Preparation of Self-Coating Al2O3 Bonded SiAlON Porous Ceramics Using Aluminum Dross and Silicon Solid Waste under Ambient Air Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
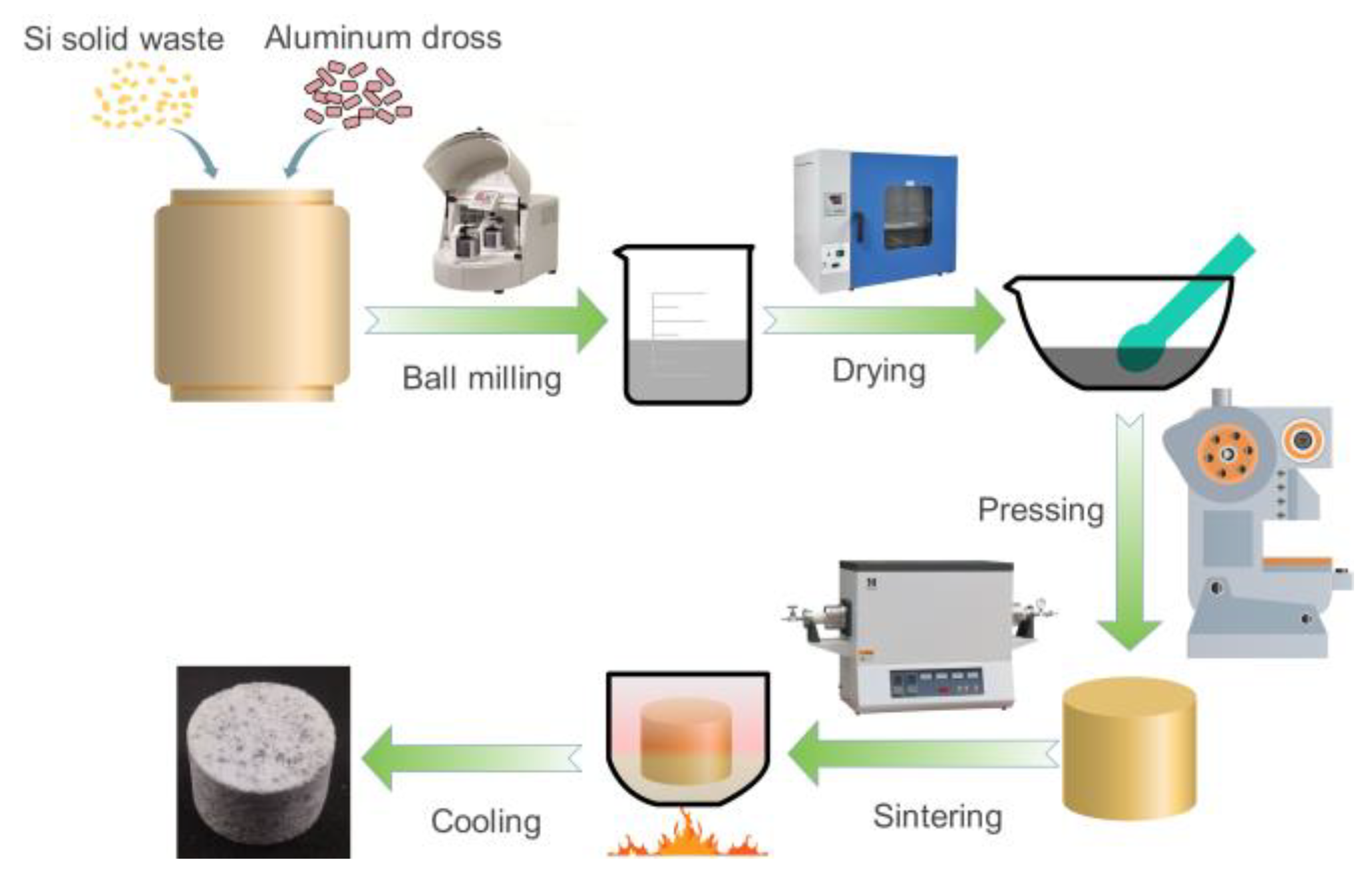
2.2. Preparation of Al2O3-Bonded SiAlON Ceramic
2.3. Characterization
3. Results and Discussion
4. Conclusions
- Higher temperature was more suitable for the formation of SiAlON phase. With the increase in temperature from 1400 to 1600 °C, the mullite phase in the Al2O3-bonded SiAlON porous ceramic disappeared, and the content of Al2O3 and SiAlON phases increased.
- A layer of Al2O3-rich coating spontaneously formed on the surface of the Al2O3-bonded SiAlON porous ceramic, which decreased in thickness with increase in temperature. The formation of the Al2O3-rich coating layer and the sealing of crucible obstructed the air flow, facilitating the nitriding of Si and Al and the formation of SiAlON phase under ambient air conditions.
- The apparent porosity of the Al2O3-bonded SiAlON porous ceramic increased with the increase in temperature, while the bulk density and cold compressive strength decreased first and then increased with the increase in temperature. These changes were mainly related to the phase evolution and the microstructure of Al2O3-bonded SiAlON porous ceramic and Al2O3-rich coating layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, Z.F.; Liu, H.; Huang, Z.; Wen, R.L.; He, C.; Yin, Z.Y.; Liu, Y.G.; Fang, M.H.; Wu, X.W.; Min, X. Growth mechanism and synchronous synthesis of 1D β-sialon nanostructures and β-sialon-Si3N4 composite powders by a process of reduction nitridation. Mater. Res. Express 2019, 6, 065054. [Google Scholar] [CrossRef]
- Xiong, Q.M.; Chen, Z.; Huang, J.; Zhang, M.; Song, H.; Hou, X.F.; Li, X.B.; Feng, Z.J. Preparation, structure and mechanical properties of Sialon ceramics by transition metal-catalyzed nitriding reaction. Rare Metals 2020, 39, 589–596. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Zhang, Q.; Zhao, F.; Liu, X.H.; Jia, Q.L. Oxidation kinetics of bauxite-based β-SiAlON with different particle sizes. Corros. Sci. 2020, 166, 108446. [Google Scholar] [CrossRef]
- Barick, P.; Saha, B.P. Effect of boron nitride addition on densification, microstructure, mechanical, thermal, and dielectric properties of b-SiAlON ceramic. J. Mater. Eng. Perform. 2021, 30, 3603–3611. [Google Scholar] [CrossRef]
- Li, X.Q.; Yao, D.X.; Zuo, K.H.; Xia, Y.F.; Yin, J.W.; Liang, H.Q.; Zeng, Y.P. The microstructure and thermal conductivity of porous beta-SiAlON ceramics fabricated by pressureless sintering with Y-alpha-SiAlON as the sintering additive. Ceram. Int. 2022, 48, 6177–6184. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Huang, J.; Wang, S.Q.; Xiong, Q.M.; Feng, Z.J.; Liu, Q.W.; Sun, Z.H.; Li, X.B. In situ nitriding reaction formation of β-Sialon with fibers using transition metal catalysts. Ceram. Int. 2019, 45, 21923–21930. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, D.H.; Cai, Y.Z.; Wang, S.Q.; Xiong, Q.M.; Feng, Z.J.; Liu, Q.W.; Sun, Z.H.; Li, X.B. Improved thermal shock resistance of beta-SiAlON/h-BN composite prepared by a precursor infiltration pyrolysis (PIP) route. Ceram. Int. 2020, 46, 16932–16937. [Google Scholar] [CrossRef]
- Acikbas, G.; Acikbas, N.C.; Islak, B.Y.; Ayas, E.; Bayrak, K.G.; Ataman, A. Copper reinforced SiAlON matrix composites produced by spark plasma sintering. Ceram. Int. 2022, 48, 13260–13270. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, Y.N.; Bai, X.L.; Shen, X.H.; He, J.Q.; Zhang, Y.; Li, A.H. Preparation of in situ growth multiscale β-Sialon grain-reinforced Al2O3-based composite ceramic tool materials. Materials 2023, 16, 2333. [Google Scholar] [CrossRef]
- Huang, J.; Miao, Y.; Zhang, M.; Feng, Z.J.; Hu, Z.H.; Li, X.B.; Luo, J.M. Hot-pressed sintered Ca-α-Sialon ceramics with grains from short prismatic to elongated morphology synthesized via carbothermal reduction and nitridation. J. Alloys Compd. 2018, 767, 90–97. [Google Scholar] [CrossRef]
- McGarrity, K.A.; Ning, K.J.; Shulman, H.S. Molecular-level composition design for efficient synthesis of SiAlON ceramics. J. Am. Ceram. Soc. 2023, 106, 888–896. [Google Scholar] [CrossRef]
- Liu, G.H.; Chen, K.X.; Li, J.T. Combustion synthesis of SiAlON ceramic powders: A review. Mater. Manuf. Process. 2013, 2, 113–125. [Google Scholar] [CrossRef]
- Guo, F.; Yin, Z.; Chen, W.; Liu, H.T.; Hong, D.B.; Yuan, J.T. Spark plasma sintering of multi-cation doped (Yb, Sm) α/β-SiAlON ceramic tool materials: Effects of cation type, composition, and sintering temperature. Ceram. Int. 2022, 48, 4371–4375. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Shi, Z.; Jin, H.Y.; Qiao, G.J.; Jin, Z.H. Synthesis of β-SiAlON/h-BN nanocomposite by a precursor infiltration and pyrolysis (PIP) route. Mater. Lett. 2015, 139, 303–306. [Google Scholar] [CrossRef]
- Tian, X.; Ouyang, D.; Su, K.; Zhao, F.; Gao, J.X.; Liu, X.H. Fabrication and oxidation behavior of β-SiAlON powders in presence of trace Y2O3. Ceram. Int. 2022, 48, 32464–32469. [Google Scholar] [CrossRef]
- Bolgaru, K.; Reger, A.; Vereshchagin, V.; Akulinkin, A. Combustion synthesis of β-SiAlON from a mixture of aluminum ferrosilicon and kaolin with nitrogen-containing additives using acid enrichment. Ceram. Int. 2023, 49, 2302–2309. [Google Scholar] [CrossRef]
- Shahien, M.; Radwan, M.; Kirihara, S.; Miyamoto, Y.; Sakurai, T. Combustion synthesis of single-phase -sialons (z = 2–4). J. Eur. Ceram. Soc. 2010, 20, 1925–1930. [Google Scholar] [CrossRef]
- Srivastava, A.; Meshram, A. On trending technologies of aluminium dross recycling: A review. Process Saf. Environ. Prot. 2023, 171, 38–54. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Guo, Y.; He, Y.Y.; Liu, J.; Liu, H. Comprehensive treatments of aluminum dross in China: A critical review. J. Environ. Manag. 2023, 345, 118575. [Google Scholar] [CrossRef]
- Huang, K.P.; Wang, L.L.; Li, M.K.; Mi, T.T.; Zhang, J.L.; Liu, J.; Yi, X.M. Mechanism of porous ceramic fabrication using second aluminum dross assisted by corn stalk as pore-forming agent. Int. J. Hydrogen Energy 2023, 31, 103195. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Lan, X.; Feng, G.L.; Guo, Z.C. An environmental-friendly method for recovery of aluminum droplets from aluminum dross: Mechanical activation and super-gravity separation. Process Saf. Environ. Prot. 2023, 175, 199–211. [Google Scholar] [CrossRef]
- Yoshimura, H.; Abreu, A.; Molisani, A.; Camargo, A.C.; Portela, J.C.S.; Narita, N.E. Evaluation of aluminum dross waste as raw material for refractories. Ceram. Int. 2008, 34, 581–591. [Google Scholar] [CrossRef]
- Sarker, M.; Alam, M.; Qadir, M.; Gafur, M.A.; Moniruzzaman, M. Extraction and characterization of alumina nanopowders from aluminum dross by acid dissolution process. Int. J. Miner. Metall. Mater. 2015, 22, 429–436. [Google Scholar] [CrossRef]
- Shen, H.; Liu, B.; Ekberg, C.; Zhang, S.G. Harmless disposal and resource utilization for secondary aluminum dross: A review. Sci. Total Environ. 2021, 760, 143968. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Li, Z.S.; Ding, Y.D.; Yang, H.L.; Zhang, J.Z.; Fu, G.F. Waste to wealth strategy: Preparation and properties of lightweight Al2O3-SiO2-rich castables using aluminum dross waste. Materials 2022, 14, 7803. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.H.; Han, Z.Y.; Xiao, X.Y. Effect of rare earth oxides doping on MgAl2O4 spinel obtained by sintering of secondary aluminium dross. J. Alloys Compd. 2018, 735, 2597–2603. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, J.; Wang, X.; Wang, J.K.; Zhang, L.; Wen, T.P.; Jia, D.B.; Yan, Z.G.; Yuan, L.; Ma, B.Y. Using Si/SiC solid waste to design urchin-like mullite whiskers for oil-water separation. Int. J. Appl. Ceram. Technol. 2022, 19, 1405–1414. [Google Scholar] [CrossRef]
- Li, J.; Lin, Y.; Wang, F.; Shi, J.; Sun, J.F.; Ban, B.Y.; Liu, G.C.; Chen, J. Progress in recovery and recycling of kerf loss silicon waste in photovoltaic industry. Sep. Purif. Technol. 2021, 254, 117581. [Google Scholar] [CrossRef]
- Wang, T.; Lin, Y.; Tai, C.; Sivakumar, R.; Rai, D.K.; Lan, C.W. A novel approach for recycling of kerf loss silicon from cutting slurry waste for solar cell applications. J. Cryst. Growth 2008, 310, 3403–3406. [Google Scholar] [CrossRef]
- Liu, W.; Tian, J.; Xiang, D.; Mao, R.P.; Wang, B.X. Fabricating superior thermal conductivity SiC–AlN composites from photovoltaic silicon waste. J. Clean. Prod. 2020, 274, 122799. [Google Scholar] [CrossRef]
- Yang, H.L.; Liu, I.T.; Liu, C.E.; Hsu, H.P.; Lan, C.W. Recycling and reuse of kerf-loss silicon from diamond wire sawing for photovoltaic industry. Waste Manag. 2019, 84, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Liu, X.M.; Yu, J.M.; Xu, C.F.; Wu, Y.F.; Pan, D.A.; Senthil, R.A. An overview of the comprehensive utilization of silicon–based solid waste related to PV industry. Resour. Conserv. Recycl. 2021, 169, 105450. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, J.; Yang, X.; Jin, E.D.; Yuan, L. Oxidation Resistance and Wetting Behavior of MgO–C Refractories: Effect of Carbon Content. Materials 2018, 11, 883. [Google Scholar] [CrossRef]
- O’Leary, B.; Mackenzie, K. Inorganic polymers (geopolymers) as precursors for carbothermal reduction and nitridation (CRN) synthesis of SiAlON ceramics. J. Eur. Ceram. Soc. 2015, 35, 2755–2764. [Google Scholar] [CrossRef]
- Tu, Z.; Lao, X.B.; Xu, X.Y.; Jiang, W.H.; Liu, J.M.; Liang, J. Effect of Si/Al ratio on in-situ synthesis of Al2O3–β–Sialon composite ceramics for solar thermal storage by aluminothermic and silicothermic nitridation. Ceram. Int. 2023, 49, 22970–22978. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, D.; Zuo, K.; Xia, Y.F.; Yin, J.W.; Liang, H.Q.; Zeng, Y.P. The synthesis of single-phase β-Sialon porous ceramics using self-propagating high-temperature processing. Ceram. Int. 2022, 48, 4371–4375. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, J.; Yue, S.; Jia, D.B.; Jin, E.D.; Ma, B.Y.; Yuan, L. Effect of carbon content on the oxidation resistance and kinetics of MgO-C refractory with the addition of Al powder. Ceram. Int. 2019, 46, 3091–3098. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yuan, L.; Yu, J.K. Improvements in the mechanical properties and oxidation resistance of MgO-C refractories with the addition of nano-Y2O3 powder. Adv. Appl. Ceram. 2019, 118, 249–256. [Google Scholar] [CrossRef]
- Zhao, L.F.; Cao, W.D.; Li, Y.J.; Jin, E.D.; Cai, Y.Z.; Ding, D.H.; Xiao, G.Q. Improved thermal shock and oxidation resistance of β-SiAlON incorporated with h-BN. J. Alloys Compd. 2023, 947, 169686. [Google Scholar] [CrossRef]
- Gu, Q.; Ma, T.; Zhao, F.; Jia, Q.L.; Liu, X.H.; Liu, G.Q.; Li, H.X. Enhancement of the thermal shock resistance of MgO–C slide plate materials with the addition of Nano-ZrO2 modified magnesia aggregates. J. Alloys Compd. 2020, 847, 156339. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yu, J.K.; Wang, X.N.; Ma, P.C.; Gu, W.B.; Wen, J.; Wei, S.; Zhang, X.F.; Yan, Z.G.; Wen, T.P.; et al. Comparative study of B4C, Mg2B2O5, and ZrB2 powder additions on the mechanical properties, oxidation, and slag corrosion resistance of MgO-C refractories. Ceram. Int. 2022, 48, 14117–14126. [Google Scholar] [CrossRef]
- Gu, Q.; Zhao, F.; Liu, X.; Jia, Q.L. Preparation and thermal shock behavior of nanoscale MgAl2O4 spinel-toughened MgO-based refractory aggregates. Ceram. Int. 2019, 45, 12093–12100. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.W.; Meng, Z.; Liu, L.L.; Wang, X.D.; Xing, Y. Integrated utilization of coal gangue for synthesis of β-Sialon multiphase ceramic materials. Ceram. Int. 2023, 49, 11275–11284. [Google Scholar] [CrossRef]
- Dou, K.; Jiang, Y.; Xue, B.; Wei, C.D.; Li, F.F. The carbon environment effects on phase composition and photoluminescence properties of β-SiAlON multiphase materials prepared from fly ash acid slag. Ceram. Int. 2019, 45, 7850–7856. [Google Scholar] [CrossRef]
- Yang, G.; Yin, L.; Fang, X.; Fang, M.H.; Liu, Y.G.; Huang, Z.H.; Liu, B.L. Fabrication and liquid–solid, two-phase erosion wear behaviour of β-Sialon ceramic from pyrophyllite by carbothermal reduction and nitridation. Ceram. Int. 2014, 40, 10737. [Google Scholar] [CrossRef]
- Zhu, W.J.; Wu, K.K.; Zhang, S.J.; Liu, J.; Yi, X.M.; Zhang, L.H. Zero-waste progress for the synthesis of high-purity β-sialon ceramics from secondary aluminum dross. Adv. Eng. Mater. 2021, 23, 2001298. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, G.; Chou, K.; Zhong, X. A comparison of oxidation kinetics of O′–SiAlON and β–SiAlON powders synthesized from bauxite. Int. J. Appl. Ceram. Tec. 2008, 5, 529–536. [Google Scholar] [CrossRef]
- Yi, X.M.; Niu, J.; Nakamura, T.; Akiyama, T. Reaction mechanism for combustion synthesis of β-SiAlON by using Si, Al, and SiO2 as raw materials. J. Alloys Compd. 2013, 561, 1–4. [Google Scholar] [CrossRef]
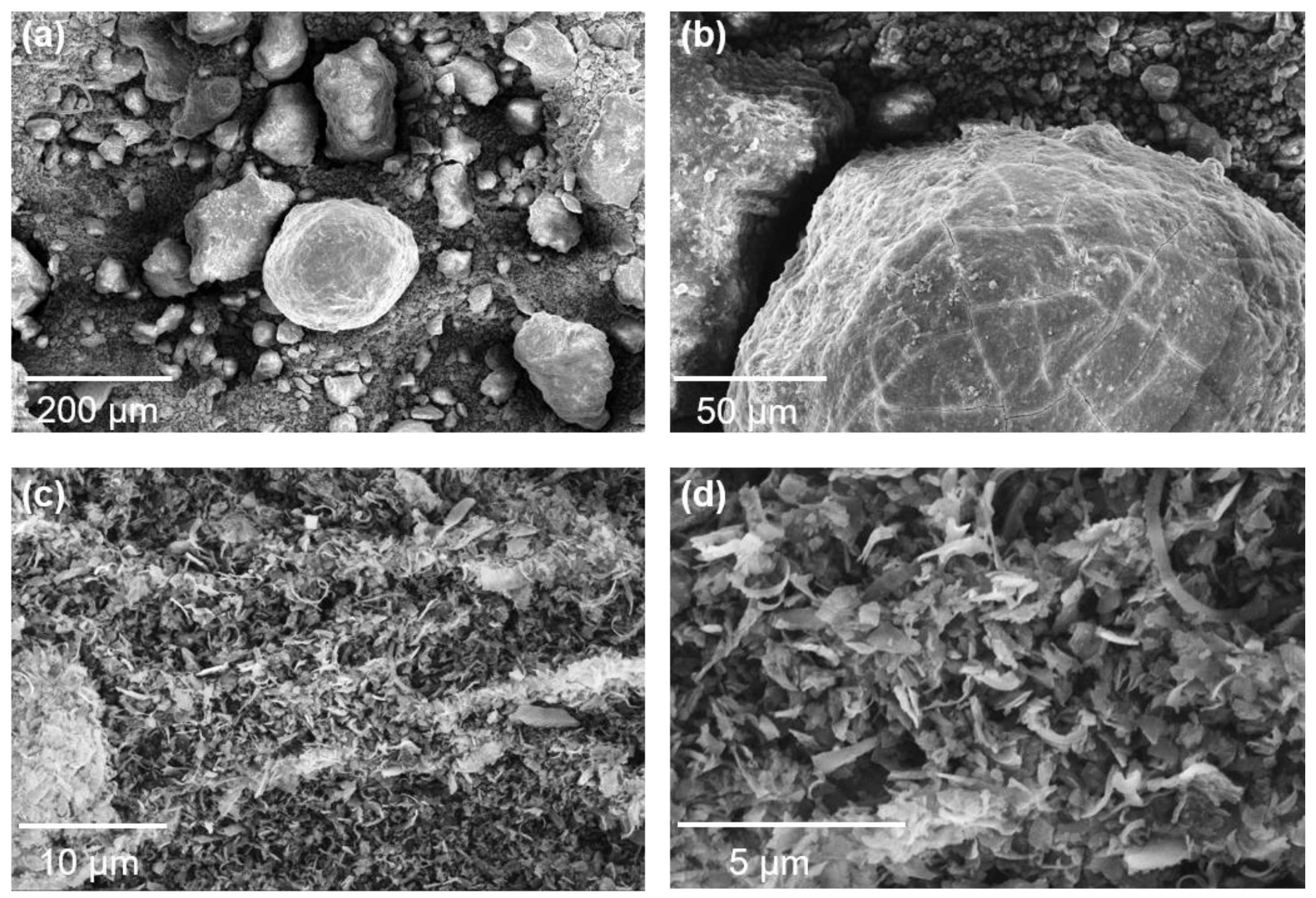
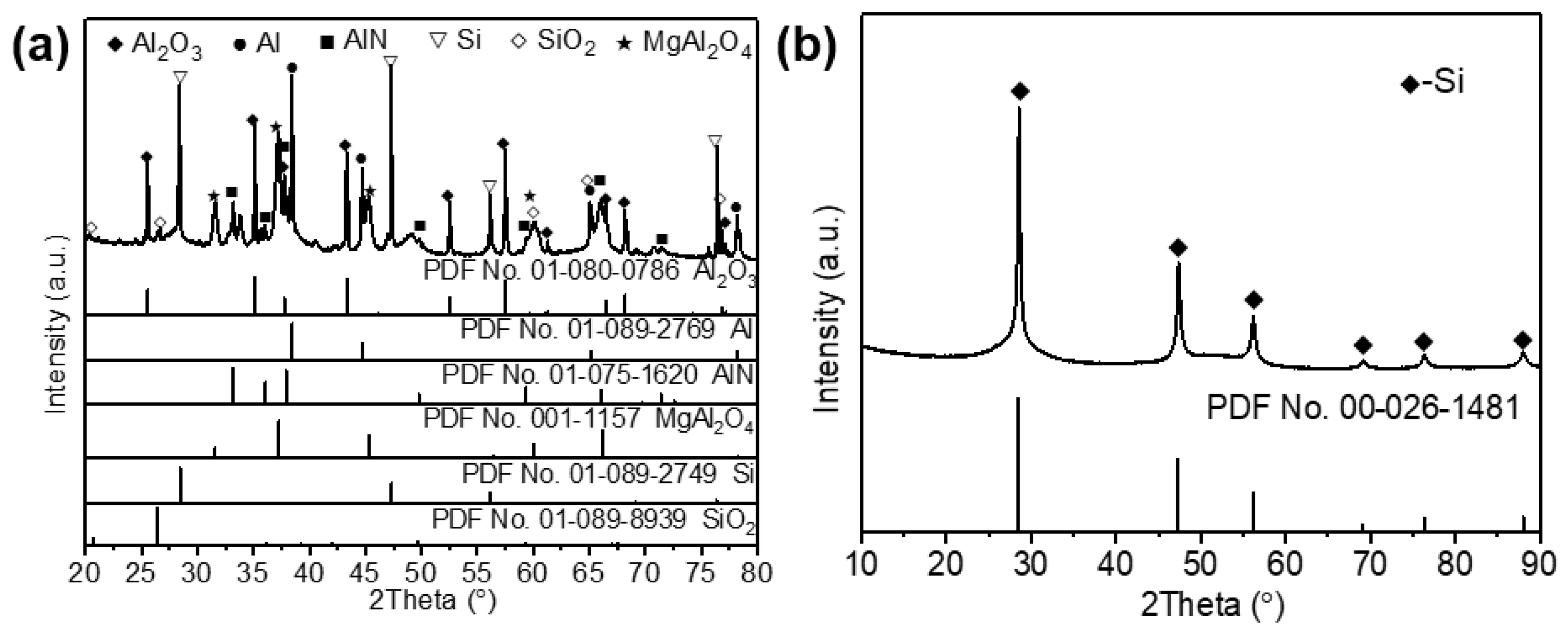
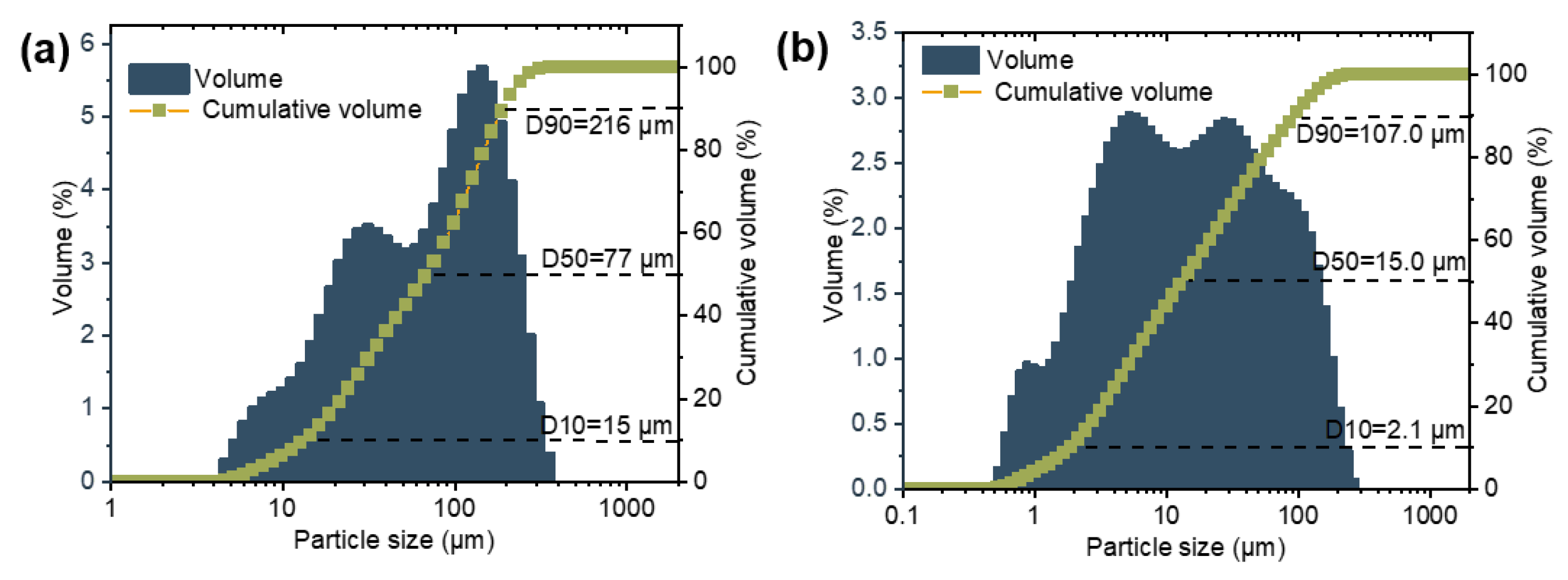
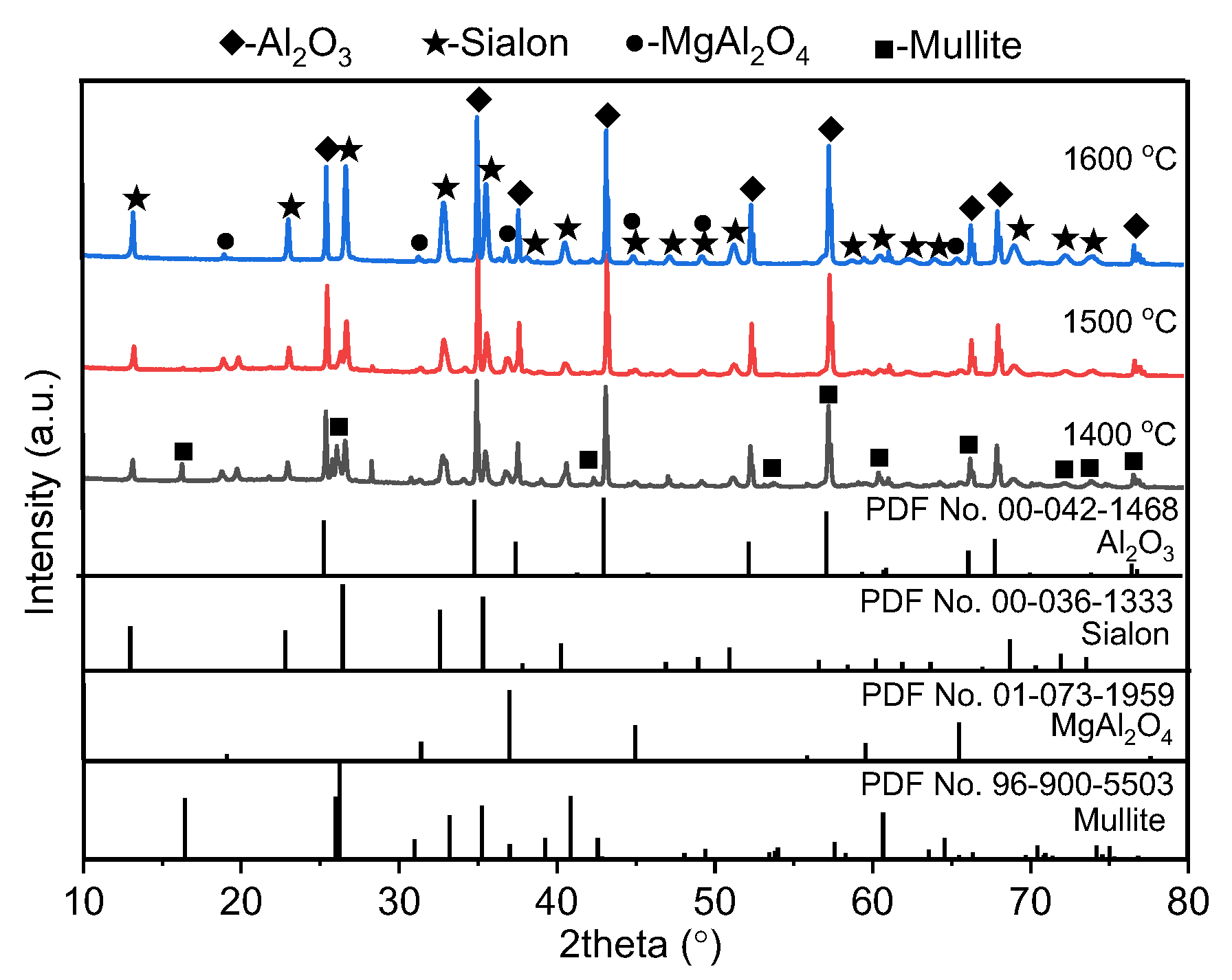



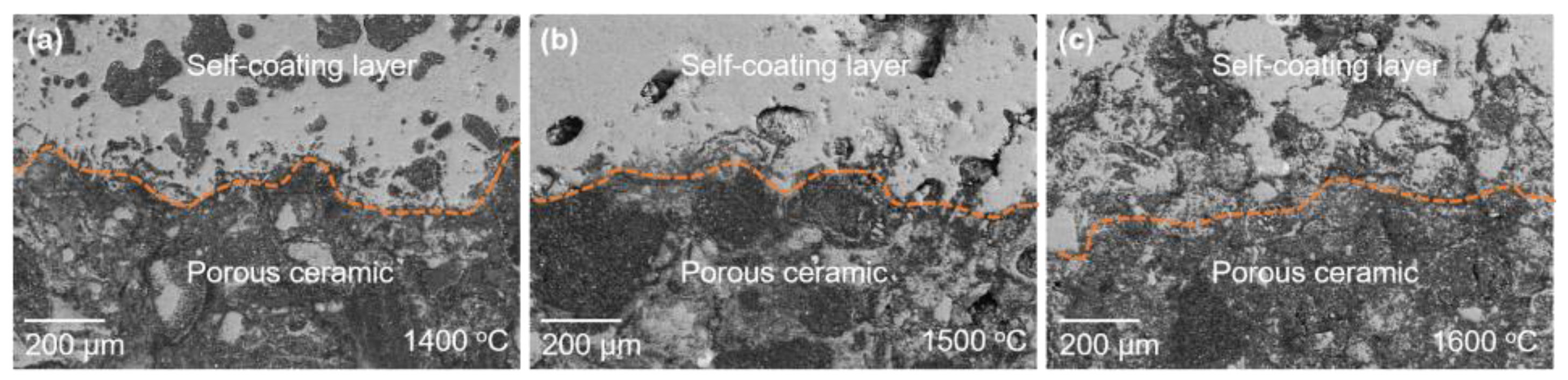
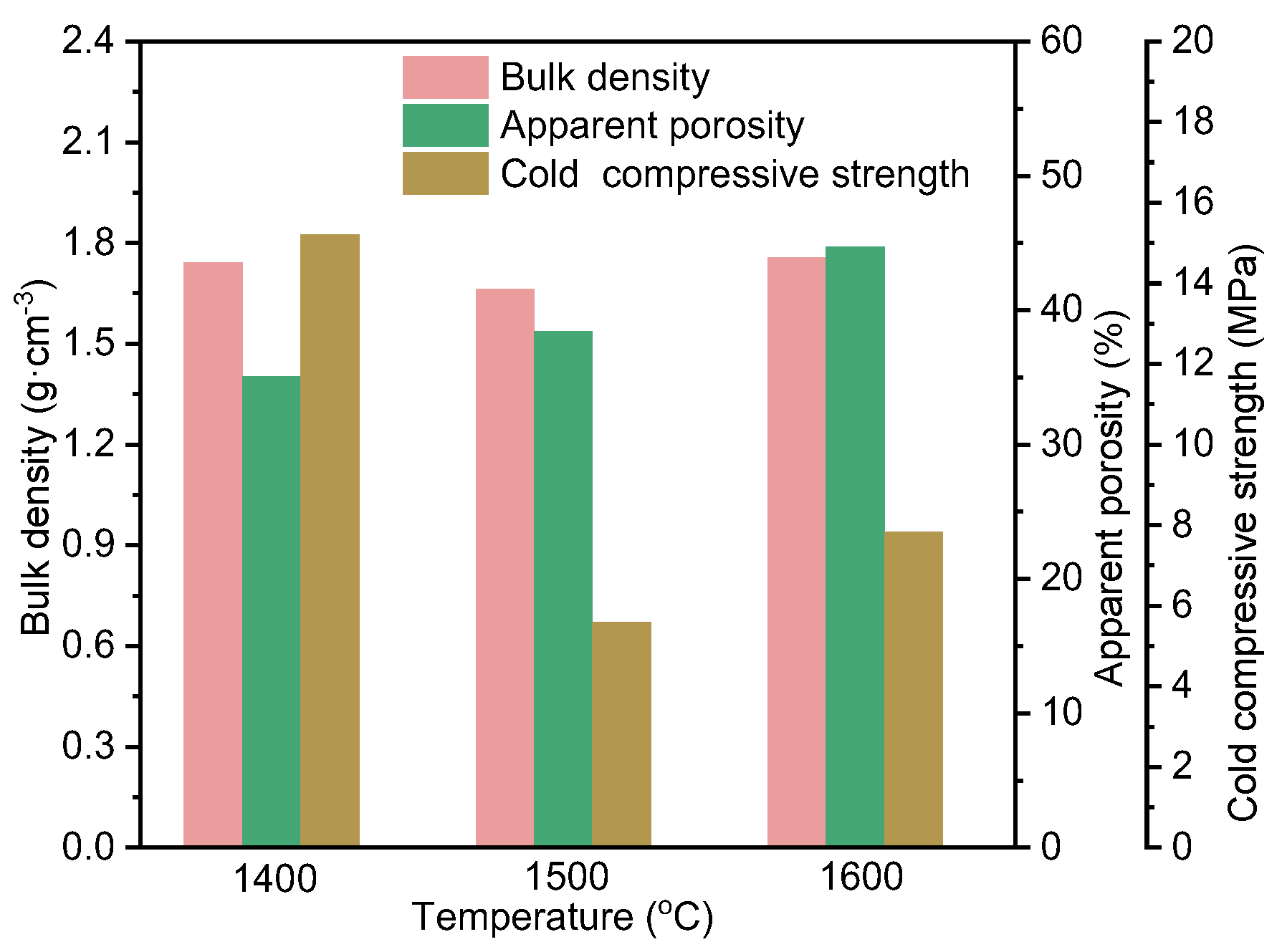
| Al | Si | Mg | Ca | K | Ti | Fe | Others | |
|---|---|---|---|---|---|---|---|---|
| Contents | 77.42 | 9.99 | 3.53 | 2.34 | 2.24 | 2.20 | 0.91 | 1.37 |
| Si | Al | O | N | Mg | Possible Phase | |
|---|---|---|---|---|---|---|
| +1 | 19.25 | 19.17 | 30.19 | 30.36 | 1.02 | Sialon |
| +2 | 15.66 | 42.22 | 41.15 | - | 0.97 | Mullite |
| +3 | 3.31 | 37.09 | 59.60 | - | - | Al2O3 |
| +4 | 24.09 | 27.29 | 46.95 | - | 1.67 | Mullite |
| +5 | 1.59 | 33.11 | 65.29 | - | - | Al2O3 |
| +6 | 20.33 | 14.83 | 31.71 | 32.17 | 0.96 | Sialon |
| +7 | 1.90 | 24.40 | 64.02 | - | 9.67 | MgAl2O4 |
| +8 | 20.37 | 14.71 | 28.44 | 35.41 | 1.06 | Sialon |
| +9 | 2.08 | 43.20 | 54.71 | - | - | Al2O3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, J.; Zhao, Z.; Yang, Q.; Qin, L.; Zhang, K.; Wang, X.; Su, N.; Wen, T.; Yuan, L.; et al. Preparation of Self-Coating Al2O3 Bonded SiAlON Porous Ceramics Using Aluminum Dross and Silicon Solid Waste under Ambient Air Atmosphere. Materials 2023, 16, 5679. https://doi.org/10.3390/ma16165679
Liu Z, Wang J, Zhao Z, Yang Q, Qin L, Zhang K, Wang X, Su N, Wen T, Yuan L, et al. Preparation of Self-Coating Al2O3 Bonded SiAlON Porous Ceramics Using Aluminum Dross and Silicon Solid Waste under Ambient Air Atmosphere. Materials. 2023; 16(16):5679. https://doi.org/10.3390/ma16165679
Chicago/Turabian StyleLiu, Zhaoyang, Junyang Wang, Zixu Zhao, Qiuyu Yang, Lihang Qin, Kaichen Zhang, Xiangnan Wang, Nan Su, Tianpeng Wen, Lei Yuan, and et al. 2023. "Preparation of Self-Coating Al2O3 Bonded SiAlON Porous Ceramics Using Aluminum Dross and Silicon Solid Waste under Ambient Air Atmosphere" Materials 16, no. 16: 5679. https://doi.org/10.3390/ma16165679
APA StyleLiu, Z., Wang, J., Zhao, Z., Yang, Q., Qin, L., Zhang, K., Wang, X., Su, N., Wen, T., Yuan, L., & Yu, J. (2023). Preparation of Self-Coating Al2O3 Bonded SiAlON Porous Ceramics Using Aluminum Dross and Silicon Solid Waste under Ambient Air Atmosphere. Materials, 16(16), 5679. https://doi.org/10.3390/ma16165679






