Controlling the Cooling Rate of Hydrothermal Synthesis to Enhance the Supercapacitive Properties of β-Nickel Hydroxide Electrode Materials
Abstract
1. Introduction
2. Experimental Section
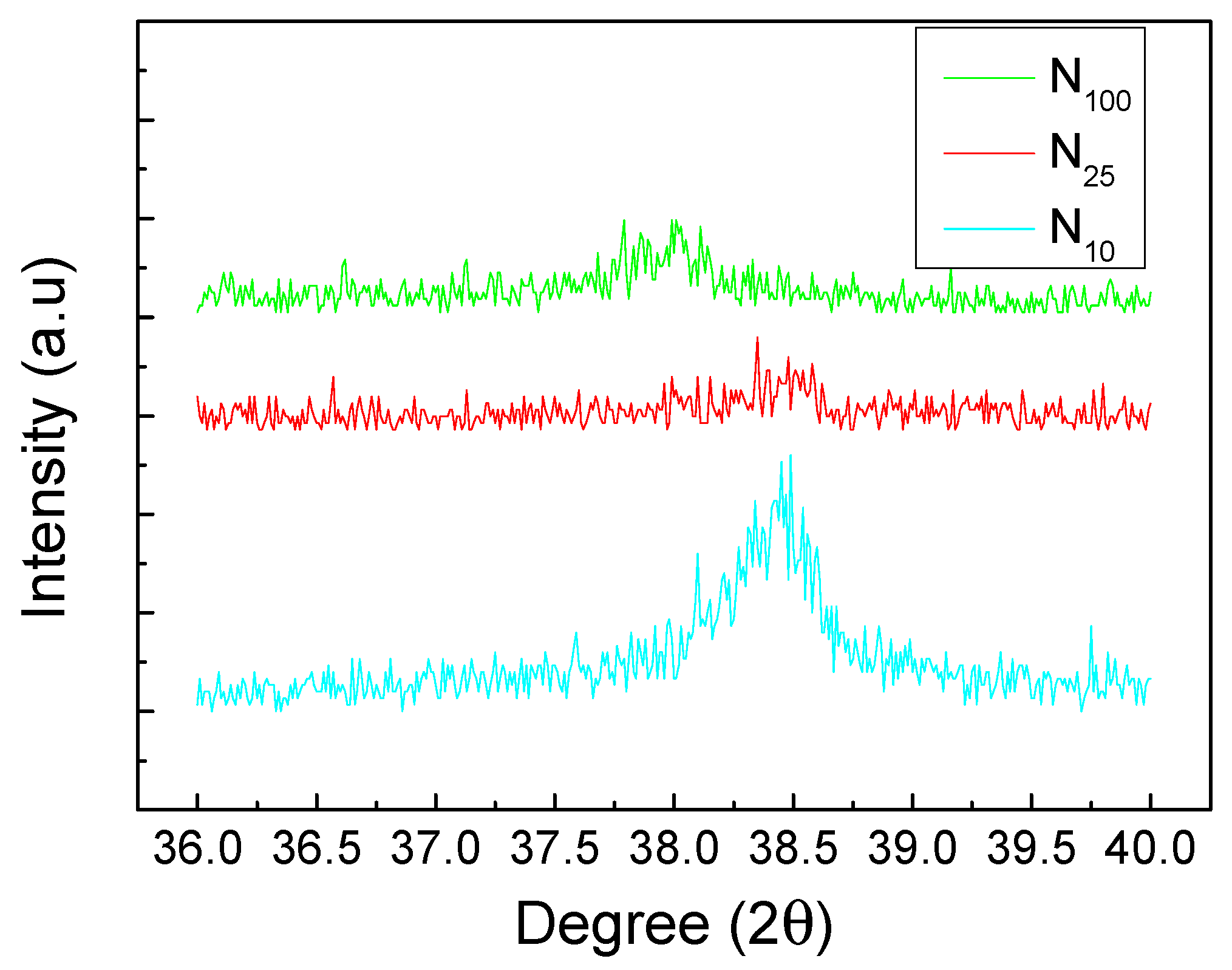
3. Results and Discussion
4. Conclusions
5. Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, C.; Wang, S.; Sha, S.; Lv, S.; Wang, G.; Wang, B.; Li, Q.; Yu, J.; Xua, X.; Zhang, L. Novel semiconductor materials for advanced supercapacitors. J. Mater. Chem. C 2023, 11, 4288–4317. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academia/Plenum: New York, NY, USA, 1999. [Google Scholar]
- Kotz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Zhao, X.; Sánchez, B.M.; Dobson, P.J.; Grant, P.S. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774. [Google Scholar] [CrossRef]
- Burke, A. R&D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083. [Google Scholar]
- Mendoza-Ponce, P.; John, B.; Schroeder, D.; Krautschneider, W.H. Super-capacitors for implantable medical devices with wireless power transmission. In Proceedings of the 14th Conference on Ph. D. Research in Microelectronics and Electronics (PRIME), Prague, Czech Republic, 2–5 July 2018; pp. 241–244. [Google Scholar] [CrossRef]
- Horn, M.; MacLeod, J.; Liu, M.; Webb, J.; Motta, N. Supercapacitors: A new source of power for electric cars? Econ. Anal. Policy 2019, 61, 93–103. [Google Scholar] [CrossRef]
- Al-Furjan, M.S.H.; Qi, Z.H.; Shan, L.; Farrokhian, A.; Shen, X.; Kolahchi, R. Nano supercapacitors with practical application in aerospace technology: Vibration and wave propagation analysis. Aerosp. Sci. Technol. 2023, 133, 108082. [Google Scholar] [CrossRef]
- Végvári, Z. Supercapacitors and Their Military Applicability. Honvédségi Szle. 2019, 147, 38–49. [Google Scholar] [CrossRef]
- Shinde, P.A.; Olabi, A.G.; Chodankar, N.R.; Patil, S.J.; Hwang, S.K.; Abdelkareem, M.A. Realizing superior redox kinetics of metal-metal carbides/carbon coordination supported heterointerface for stable solid-state hybrid supercapacitor. Chem. Eng. J. 2023, 454, 140246. [Google Scholar] [CrossRef]
- Lu, J.L.; Li, J.E.; Wan, J.; Han, X.Y.; Ji, P.Y.; Luo, S.; Gu, M.X.; Wei, D.P.; Hu, C.G. A Facile Strategy of In-situ Anchoring of Co3O4 on N Doped Carbon Cloth for an Ultrahigh Electrochemical Performance. Nano Res. 2020, 14, 2410–2417. [Google Scholar] [CrossRef]
- Chatterjee, M.; Sain, S.; Roy, A.; Das, S.; Pradhan, S.W. Enhanced Electrochemical Properties of Co3O4 with Morphological Hierarchy for Energy Storage Application: A Comparative Study with Different Electrolytes. J. Phys. Chem. Solids 2021, 148, 109733. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef]
- Bello, A.; Makgopa, K.; Fabiane, M.; Dodoo-Ahrin, D.; Ozoemena, K.I.; Manyala, N. Chemical adsorption of NiO nanostructures on nickel foam-graphene for supercapacitor applications. J. Mater. Sci. 2013, 48, 6707–6712. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, X.; Wang, L.; Song, H.; Zhang, H.; Huang, W.; Chen, P. 3D Graphene Foam as a Monolithic and Macroporous Carbon Electrode for Electrochemical Sensing. ACS Appl. Mater. Interfaces 2012, 4, 3129–3133. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wang, T.; Héroguel, F.; Krammer, A.; Lee, S.; Yao, L.; Schuler, A.; Luterbacher, J.S.; Yan, Y.; Hu, X. An efficient nickel hydrogen oxidation catalyst for hydroxide exchange membrane fuel cells. Nat. Mater. 2022, 21, 804–810. [Google Scholar] [CrossRef]
- Pei, P.; Huang, S.; Chen, D.; Li, Y.; Wu, Z.; Ren, P.; Wang, K.; Jia, X. A high-energy-density and long-stable-performance zinc-air fuel cell system. Appl. Energy 2019, 241, 124–129. [Google Scholar] [CrossRef]
- Lee, D.J.; Yu, S.-H.; Lee, H.S.; Jin, A.; Lee, J.; Lee, J.E.; Sung, Y.-E.; Hyeon, T. Facile synthesis of metal hydroxide nanoplates and their application as lithium-ion battery anodes. J. Mater. Chem. A 2017, 5, 8744–8751. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Hashmi, S.A.; Khan, M.; Choi, D.; Qurashi, A. Frontiers and recent developments on supercapacitor’s materials, design, and applications: Transport and power system applications. J. Energy Storage 2023, 58, 106104. [Google Scholar] [CrossRef]
- Shi, F.; Li, L.; Wang, X.-L.; Gua, C.-D.; Tu, J.-P. Metal oxide/hydroxide-based materials for supercapacitors. RCS Adv. 2014, 4, 41910–41921. [Google Scholar] [CrossRef]
- Naeem, S.; Patil, A.V.; Shaikh, A.V.; Shinde, U.P.; Husain, D.; Alam, M.T.; Sharma, M.; Tewari, K.; Ahmad, S.; Shah, A.A.; et al. A Review of Cobalt-Based Metal Hydroxide Electrode for Applications in Supercapacitors. Adv. Mater. Sci. Eng. 2023, 2023, 1133559. [Google Scholar] [CrossRef]
- Nilimapriyadarsini, S.; Balasubramaniam, S.; Manab, K.; Lukas, S.M.; Ananthakumar, R. Recent trends in template assisted 3D porous materials for electrochemical supercapacitors. J. Mater. Chem. A 2021, 9, 25286–25324. [Google Scholar]
- Hu, C.C.; Chen, W.C. Effects of substrates on the capacitive performance of RuOx·nH2O and activated carbon–RuOx electrodes for supercapacitors. Electrochim. Acta 2004, 49, 3469–3477. [Google Scholar] [CrossRef]
- Hu, C.C.; Chen, W.C.; Chang, K.H. How to Achieve Maximum Utilization of Hydrous Ruthenium Oxide for Supercapacitors. J. Electrochem. Soc. 2004, 151, A281. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, K.H.; Lin, M.C.; Wu, Y.T. Design and Tailoring of the Nanotubular Arrayed Architecture of Hydrous RuO2 for Next Generation Supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, C.; Qi, Z.; Hu, W.; Zhang, Z. Nanostructure Nickel-Based Selenides as Cathode Materials for Hybrid Battery-Supercapacitors. Front. Chem. 2021, 8, 611032. [Google Scholar] [CrossRef]
- Liuqin, L.; Rong, L.; Siyu, S.; Liang, Z.; Yifan, C.; Naili, G.; Wei, S.; Xiaohong, Z. Controllable synthesis of reduced graphene oxide/nickel hydroxide composites with different morphologies for high performance supercapacitors. J. Alloys Compd. 2006, 6, 2690–2695. [Google Scholar]
- Bai, X.L.; Gao, Y.L.; Gao, Z.Y.; Ma, J.Y.; Tong, X.L.; Sun, H.B.; Wang, J.A. Supercapacitor performance of 3D-graphene/MnO2 foam synthesized via the combination of chemical vapor deposition with hydrothermal method. Appl. Phys. Lett. 2020, 117, 18. [Google Scholar] [CrossRef]
- Qi, J.; Xu, P.; Lv, Z.; Liu, X.; Wen, A. Effect of crystallinity on the electrochemical performance of nanometer Al-stabilized α-nickel hydroxide. J. Alloys Compd. 2008, 462, 164–169. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Liao, C.-H.; Hsu, C.-S.; Yougbaré, S.; Lin, L.-Y.; Wu, Y.-F. Novel synthesis of manganese cobalt layered double hydroxide and sulfur-doped nickel cobalt layered double hydroxide composite as efficient active material of battery supercapacitor hybrids. J. Energy Storage 2023, 57, 106171. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Guo, X.; Li, R.; Peng, Z.; Zhang, W.; Zheng, Y.; Xie, H.; Zhang, Y.; Zhao, Y. High-Performance Nickel Cobalt Hydroxide Nanosheets/Graphene/Ni foam Composite Electrode for Supercapacitor Applications. J. Electroanal. Chem. 2021, 897, 115543. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Wang, C.; Neville, A.; Hua, Y. Fundamental Insight into the Degradation Mechanism of an rGO-Fe3O4 Supercapacitor and Improving Its Capacity Behavior via Adding an Electrolyte Additive. Energy Fuels 2021, 35, 8406–8416. [Google Scholar] [CrossRef]
- Erdemutu, E.; Bai, C.; Ding, L. Electrospun Ni-Ni(OH)2/Carbon Nanofibers as Flexible Binder-Free Supercapacitor Electrode with Enhanced Specific Capacitance. J. Electron. Mater. 2020, 49, 7211–7218. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, C.; Wang, Y.; Hong, Y.; Wu, H.; Wang, K.; Niu, D.; Zhang, C.; Zhang, Q. Cu/CuxO@C nanocomposites as efficient electrodes for high-performance supercapacitor devices. Dalton Trans 2022, 51, 14551–14556. [Google Scholar] [CrossRef] [PubMed]
- Kasap, S.; Kaya, I.I.; Repp, S.; Erdem, E. Superbat: Battery-like supercapacitor utilized by graphene foam and zinc oxide (ZnO) electrodes induced by structural defects. Nanoscale Adv. 2019, 1, 2586–2597. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, Y.I.; Aldalbahi, A.; Rahaman, M.; Yoon, S.S. ZnO/MnOx Nanoflowers for High-Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 3697–3708. [Google Scholar] [CrossRef]
- Yi, X.; Sun, H.; Robertson, N.; Kirk, C. Nanoflower Ni(OH)2 grown in situ on Ni foam for high-performance supercapacitor electrodematerials. Sustain. Energy Fuels 2021, 5, 5236–5246. [Google Scholar] [CrossRef]
- Kim, B.K.; Chabot, V.; Yu, A. Carbon nanomaterials supported Ni(OH)2/NiO hybrid flower structure for supercapacitor. Electrochim. Acta 2013, 109, 370–380. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Eftekhari, A. Energy efficiency: A critically important but neglected factor in battery research. Sustain. Energy Fuels 2017, 1, 2053–2060. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, F.; Yang, L.; Gao, L.; Burke, A.F. A measurement method for determination of dc internal resistance of batteries and supercapacitors. Electrochem. Commun. 2010, 12, 242–245. [Google Scholar] [CrossRef]
- Negroiu, R.; Svasta, P.; Pirvu, C.; Vasile, A.; Marghescu, C. Electrochemical impedance spectroscopy for different types of supercapacitors. In Proceedings of the 2017 40th International Spring Seminar on Electronics Technology (ISSE), Sofia, Bulgaria, 10–14 May 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Wiston, B.R.; Ashok, M. Electrochemical performance of hydrothermally synthesized flower-like α-nickel hydroxide. Vacuum 2018, 160, 12–17. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Hu, Z.-A.; Wu, H.-Y. Preparation and electrochemical performance of alpha-nickel hydroxide nanowire. Mater. Chem. Phys. 2011, 126, 580–583. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Yen, S.-C.; Kubendhiran, S.; Yougbaré, S.; Lin, L.-Y.; Wu, Y.-F. Tailoring morphology of pure terephthalic acid induced beta nickel hydroxide using ultrasonication and alkalization for efficient energy storage. J. Energy Storage 2022, 56, 106117. [Google Scholar] [CrossRef]
- Xie, M.; Xu, Z.; Duan, S.; Tian, Z.; Zhang, Y.; Xiang, K.; Lin, M.; Guo, X.; Ding, W. Facile growth of homogeneous Ni(OH)2 coating on carbon nanosheets for high-performance asymmetric supercapacitor applications. Nano Res. 2018, 11, 216–224. [Google Scholar] [CrossRef]
- Attia, S.Y.; Bedir, A.G.; Barakat, Y.F.; Mohamed, S.G. A two-dimensional nickel-doped bismuth-layered double hydroxide structure as a bifunctional efficient electrode material for symmetric supercapacitors. Sustain. Mater. Technol. 2023, 36, e00595. [Google Scholar] [CrossRef]
- Berrabah, S.E.; Benchettara, A.; Smaili, F.; Benchettara, A.; Mahieddine, A. High performance hybrid supercapacitor based on electrochemical deposed of nickel hydroxide on zinc oxide supported by graphite electrode. J. Alloys Compd. 2023, 942, 169112. [Google Scholar] [CrossRef]
- Emin, A.; Li, J.; Dong, Y.; Fu, Y.; He, D.; Li, Y. Facilely prepared nickel-manganese layered double hydroxide-supported manganese dioxide on nickel foam for aqueous asymmetric supercapacitors with high performance. J. Energy Storage 2023, 65, 107340. [Google Scholar] [CrossRef]
- Mollajafari, M. An efficient lightweight algorithm for scheduling tasks onto dynamically reconfigurable hardware using graph-oriented simulated annealing. Neural Comput. Appl. 2023, 35, 18035–18057. [Google Scholar] [CrossRef]











| Electrode Material | Preparation Method | GCD Current Density (A/g) | Specific Capacitance Value (F/g) | Reference in This Work |
|---|---|---|---|---|
| MnO2/graphene | CVD, hydrothermal | 333.4 | [30] | |
| Hydrothermal | 1581.3 | [32] | ||
| CVD, hydrothermal | 2023.5 | [33] | ||
| Hydrothermal (with rGO) | 186.6 | [34] | ||
| Electrospun | 1 | 763 | [35] | |
| Thermal reduction and oxidation with MOF | 3 | 400 | [36] | |
| Solid-state (SS) synthesis | 1 | 448 | [37] | |
| Electrodeposition, chemical bath | 556 | [38] | ||
| Ni(OH)2/graphene | CVD, hydrothermal | 3 | 539 | This work |
| Sample Number | N100 | N25 | N10 |
|---|---|---|---|
| Energy efficiency | 73.4% | 81.2% | 90.4% |
| Preparation Method | Phase of Crystalline Nickel Hydroxide | GCD Current Density(A/g) | Specific Capacitance Value (F/g) | References |
|---|---|---|---|---|
| Hydrothermal | 516 | [45] | ||
| AAO-template-supported precipitation | 833 | [46] | ||
| Ultrasonication and alkalization | 1171 | [47] | ||
| Electrospun | β | 763 | [48] | |
| Hydrothermal | 539 | In this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-M.; Hong, S.-H. Controlling the Cooling Rate of Hydrothermal Synthesis to Enhance the Supercapacitive Properties of β-Nickel Hydroxide Electrode Materials. Materials 2023, 16, 5576. https://doi.org/10.3390/ma16165576
Lu Y-M, Hong S-H. Controlling the Cooling Rate of Hydrothermal Synthesis to Enhance the Supercapacitive Properties of β-Nickel Hydroxide Electrode Materials. Materials. 2023; 16(16):5576. https://doi.org/10.3390/ma16165576
Chicago/Turabian StyleLu, Yang-Ming, and Sheng-Huai Hong. 2023. "Controlling the Cooling Rate of Hydrothermal Synthesis to Enhance the Supercapacitive Properties of β-Nickel Hydroxide Electrode Materials" Materials 16, no. 16: 5576. https://doi.org/10.3390/ma16165576
APA StyleLu, Y.-M., & Hong, S.-H. (2023). Controlling the Cooling Rate of Hydrothermal Synthesis to Enhance the Supercapacitive Properties of β-Nickel Hydroxide Electrode Materials. Materials, 16(16), 5576. https://doi.org/10.3390/ma16165576







