CO2-Assisted Sugar Cane Gasification Using Transition Metal Catalysis: An Impact of Metal Loading on the Catalytic Behavior
Abstract
:1. Introduction
2. Materials and Methods
Synthetic Procedure
3. Results and Discussion
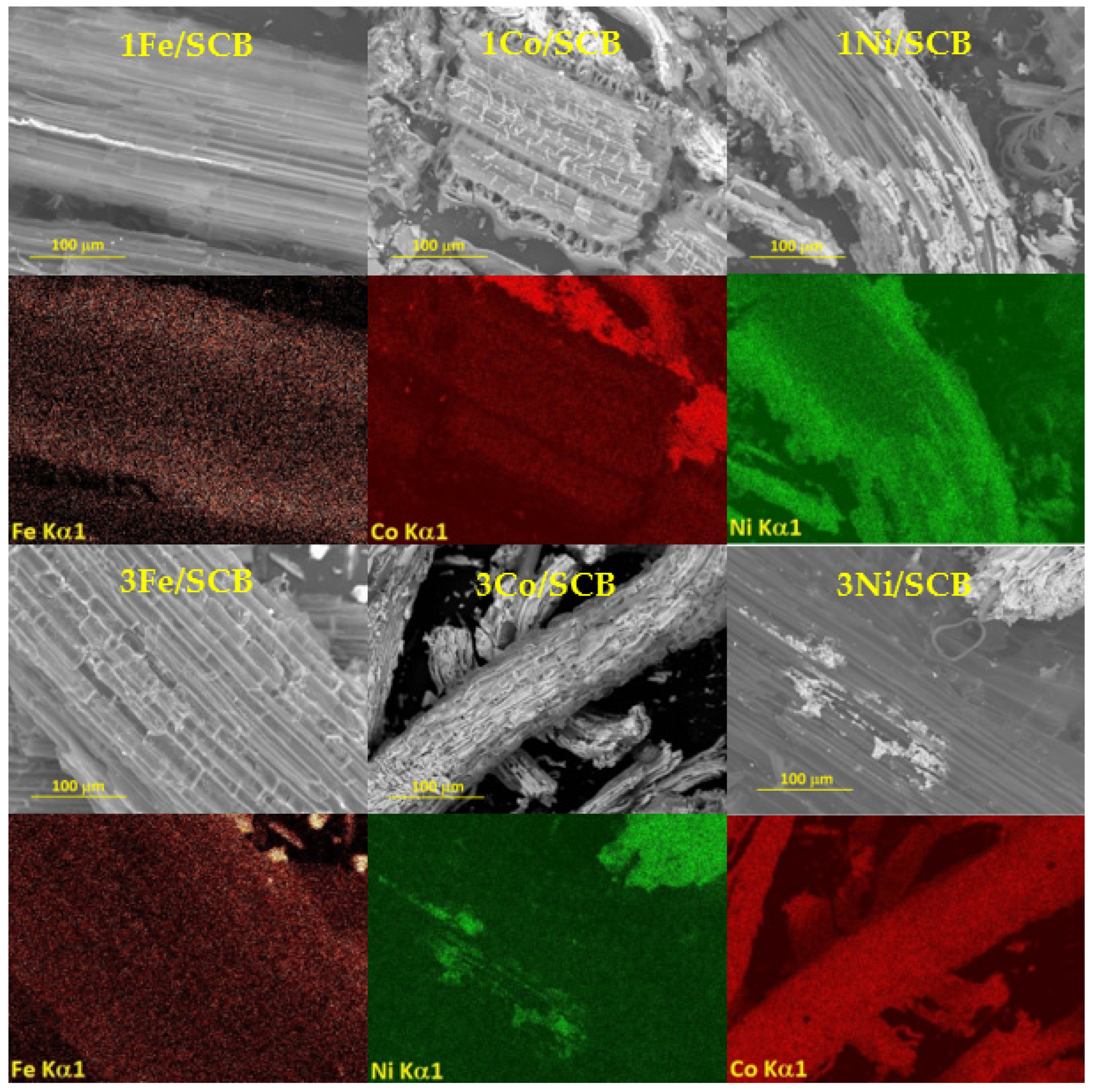
3.1. Scanning Electron Microscopy
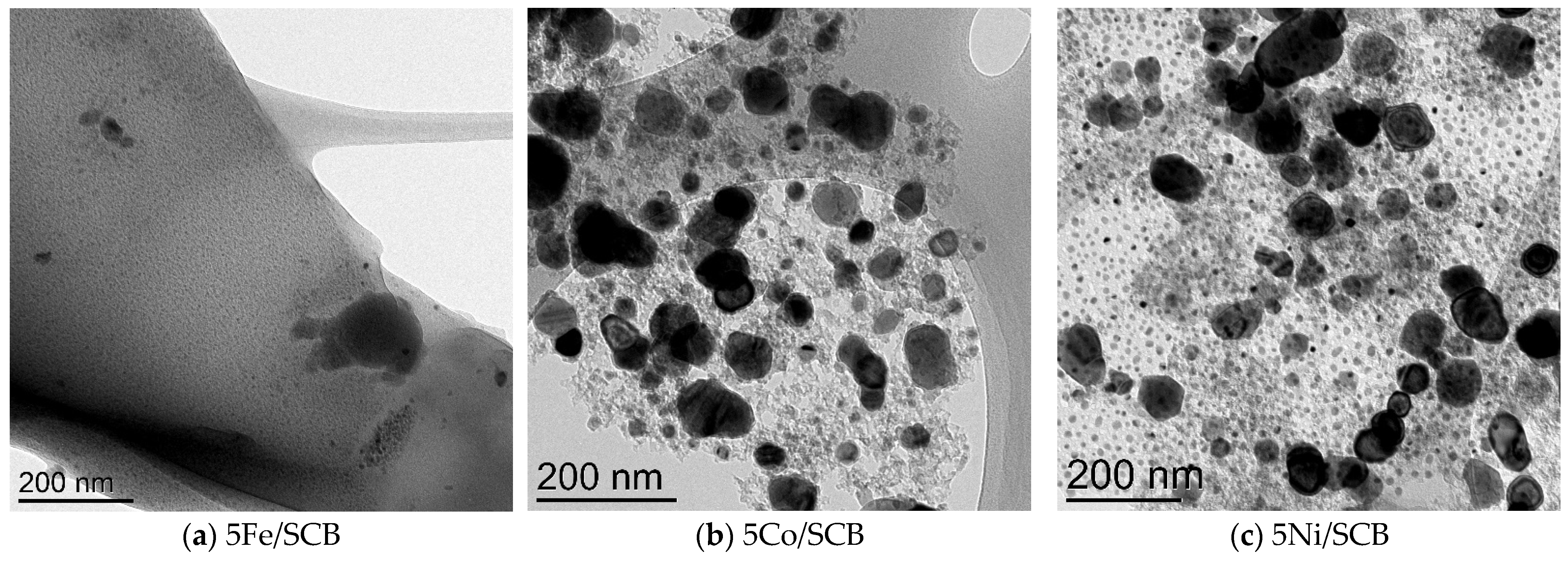
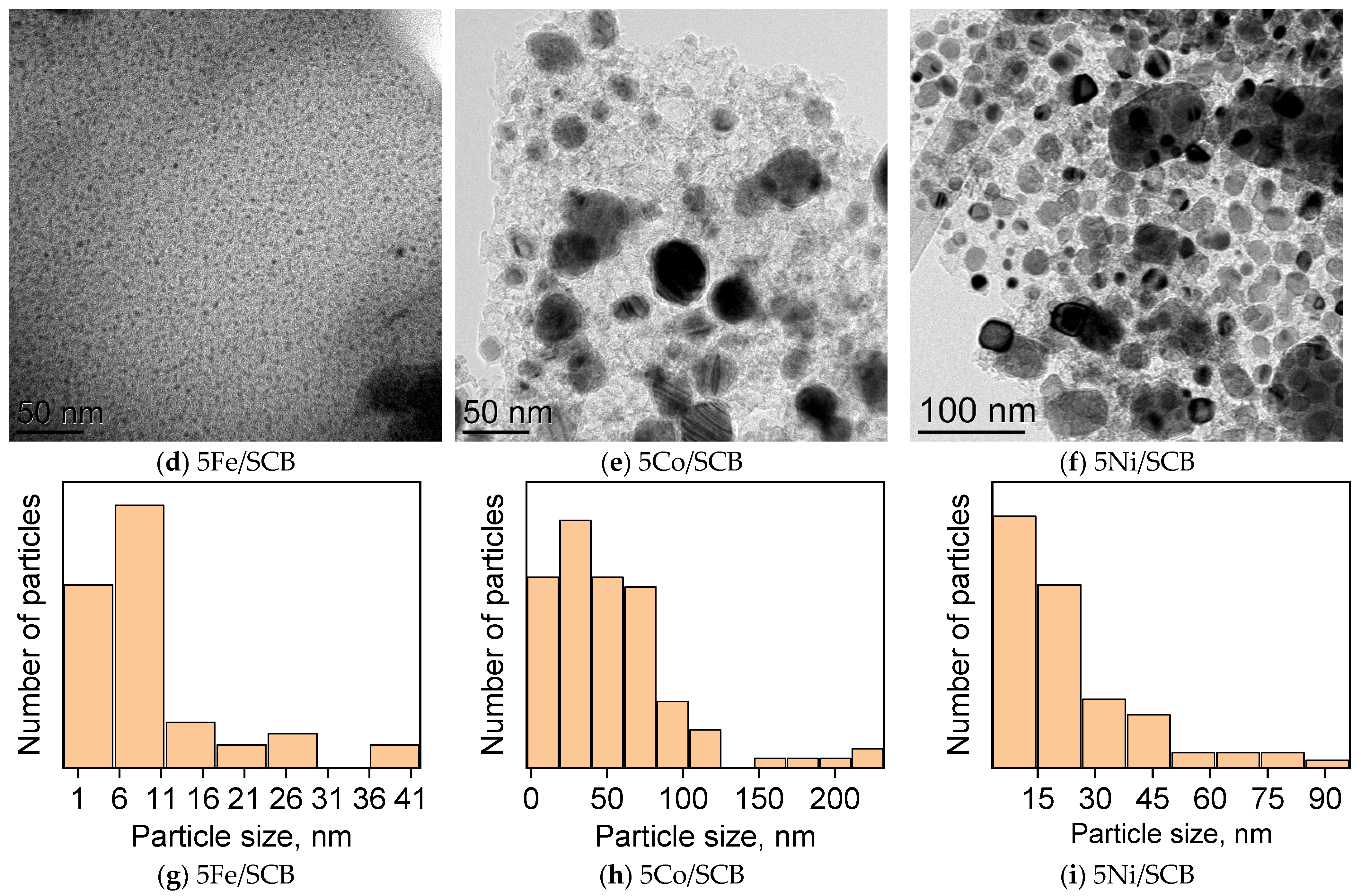
3.2. Transmission Electron Microscopy
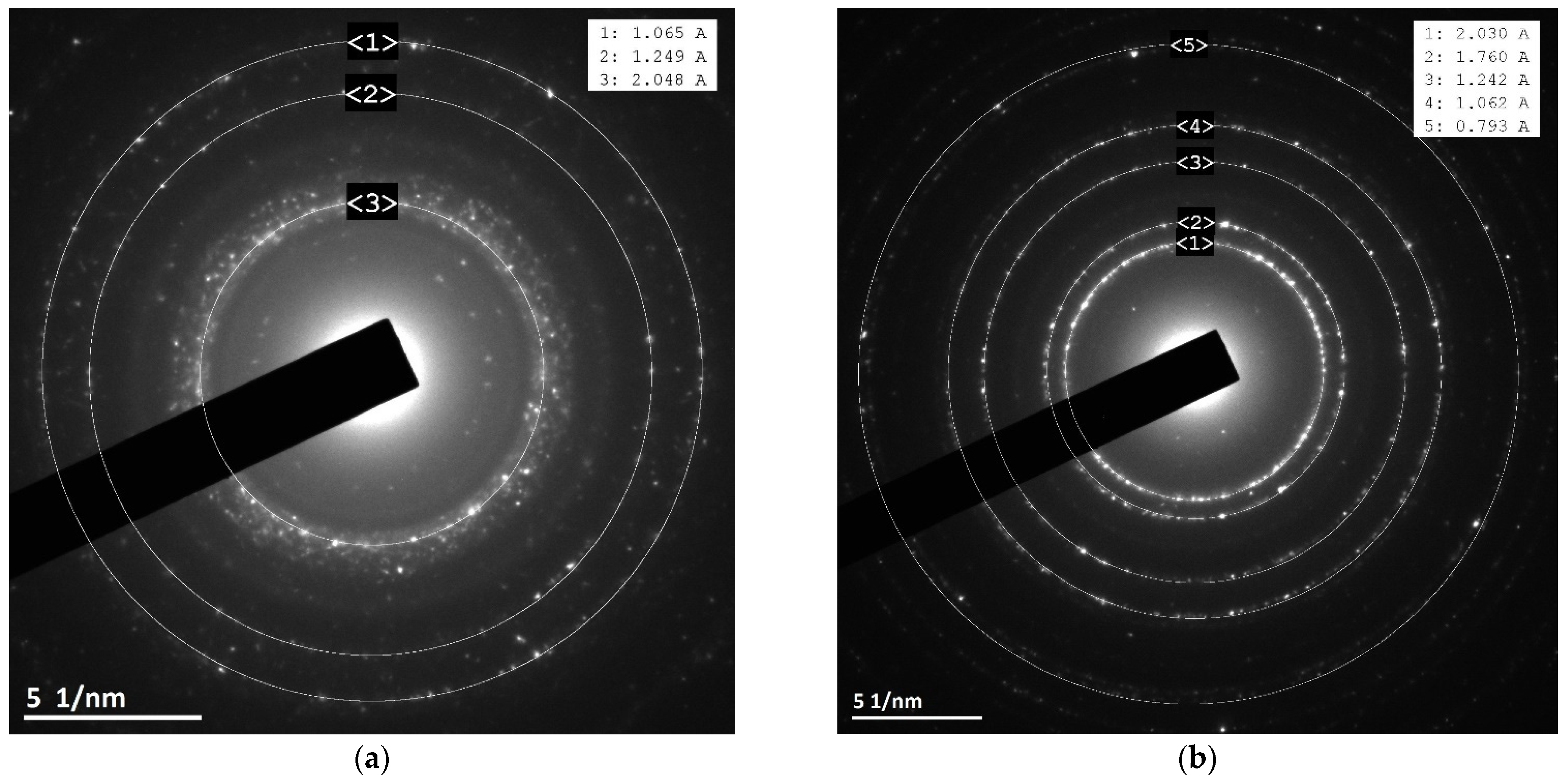
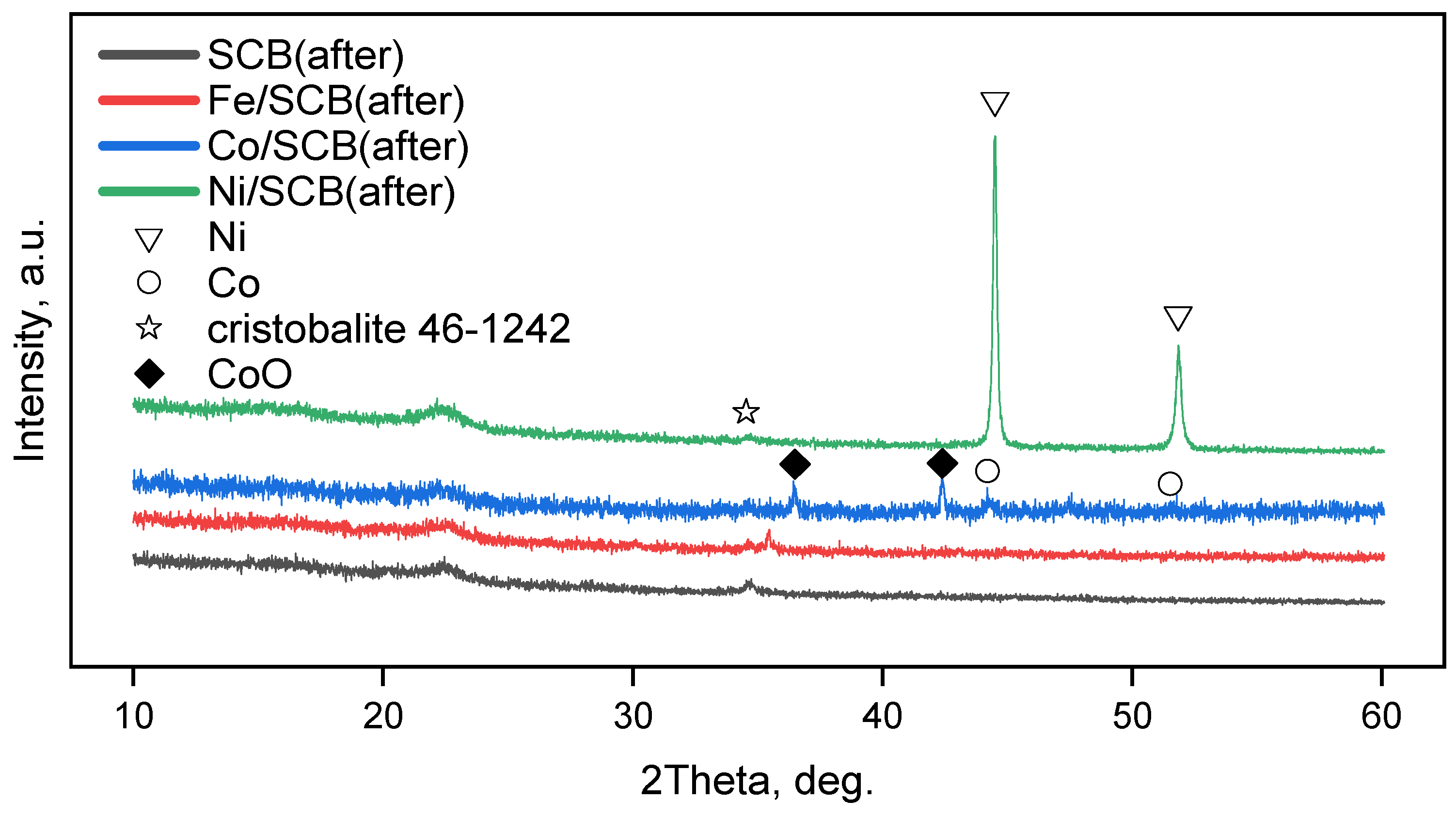
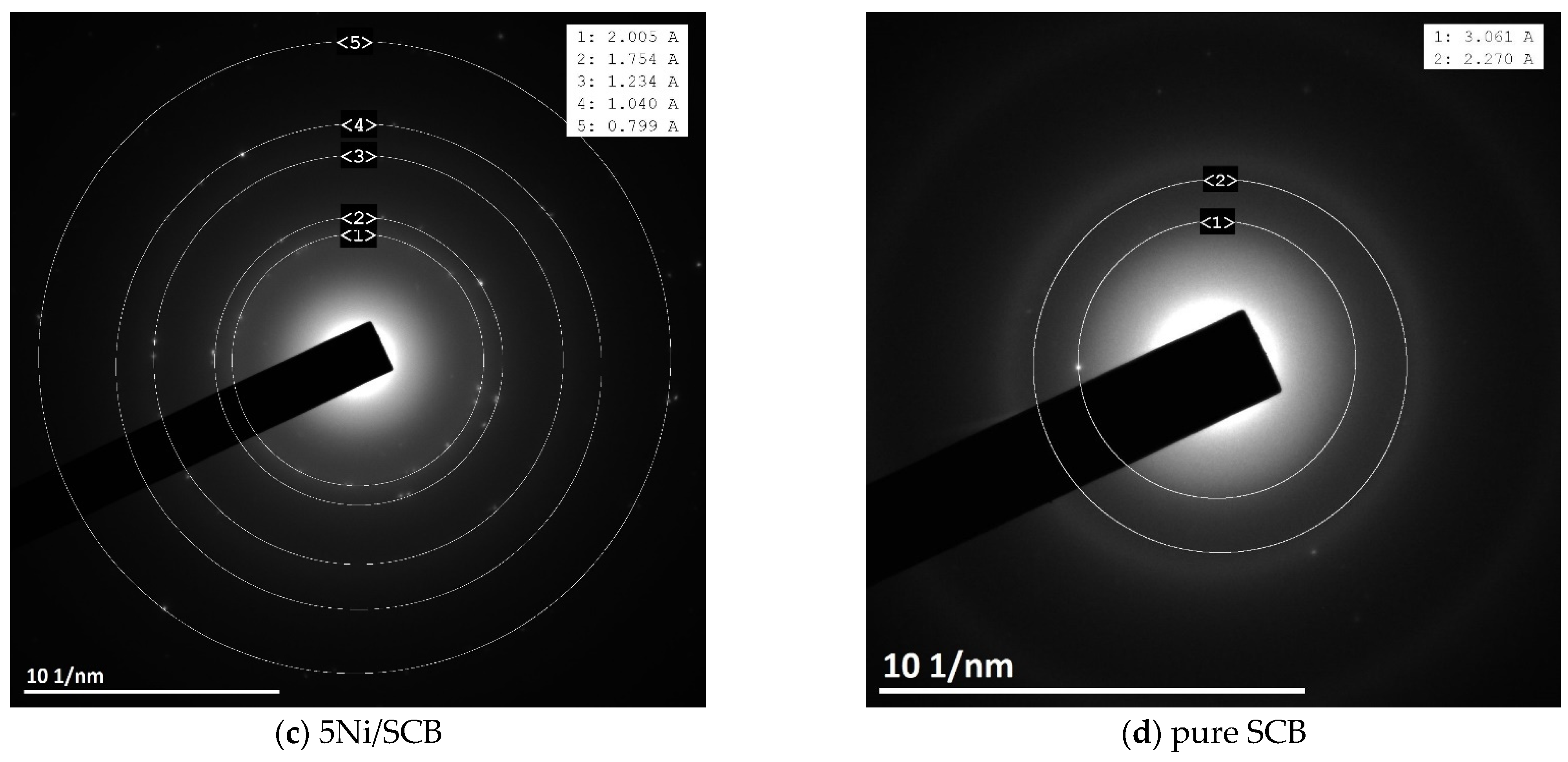
3.3. XRD and Electron Diffraction Patterns
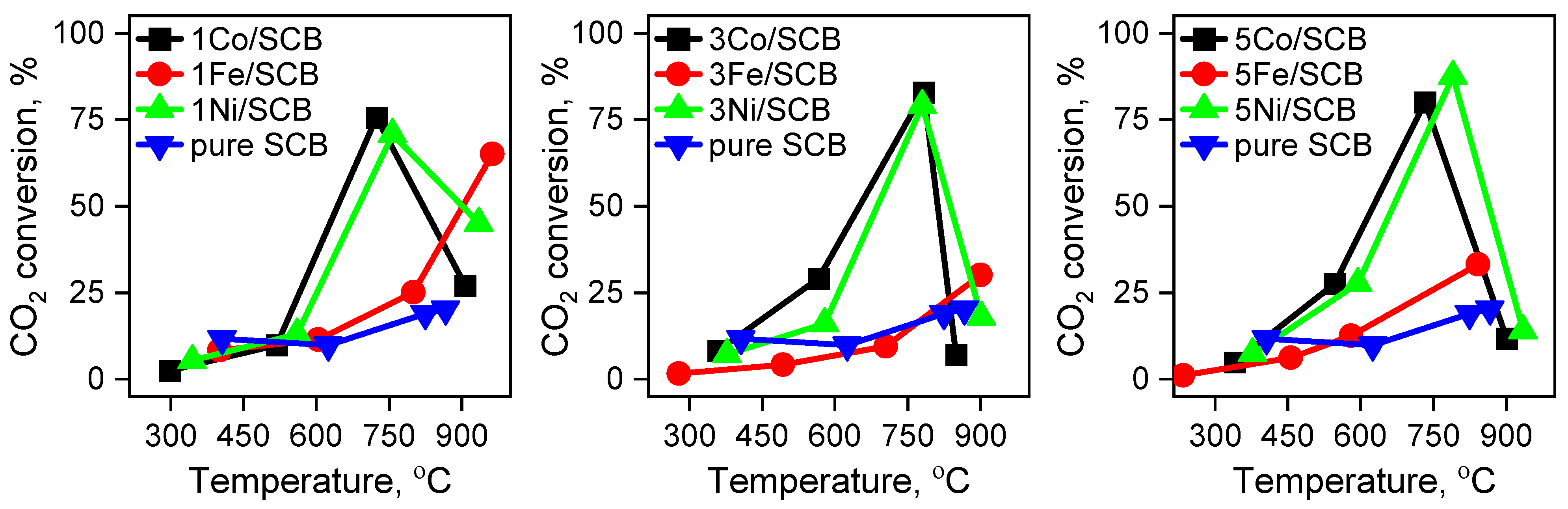
3.4. Catalytic Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafiee, A.; Rajab Khalilpour, K.; Milani, D.; Panahi, M. Trends in CO2 Conversion and Utilization: A Review from Process Systems Perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Mashkin, M.; Tedeeva, M.; Fedorova, A.; Vasiliev, A.; Egorov, A.; Pribytkov, P.; Kalmykov, K.; Kapustin, G.; Morozov, I.; Kustov, L.; et al. CrOx/SiO2 Mesoporous Catalysts Prepared Using Beta-Cyclodextrin as a Template and Their Catalytic Properties in Propane Oxidative Dehydrogenation in the Presence of Carbon Dioxide. Microporous Mesoporous Mater 2022, 338, 111967. [Google Scholar] [CrossRef]
- Igonina, M.; Tedeeva, M.; Kalmykov, K.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Pribytkov, P.; Dunaev, S.; Kustov, L.; Kustov, A. Properties of CrOx/MCM-41 and Its Catalytic Activity in the Reaction of Propane Dehydrogenation in the Presence of CO2. Catalysts 2023, 13, 906. [Google Scholar] [CrossRef]
- Mashkin, M.Y.; Tedeeva, M.A.; Fedorova, A.A.; Fatula, E.R.; Egorov, A.V.; Dvoryak, S.V.; Maslakov, K.I.; Knotko, A.V.; Baranchikov, A.E.; Kapustin, G.I.; et al. Synthesis of CexZr1-XO2/SiO2 Supports for Chromium Oxide Catalysts of Oxidative Dehydrogenation of Propane with Carbon Dioxide. J. Chem. Technol. Biotechnol. 2023, 98, 1247–1259. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Kuśtrowski, P.; Chmielarz, L. Chromium Oxide Supported on MCM-41 as a Highly Active and Selective Catalyst for Dehydrogenation of Propane with CO2. Appl. Catal. A Gen. 2008, 349, 62–69. [Google Scholar] [CrossRef]
- Ma, Y.; Zha, Z.; Huang, C.; Ge, Z.; Zeng, M.; Zhang, H. Gasification Characteristics and Synergistic Effects of Typical Organic Solid Wastes under CO2/Steam Atmospheres. Waste Manag. 2023, 168, 35–44. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Teplyakov, V.V.; Tsodikov, M.V.; Chistjakov, A.V.; Zharova, P.A.; Shalygin, M.G. Laboratory Scale Production of Hydrocarbon Motor Fuel Components from Lignocellulose: Combination of New Developments of Membrane Science and Catalysis. Biomass Bioenergy 2020, 135, 105506. [Google Scholar] [CrossRef]
- Lopez, G.; Santamaria, L.; Lemonidou, A.; Zhang, S.; Wu, C.; Sipra, A.T.; Gao, N. Hydrogen Generation from Biomass by Pyrolysis. Nat. Rev. Methods Prim. 2022, 2, 20. [Google Scholar] [CrossRef]
- Koklin, A.E.; Bobrova, N.A.; Bogdan, T.V.; Mishanin, I.I.; Bogdan, V.I. Conversion of Phenol and Lignin as Components of Renewable Raw Materials on Pt and Ru-Supported Catalysts. Molecules 2022, 27, 1494. [Google Scholar] [CrossRef]
- Naranov, E.; Sadovnikov, A.; Arapova, O.; Kuchinskaya, T.; Usoltsev, O.; Bugaev, A.; Janssens, K.; De Vos, D.; Maximov, A. The In-Situ Formation of Supported Hydrous Ruthenium Oxide in Aqueous Phase during HDO of Lignin-Derived Fractions. Appl. Catal. B Environ. 2023, 334, 122861. [Google Scholar] [CrossRef]
- Bogdan, T.V.; Bobrova, N.A.; Koklin, A.E.; Mishanin, I.I.; Odintsova, E.G.; Antipova, M.L.; Petrenko, V.E.; Bogdan, V.I. Structure of Aqueous Solutions of Lignin Treated by Sub- and Supercritical Water: Experiment and Simulation. J. Mol. Liq. 2023, 383, 122030. [Google Scholar] [CrossRef]
- Singh, O.; Sharma, T.; Ghosh, I.; Dasgupta, D.; Vempatapu, B.P.; Hazra, S.; Kustov, A.L.; Sarkar, B.; Ghosh, D. Converting Lignocellulosic Pentosan-Derived Yeast Single Cell Oil into Aromatics: Biomass to Bio-BTX. ACS Sustain. Chem. Eng. 2019, 7, 13437–13445. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem.-Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane Bagasse and Leaves: Foreseeable Biomass of Biofuel and Bio-Products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Kulikov, L.A.; Bazhenova, M.A.; Makeeva, D.A.; Terenina, M.V.; Maximov, A.L.; Karakhanov, E.A. Hydrogenation of Lignin Bio-Oil Components over Catalysts Based on Porous Aromatic Frameworks. Pet. Chem. 2022, 62, 1096–1106. [Google Scholar] [CrossRef]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane Bagasse Pyrolysis: A Review of Operating Conditions and Products Properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Pellegrini, L.F.; de Oliveira, S. Exergy Analysis of Sugarcane Bagasse Gasification. Energy 2007, 32, 314–327. [Google Scholar] [CrossRef]
- Deshmukh, R.; Jacobson, A.; Chamberlin, C.; Kammen, D. Thermal Gasification or Direct Combustion? Comparison of Advanced Cogeneration Systems Inthe Sugarcane Industry. Biomass Bioenergy 2013, 55, 163–174. [Google Scholar] [CrossRef]
- Erlich, C.; Björnbom, E.; Bolado, D.; Giner, M.; Fransson, T.H. Pyrolysis and Gasification of Pellets from Sugar Cane Bagasse and Wood. Fuel 2006, 85, 1535–1540. [Google Scholar] [CrossRef]
- Motta, I.L.; Miranda, N.T.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane Bagasse Gasification: Simulation and Analysis of Different Operating Parameters, Fluidizing Media, and Gasifier Types. Biomass Bioenergy 2019, 122, 433–445. [Google Scholar] [CrossRef]
- Higman, C. Gasification. In Combustion Engineering Issues for Solid Fuel Systems; Elsevier: Amsterdam, The Netherlands, 2008; pp. 423–468. [Google Scholar] [CrossRef]
- Anukam, A.; Mamphweli, S.; Reddy, P.; Meyer, E.; Okoh, O. Pre-Processing of Sugarcane Bagasse for Gasification in a Downdraft Biomass Gasifier System: A Comprehensive Review. Renew. Sustain. Energy Rev. 2016, 66, 775–801. [Google Scholar] [CrossRef]
- Zharova, P.; Arapova, O.V.; Konstantinov, G.I.; Chistyakov, A.V.; Tsodikov, M.V. Kraft Lignin Conversion into Energy Carriers under the Action of Electromagnetic Radiation. J. Chem. 2019, 2019, 6480354. [Google Scholar] [CrossRef]
- Ye, D.P.; Agnew, J.B.; Zhang, D.K. Gasification of a South Australian Low-Rank Coal with Carbon Dioxide and Steam: Kinetics and Reactivity Studies. Fuel 1998, 77, 1209–1219. [Google Scholar] [CrossRef]
- Matsunami, J.; Yoshida, S.; Oku, Y.; Yokota, O.; Tamaura, Y.; Kitamura, M. Coal Gasification by CO2 Gas Bubbling in Molten Salt for Solar/Fossil Energy Hybridization. Sol. Energy 2000, 68, 257–261. [Google Scholar] [CrossRef]
- Popa, T.; Fan, M.; Argyle, M.D.; Slimane, R.B.; Bell, D.A.; Towler, B.F. Catalytic Gasification of a Powder River Basin Coal. Fuel 2013, 103, 161–170. [Google Scholar] [CrossRef]
- Namkung, H.; Yuan, X.; Lee, G.; Kim, D.; Kang, T.J.; Kim, H.T. Reaction Characteristics through Catalytic Steam Gasification with Ultra Clean Coal Char and Coal. J. Energy Inst. 2014, 87, 253–262. [Google Scholar] [CrossRef]
- Alam, M.; DebRoy, T. Reaction between CO2 and Coke Doped with NaCN. Carbon N. Y. 1987, 25, 279–288. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Zhu, H.; Chen, X.; Yu, G. In-Situ Study on Structure Evolution and Gasification Reactivity of Biomass Char with K and Ca Catalysts at Carbon Dioxide Atmosphere. Carbon Resour. Convers. 2023, 6, 27–33. [Google Scholar] [CrossRef]
- Devi, T.G.; Kannan, M.P. Calcium Catalysis in Air Gasification of Cellulosic Chars. Fuel 1998, 77, 1825–1830. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Mechanism of Alkali and Alkaline Earth Catalyzed Gasification of Graphite by CO2 and H2O Studied by Electron Microscopy. J. Catal. 1992, 138, 12–23. [Google Scholar] [CrossRef]
- Hengel, T.; Late, T.D.; Walker, P.L. Catalysis of Lignite Char Gasification by Exchangeable Calcium and Magnesium. Fuel 1984, 63, 1214–1220. [Google Scholar] [CrossRef]
- Kurbatova, N.A.; El’Man, A.R.; Bukharkina, T.V. Application of Catalysts to Coal Gasification with Carbon Dioxide. Kinet. Catal. 2011, 52, 739–748. [Google Scholar] [CrossRef]
- Kodama, T.; Funatoh, A.; Shimizu, K.; Kitayama, Y. Kinetics of Metal Oxide-Catalyzed CO2 Gasification of Coal in a Fluidized-Bed Reactor for Solar Thermochemical Process. Energy Fuels 2001, 15, 1200–1206. [Google Scholar] [CrossRef]
- Furimsky, E.; Sears, P.; Suzuki, T. Iron-Catalyzed Gasification of Char in CO2. Energy Fuels 1988, 2, 634–639. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Rivera-Utrilla, J.; Ferro-Garcia, M.A. Gasification of Active Carbons of Different Texture Impregnated with Nickel, Cobalt and Iron. Carbon N. Y. 1987, 25, 703–708. [Google Scholar] [CrossRef]
- Gokon, N.; Hasegawa, N.; Kaneko, H.; Aoki, H.; Tamaura, Y.; Kitamura, M. Photocatalytic Effect of ZnO on Carbon Gasification with CO2 for High Temperature Solar Thermochemistry. Sol. Energy Mater. Sol. Cells 2003, 80, 335–341. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Kravtsov, A.V.; Kalmykov, K.B.; Sarkar, B.; Kostyukhin, E.M.; Kustov, L.M. Gasification of Hydrolysis Lignin with CO2 in the Presence of Fe and Co Compounds. Mendeleev Commun. 2022, 32, 402–404. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Beldova, D.A.; Kalmykov, K.B.; Kravtsov, A.V.; Tedeeva, M.A.; Kustov, L.M.; Dunaev, S.F.; Kustov, A.L. Carbon Dioxide Assisted Conversion of Hydrolysis Lignin Catalyzed by Nickel Compounds. Energies 2022, 15, 6774. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Kalmykov, K.B.; Mashkin, M.Y.; Shesterkina, A.A.; Dunaev, S.F.; Kustov, L.M. Influence of the Method of Fe Deposition on the Surface of Hydrolytic Lignin on the Activity in the Process of Its Conversion in the Presence of CO2. Int. J. Mol. Sci. 2023, 24, 1279. [Google Scholar] [CrossRef]
- Tolkachev, N.N.; Koklin, A.E.; Laptinskaya, T.V.; Lunin, V.V.; Bogdan, V.I. Influence of Heat Treatment on the Size of Sodium Lignosulfonate Particles in Water—Ethanol Media. Russ. Chem. Bull. 2019, 68, 1613–1620. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Nikolaev, S.A.; Chistyakov, A.V.; Bukhtenko, O.V.; Fomkin, A.A. Formation of Adsorbents from Fe-Containing Processing Residues of Lignin. Microporous Mesoporous Mater. 2020, 298, 110089. [Google Scholar] [CrossRef]
- Tsodikov, M.D.; Ellert, O.G.; Arapova, O.V.; Nikolaev, S.A.; Chistyakov, A.V.; Maksimov, Y.V. Benefit of Fe-Containing Catalytic Systems for Dry Reforming of Lignin to Syngas under Microwave Radiation. Chem. Eng. Trans. 2018, 65, 367–372. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Chen, G. Catalytic Gasification Characteristics of Cellulose, Hemicellulose and Lignin. Renew. Energy 2018, 121, 559–567. [Google Scholar] [CrossRef]
- Pan, Y.; Tursun, Y.; Abduhani, H.; Turap, Y.; Abulizi, A.; Talifua, D. Chemical Looping Gasification of Cotton Stalk with Bimetallic Cu/Ni/Olivine as Oxygen Carrier. Int. J. Energy Res. 2020, 44, 7268–7282. [Google Scholar] [CrossRef]
- Ruiz, M.; Schnitzer, A.; Courson, C.; Mauviel, G. Fe-Doped Olivine and Char for in-Bed Elimination of Gasification Tars in an Air-Blown Fluidised Bed Reactor Coupled with Oxidative Hot Gas Filtration. Carbon Resour. Convers. 2022, 5, 271–288. [Google Scholar] [CrossRef]
- Mastuli, M.S.; Kamarulzaman, N.; Kasim, M.F.; Sivasangar, S.; Saiman, M.I.; Taufiq-Yap, Y.H. Catalytic Gasification of Oil Palm Frond Biomass in Supercritical Water Using MgO Supported Ni, Cu and Zn Oxides as Catalysts for Hydrogen Production. Int. J. Hydrogen Energy 2017, 42, 11215–11228. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Ji, G.; Gao, Y.; Khushk, S. Hydrogen-Rich Syngas from Wet Municipal Solid Waste Gasification Using Ni/Waste Marble Powder Catalyst Promoted by Transition Metals. Waste Manag. 2021, 132, 96–104. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Wang, M.; Chen, C.; Khushk, S. Production of Hydrogen Enriched Syngas from Municipal Solid Waste Gasification with Waste Marble Powder as a Catalyst. Int. J. Hydrogen Energy 2019, 44, 8051–8061. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Catalytic Pyrolysis of Rice Husk with SnCl2, Al2O3.4SiO2.H2O, and MoS2 for Improving the Chemical Composition of Pyrolysis Oil and Gas. J. Indian Chem. Soc. 2022, 99, 100728. [Google Scholar] [CrossRef]
- Li, B.; Magoua Mbeugang, C.F.; Xie, X.; Wei, J.; Zhang, S.; Zhang, L.; El Samahy, A.A.; Xu, D.; Wang, Q.; Zhang, S.; et al. Catalysis/CO2 Sorption Enhanced Pyrolysis-Gasification of Biomass for H2-Rich Gas Production: Effects of Activated Carbon, NiO Active Component and Calcined Dolomite. Fuel 2023, 334 Pt 2, 126842. [Google Scholar] [CrossRef]
- Mei, Y.; Zhang, Q.; Wang, Z.; Gao, S.; Fang, Y. Novel Re-Utilization of High-Temperature Catalytic Gasification Ash with Sodium Recovery, Aluminum Extraction, Aragonite and Mesoporous SiO2 Synthesis. Fuel 2023, 331 Pt 1, 125727. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Ye, L.; Li, S.; Su, Y.; Zhang, H. Investigation into Biochar Supported Fe-Mo Carbides Catalysts for Efficient Biomass Gasification Tar Cracking. Chem. Eng. J. 2023, 454 Pt 1, 140072. [Google Scholar] [CrossRef]
- Faust, R.; Valizadeh, A.; Qiu, R.; Tormachen, A.; Maric, J.; Vilches, T.B.; Skoglund, N.; Seemann, M.; Halvarsson, M.; Öhman, M.; et al. Role of Surface Morphology on Bed Material Activation during Indirect Gasification of Wood. Fuel 2023, 333, 126387. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.; Li, Z.J.; Dariva, C.; Cao, Y.; Ellis, N. Predicting and Optimizing Syngas Production from Fluidized Bed Biomass Gasifiers: A Machine Learning Approach. Energy 2023, 263, 125900. [Google Scholar] [CrossRef]
- Varga, G.; Sápi, A.; Varga, T.; Baán, K.; Szenti, I.; Halasi, G.; Mucsi, R.; Óvári, L.; Kiss, J.; Fogarassy, Z.; et al. Ambient Pressure CO2 Hydrogenation over a Cobalt/Manganese-Oxide Nanostructured Interface: A Combined in Situ and Ex Situ Study. J. Catal. 2020, 386, 70–80. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Polikarpova, S.B.; Ponomarev, V.E.; Murashova, E.V.; Sokolovskiy, P.V.; Kustov, L.M. A Synergistic Effect of Potassium and Transition Metal Compounds on the Catalytic Behaviour of Hydrolysis Lignin in CO2-Assisted Gasification. Energies 2023, 16, 4335. [Google Scholar] [CrossRef]
- Zahara, Z.F.; Kudo, S.; Daniyanto; Ashik, U.P.M.; Norinaga, K.; Budiman, A.; Hayashi, J.I. CO2 Gasification of Sugar Cane Bagasse: Quantitative Understanding of Kinetics and Catalytic Roles of Inherent Metallic Species. Energy Fuels 2018, 32, 4255–4268. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Kinetics and Mechanism of Catalytic Carbon Gasification. Fuel 2016, 183, 457–469. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent Radii Revisited. J. Chem. Soc. Dalt. Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef]




| C, wt. % | H, wt. % | N, % | S, % |
|---|---|---|---|
| 46.81 | 6.25 | 0.48 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldova, D.A.; Medvedev, A.A.; Kustov, A.L.; Mashkin, M.Y.; Kirsanov, V.Y.; Vysotskaya, I.V.; Sokolovskiy, P.V.; Kustov, L.M. CO2-Assisted Sugar Cane Gasification Using Transition Metal Catalysis: An Impact of Metal Loading on the Catalytic Behavior. Materials 2023, 16, 5662. https://doi.org/10.3390/ma16165662
Beldova DA, Medvedev AA, Kustov AL, Mashkin MY, Kirsanov VY, Vysotskaya IV, Sokolovskiy PV, Kustov LM. CO2-Assisted Sugar Cane Gasification Using Transition Metal Catalysis: An Impact of Metal Loading on the Catalytic Behavior. Materials. 2023; 16(16):5662. https://doi.org/10.3390/ma16165662
Chicago/Turabian StyleBeldova, Daria A., Artem A. Medvedev, Alexander L. Kustov, Mikhail Yu. Mashkin, Vladislav Yu. Kirsanov, Irina V. Vysotskaya, Pavel V. Sokolovskiy, and Leonid M. Kustov. 2023. "CO2-Assisted Sugar Cane Gasification Using Transition Metal Catalysis: An Impact of Metal Loading on the Catalytic Behavior" Materials 16, no. 16: 5662. https://doi.org/10.3390/ma16165662
APA StyleBeldova, D. A., Medvedev, A. A., Kustov, A. L., Mashkin, M. Y., Kirsanov, V. Y., Vysotskaya, I. V., Sokolovskiy, P. V., & Kustov, L. M. (2023). CO2-Assisted Sugar Cane Gasification Using Transition Metal Catalysis: An Impact of Metal Loading on the Catalytic Behavior. Materials, 16(16), 5662. https://doi.org/10.3390/ma16165662







