Impact of Sintering Temperature Variation on Porous Structure of Mo2TiAlC2 Ceramics
Abstract
:1. Introduction
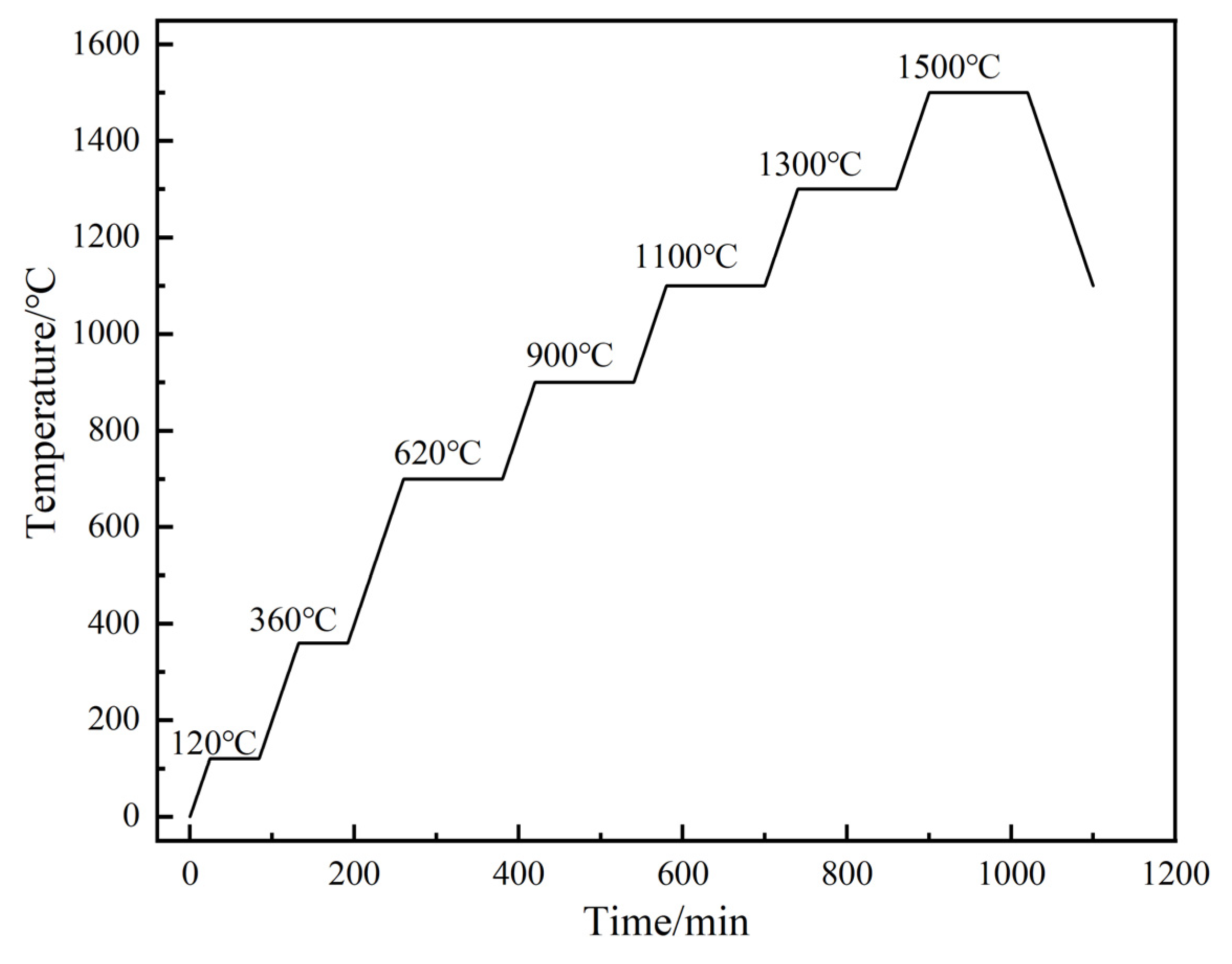
2. Experimental Preparation
3. Results and Discussion
3.1. X-ray Diffraction Analysis (XRD)
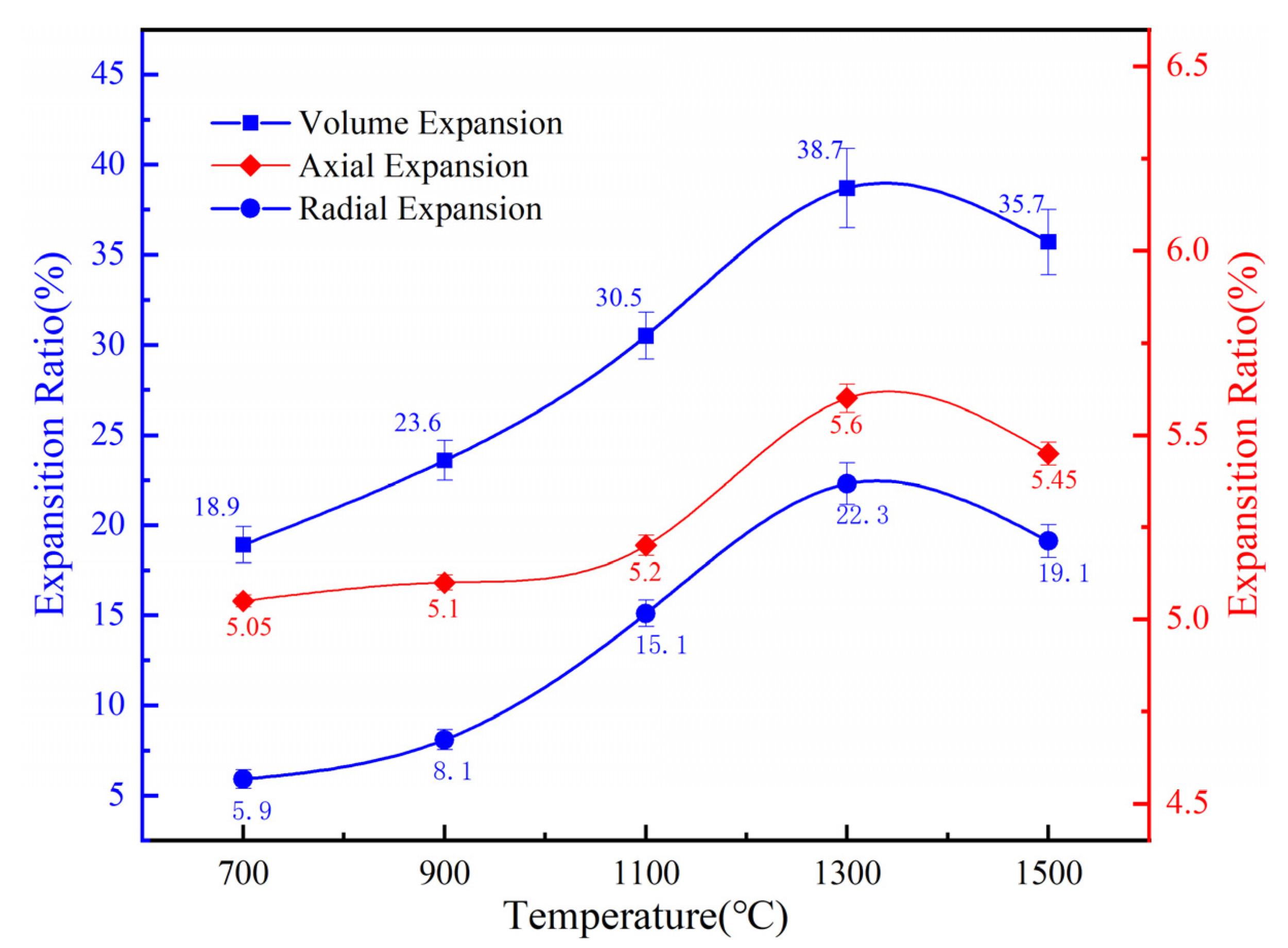
3.2. Effects of Temperature on Volume Evolution
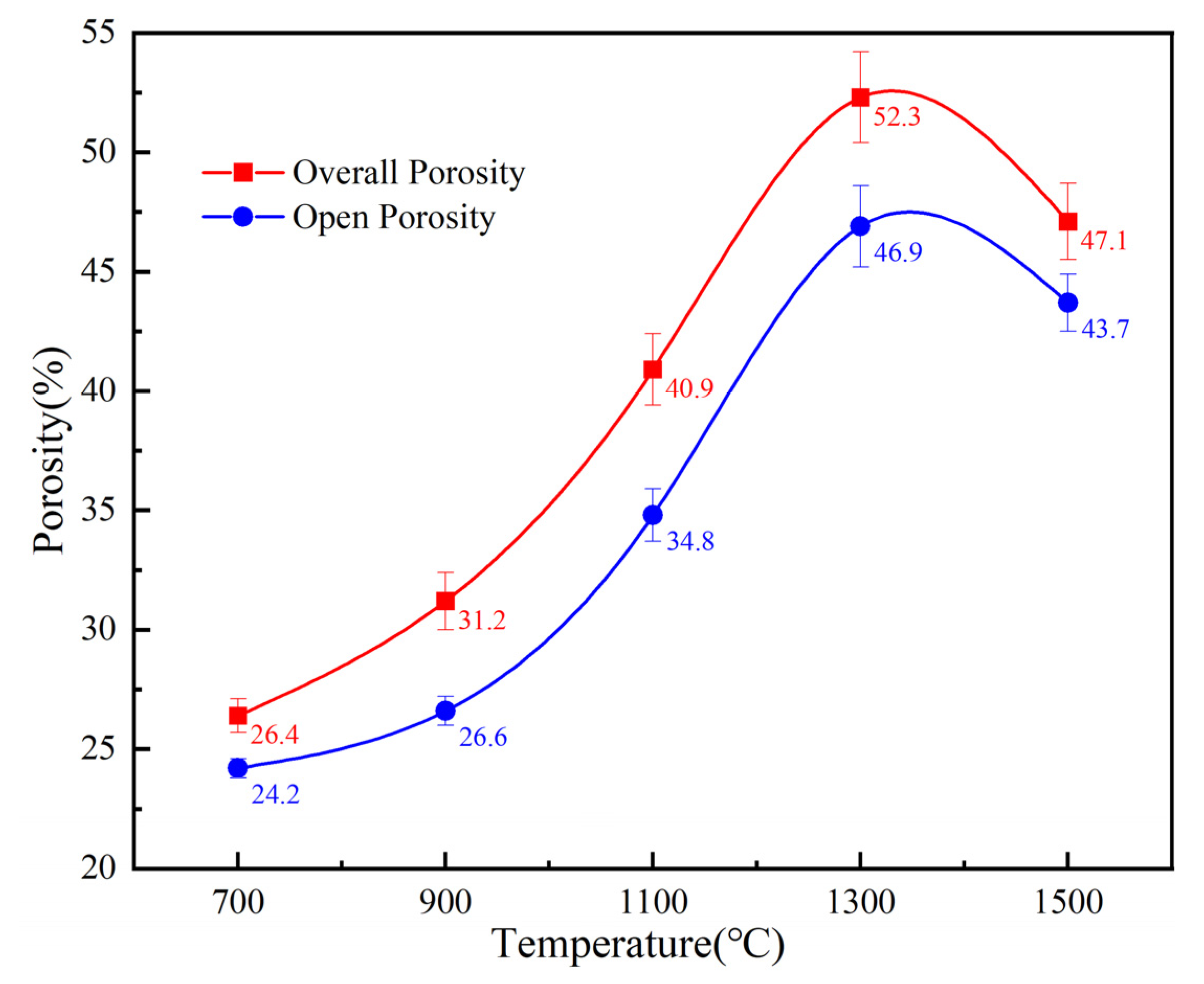
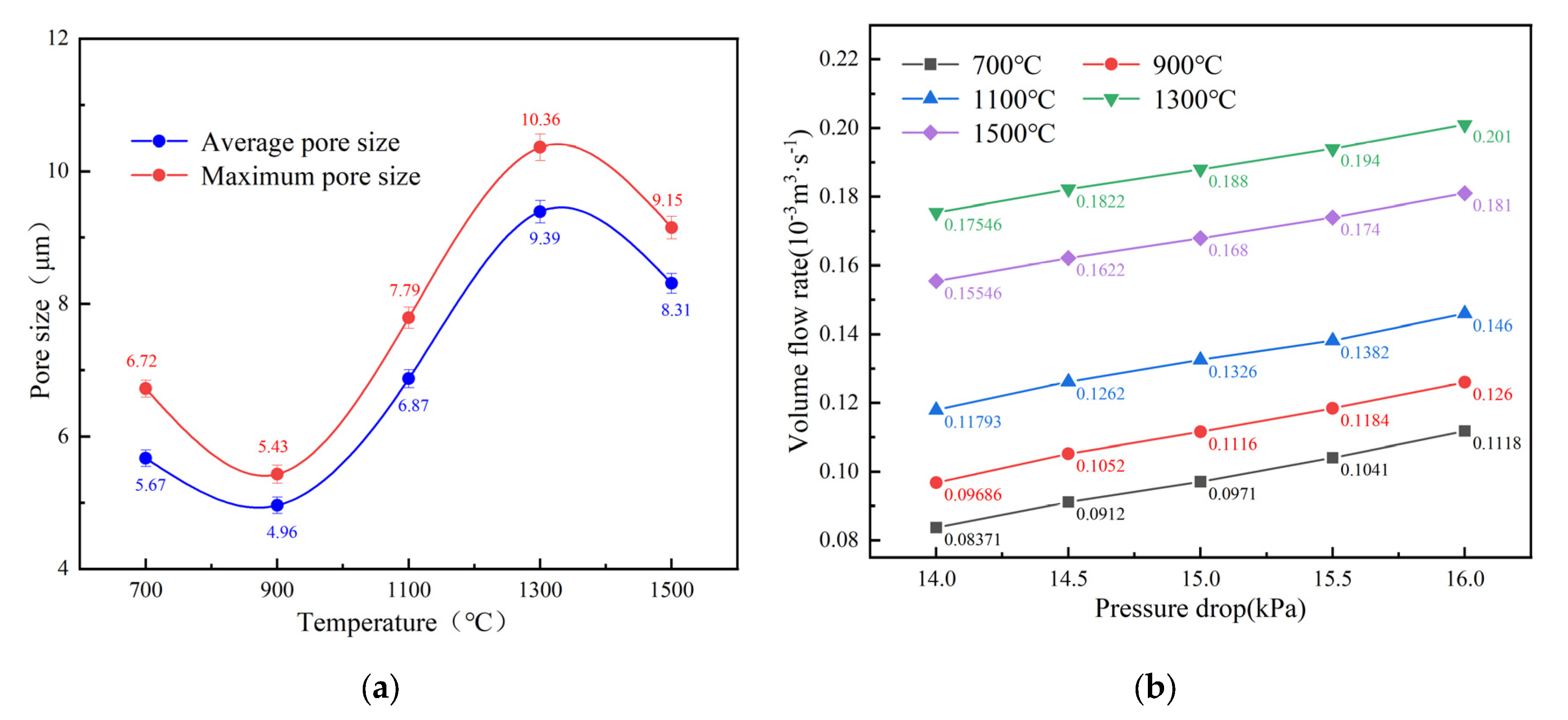
3.3. Pore Structure Parameters
3.4. Micromorphology of Mo2TiAlC2 Ceramic
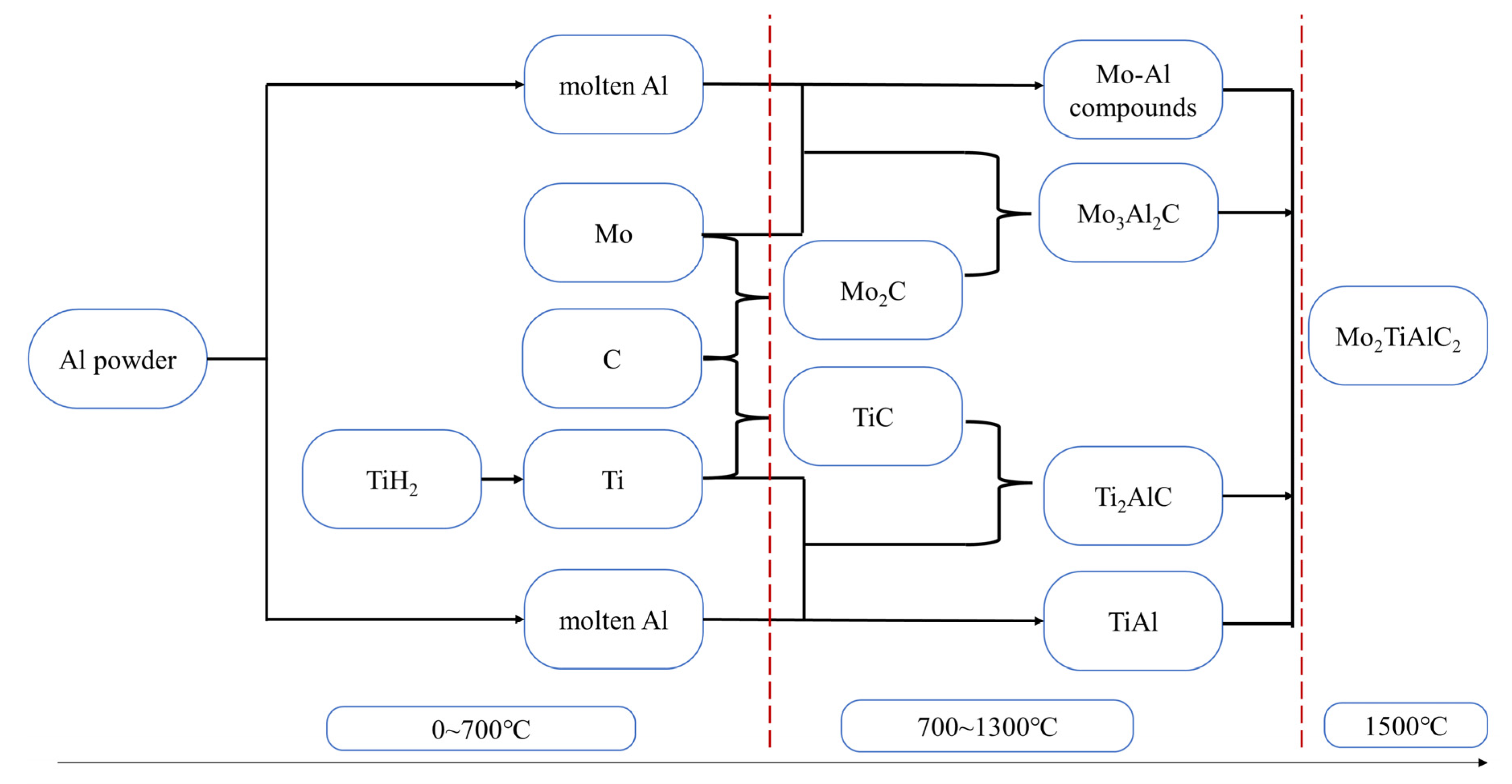
3.5. Pore Forming Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azina, C.; Tunca, B.; Petruhins, A.; Xin, B.; Yildizhan, M.; Persson, P.O.Å.; Vleugels, J.; Lambrinou, K.; Rosen, J.; Eklund, P. Deposition of MAX phase-containing thin films from a (Ti,Zr)2AlC compound target. Appl. Surf. Sci. 2021, 551, 149370. [Google Scholar] [CrossRef]
- Azzouz-Rached, A.; Hadi, M.A.; Rached, H.; Hadji, T.; Rached, D.; Bouhemadou, A. Pressure effects on the structural, elastic, magnetic and thermodynamic properties of Mn2AlC and Mn2SiC MAX phases. J. Alloys Compd. 2021, 885, 160998. [Google Scholar] [CrossRef]
- Badie, S.; Sebold, D.; Vaßen, R.; Guillon, O.; Gonzalez-Julian, J. Mechanism for breakaway oxidation of the Ti2AlC MAX phase. Acta Mater. 2021, 215, 117025. [Google Scholar] [CrossRef]
- Ghasali, E.; Derakhshandeh, M.R.; Orooji, Y.; Alizadeh, M.; Ebadzadeh, T. Effects of 211 and 413 ordering on the corrosion behavior of V-Al-C MAX phases prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2021, 41, 4774–4787. [Google Scholar] [CrossRef]
- Hadi, M.A. Superconducting phases in a remarkable class of metallic ceramics. J. Phys. Chem. Solids 2020, 138, 109275. [Google Scholar] [CrossRef]
- Rud, A.D.; Lakhnik, A.M.; Kirian, I.M.; Sizonenko, O.N.; Zaychenko, A.D.; Pristash, N.S.; Rud, N.D. Mechanochemical synthesis and structure of metal-carbon composites based on the MAX phases. Mater. Today: Proc. 2018, 5, 26084–26088. [Google Scholar] [CrossRef]
- Hossein-Zadeh, M.; Ghasali, E.; Mirzaee, O.; Mohammadian-Semnani, H.; Alizadeh, M.; Orooji, Y.; Ebadzadeh, T. An investigation into the microstructure and mechanical properties of V2AlC MAX phase prepared by microwave sintering. J. Alloys Compd. 2019, 795, 291–303. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Goulbourne, N.C. Heterogeneous strain evolution in representative polycrystalline MAX phases. Int. J. Solids Struct. 2016, 81, 13–22. [Google Scholar] [CrossRef]
- Tallman, D.J.; Naguib, M.; Anasori, B.; Barsoum, M.W. Tensile creep of Ti2AlC in air in the temperature range 1000–1150 °C. Scr. Mater. 2012, 66, 805–808. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, E.; Wang, J.; Qian, Y.; Xiang, H.; Li, X.; Jin, Q.; Sun, G.; Chen, X.; Wang, J.; et al. Crystal structure and formation mechanism of (Cr2/3Ti1/3)3AlC2 MAX phase. Acta Mater. 2014, 73, 186–193. [Google Scholar] [CrossRef]
- Caspi, E.a.N.; Chartier, P.; Porcher, F.; Damay, F.; Cabioc’h, T. Ordering of (Cr,V) Layers in Nanolamellar (Cr0.5V0.5)n+1AlCn Compounds. Mater. Res. Lett. 2014, 3, 100–106. [Google Scholar] [CrossRef]
- Zou, H.; Li, X.; Zhang, C.; Wen, Y.; Fan, Y.; Liu, Y.; Xiong, L.; Zheng, X.; Yang, J. Reactive synthesis for porous TiVAlC ceramics by TiH2,V, Al and graphite powders. Ceram. Int. 2021, 47, 28288–28295. [Google Scholar] [CrossRef]
- Anasori, B.; Halim, J.; Lu, J.; Voigt, C.A.; Hultman, L.; Barsoum, M.W. Mo2TiAlC2: A new ordered layered ternary carbide. Scr. Mater. 2015, 101, 5–7. [Google Scholar] [CrossRef]
- Qing-He, G.; Zhi-Jun, X.; Ling, T.; Xianjun, Z.; Guozhu, J.; An, D.; Rong-Feng, L.; Yun-Dong, G.; Ze-Jin, Y. Evidence of the stability of Mo2TiAlC2 from first principles calculations and its thermodynamical and optical properties. Comput. Mater. Sci. 2016, 118, 77–86. [Google Scholar] [CrossRef]
- Anasori, B.; Dahlqvist, M.; Halim, J.; Moon, E.J.; Lu, J.; Hosler, B.C.; Caspi, E.a.N.; May, S.J.; Hultman, L.; Eklund, P.; et al. Experimental and theoretical characterization of ordered MAX phases Mo2TiAlC2 and Mo2Ti2AlC3. J. Appl. Phys. 2015, 118, 094304. [Google Scholar] [CrossRef]
- Hadi, M.A.; Ali, M.S. New ordered MAX phase Mo2TiAlC2: Elastic and electronic properties from first-principles. Chin. Phys. B 2016, 25, 107103. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Li, C.; Zhang, C.; Feng, K.; Wang, Z.; Wang, X.; Meira, D.M.; Cai, M.; Zhang, D.; et al. Mo2TiC2 MXene-Supported Ru Clusters for Efficient Photothermal Reverse Water-Gas Shift. ACS Nano 2022, 17, 1550–1559. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Zhuo, H.; Sun, X.; Gu, Y.; Zhuang, G.; Deng, S.; Zhong, X.; Wei, Z.; Li, X.; et al. Mo2TiC2 MXene: A Promising Catalyst for Electrocatalytic Ammonia Synthesis. Catal. Today 2020, 339, 120–126. [Google Scholar] [CrossRef]
- Maughan, P.A.; Bouscarrat, L.; Seymour, V.R.; Shao, S.; Haigh, S.J.; Dawson, R.; Tapia-Ruiz, N.; Bimbo, N. Pillared Mo2TiC2 MXene for high-power and long-life lithium and sodium-ion batteries. Nanoscale Adv. 2021, 3, 3145–3158. [Google Scholar] [CrossRef]
- Yu, L.; Lei, T.; Nan, B.; Jiang, Y.; He, Y.; Liu, C.T. Characteristics of a sintered porous Ni–Cu alloy cathode for hydrogen production in a potassium hydroxide solution. Energy 2016, 97, 498–505. [Google Scholar] [CrossRef]
- Wu, L.; He, Y.; Lei, T.; Nan, B.; Xu, N.; Zou, J.; Huang, B.; Liu, C.T. The stability of hydrogen evolution activity and corrosion behavior of porous Ni3Al–Mo electrode in alkaline solution during long-term electrolysis. Energy 2014, 67, 19–26. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Superelastic behaviors of biomedical porous NiTi alloy with high porosity and large pore size prepared by spark plasma sintering. J. Alloys Compd. 2015, 644, 513–522. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Wang, Y.; Dong, S.; Fan, Y.; Liu, W.; Kuang, Y.; Tan, S.; Xiao, G.; Wang, B.; et al. Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution. Metals 2022, 12, 2123. [Google Scholar] [CrossRef]
- Yang, J.; Wen, Y.; Li, X.; Fan, Y.; Zou, H.; Zhang, C.; Xiong, L.; Liu, Y. The influence of Al content on pore structures of porous Mo2TiAlC2 ceramics through powder metallurgy technique. J. Asian Ceram. Soc. 2022, 10, 120–129. [Google Scholar] [CrossRef]
- Li, G.; Zhou, B.; Wang, P.; He, M.; Fang, Z.; Yuan, X.; Wang, W.; Sun, X.; Li, Z. High-Efficiency Oxygen Reduction to Hydrogen Peroxide Catalyzed by Oxidized Mo2TiC2 MXene. Catalysts 2022, 12, 850. [Google Scholar] [CrossRef]
- DIN EN ISO 4022:2006. Permeable Sintered Metal Materials—Determination of Bubble Test Pore Size. Available online: https://webstore.ansi.org/standards/din/dineniso40222006 (accessed on 11 May 2023).
- Liu, X.; Zhang, H.; Jiang, Y.; He, Y. Characterization and application of porous Ti3SiC2 ceramic prepared through reactive synthesis. Mater. Des. 2015, 79, 94–98. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Z. Electronic structure and bonding properties of layered machinableTi2AlC and Ti2AlNceramics. Phys. Rev. B 2000, 61, 12570–12573. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.H.; Xu, N.P.; Zou, J.; Huang, B.Y.; Liu, C.T. Effects of the Al content on pore structures of porous Ti–Al alloys. Intermetallics 2008, 16, 327–332. [Google Scholar] [CrossRef]
- Neumann, G.; Tuijn, C. Self-Diffusion and Impurity Diffusion in Pure Metals; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- He, Y.H.; Jiang, Y.; Xu, N.P.; Zou, J.; Huang, B.Y.; Liu, C.T.; Liaw, P.K. Fabrication of Ti–Al Micro/ Nanometer-Sized Porous Alloys through the Kirkendall Effect. Adv. Mater. 2007, 19, 2102–2106. [Google Scholar] [CrossRef]
- Yang, J.; Liao, C.; Wang, J.; Jiang, Y.; He, Y. Effects of the Al content on pore structures of porous Ti3AlC2 ceramics by reactive synthesis. Ceram. Int. 2014, 40, 4643–4648. [Google Scholar] [CrossRef]



| Temperature/°C | Phase Constitution | Major Phase |
|---|---|---|
| 700 | Mo, Ti, Al, C | Single phase |
| 900 | Mo, AlMo3, TiC, C | AlMo3 |
| 1100 | Mo2C, TiC, AlMo3, TiAl, C | Mo2C, TiC |
| 1300 | Mo2C, TiC, AlMo3, Mo3Al2C, Ti2AlC, Mo2TiAlC2 | Mo2C, TiC, Mo3Al2C |
| 1500 | Mo2TiAlC2 | Mo2TiAlC2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Fan, Y.; Tan, H.; Liu, W.; Kuang, Y.; Yang, X.; Cao, M.; Li, J. Impact of Sintering Temperature Variation on Porous Structure of Mo2TiAlC2 Ceramics. Materials 2023, 16, 5682. https://doi.org/10.3390/ma16165682
Yang J, Fan Y, Tan H, Liu W, Kuang Y, Yang X, Cao M, Li J. Impact of Sintering Temperature Variation on Porous Structure of Mo2TiAlC2 Ceramics. Materials. 2023; 16(16):5682. https://doi.org/10.3390/ma16165682
Chicago/Turabian StyleYang, Junsheng, Yiquan Fan, Hua Tan, Wenkang Liu, Yijian Kuang, Xuejin Yang, Meili Cao, and Jie Li. 2023. "Impact of Sintering Temperature Variation on Porous Structure of Mo2TiAlC2 Ceramics" Materials 16, no. 16: 5682. https://doi.org/10.3390/ma16165682





