Formation and Characterization of Stable TiO2/CuxO-Based Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
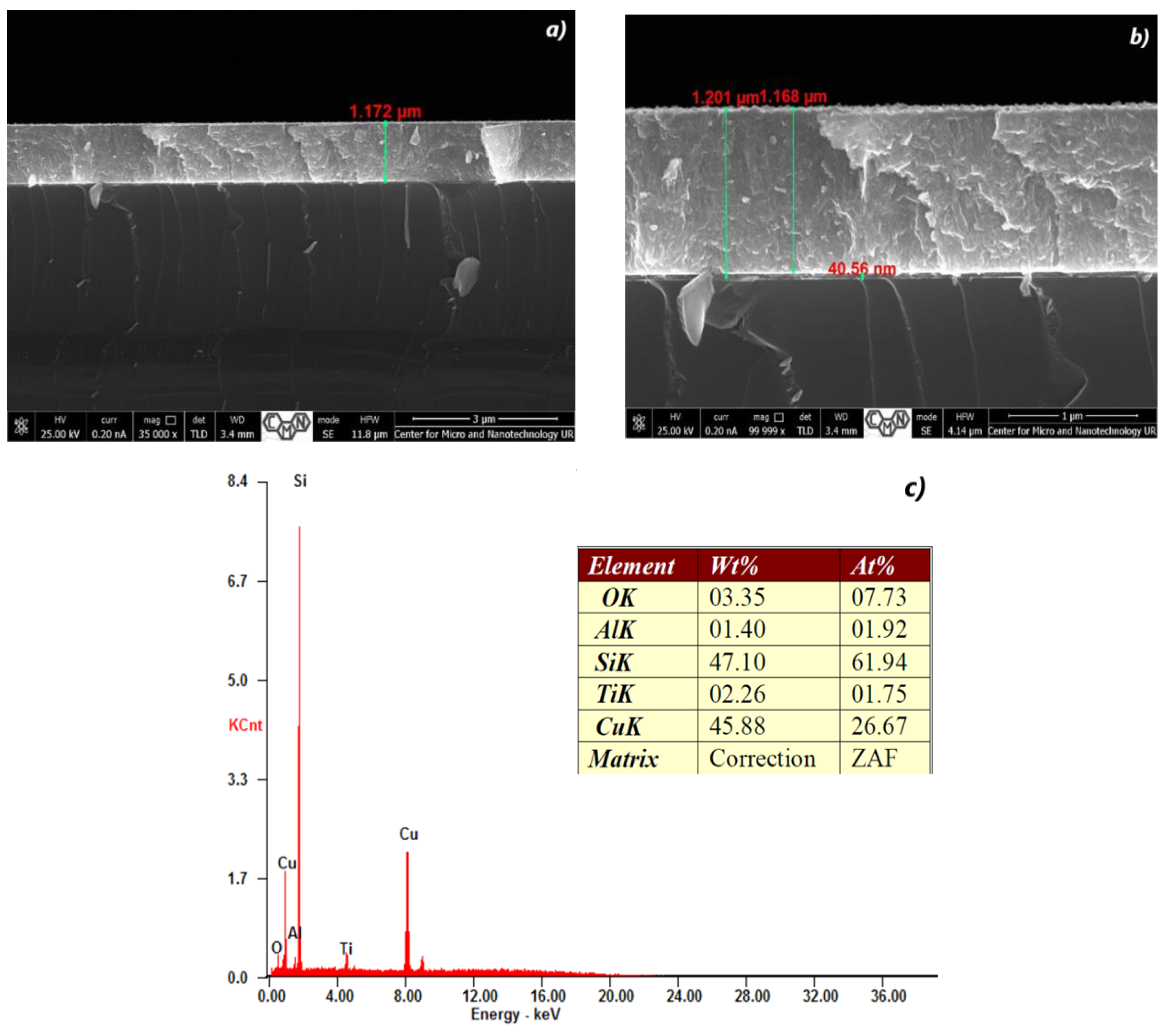
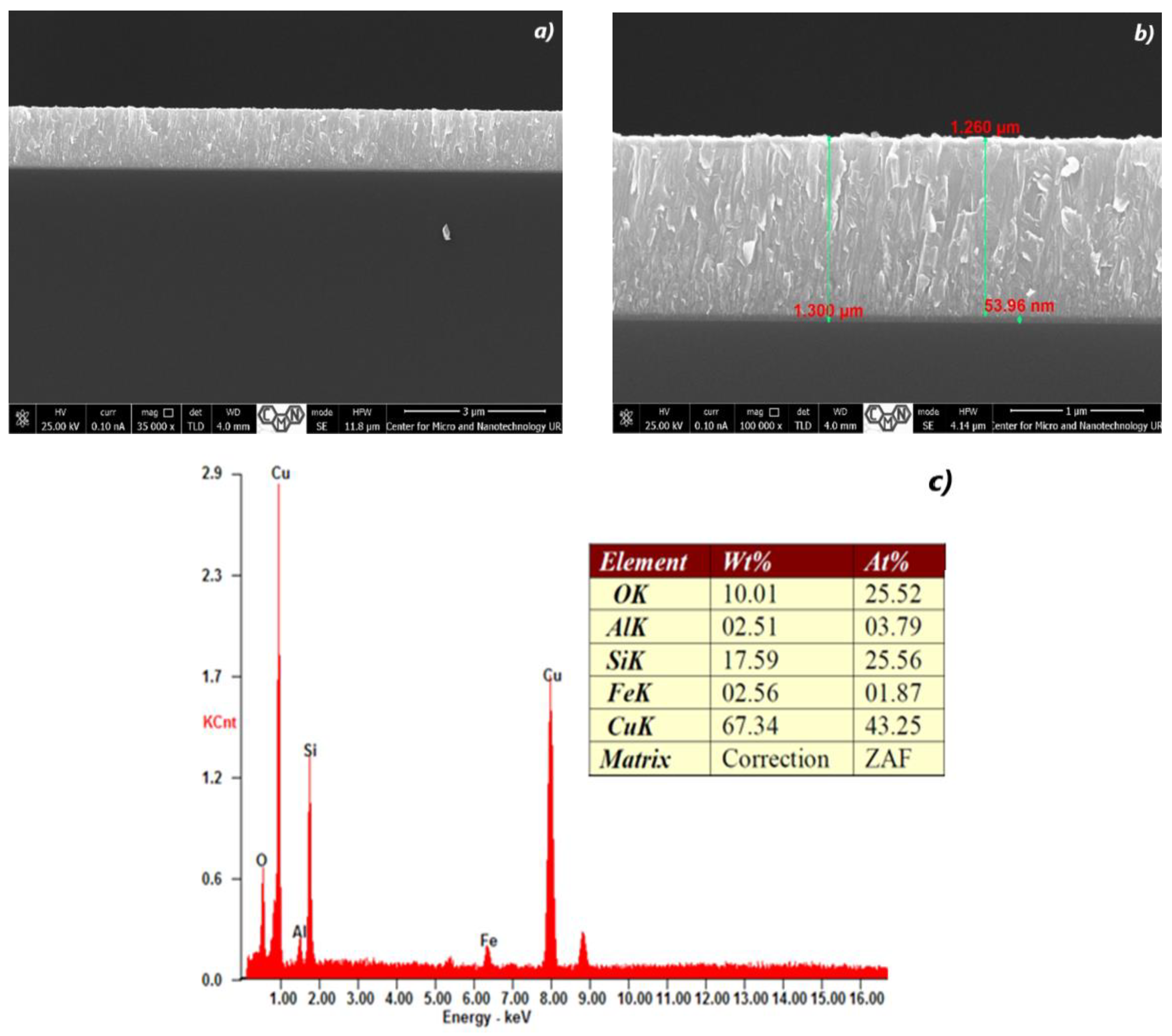
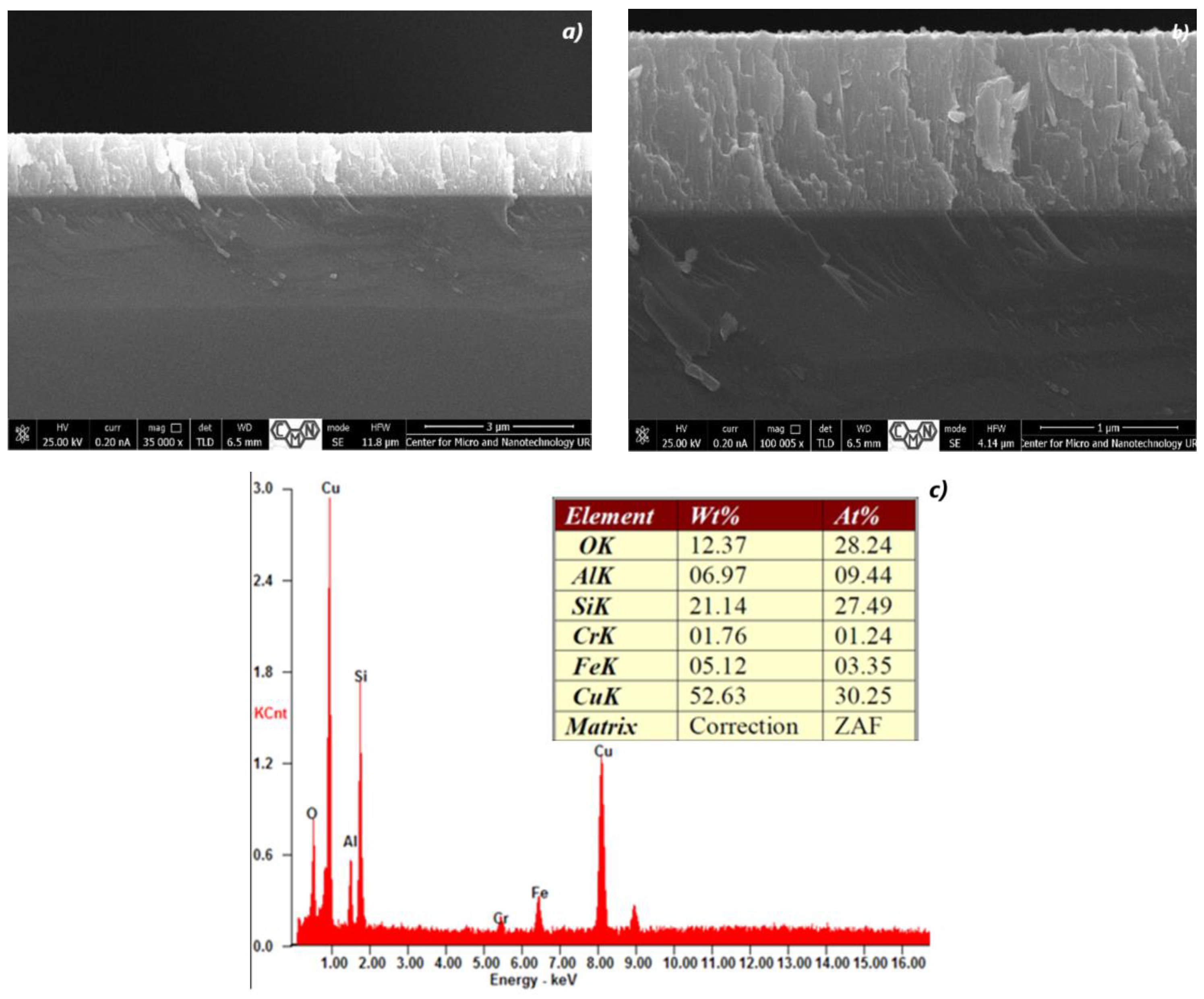
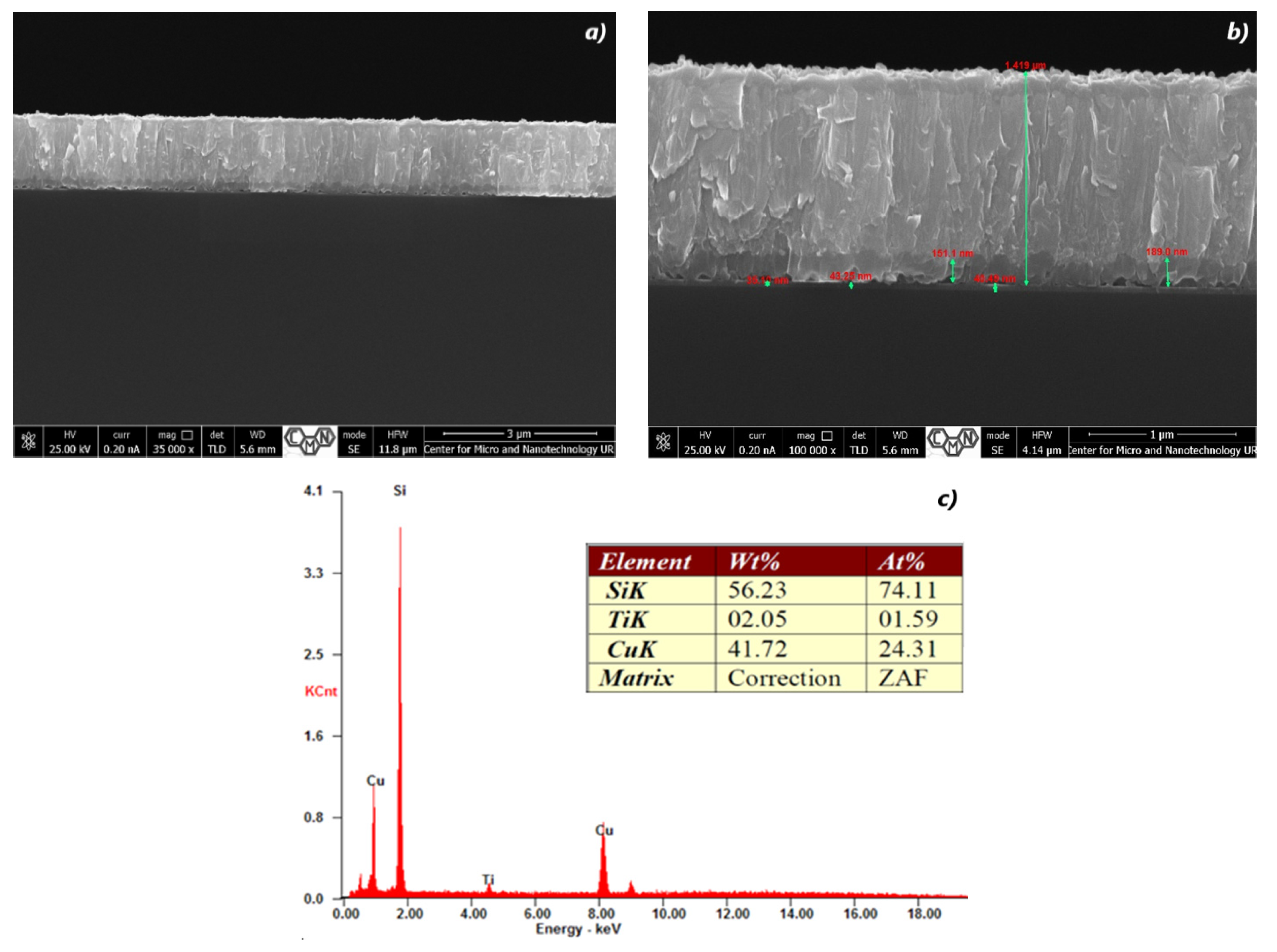
3.1. Cross Section Analysis of TiO2/CuxO Thin Films
3.2. Solar Cell Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Ji, X.; Li, M.; Lin, W.; Luo, X.; Zhang, H. A Review on Development Prospect of CZTS Based Thin Film Solar Cells. Int. J. Photoenergy 2014, 2014, 613173. [Google Scholar] [CrossRef]
- GuGuo, H.; Ma, C.; Zhang, K.; Jia, X.; Wang, X.; Yuan, N.; Ding, J. Dual function of ultrathin Ti intermediate layers in CZTS solar cells: Sulfur blocking and charge enhancement. Sol. Energy Mater. Sol. Cells 2018, 175, 20–28. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.D. Role of contact work function, back surface field, and conduction band offset in Cu2ZnSnS4 solar cell. Jpn. J. Appl. Phys. 2018, 57, 8RC05. [Google Scholar] [CrossRef]
- Lee, D.; Yang, J.Y. Superstrate structured FTO/TiO2/In2S3/Cu2ZnSnS4 solar cells fabricated by a spray method with aqueous solutions. Coatings 2020, 10, 548. [Google Scholar] [CrossRef]
- Satale, V.V.; Bhat, S.V. Superstrate type CZTS solar cell with all solution processed functional layers at low temperature. Sol. Energy 2020, 208, 220–226. [Google Scholar] [CrossRef]
- Khalil, M.I.; Bernasconi, R.; Lucotti, A.; Le Donne, A.; Mereu, R.A.; Binetti, S.; Hart, J.L.; Taheri, M.L.; Nobili, L.; Magagnin, L. CZTS thin film solar cells on flexible Molybdenum foil by electrodeposition-annealing route. J. Appl. Electrochem. 2021, 51, 209–218. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Yuan, X.; Huang, J.; Green, M.A.; Hao, X. Emergence of flexible kesterite solar cells: Progress and perspectives. Npj Flex. Electron. 2023, 7, 16. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, C.A.; Morales-Acevedo, A.; Cardona, D.; Contreras-Puente, G.; López-López, M. Analysis of the performance of InxGa1−xN based solar cells. SN Appl. Sci. 2019, 1, 628. [Google Scholar] [CrossRef]
- Jose, R.; Velmurugan, T.; Seeram, R. Metal oxides for dye-sensitized solar cells. J. Am. Ceram. Soc. 2009, 92, 289–301. [Google Scholar] [CrossRef]
- Joni, H.; Jyrki, L.; Jarkko, P.; Tomi, H.; Anita, S.; Samuli, K. Pulsed laser deposition of metal oxide nanoparticles, agglomerates, and nanotrees for chemical sensors. Proc. Eng. 2015, 120, 1158–1161. [Google Scholar]
- Ho, S.M. Synthesis and properties of cadmium oxide thin films: A review. Int. J. Curr. Adv. Res. 2016, 5, 1038–1041. [Google Scholar]
- Son, M.-K.; Steier, L.; Schreier, M.; Mayer, M.T.; Luo, J.; Grätzel, M. A copper nickel mixed oxide hole selective layer for Au-free transparent cuprous oxide photocathodes. Energy Environ. Sci. 2017, 4, 912–918. [Google Scholar] [CrossRef]
- Pérez-Tomás, A.; Mingorance, A.; Tanenbaum, D.; Lira-Cantú, M. Chapter 8—Metal Oxides in Photovoltaics: All-Oxide, Ferroic, and Perovskite Solar Cells. In Metal Oxides Series: The Future of Semiconductor Oxides in Next-Generation Solar Cells; Lira-Cantú, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–365. [Google Scholar]
- He, H. 2—Metal oxide semiconductors and conductors. In Metal Oxide Series: Solution Processed Metal Oxide Thin Films for Electronic Applications; Cui, Z., Korotcenkov, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–30. [Google Scholar]
- Horta, I.M.; Godoy, A., Jr.; Damasceno, B.S.; de Jesus Pereira, D.L.; Leite, D.M.G.; da Silva Sobrinho, A.S. 10—Development of metal oxide heterostructures for photovoltaic and solar cell applications. In Metal Oxide-Based Heterostructures Fabrication and Applications; Kumar, N., Soucase, B.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 359–389. [Google Scholar]
- Lee, Y.S.; Chua, D.; Brandt, R.E.; Siah, S.C.; Li, J.V.; Mailoa, J.P.; Lee, S.W.; Gordon, R.G.; Buonassisi, T. Atomic layer deposited gallium oxide buffer layer enables 1.2 V open-circuit voltage in cuprous oxide solar cells. Adv. Mater. 2014, 26, 4704–4710. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.; Rühle, S.; Ginsburg, A.; Keller, D.A.; Barad, H.-N.; Sberna, P.M.; Nunes, D.; Martins, R.; Anderson, A.Y.; Zaban, A.; et al. TiO2/Cu2O all-oxide heterojunction solar cells produced by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2015, 132, 549–556. [Google Scholar] [CrossRef]
- Rokhmat, M.; Wibowo, E.; Sutisna, K.; Abdullah, M. Performance improvement of TiO2/CuO solar cell by growing copper particle using fix current electroplating method. Procedia Eng. 2017, 170, 72–77. [Google Scholar] [CrossRef]
- Iivonen, T.; Heikkilä, M.J.; Popov, G.; Nieminen, H.-E.; Kaipio, M.; Kemell, M.; Mattinen, M.; Meinander, K.; Mizohata, K.; Räisänen, J.; et al. Atomic layer deposition of photoconductive Cu2O thin films. ACS Omega 2019, 4, 11205–11214. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Obstarczyk, A.; Domaradzki, J.; Kaczmarek, D.; Zakrzewska, K.; Pastuszek, R. Investigations of structure and electrical properties of TiO2/CuO thin film heterostructures. Thin Solid Films 2019, 690, 137538. [Google Scholar] [CrossRef]
- Patel, D.B.; Chauhan, K.R. 50% transparent solar cells of CuOx/TiO2: Device aspects. J. Alloys Compd. 2020, 842, 155594. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Rybak-Wilusz, E.; Cholewa, M.; Wisz, G.; Yavorskyi, R. Review of the development of copper oxides with titanium dioxide thin film solar cells. AIP Adv. 2020, 10, 10701. [Google Scholar] [CrossRef]
- Ghrib, T.; AL-Saleem, N.K.; AL-Naghmaish, A.; Elshekhipy, A.A.; Brini, S.; Briki, K.; Elsayed, K.A. Annealing effect on the microstructural, optical, electrical, and thermal properties of Cu2O/TiO2/Cu2O/TiO2/Si heterojunction prepared by sol-gel technique. Micro Nanostr. 2022, 164, 107119. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tareja, R.K.; Wilker, U.; Schade, W. Phase transformation in room temperature pulsed laser deposited TiO2 thin films. Appl. Surf. Sci. 2003, 206, 137–148. [Google Scholar] [CrossRef]
- Hussain, S.; Cao, C.; Usman, Z.; Chen, Z.; Nabi, G.; Khan, W.S.; Ali, Z.; Butt, F.K.; Mahmood, T. Fabrication and photovoltaic characteristics of Cu2O/TiO2 thin film heterojunction solar cell. Thin Solid Films 2012, 522, 430–434. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zheng, G.; Pei, S.; Wang, J. Investigation of optical bandgap variation and photoluminescence behavior in nanocrystalline CuO thin films. Optik 2018, 158, 382–390. [Google Scholar] [CrossRef]
- Tripathi, T.S.; Terasaki, I.; Karppinen, M. Anomalous thickness-dependent optical energy gap of ALD-grown ultra-thin CuO films. J. Phys. Condens. Matter 2016, 28, 475801. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, Y.; Peng, X.; Zhong, K.; Lin, Y.; Huang, Z. The phase evolution and physical properties of binary copper oxide thin films prepared by reactive magnetron sputtering. Materials 2018, 11, 1253. [Google Scholar] [CrossRef]
- Diachenko, O.; Kováč, J., Jr.; Dobrozhan, O.; Novák, P.; Kováč, J.; Skriniarova, J.; Opanasyuk, A. Structural and optical properties of CuO thin films, synthesized using spray pyrolysis method. Coatings 2021, 11, 1392. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Alex, Z.C.; Meher, S.R. Recent advances in cuprous oxide thin film based photovoltaics. Mater. Today Sustain. 2022, 20, 100244. [Google Scholar] [CrossRef]
- Ichimura, M.; Kato, Y. Fabrication of TiO2/Cu2O heterojunction solar cells by electrophoretic deposition and electrodeposition. Mat. Sci. Semicon. Proc. 2013, 16, 1538–1541. [Google Scholar] [CrossRef]
- Tahir, D.; Tougaard, S. Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J. Phys. Condens. Matter 2012, 24, 175002. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.; Alex, Z.C.; Meher, S.R. Cu2O thin films grown by magnetron sputtering as solar cell absorber layers. Mat. Sci. Semicon. Proc. 2022, 148, 106818. [Google Scholar] [CrossRef]
- Pagare, P.K.; Torane, A.P. Band gap varied cuprous oxide (Cu2O) thin films as a tool for glucose sensing. Microchim. Acta 2016, 183, 2983–2989. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Wal, A.; Potera, P.; Bester, M.; Płoch, D.; Sibiński, M.; Cholewa, M.; Ruszała, M. TiO2:ZnO/CuO thin film solar cells prepared via reactive direct-current (DC) magnetron sputtering. Appl. Mater. Today 2022, 29, 101673. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Sibiński, M.; Starowicz, Z.; Płoch, D.; Góral, A.; Bester, M.; Cholewa, M.; Woźny, J.; Sosna-Głębska, A. Solar cells based on copper oxide and titanium dioxide prepared by reactive direct-current magnetron sputtering. Opto-Electron. Rev. 2021, 29, 97–104. [Google Scholar]
- Bremner, S.P.; Yi, C.; Almansouri, I.; Ho-Baillie, A.; Green, M.A. Optimum band gap combinations to make best use of new photovoltaic materials. Sol. Energy 2016, 135, 750–757. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Wal, A.; Sibiński, M.; Potera, P.; Yavorskyi, R.; Nykyruy, L.; Płoch, D.; Bester, M.; Cholewa, M.; et al. Structure defects and photovoltaic properties of TiO2:ZnO/CuO solar cells prepared by reactive DC magnetron sputtering. Appl. Sci. 2023, 13, 3613. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Tsai, C.H.; Fei, P.H.; Chen, C.H. Investigation of coral-like Cu2O nano/microstructures as counter electrodes for dye-sensitized solar cells. Materials 2015, 8, 5715–5729. [Google Scholar] [CrossRef]
- Baptayev, B.; Salimgerey, A.; Balanay, M.P. Surface modification of TiO2 photoanodes with In3+ using a simple soaking technique for enhancing the efficiency of dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2020, 394, 112468. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Gratzel, M. Enhance the optical absorptivity of nanocrystalline TiO2 film with high molar extinction coefficient ruthenium sensitizers for high performance dye sensitized solar cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Song, D.M.; Qiang, Y.H.; Gu, X.Q.; Zhu, L.; Song, C.B. Dye-sensitized solar cells based on TiO2 hollow spheres/TiO2 nanotube array composite films. Appl. Surf. Sci. 2014, 309, 85–89. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Yu, Q.; Wang, J.; Yu, C.; Wang, J.; Gao, S.; Jiao, S.; Zhang, X.; Wang, P. Single-layer TiO2 film composed of mesoporous spheres for high-efficiency and stable dye-sensitized solar sells. ACS Sustain. Chem. Eng. 2018, 6, 3411–3418. [Google Scholar] [CrossRef]
- Ursu, D.; Vajda, M.; Miclau, M. Investigation of the p-type dye-sensitized solar cell based on full Cu2O electrodes. J. Alloys Compd. 2019, 802, 86–92. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, J.; Zhang, Y.; Huang, Z.; Gao, Y.; Li, X.; Du, Y. Unassisted solar water splitting using a Cu2O/Ni(OH)2-ZnO/Au tandem photoelectrochemical cell. J. Solid State Electr. 2020, 24, 321–328. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yang, Z.-L.; Hsu, Y.-K. Unassisted solar water splitting by dual Cu2O–based tandem device with complementary wavelength–dependent quantum efficiency and antipodal conductivity. Renew. Energy 2023, 212, 166–174. [Google Scholar] [CrossRef]
- Shahvaranfard, F.; Li, N.; Hosseinpour, S.; Hejazi, S.; Zhang, K.; Altomare, M.; Schmuki, P.; Brabec, C.J. Comparison of the sputtered TiO2 anatase and rutile thin films as electron transporting layers in perovskite solar cells. Nanoselect 2022, 3, 990–997. [Google Scholar]
- Zhu, H.; Zhang, T.-H.; Wei, Q.-Y.; Yu, S.-J.; Gao, H.; Guo, P.-C.; Li, J.-K.; Wang, Y.-X. Preparation of TiO2 electron transport layer by magnetron sputtering and its effect on the properties of perovskite solar cells. Energy Rep. 2022, 8, 3166–3175. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, Y.; Wang, Q.; Liu, Z.; Geng, R.; Wang, H.; Jiang, W.; Ding, W. Improving the performance of perovskite solar cells via TiO2 electron transport layer prepared by direct current pulsed magnetron sputtering. J. Alloys Compd. 2022, 929, 167278. [Google Scholar] [CrossRef]
- Valastro, S.; Smecca, E.; Mannino, G.; Bongiorno, C.; Fisicaro, G.; Goedecker, S.; Arena, V.; Spampinato, C.; Deretzis, I.; Dattilo, S.; et al. Preventing lead leakage in perovskite solar cells with a sustainable titanium dioxide sponge. Nat. Sustain. 2023, 6, 974–983. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Ye, S.; Rao, H.; Yan, W.; Peng, H.; Li, Y.; Liu, Z.; Wang, S.; Chen, Z.; et al. High-performance inverted planar heterojunction perovskite solar cells based on a solution-processed CuOx hole transport layer. Nanoscale 2016, 8, 10806–10813. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Chen, C.-C.; Liang, K.-C.; Chang, S.H.; Tseng, Z.-L.; Yeh, S.-C.; Chen, C.-T.; Wu, W.-T.; Wu, C.-G. Nano-structured CuO-Cu2O complex thin film for application in CH3NH3PbI3 perovskite solar cells. Nanoscale Res. Lett. 2016, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Arjun, V.; Muthukumaran, K.P.; Ramachandran, K.; Nithya, A.; Karuppuchamy, S. Fabrication of efficient and stable planar perovskite solar cell using copper oxide as hole transport material. J. Alloys Compd. 2022, 923, 166285. [Google Scholar] [CrossRef]
- Minami, T.; Nishi, Y.; Miyata, T. Effect of the thin Ga2O3 layer in n+-ZnO/n-Ga2O3/p-Cu2O heterojunction solar cells. Thin Solid Films 2013, 549, 65–69. [Google Scholar] [CrossRef]
- Minami, T.; Nishi, Y.; Miyata, T. High-efficiency Cu2O-based heterojunction solar cells fabricated using a Ga2O3 thin film as n-type layer. Appl. Phys. Express 2013, 6, 44101. [Google Scholar] [CrossRef]
- Rizi, M.T.; Abadi, M.H.S.; Ghaneii, M. Two dimensional modeling of Cu2O heterojunction solar cells based-on β-Ga2O3 buffer. Optik 2018, 155, 121–132. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, C.A.; Casallas-Moreno, Y.L.; Cardona, D.; Kudriavtsev, Y.; Santana-Rodríguez, G.; Mendoza-Pérez, R.; Contreras-Puente, G.; Mendez-Garcia, V.H.; Gallardo-Hernández, S.; Quevedo-Lopez, M.A.; et al. Characterization of n-GaN/p-GaAs NP heterojunctions. Superlattices Microstruct. 2019, 136, 106298. [Google Scholar] [CrossRef]
- Miyata, T.; Tokunaga, H.; Watanabe, K.; Ikenaga, N.; Minami, T. Photovoltaic properties of low-damage magnetron-sputtered n-type ZnO thin film/p-type Cu2O sheet heterojunction solar cells. Thin Solid Films 2020, 697, 137825. [Google Scholar] [CrossRef]
- Sekkat, A.; Bellet, D.; Chichignoud, G.; Muñoz-Rojas, D.; Kaminski-Cachopo, A. Unveiling key limitations of ZnO/Cu2O all-oxide solar cells through numerical simulations. ACS Appl. Energy Mater. 2022, 5, 5423–5433. [Google Scholar] [CrossRef]
- Mehta, M.; Avasthi, S. The possibility of gallium oxide (β-Ga2O3) heterojunction bipolar transistors. Phys. Scr. 2023, 98, 25013. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Pawełek, R.; Wisz, G.; Cieniek, B.; Potera, P.; Szczepan, P.; Adamiak, S.; Cholewa, M.; Głowa, Ł. Characteristics of TiO2, Cu2O and TiO2/Cu2O thin films for application in PV devices. AIP Adv. 2019, 9, 2158–3226. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Wisz, G.; Sibiński, M.; Starowicz, Z.; Głowa, Ł.; Szczerba, M.; Cholewa, M. Performance improvement of TiO2/CuO by increasing oxygen flow rates and substrate temperature using DC reactive magnetron sputtering method. Optik 2020, 206, 164297. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Sibiński, M.; Płoch, D.; Bester, M.; Cholewa, M.; Woźny, J.; Yavorskyi, R.; Nykyruy, L.; Ruszała, M. TiO2/CuO/Cu2O Photovoltaic Nanostructures Prepared by DC Reactive Magnetron Sputtering. Nanomaterials 2022, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399. [Google Scholar] [CrossRef]
- Saliy, Y.P.; Nykyruy, L.I.; Yavorskyi, R.S.; Adamiak, S. The Surface Morphology of CdTe Thin Films Obtained by Open Evaporation in Vacuum. J. Nano Electron. Phys. 2017, 9, 410–416. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of growth defects in thin films prepared by PVD techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Yavorskyi, R.S. Features of optical properties of high stable CdTe photovoltaic absorber layer. Phys. Chem. Solid State 2020, 21, 243–253. [Google Scholar] [CrossRef]
- Venables, J.A. Nucleation and growth processes in thin film formation. J. Vac. Sci. Technol. B 1986, 4, 870–873. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Yavorskyi, R.; Potera, P.; Bester, M. TiO2/Cu2O heterojunctions for photovoltaic cells application produced by reactive magnetron sputtering. Mater. Today Proc. 2021, 35, 552–557. [Google Scholar] [CrossRef]
- Venables, J.A. Nucleation and growth of thin films: Recent progress. Vacuum 1983, 33, 701–705. [Google Scholar] [CrossRef]
- Robins, J.L. Problems and progress in describing quantitatively the development of thin film deposits. Surf. Sci. 1979, 86, 1–13. [Google Scholar] [CrossRef]
- Usher, B.F.; Robins, J.L. The mobility of gold clusters on sodium chloride at temperatures between 123 K and ambient. Thin Solid Films 1982, 90, 15–18. [Google Scholar] [CrossRef]
- Gates, A.D.; Robins, J.L. Heterogeneous nucleation on cleavage steps: I. Theory. Surf. Sci. 1982, 116, 188–204. [Google Scholar] [CrossRef]
- Harsdorff, M. Heterogeneous nucleation and growth of thin films. Thin Solid Films 1982, 90, 1–14. [Google Scholar] [CrossRef]
- Harsdorff, M. The influence of charged point defects and contamination of substrate surfaces on nucleation. Thin Solid Films 1984, 116, 55–74. [Google Scholar] [CrossRef]



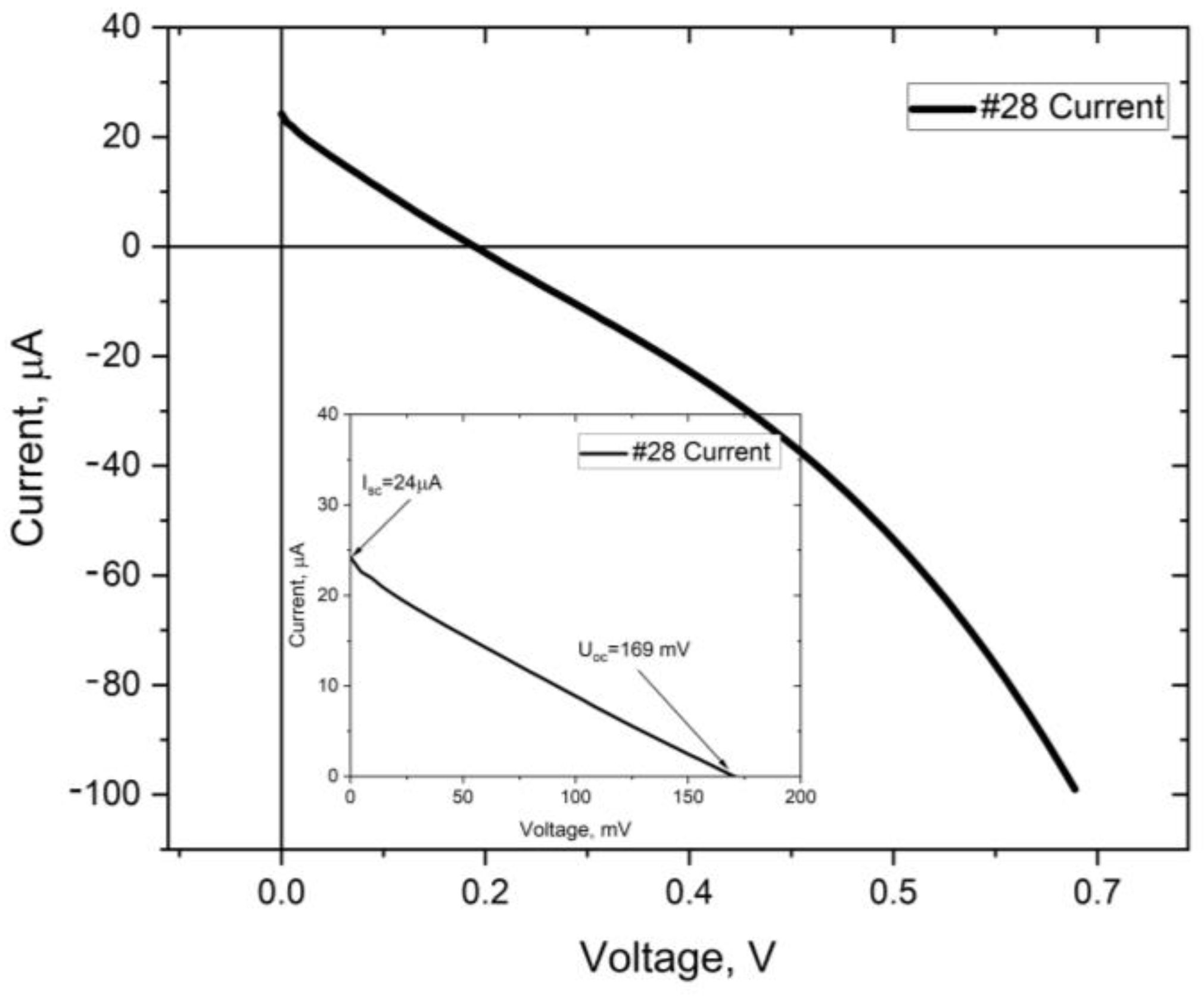
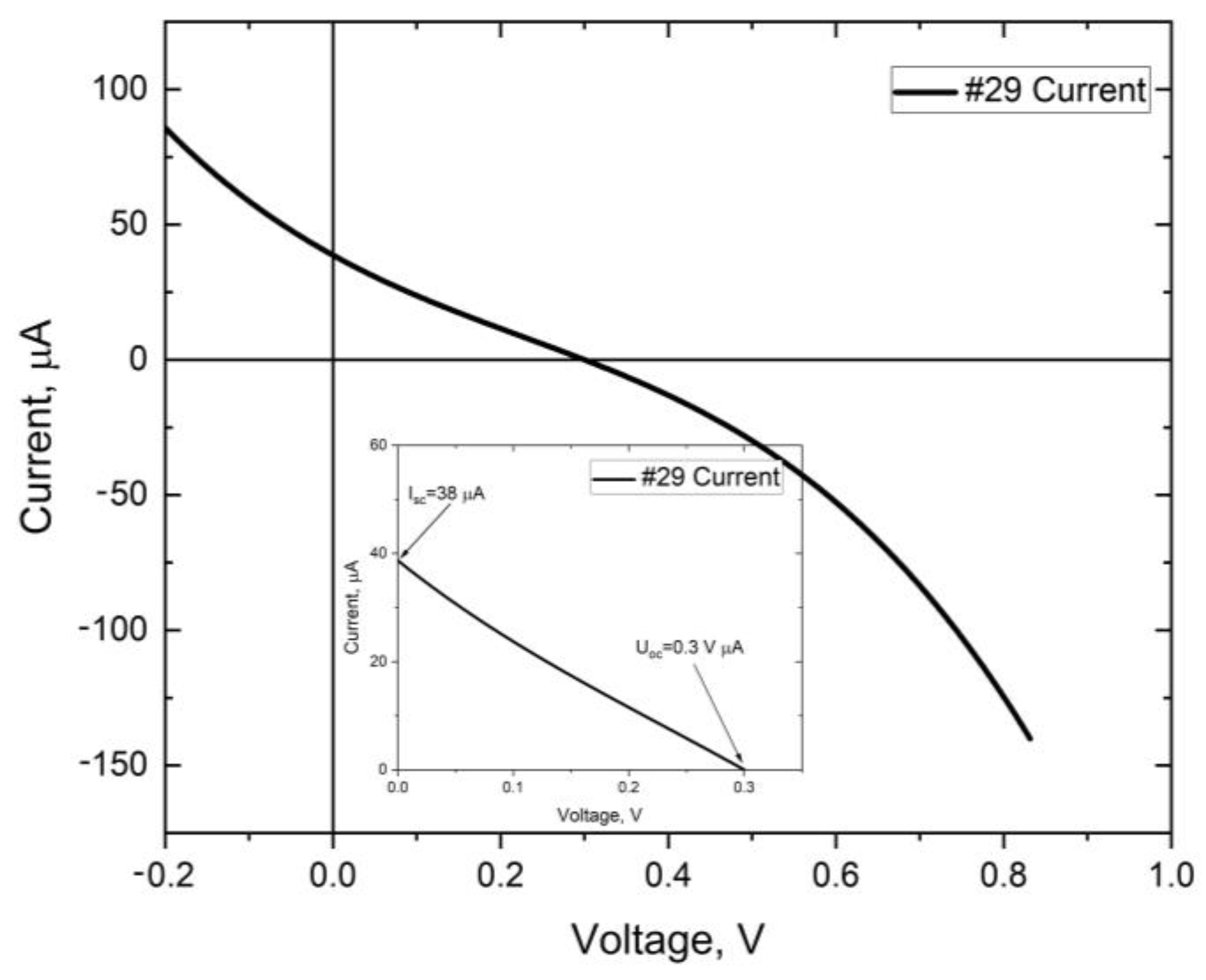
| #28 | #29 | #32 | #35 | #37 | #38 | |
|---|---|---|---|---|---|---|
| Time [min] | 25 | 24 | ||||
| Power [W] | 100 | |||||
| Pressure [mbar] | 1.15 × 10−2 | 1.05 × 10−2 | 1.11 × 10−2 | 1.08 × 10−2 | 1.06 × 10−2 | 1.11 × 10−2 |
| Distance between the source and substrate [mm] | 58 | |||||
| Oxygen flow rate [cm3/s] | 2.5 | |||||
| Argon flow rate [cm3/s] | 1 | |||||
| Substrate temperature [°C] | 300 | |||||
| Thickness [nm] | 49 | 41 | 54 | 43 | 49 | 45 |
| #28 | #29 | #32 | #35 | #37 | #38 | |
|---|---|---|---|---|---|---|
| Time [min] | 30 | 22 | 25 | 22 | ||
| Power [W] | 70 | |||||
| Pressure [mbar] | 1.11 × 10−2 | 1.11 × 10−2 | 1.14 × 10−2 | 1.11 × 10−2 | 1.11 × 10−2 | 1.10 × 10−2 |
| Distance between the source and substrate [mm] | 58 | |||||
| Oxygen flow rate [cm3/s] | 3.5 | |||||
| Argon flow rate [cm3/s] | 1 | |||||
| Substrate temperature [°C] | 300 | |||||
| Thickness [nm] | 1654 | 1201 | 1260 | 1305 | 1419 | 318 |
| #28 | #29 | #32 | #35 | #37 | #38 | |
|---|---|---|---|---|---|---|
| Voc [mV] | 176 | 300 | 75 | 14 | 23 | 71 |
| Isc [µA] | 21 | 38 | 4.3 | 7 | 1.5 | 5.3 |
| µ [%] | 0.01 | 0.01 | ||||
| Contact area [mm2] | 30 | 35 | 45 | 36 | 40 | |
| Voc/CuO thickness [mV/nm] | 0.10 | 0.25 | 0.06 | 0.01 | 0.02 | 0.22 |
| Voc/TiO2 thickness [mV/nm] | 3.59 | 7.32 | 1.39 | 0.33 | 0.47 | 1.58 |
| #28 | #29 | |
|---|---|---|
| Rs [Ω] | 27 k | 55 k |
| Rsh [Ω] | 6.2 k | 270 M |
| FF [%] | 18.5 | 16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisz, G.; Sawicka-Chudy, P.; Sibiński, M.; Yavorskyi, R.; Łabuz, M.; Płoch, D.; Bester, M. Formation and Characterization of Stable TiO2/CuxO-Based Solar Cells. Materials 2023, 16, 5683. https://doi.org/10.3390/ma16165683
Wisz G, Sawicka-Chudy P, Sibiński M, Yavorskyi R, Łabuz M, Płoch D, Bester M. Formation and Characterization of Stable TiO2/CuxO-Based Solar Cells. Materials. 2023; 16(16):5683. https://doi.org/10.3390/ma16165683
Chicago/Turabian StyleWisz, Grzegorz, Paulina Sawicka-Chudy, Maciej Sibiński, Rostyslav Yavorskyi, Mirosław Łabuz, Dariusz Płoch, and Mariusz Bester. 2023. "Formation and Characterization of Stable TiO2/CuxO-Based Solar Cells" Materials 16, no. 16: 5683. https://doi.org/10.3390/ma16165683
APA StyleWisz, G., Sawicka-Chudy, P., Sibiński, M., Yavorskyi, R., Łabuz, M., Płoch, D., & Bester, M. (2023). Formation and Characterization of Stable TiO2/CuxO-Based Solar Cells. Materials, 16(16), 5683. https://doi.org/10.3390/ma16165683










