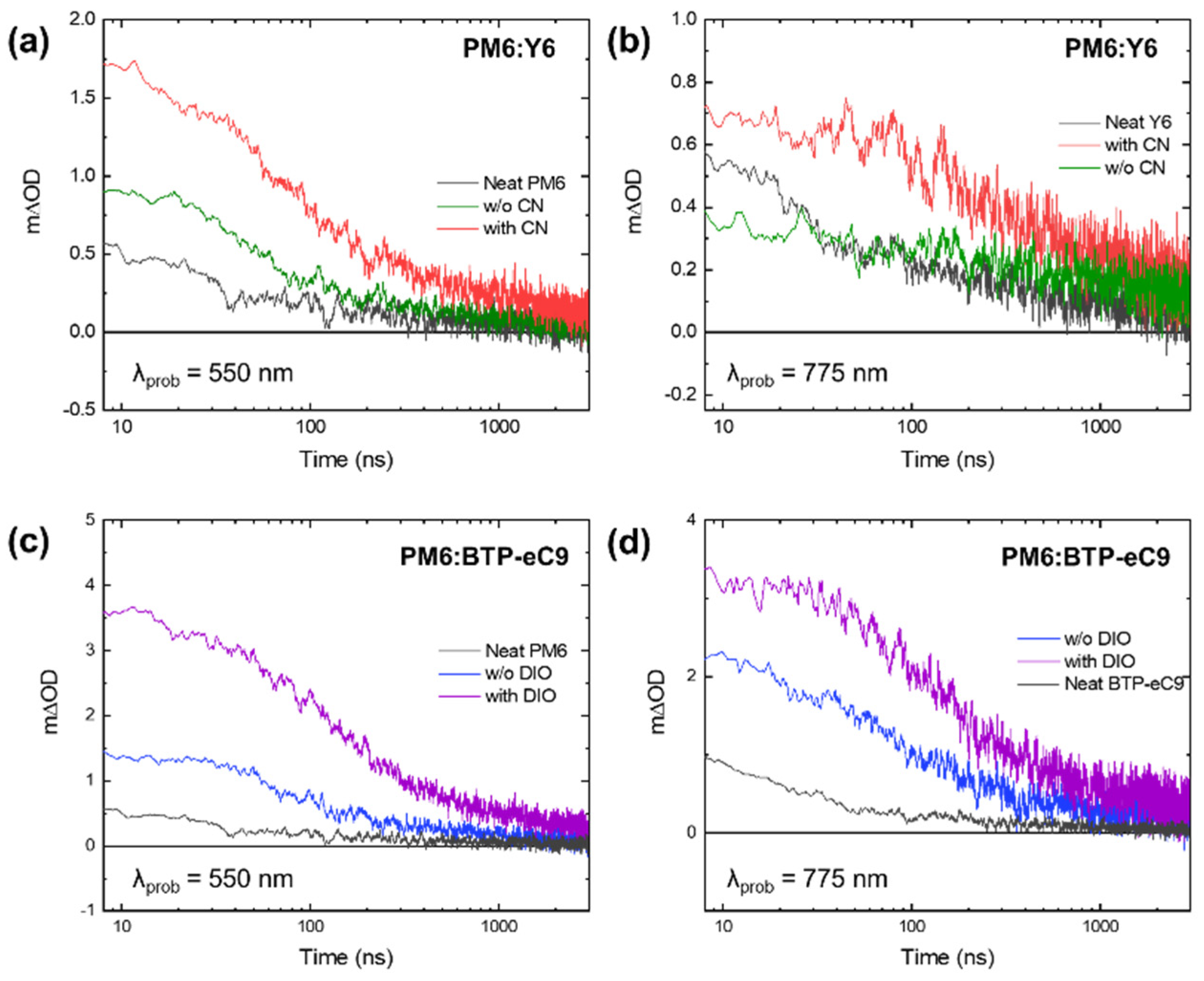
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Granström, M.; Petritsch, K.; Arias, A.C.; Lux, A.; Andersson, M.R.; Friend, R.H. Laminated fabrication of polymeric photovoltaic diodes. Nature 1998, 395, 257. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer: Fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Zhong, W.; Hao, T.; Song, J.; Xue, X.; et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2021, 6, 171. [Google Scholar] [CrossRef]
- Han, C.; Wang, J.; Zhang, S.; Chen, L.; Bi, F.; Wang, J.; Yang, C.; Wang, P.; Li, Y.; Bao, X. Over 19% Efficiency Organic Solar Cells by Regulating Multidimensional Intermolecular Interactions. Adv. Mater. 2023, 35, 2208986. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Z.; Zu, Y.; Wang, Y.; Liu, X.; Zhang, S.; Zhang, M.; Hou, J. A Tandem Organic Photovoltaic Cell with 19.6% Efficiency Enabled by Light Distribution Control. Adv. Mater. 2021, 33, 2102787. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497. [Google Scholar] [CrossRef]
- Karuthedath, S.; Gorenflot, J.; Firdaus, Y.; Chaturvedi, N.; De Castro, C.S.P.; Harrison, G.T.; Khan, J.I.; Markina, A.; Balawi, A.H.; Pena, T.A.D.; et al. Intrinsic efficiency limits in low-bandgap non-fullerene acceptor organic solar cells. Nat. Mater. 2021, 20, 378–384. [Google Scholar] [CrossRef]
- Classen, A.; Chochos, C.L.; Lüer, L.; Gregoriou, V.G.; Wortmann, J.; Osvet, A.; Forberich, K.; McCulloch, I.; Heumüller, T.; Brabec, C.J. The Role of Exciton Lifetime for Charge Generation in Organic Solar Cells at Negligible Energy-Level Offsets. Nat. Energy 2020, 5, 711–719. [Google Scholar] [CrossRef]
- Wei, Q.; Yuan, J.; Yi, Y.; Zhang, C.; Zou, Y. Y6 and its derivatives: Molecular design and physical mechanism. Natl. Sci. Rev. 2021, 8, nwab121. [Google Scholar] [CrossRef] [PubMed]
- Natsuda, S.-I.; Sakamoto, Y.; Takeyama, T.; Shirouchi, R.; Saito, T.; Tamai, Y.; Ohkita, H. Singlet and Triplet Excited-State Dynamics of a Nonfullerene Electron Acceptor Y6. J. Phys. Chem. C 2021, 125, 20806–20813. [Google Scholar] [CrossRef]
- Natsuda, S.; Saito, T.; Shirouchi, R.; Sakamoto, Y.; Takeyama, T.; Tamai, Y.; Ohkita, H. Cascaded energy landscape as a key driver for slow yet efficient charge separation with small energy offset in organic solar cells. Energy Environ. Sci. 2022, 15, 1545–1555. [Google Scholar] [CrossRef]
- Armin, A.; Li, W.; Sandberg, O.J.; Xiao, Z.; Ding, L.; Nelson, J.; Neher, D.; Vandewal, K.; Shoaee, S.; Wang, T.A.; et al. History and Perspective of Non-Fullerene Electron Acceptors for Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003570. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G. Non-Fullerene Acceptors with Branched Side Chains and Improved Molecular Packing to Exceed 18% Efficiency in Organic Solar Cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Li, G.; Feng, L.-W.; Mukherjee, S.; Jones, L.O.; Jacobberger, R.M.; Huang, W.; Young, R.M.; Pankow, R.M.; Zhu, W.; Lu, N.; et al. Non-Fullerene Acceptors with Direct and Indirect Hexa-Fluorination Afford > 17% Efficiency in Polymer Solar Cells. Energy Environ. Sci. 2022, 15, 645–659. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Zhong, H.; Everett, C.R.; Jiang, X.; Reus, M.A.; Chumakov, A.; Roth, S.V.; Adedeji, M.A.; Jili, N.; et al. Control of the Crystallization and Phase Separation Kinetics in Sequential Blade-Coated Organic Solar Cells by Optimizing the Upper Layer Processing Solvent. Adv. Energy Mater. 2023, 13, 2203496. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Gasparini, N.; Jiao, X.; Heumueller, T.; Baran, D.; Matt, G.J.; Fladischer, S.; Spiecker, E.; Ade, H.; Brabec, C.J.; Ameri, T. Designing ternary blend bulk heterojunction solar cells with reduced carrier recombination and a fill factor of 77%. Nat. Energy 2016, 1, 16118. [Google Scholar] [CrossRef]
- Clark, T.M.; Durrant, J.R. Charge Photogeneration in Organic Solar Cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Ledari, R.; Ganjali, F.; Zarei-Shokat, S.; Saeidirad, M.; Ansari, F.; Forouzandeh-Malati, M.; Hassanzadeh-Afruzi, F.; Hashemi, S.M.; Maleki, A. A Review of Metal-Free Organic Halide Perovskite: Future Directions for the Next Generation of Solar Cells. Energy Fuels 2022, 36, 10702–10720. [Google Scholar] [CrossRef]
- Pezhooli, N.; Rahimi, J.; Hasti, J.; Maleki, A. Synthesis and evaluation of composite TiO2@ZnO quantum dots on hybrid nanostructure perovskite solar cell. Sci. Rep. 2022, 12, 9885. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ma, W.L.; Brabec, C.J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing Additives for Improved Efficiency from Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2008, 130, 3619. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.J.; Szarko, J.M.; Xu, T.; Yu, L.; Marks, T.J.; Chen, L.X. Effects of Additives on the Morphology of Solution Phase Aggregates Formed by Active Layer Components of High-Efficiency Organic Solar Cells. J. Am. Chem. Soc. 2011, 133, 20661. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, C.; Russell, T.P. Multi-Length-Scale Morphologies in PCPDTBT/PCBM Bulk-Heterojunction Solar Cells. Adv. Energy Mater. 2012, 2, 683. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 2012, 11, 44. [Google Scholar] [CrossRef]
- Lee, T.K.; Park, S.Y.; Walker, B.; Ko, S.-J.; Heo, J.; Woo, H.Y.; Choi, H.; Kim, J.Y. A universal processing additive for high-performance polymer solar cells. RSC Adv. 2017, 7, 7476–7482. [Google Scholar] [CrossRef]
- Guo, X.; Cui, C.; Zhang, M.; Huo, L.; Huang, Y.; Hou, J.; Li, Y. High efficiency polymer solar cells based on poly(3-hexylthiophene)/indene-C70 bisadduct with solvent additive. Energy Environ. Sci. 2012, 5, 7943. [Google Scholar] [CrossRef]
- Wen, Z.-C.; Yin, H.; Hao, X.-T. Recent progress of PM6:Y6-based high efficiency organic solar cells. Surf. Interfaces 2021, 23, 100921. [Google Scholar] [CrossRef]
- Wu, J.; Lee, J.; Chin, Y.-C.; Yao, H.; Cha, H.; Luke, J.; Hou, J.; Kim, J.-S.; Durrant, J.R. Exceptionally Low Charge Trapping Enables Highly Efficient Organic Bulk Heterojunction Solar Cells. Energy Environ. Sci. 2020, 13, 2422–2430. [Google Scholar] [CrossRef]
- Perdigón-Toro, L.; Zhang, H.; Markina, A.; Yuan, J.; Hosseini, S.M.; Wolff, C.M.; Zuo, G.; Stolterfoht, M.; Zou, Y.; Gao, F.; et al. Barrierless Free Charge Generation in the High-Performance PM6:Y6 Bulk Heterojunction Non-Fullerene Solar Cell. Adv. Mater. 2020, 32, 1906763. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lee, T.H.; Chin, Y.-C.; Pacalaj, R.A.; Labanti, C.; Park, S.Y.; Dong, Y.; Cho, H.W.; Kim, J.Y.; Minami, D.; et al. Molecular orientation-dependent energetic shifts in solution-processed non-fullerene acceptors and their impact on organic photovoltaic performance. Nat. Commun. 2023, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Eisner, F.; Azzouzi, M.; Fei, Z.; Hou, X.; Anthopoulos, T.D.; Dennis, T.J.S.; Heeney, M.J.; Nelson, J. Hybridization of Local Exciton and Charge-Transfer States Reduces Non-Radiative Voltage Losses in Organic Solar Cells. J. Am. Chem. Soc. 2019, 141, 6362–6374. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Li, Q.; Zhang, Z.; Wang, X.; Xiao, M. Charge Separation from an Intra-Moiety Intermediate State in the High-Performance PM6:Y6 Organic Photovoltaic Blend. J. Am. Chem. Soc. 2020, 142, 12751–12759. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, G.; Lee, D.; Park, C.; Cha, H. Charge Carrier Dynamics in Non-Fullerene Acceptor-Based Organic Solar Cells: Investigating the Influence of Processing Additives Using Transient Absorption Spectroscopy. Materials 2023, 16, 5712. https://doi.org/10.3390/ma16165712
Ham G, Lee D, Park C, Cha H. Charge Carrier Dynamics in Non-Fullerene Acceptor-Based Organic Solar Cells: Investigating the Influence of Processing Additives Using Transient Absorption Spectroscopy. Materials. 2023; 16(16):5712. https://doi.org/10.3390/ma16165712
Chicago/Turabian StyleHam, Gayoung, Damin Lee, Changwoo Park, and Hyojung Cha. 2023. "Charge Carrier Dynamics in Non-Fullerene Acceptor-Based Organic Solar Cells: Investigating the Influence of Processing Additives Using Transient Absorption Spectroscopy" Materials 16, no. 16: 5712. https://doi.org/10.3390/ma16165712
APA StyleHam, G., Lee, D., Park, C., & Cha, H. (2023). Charge Carrier Dynamics in Non-Fullerene Acceptor-Based Organic Solar Cells: Investigating the Influence of Processing Additives Using Transient Absorption Spectroscopy. Materials, 16(16), 5712. https://doi.org/10.3390/ma16165712





