Three-Dimensional Peri-Implant Tissue Changes in Immediately vs. Early Placed Tapered Implants Restored with Two Different Ceramic Materials—1 Year Results
Abstract
1. Introduction
2. Materials and Methods
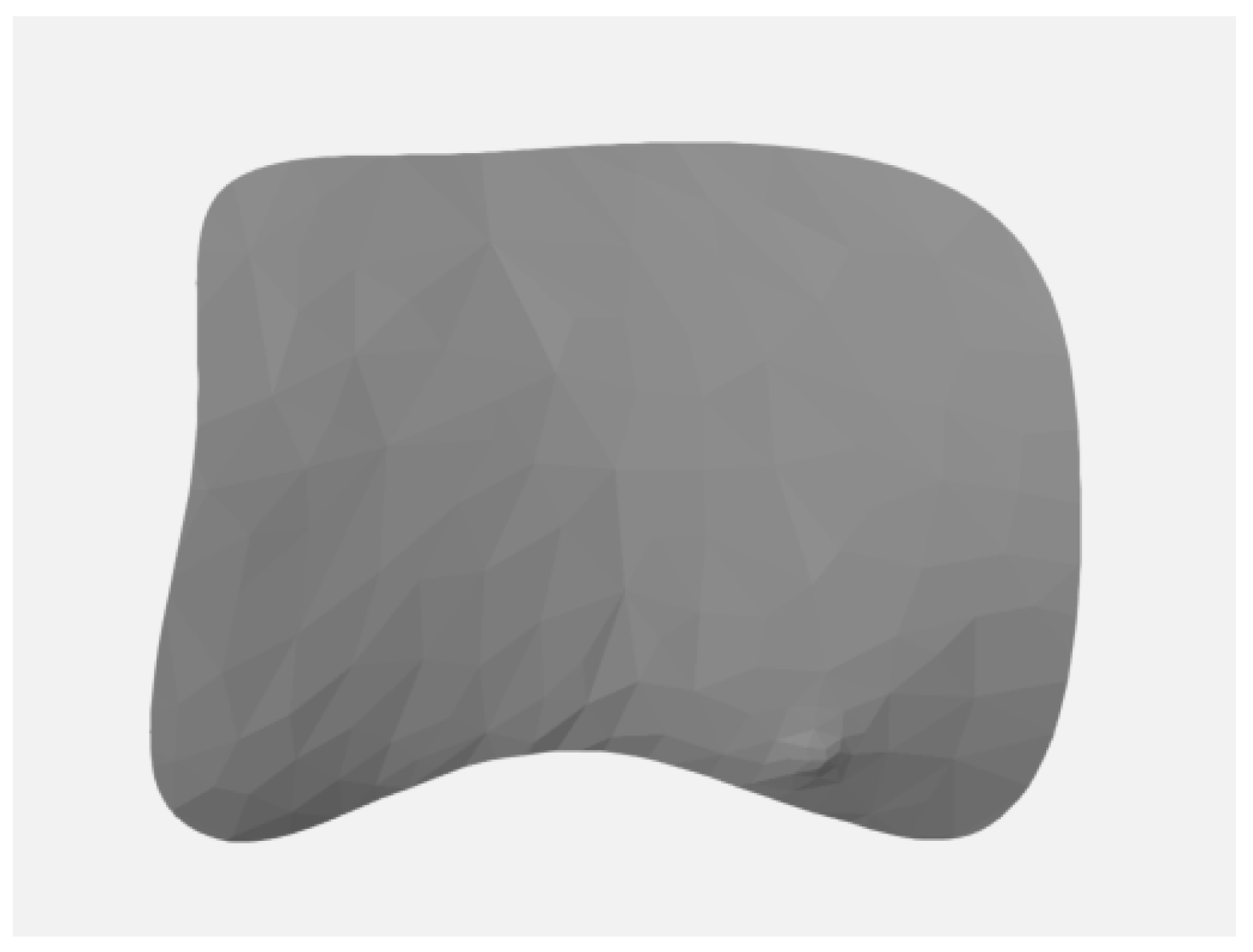
2.1. Definition of the Area of Interest (AOI)
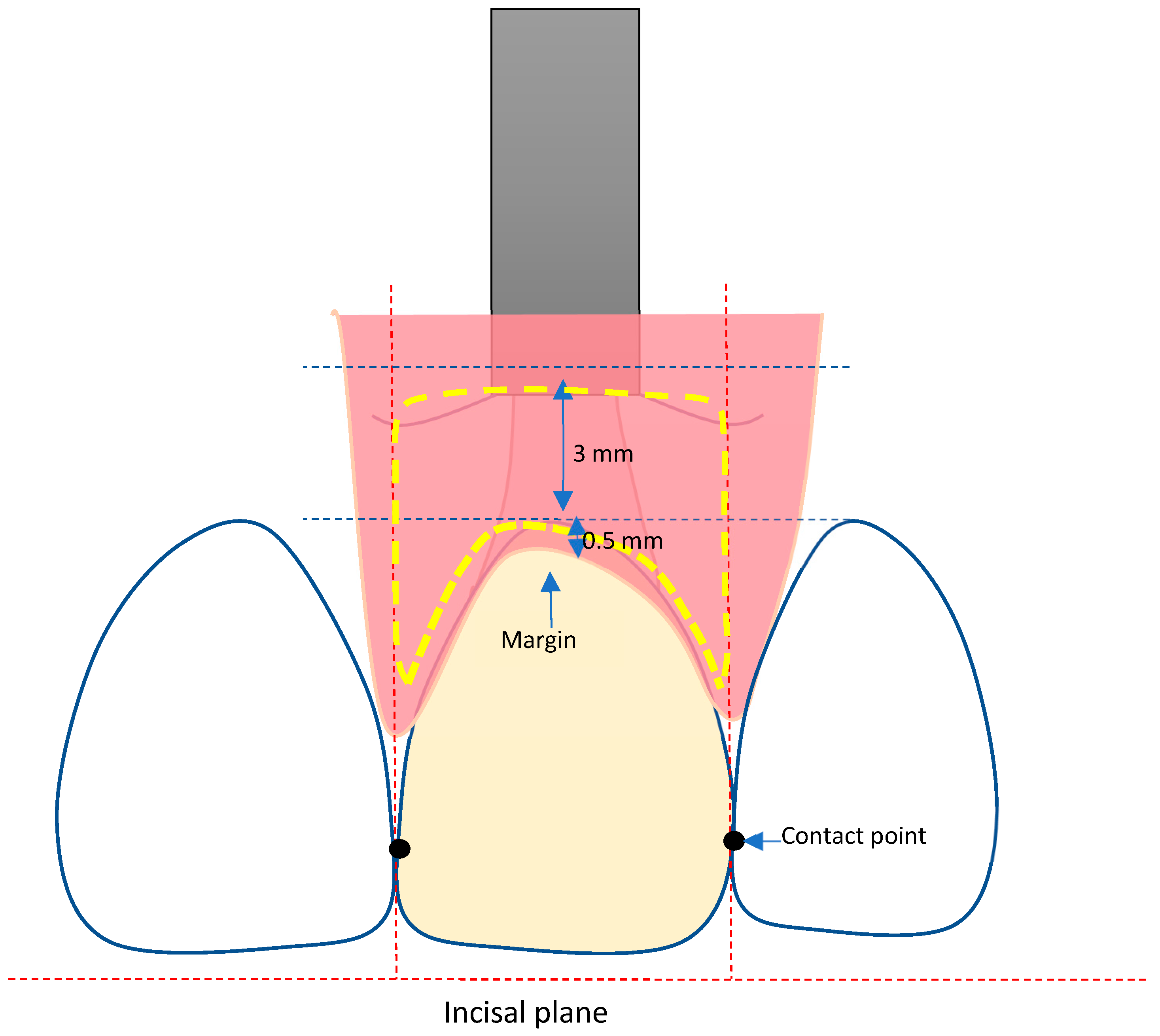
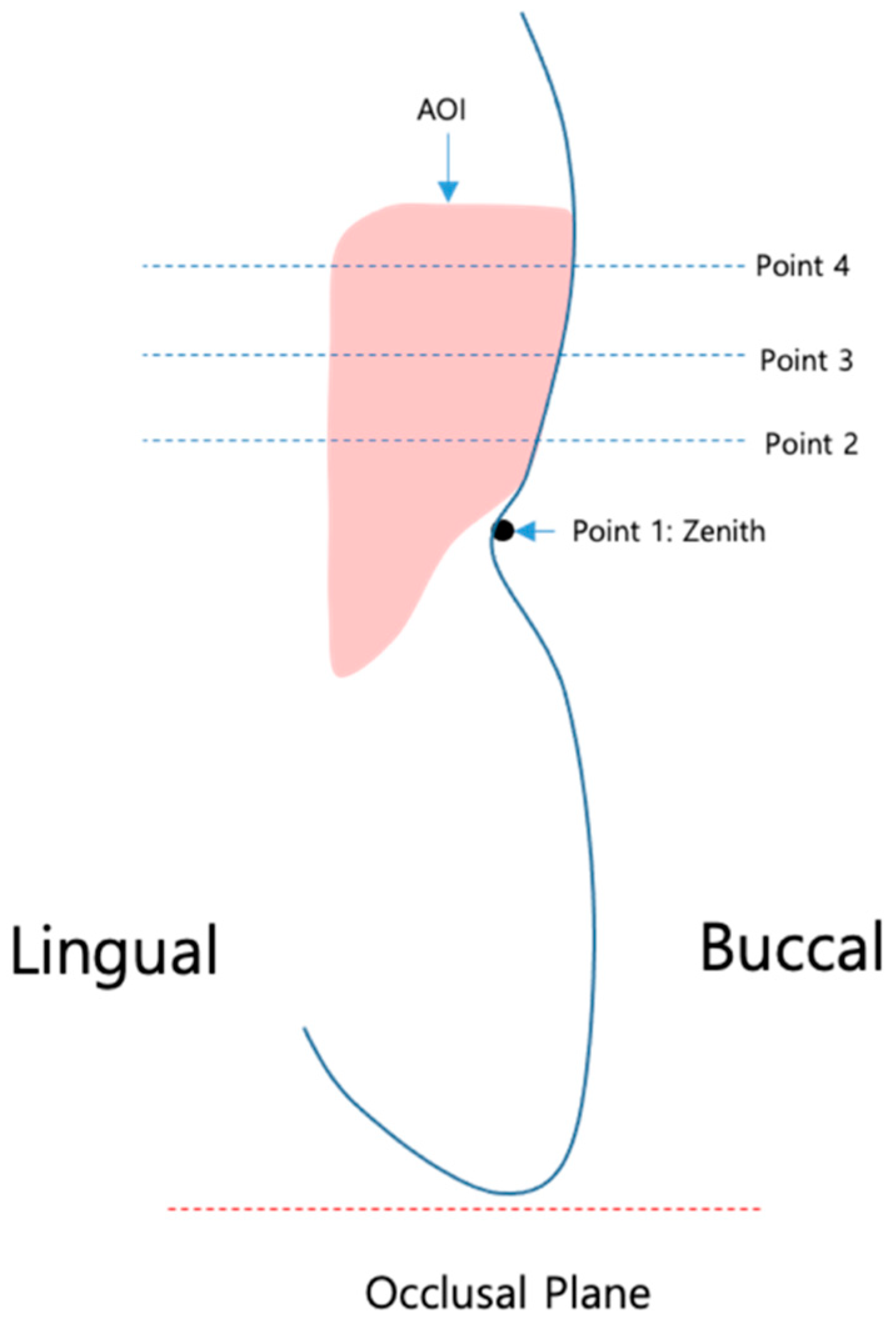
2.2. Definition of the Reference Points (Figure 2)
2.3. Volumetric Analysis
2.4. Analysis Procedure
- Superimposition of STL data using best-fit algorithm focusing on the area of implant placement.
- Alignment of the superimposed files with the x-, y-, and z-axes by each surface of the restoration of the implant.
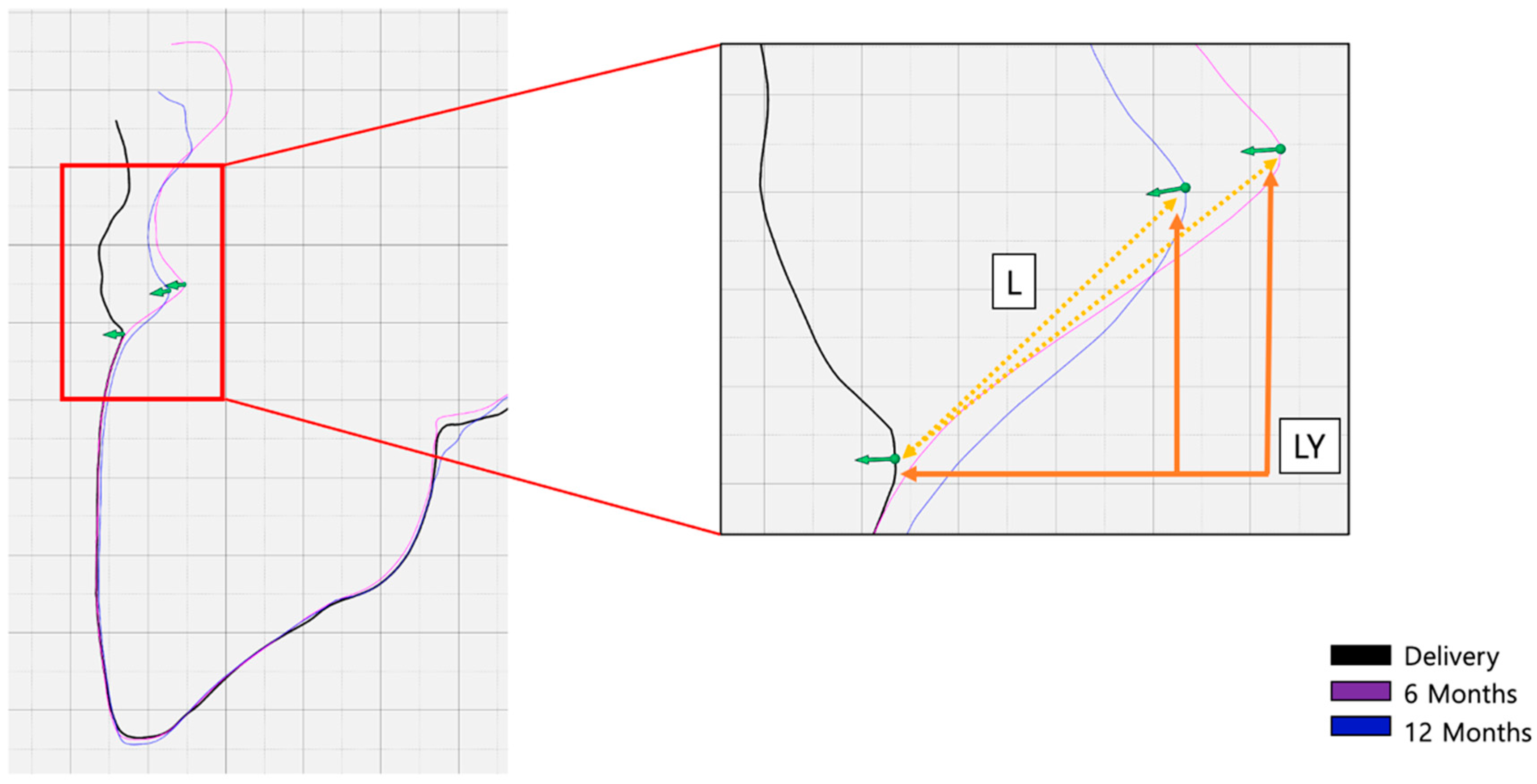
- Definition and marking of area of interest (AOI) in baseline STL and STL files at 6 and 12 months (Figure 3).
- Separation of the AOI (Figure 4) from the original STL files.
- Measurement of the AOI
- Analysis by comparison of the AOI of each STL file: the separated AOI of the baseline visit was projected onto the AOI of the STL at 6-month and 12-month follow up to analyze any volume changes.
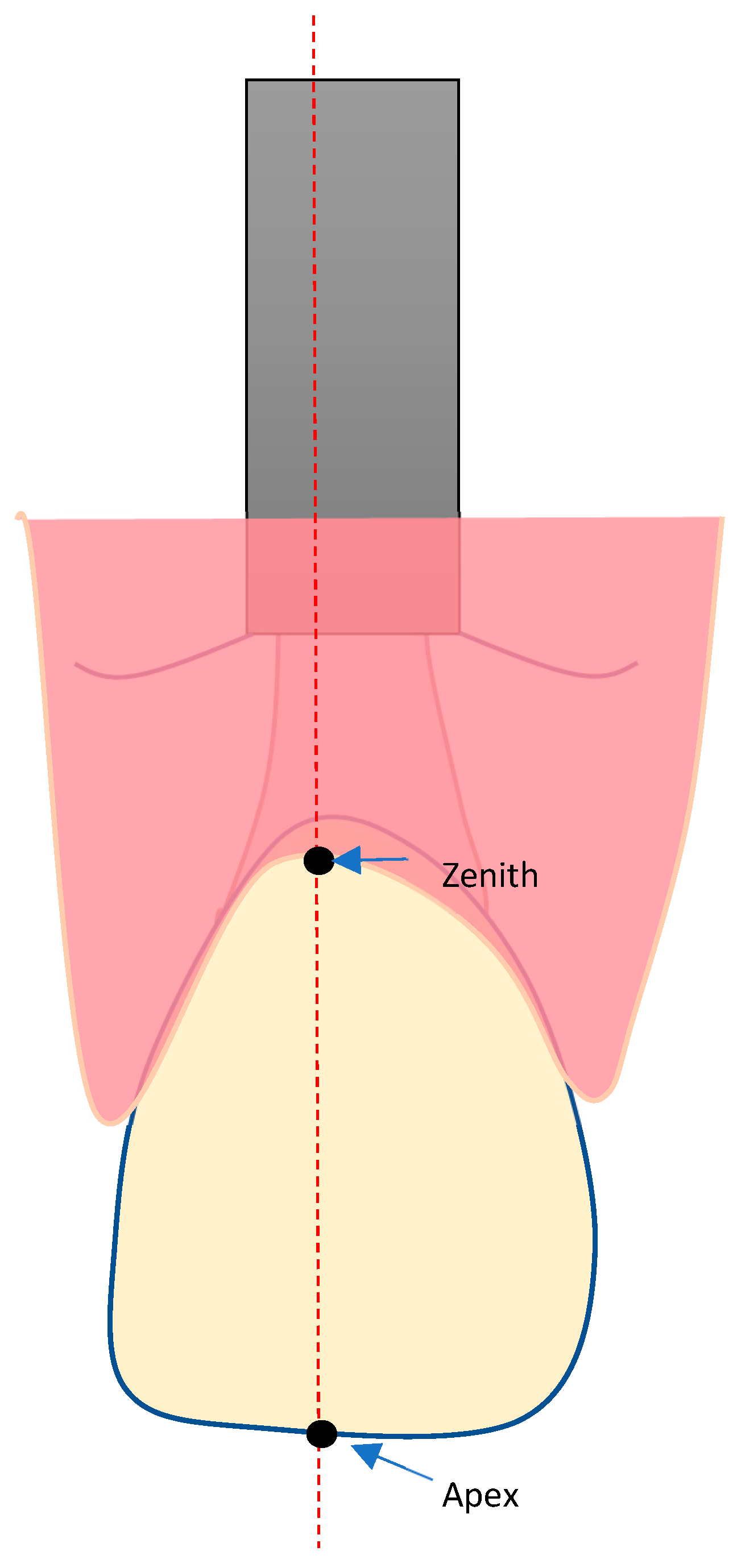
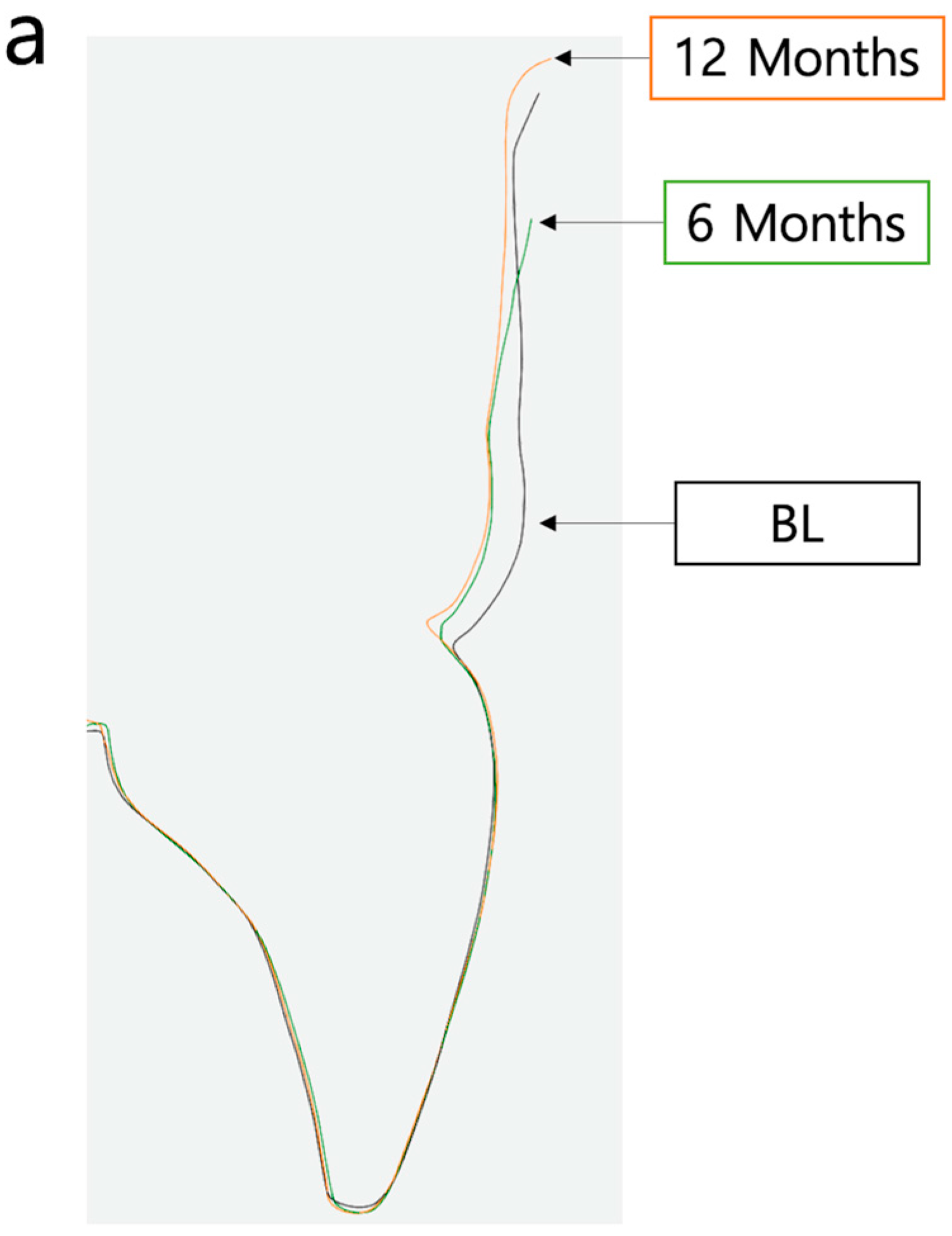
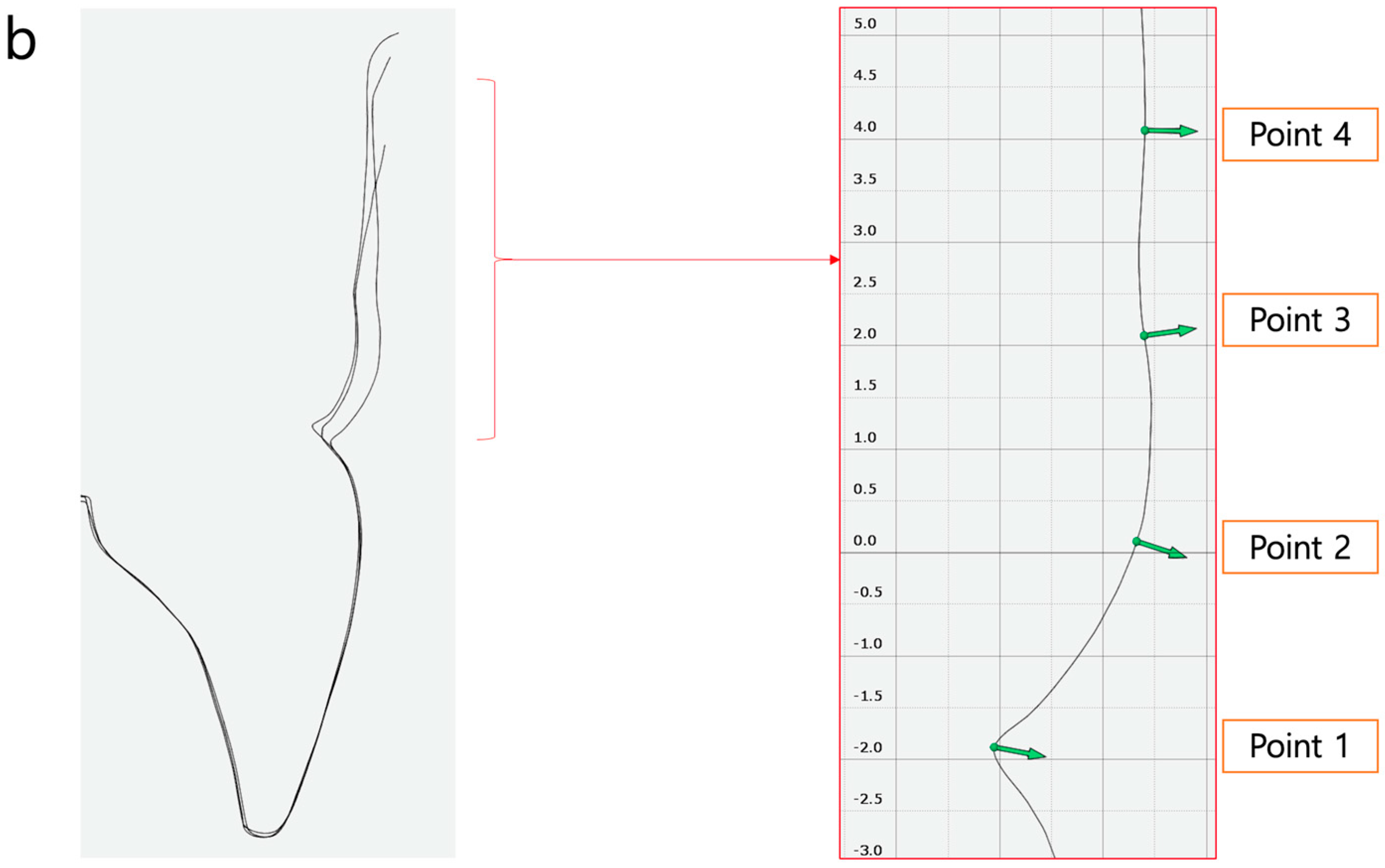
- Vertical marginal height analysis using sectional analysis method. A vertical line was drawn from the zenith to the cusp/incisal edge of the tooth (Figure 5). Selection of reference points (points 1–4) on BL-STL was realized for analysis of horizontal changes in thickness at points 2, 3, and 4 and measurement of zenith changes (point 1) from BL to 6- and 12-month follow up (Figure 6 and Figure 7).
- Analysis of volume changes from AOI of BL to AOI of 6 and 12 months, using the sectional analysis method described earlier [23].
2.5. Statistical Analysis
3. Results
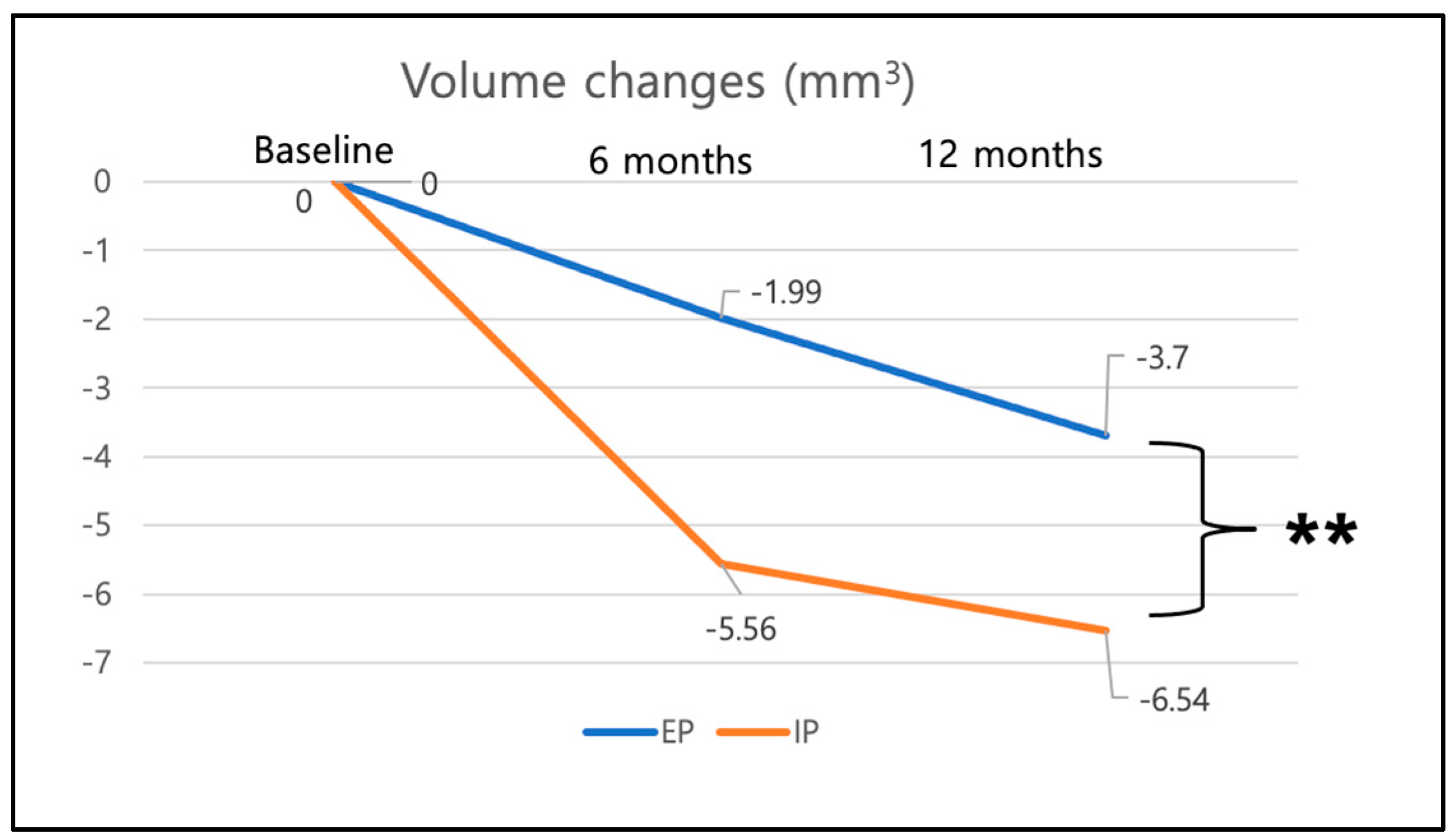
3.1. Volume Changes
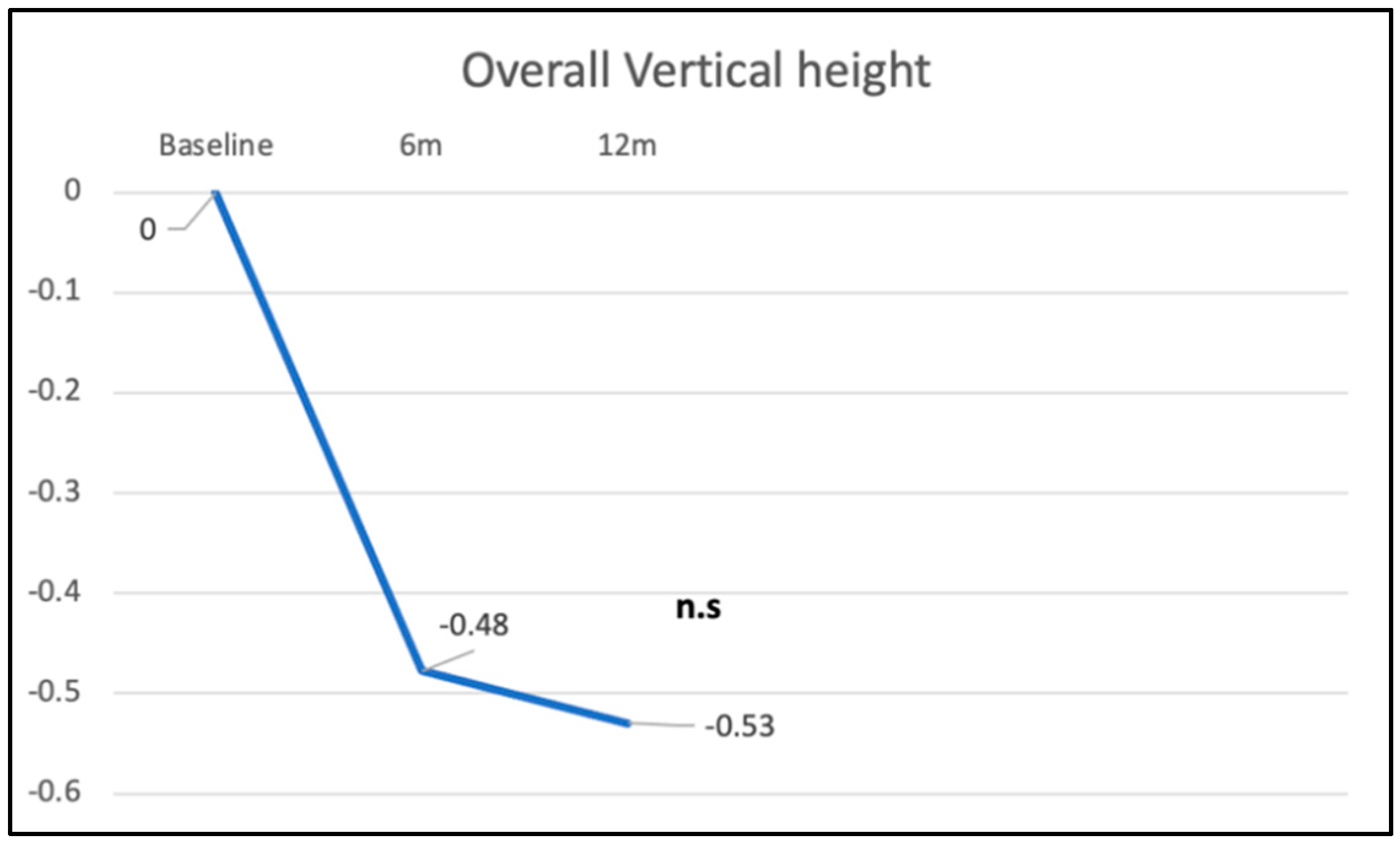
3.2. Overall Vertical Height Changes (Point 1)
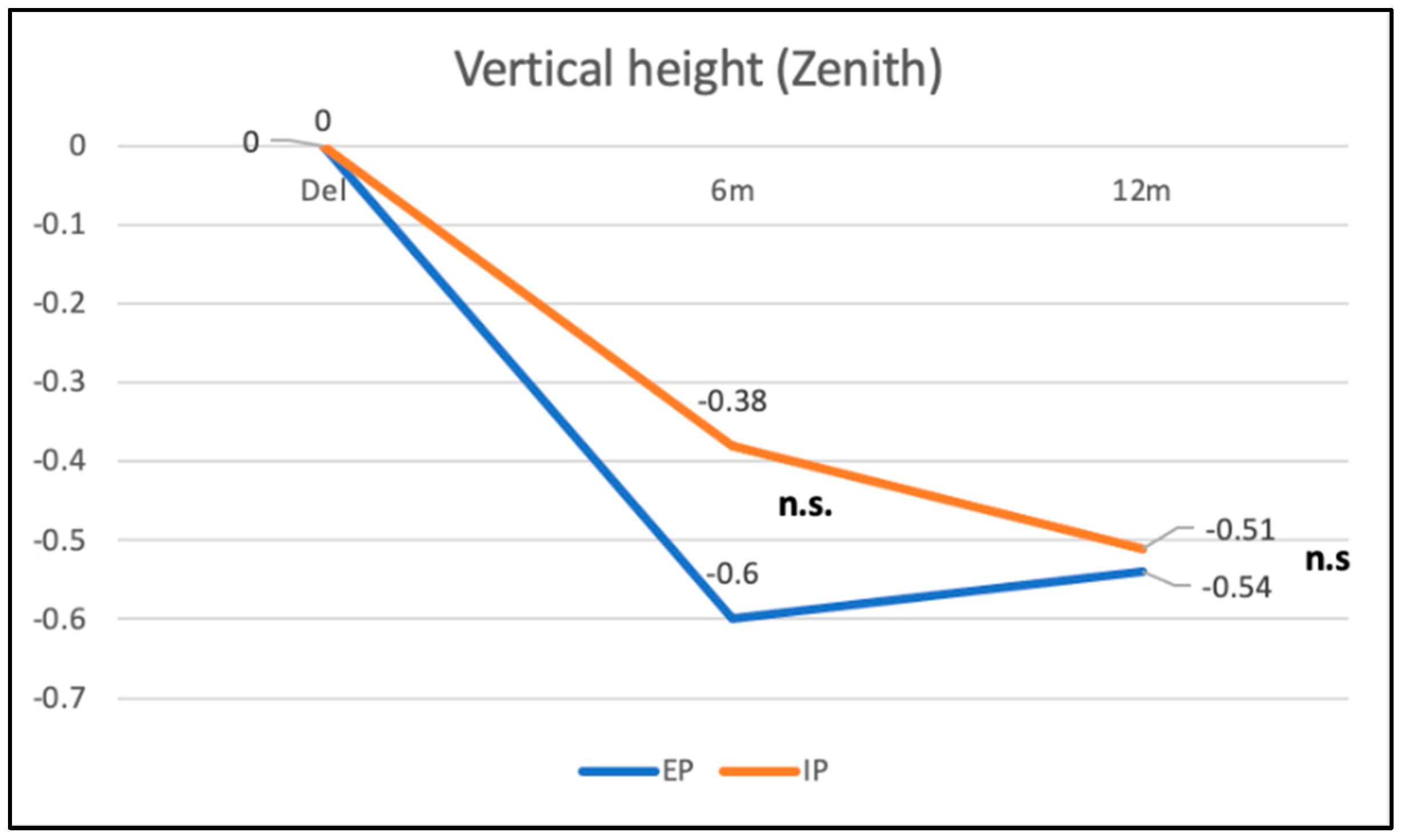
3.3. Vertical Height of Zenith Point in Immediate vs. Early Placement and Zirconia vs. Lithium Disilicate
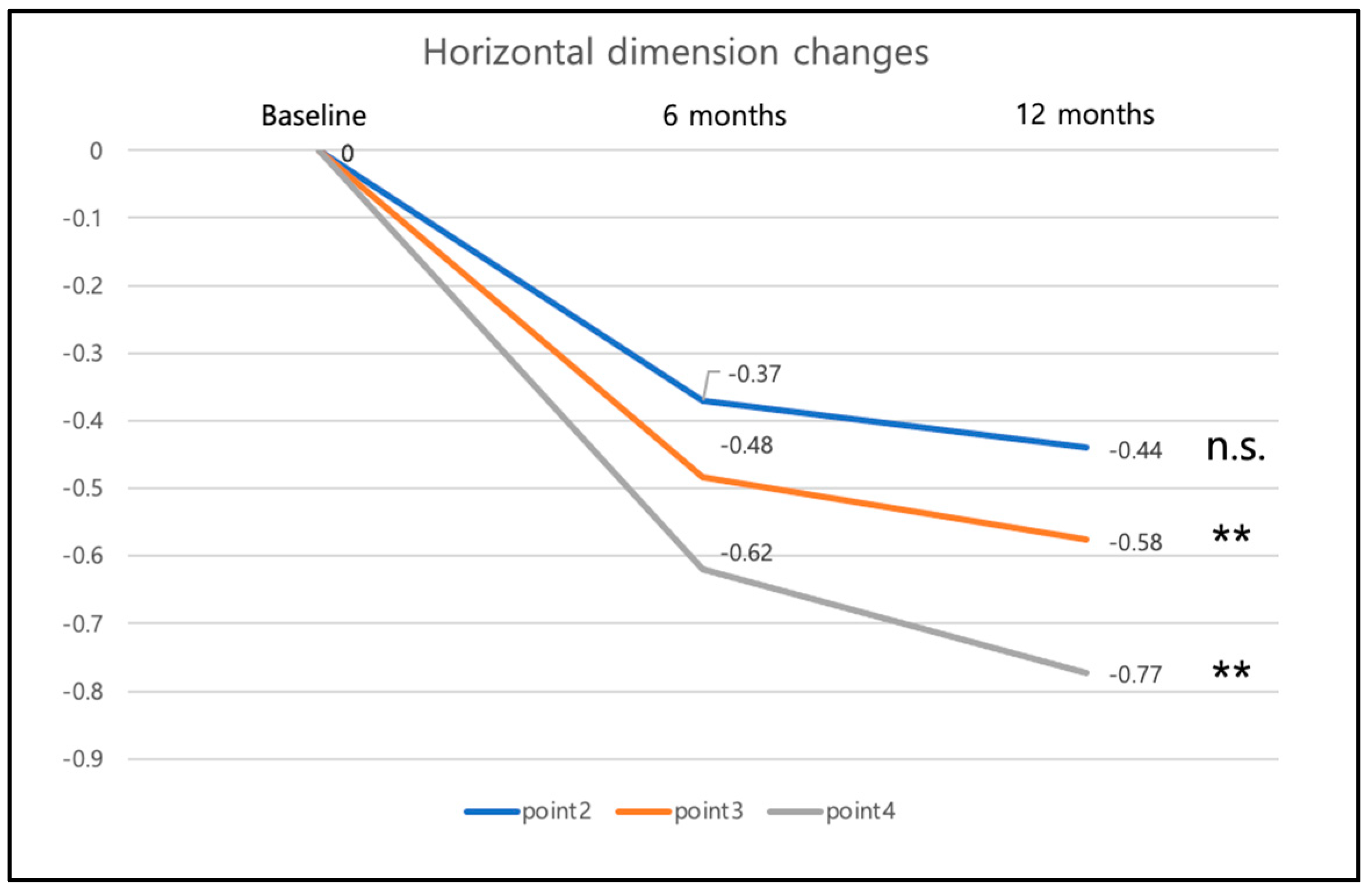
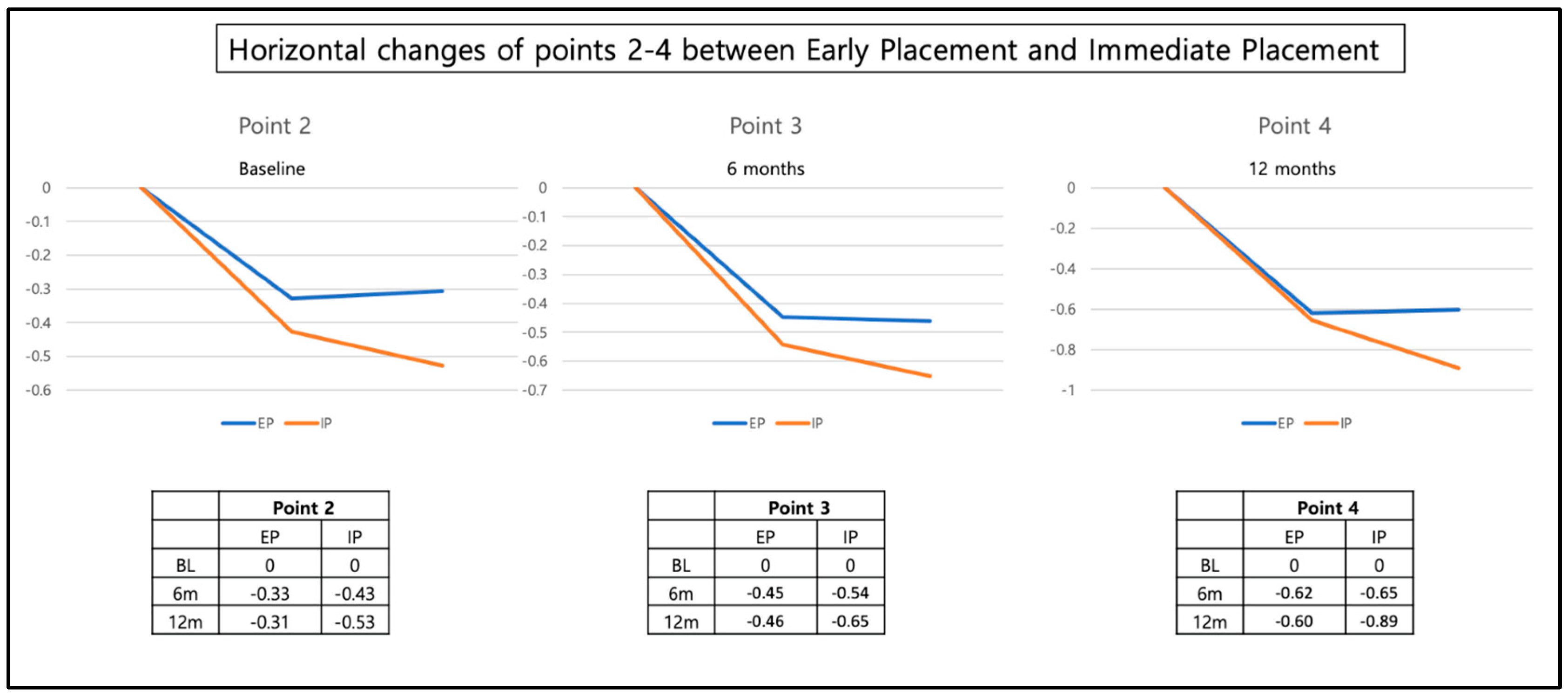
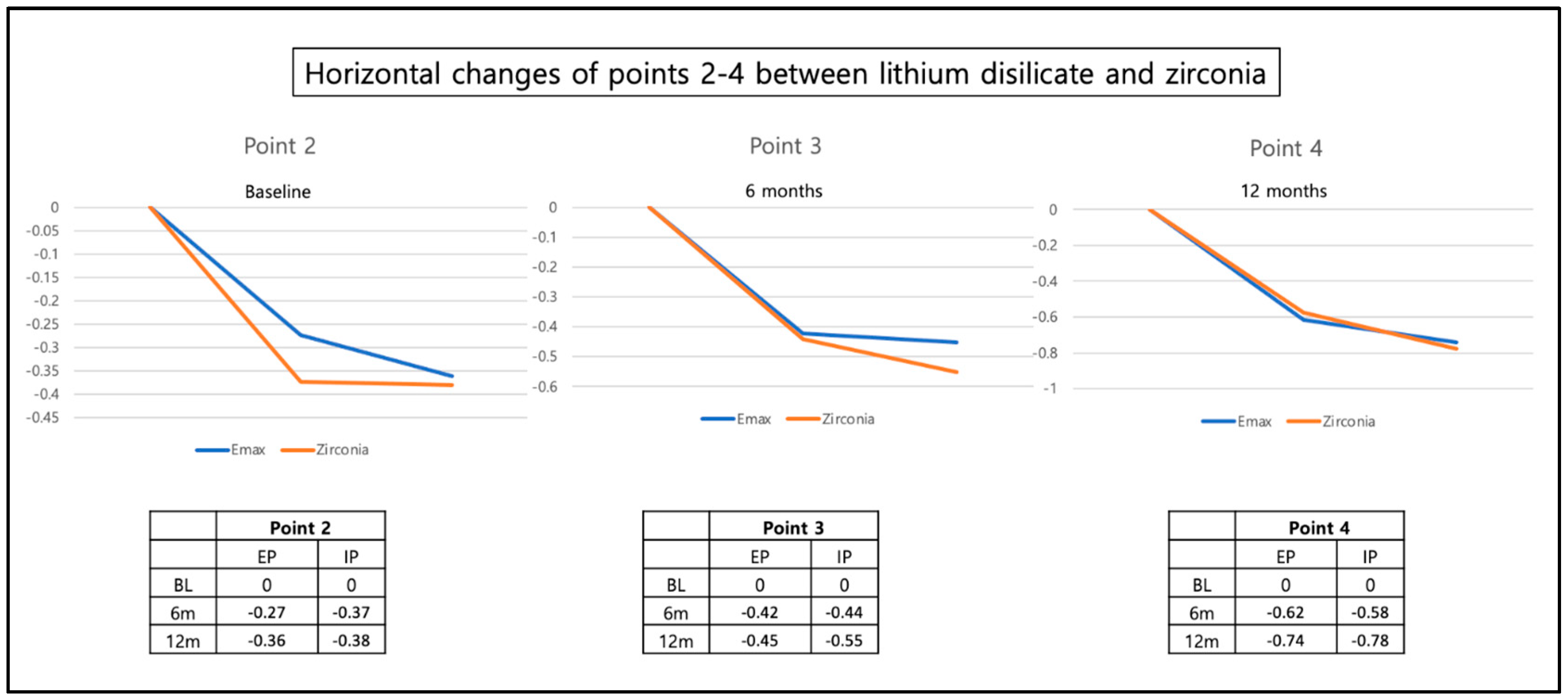
3.4. Changes in Horizontal Width
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Bragger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Albrektsson, T. Long-term clinical success of minimally and moderately rough oral implants: A review of 71 studies with 5 years or more of follow-up. Implant Dent. 2015, 24, 62–69. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Zarauz, C.; Strasding, M.; Sailer, I.; Zwahlen, M.; Zembic, A. A systematic review of the influence of the implant-abutment connection on the clinical outcomes of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 160–183. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 199–214. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Strasding, M.; Valente, N.A.; Zwahlen, M.; Liu, S.; Pjetursson, B.E. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 184–198. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Persson, L.G.; Lindhe, J. Bone regeneration at implants with turned or rough surfaces in self-c ontained defects. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Schmid, B.; Belser, U.C.; Lussi, A.; Buser, D. Early loading of non-submerged titanium implants with a sandblasted and acid-etched surface. 5-year results of a prospective study in partially edentulous patients. Clin. Oral Implants Res. 2005, 16, 631–638. [Google Scholar] [CrossRef]
- Suzuki, S.; Kobayashi, H.; Ogawa, T. Implant stability change and osseointegration speed of immediately loaded photofunctionalized implants. Implant Dent. 2013, 22, 481–490. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Grunder, U.; Polizzi, G.; Goene, R.; Hatano, N.; Henry, P.; Jackson, W.J.; Kawamura, K.; Kohler, S.; Renouard, F.; Rosenberg, R.; et al. A 3-year prospective multicenter follow-up report on the immediate and delayed-immediate placement of implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 210–216. [Google Scholar]
- Chen, S.T.; Wilson, T.G., Jr.; Hammerle, C.H. Immediate or early placement of implants following tooth extraction: Review of biologic basis, clinical procedures, and outcomes. Int. J. Oral Maxillofac. Implant. 2004, 19, 12–25. [Google Scholar]
- Lang, N.P.; Pun, L.; Lau, K.Y.; Li, K.Y.; Wong, M.C. A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 39–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Buser, D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla—A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 186–215. [Google Scholar] [CrossRef]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 106–134. [Google Scholar] [CrossRef]
- Buser, D.; Chappuis, V.; Bornstein, M.M.; Wittneben, J.G.; Frei, M.; Belser, U.C. Long-term stability of contour augmentation with early implant placement following single tooth extraction in the esthetic zone: A prospective, cross-sectional study in 41 patients with a 5- to 9-year follow-up. J. Periodontol. 2013, 84, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Wittneben, J.; Bornstein, M.M.; Grutter, L.; Chappuis, V.; Belser, U.C. Stability of contour augmentation and esthetic outcomes of implant-supported single crowns in the esthetic zone: 3-year results of a prospective study with early implant placement postextraction. J. Periodontol. 2011, 82, 342–349. [Google Scholar] [CrossRef]
- Furhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: The pink esthetic score. Clin. Oral Implant. Res. 2005, 16, 639–644. [Google Scholar] [CrossRef]
- Jemt, T. Restoring the gingival contour by means of provisional resin crowns after single-implant treatment. Int. J. Periodontics Restor. Dent. 1999, 19, 20–29. [Google Scholar]
- Meijer, H.J.; Stellingsma, K.; Meijndert, L.; Raghoebar, G.M. A new index for rating aesthetics of implant-supported single crowns and adjacent soft tissues—The Implant Crown Aesthetic Index. Clin. Oral Implant. Res. 2005, 16, 645–649. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Wang, H.L. Esthetic index for anterior maxillary implant-supported restorations. J. Periodontol. 2010, 81, 34–42. [Google Scholar] [CrossRef]
- Hosseini, M.; Gotfredsen, K. A feasible, aesthetic quality evaluation of implant-supported single crowns: An analysis of validity and reliability. Clin. Oral Implant. Res. 2012, 23, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.B.; Pette, G.A.; Parker, W.B.; Hardigan, P. Gingival margin changes in maxillary anterior sites after single immediate implant placement and provisionalization: A 5-year retrospective study of 47 patients. Int. J. Oral Maxillofac. Implant. 2014, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Fehmer, V.; Hicklin, S.; Noh, G.; Hong, S.J.; Sailer, I. Three-Dimensional Evaluation of Peri-implant Soft Tissue When Tapered Implants Are Placed: Pilot Study with Implants Placed Immediately or Early Following Tooth Extraction. Int. J. Oral Maxillofac. Implant. 2020, 35, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Calvo-Guirado, J.L.; Negri, B.; Di Felice, R.; Covani, U. Volumetric analysis of remodelling pattern after ridge preservation comparing use of two types of xenografts. A multicentre randomized clinical trial. Clin. Oral Implant. Res. 2016, 27, e105–e115. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Chappuis, V.; Kuchler, U.; Bornstein, M.M.; Wittneben, J.G.; Buser, R.; Cavusoglu, Y.; Belser, U.C. Long-term stability of early implant placement with contour augmentation. J. Dent. Res. 2013, 92, 176S–182S. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Omran, M.; Bakhshalian, N.; Tarnow, D.; Zadeh, H.H. An open randomized controlled clinical trial to evaluate ridge preservation and repair using SocketKAP(™) and SocketKAGE(™): Part 2—Three-dimensional alveolar bone volumetric analysis of CBCT imaging. Clin. Oral Implant. Res. 2016, 27, 631–639. [Google Scholar] [CrossRef]
- Benic, G.I.; Mokti, M.; Chen, C.J.; Weber, H.P.; Hammerle, C.H.; Gallucci, G.O. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: A clinical and cone beam computed tomography study. Clin. Oral Implant. Res. 2012, 23, 560–566. [Google Scholar] [CrossRef]
- Omran, M.; Min, S.; Abdelhamid, A.; Liu, Y.; Zadeh, H.H. Alveolar ridge dimensional changes following ridge preservation procedure: Part-2—CBCT 3D analysis in non-human primate model. Clin. Oral Implant. Res. 2016, 27, 859–866. [Google Scholar] [CrossRef]
- Jung, R.E.; Benic, G.I.; Scherrer, D.; Hammerle, C.H. Cone beam computed tomography evaluation of regenerated buccal bone 5 years after simultaneous implant placement and guided bone regeneration procedures—A randomized, controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Botilde, G.; Colin, P.E.; Gonzalez-Martin, O.; Lecloux, G.; Rompen, E.; Lambert, F. Hard and soft tissue analysis of alveolar ridge preservation in esthetic zone using deproteinized bovine bone mineral and a saddle connective tissue graft: A long-term prospective case series. Clin. Implant Dent. Relat. Res. 2020, 22, 387–396. [Google Scholar] [CrossRef]
- Jung, R.E.; Brugger, L.V.; Bienz, S.P.; Husler, J.; Hammerle, C.H.F.; Zitzmann, N.U. Clinical and radiographical performance of implants placed with simultaneous guided bone regeneration using resorbable and nonresorbable membranes after 22–24 years, a prospective, controlled clinical trial. Clin. Oral Implant. Res. 2021, 32, 1455–1465. [Google Scholar] [CrossRef]
- Martin, W.C.; Pollini, A.; Morton, D. The influence of restorative procedures on esthetic outcomes in implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.; Blatz, M.B.; Hegenbarth, E.; Wichmann, M.; Eitner, S. Prosthodontic considerations for predictable single-implant esthetics in the anterior maxilla. J. Oral Maxillofac. Surg. 2005, 63, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.; Ali, I.A.A.; Khan, K.; Mattheos, N.; Murbay, S.; Matinlinna, J.P.; Neelakantan, P. The Influence of Surface Roughening and Polishing on Microbial Biofilm Development on Different Ceramic Materials. J. Prosthodont. 2021, 30, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Corvino, E.; Pesce, P.; Mura, R.; Marcano, E.; Canullo, L. Influence of Modified Titanium Abutment Surface on Peri-implant Soft Tissue Behavior: A Systematic Review of In Vitro Studies. Int. J. Oral Maxillofac. Implant. 2020, 35, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Wittneben, J.G.; Joda, T.; Weber, H.P.; Bragger, U. Screw retained vs. cement retained implant-supported fixed dental prosthesis. Periodontology 2000 2017, 73, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Nothdurft, F.; Pospiech, P. Prefabricated zirconium dioxide implant abutments for single-tooth replacement in the posterior region: Evaluation of peri-implant tissues and superstructures after 12 months of function. Clin. Oral Implant. Res. 2010, 21, 857–865. [Google Scholar] [CrossRef]
- Ekfeldt, A.; Furst, B.; Carlsson, G.E. Zirconia abutments for single-tooth implant restorations: A retrospective and clinical follow-up study. Clin. Oral Implant. Res. 2011, 22, 1308–1314. [Google Scholar] [CrossRef]
- Strasding, M.; Hicklin, S.P.; Todorovic, A.; Fehmer, V.; Mojon, P.; Sailer, I. A multicenter randomized controlled clinical pilot study of buccally micro-veneered lithium-disilicate and zirconia crowns supported by titanium base abutments: 1-year outcomes. Clin. Oral Implant. Res. 2023, 34, 56–65. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Schneider, D.; Grunder, U.; Ender, A.; Hammerle, C.H.; Jung, R.E. Volume gain and stability of peri-implant tissue following bone and soft tissue augmentation: 1-year results from a prospective cohort study. Clin. Oral Implant. Res. 2011, 22, 28–37. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- van Nimwegen, W.G.; Raghoebar, G.M.; Zuiderveld, E.G.; Jung, R.E.; Meijer, H.J.A.; Muhlemann, S. Immediate placement and provisionalization of implants in the aesthetic zone with or without a connective tissue graft: A 1-year randomized controlled trial and volumetric study. Clin. Oral Implant. Res. 2018, 29, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Palattella, P.; Torsello, F.; Cordaro, L. Two-year prospective clinical comparison of immediate replacement vs. immediate restoration of single tooth in the esthetic zone. Clin. Oral Implant. Res. 2008, 19, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.D.; Chen, S.T. Esthetic outcomes of immediate implant placements. Clin. Oral Implant. Res. 2008, 19, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Darby, I.B.; Reynolds, E.C. A prospective clinical study of non-submerged immediate implants: Clinical outcomes and esthetic results. Clin. Oral Implant. Res. 2007, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Enkling, N.; Marder, M.; Bayer, S.; Gotz, W.; Stoilov, M.; Kraus, D. Soft tissue response to different abutment materials: A controlled and randomized human study using an experimental model. Clin. Oral Implant. Res. 2022, 33, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Dethier, F.; Bacevic, M.; Lecloux, G.; Seidel, L.; Rompen, E.; Lambert, F. The Effects of Abutment Materials on Peri-Implant Soft Tissue Integration: A Study in Minipigs. J. Prosthodont. 2022, 31, 585–592. [Google Scholar] [CrossRef]
- Mehl, C.; Gassling, V.; Schultz-Langerhans, S.; Acil, Y.; Bahr, T.; Wiltfang, J.; Kern, M. Influence of Four Different Abutment Materials and the Adhesive Joint of Two-Piece Abutments on Cervical Implant Bone and Soft Tissue. Int. J. Oral Maxillofac. Implant. 2016, 31, 1264–1272. [Google Scholar] [CrossRef]
- Zembic, A.; Bosch, A.; Jung, R.E.; Hammerle, C.H.; Sailer, I. Five-year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions. Clin. Oral Implant. Res. 2013, 24, 384–390. [Google Scholar] [CrossRef]
- Sailer, I.; Philipp, A.; Zembic, A.; Pjetursson, B.E.; Hammerle, C.H.; Zwahlen, M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin Oral Implant. Res 2009, 20 (Suppl. S4), 4–31. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Vaitelis, J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2015, 26 (Suppl. S11), 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.; Rudolph, H.; Graf, M.; Moldan, M.; Zhou, S.; Udart, M.; Bohmler, A.; Luthardt, R.G. Interaction of titanium, zirconia and lithium disilicate with peri-implant soft tissue: Study protocol for a randomized controlled trial. Trials 2015, 16, 467. [Google Scholar] [CrossRef]
- Karasan, D.; Sailer, I.; Lee, H.; Demir, F.; Zarauz, C.; Akca, K. Occlusal adjustment of 3-unit tooth-supported fixed dental prostheses fabricated with complete-digital and -analog workflows: A crossover clinical trial. J. Dent. 2023, 128, 104365. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L.; Sailer, I.; Lee, H.; Mojon, P.; Pitta, J. Digital wear analysis of different CAD/CAM fabricated monolithic ceramic implant-supported single crowns using two optical scanners. Int. J. Prosthodont. 2022, 35, 357–364. [Google Scholar] [CrossRef]
- Happe, A.; Debring, L.; Schmidt, A.; Fehmer, V.; Neugebauer, J. Immediate Implant Placement in Conjunction with Acellular Dermal Matrix or Connective Tissue Graft: A Randomized Controlled Clinical Volumetric Study. Int. J. Periodontics Restor. Dent. 2022, 42, 381–390. [Google Scholar] [CrossRef]
- Shanbhag, S.; Shanbhag, V.; Stavropoulos, A. Volume changes of maxillary sinus augmentations over time: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 881–892. [Google Scholar] [CrossRef]
- Kuhl, S.; Brochhausen, C.; Gotz, H.; Filippi, A.; Payer, M.; d’Hoedt, B.; Kreisler, M. The influence of bone substitute materials on the bone volume after maxillary sinus augmentation: A microcomputerized tomography study. Clin. Oral Investig. 2013, 17, 543–551. [Google Scholar] [CrossRef]
- Kim, H.; Mun, G.H.; Wiraatmadja, E.S.; Lim, S.Y.; Pyon, J.K.; Oh, K.S.; Lee, J.E.; Nam, S.J.; Bang, S.I. Preoperative magnetic resonance imaging-based breast volumetry for immediate breast reconstruction. Aesthetic Plast. Surg. 2015, 39, 369–376. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasding, M.; Jeong, Y.; Marchand, L.; Hicklin, S.P.; Sailer, I.; Sun, M.; Lee, H. Three-Dimensional Peri-Implant Tissue Changes in Immediately vs. Early Placed Tapered Implants Restored with Two Different Ceramic Materials—1 Year Results. Materials 2023, 16, 5636. https://doi.org/10.3390/ma16165636
Strasding M, Jeong Y, Marchand L, Hicklin SP, Sailer I, Sun M, Lee H. Three-Dimensional Peri-Implant Tissue Changes in Immediately vs. Early Placed Tapered Implants Restored with Two Different Ceramic Materials—1 Year Results. Materials. 2023; 16(16):5636. https://doi.org/10.3390/ma16165636
Chicago/Turabian StyleStrasding, Malin, Yuwon Jeong, Laurent Marchand, Stefan P. Hicklin, Irena Sailer, Minji Sun, and Hyeonjong Lee. 2023. "Three-Dimensional Peri-Implant Tissue Changes in Immediately vs. Early Placed Tapered Implants Restored with Two Different Ceramic Materials—1 Year Results" Materials 16, no. 16: 5636. https://doi.org/10.3390/ma16165636
APA StyleStrasding, M., Jeong, Y., Marchand, L., Hicklin, S. P., Sailer, I., Sun, M., & Lee, H. (2023). Three-Dimensional Peri-Implant Tissue Changes in Immediately vs. Early Placed Tapered Implants Restored with Two Different Ceramic Materials—1 Year Results. Materials, 16(16), 5636. https://doi.org/10.3390/ma16165636






