Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review
Abstract
1. Introduction
2. Basic Properties of Zr
- (1)
- H, N, C, O, Ti, Al Ca, Mg, Cl, Si Pb, Mo, Zn, La, Ce, Ga, V, Be and Ta accelerate corrosion;
- (2)
- Sn, Sb, Fe, Cr and Ni weaken the harmful effect of the elements of the first group;
- (3)
- Hf, Y, Cu and W are neutral.
- Relatively low mechanical properties, especially at temperatures above 300 °C.
- Insufficient corrosion resistance:
- -
- in water and steam at 300 °C or more;
- -
- in dry and especially humid air and carbon dioxide from 500 to 550 °C.
- Embrittlement when saturated with atmospheric gases (nitrogen and oxygen).
- Presence of allotropic transformation at 863 °C.
- Low temperature of the beginning of recrystallization (from 500 °C), leading to softening.
3. Basic Properties of ZrO2
4. Formation Oxide-Ceramic Surface Layers on Zr
4.1. Vacuum-Deposited Coatings
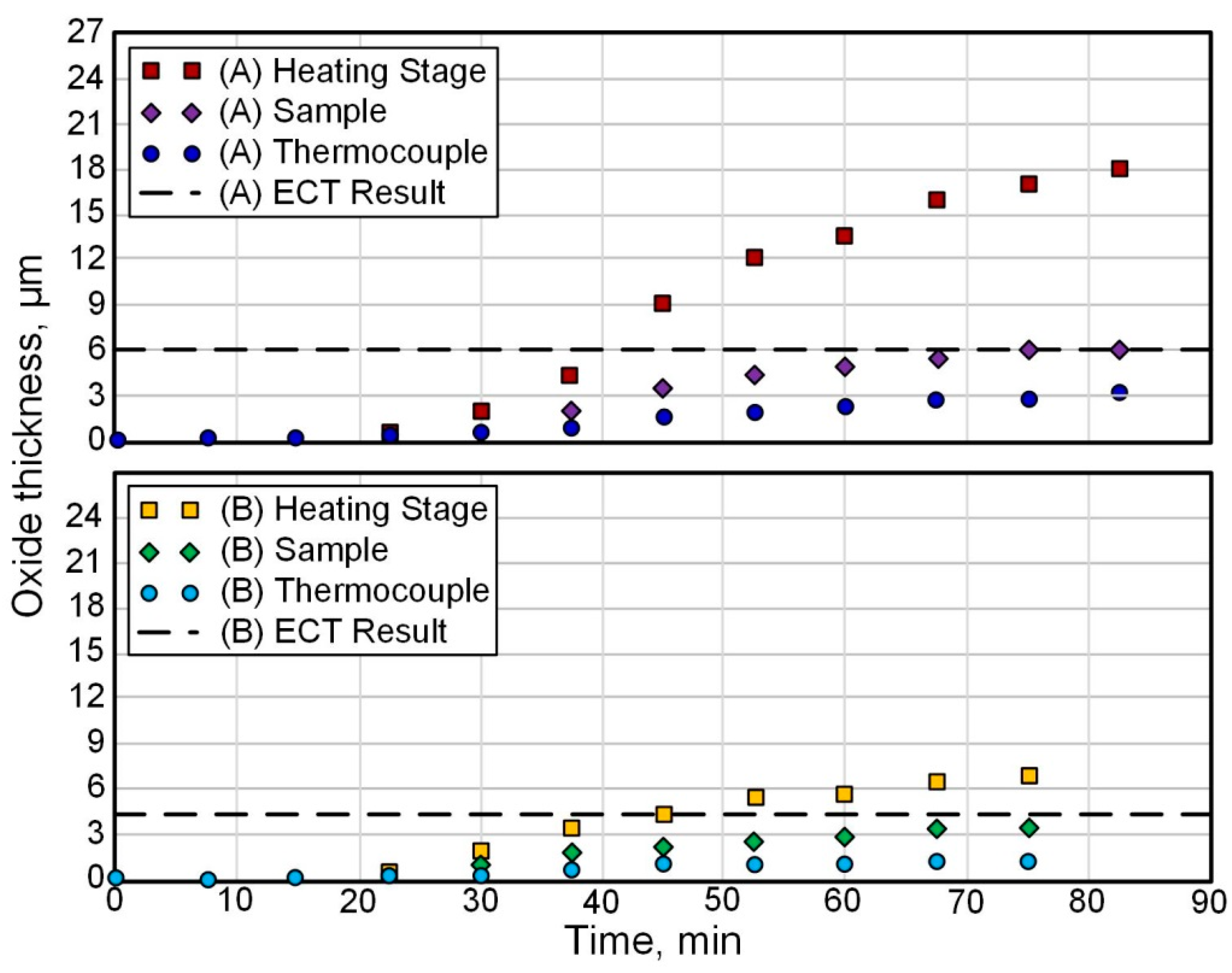
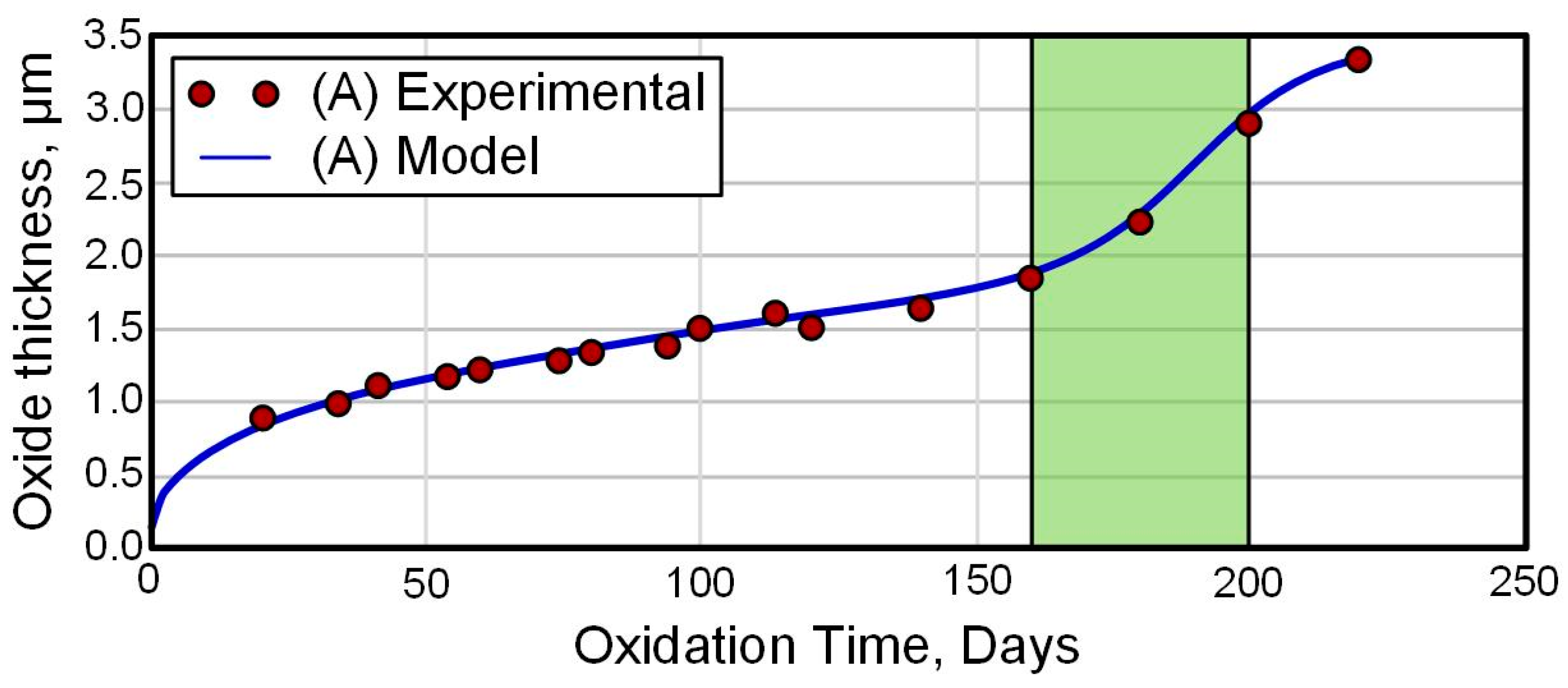
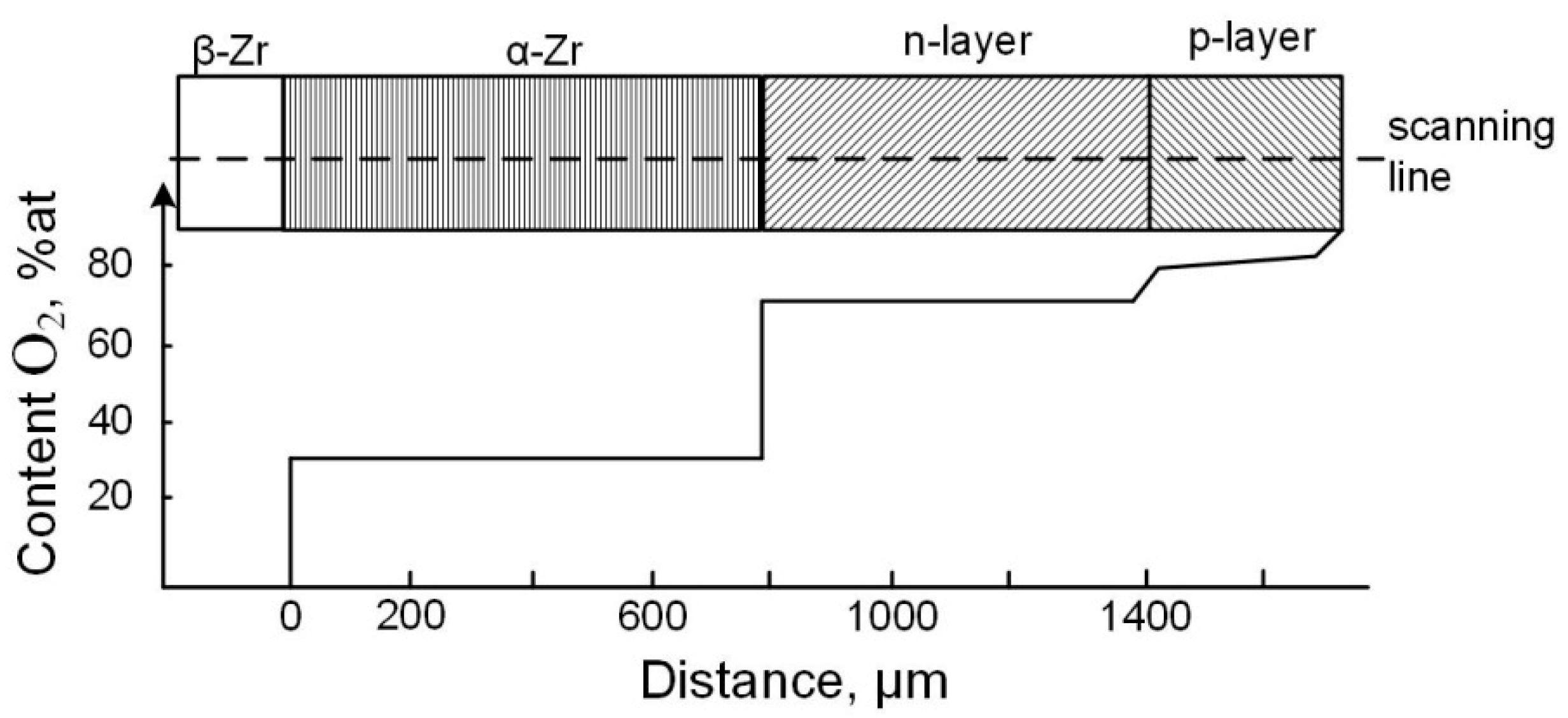
4.2. Chemical–Thermal Oxidation
5. Formation of Oxide-Ceramic Layers on the Surface of Zr and Its Alloys by PEM
- -
- The best coatings from the perspective of density and continuity were produced at higher Na2SiO3+KOH ratios (10:1 cf 1:1, Table 3), i.e., lower pH values; lower current densities; and longer treatment duration.
- -
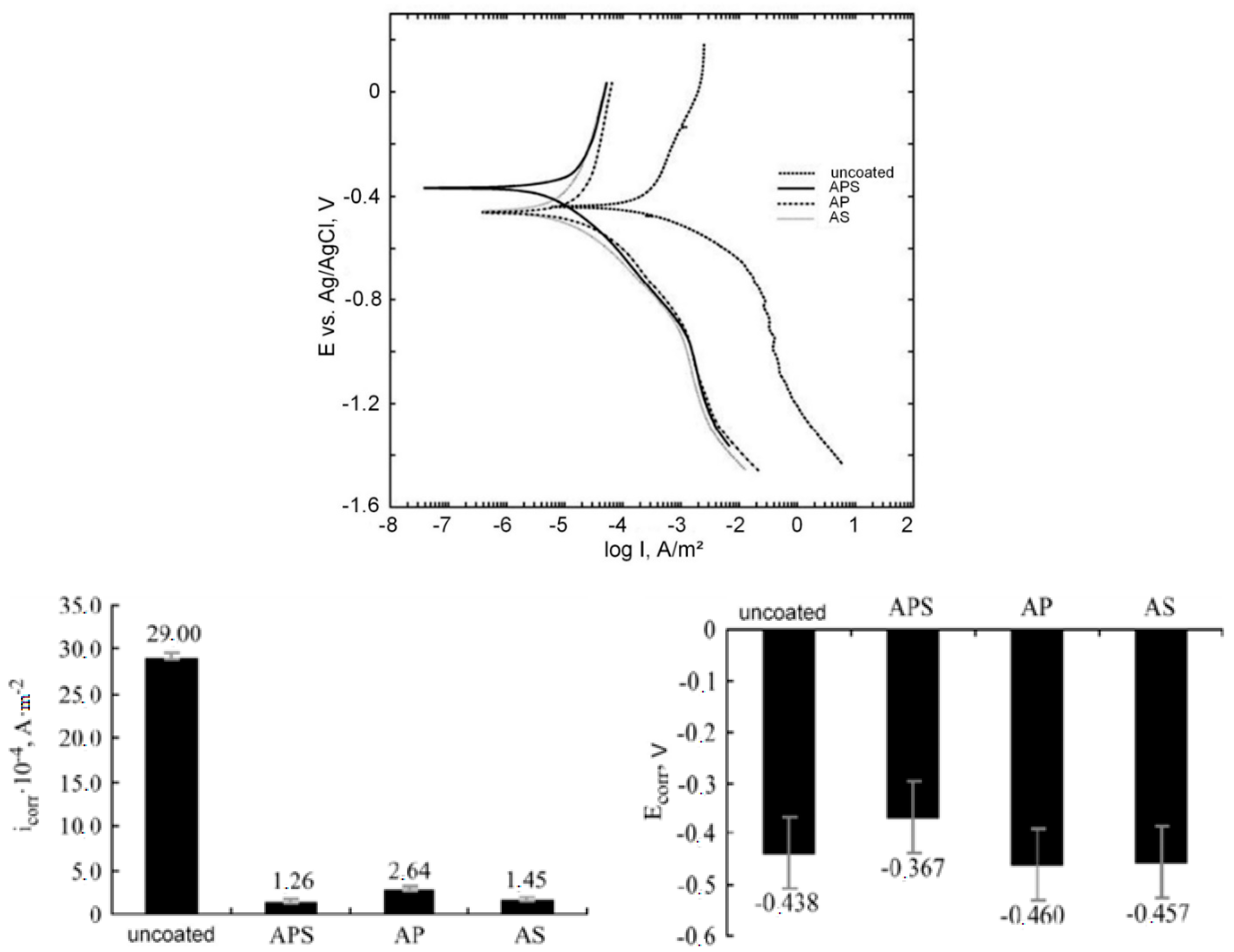
- PEM coatings improved the corrosion resistance of the Zr-2.5Nb substrate (the polarization resistance (Rp) was one to two orders of magnitude higher than that of the substrate).
- -
- PEM coatings of 5 μm thickness have a better wear resistance than the commercial autoclaved black oxide coating under both dry and water-lubricated conditions.
- -
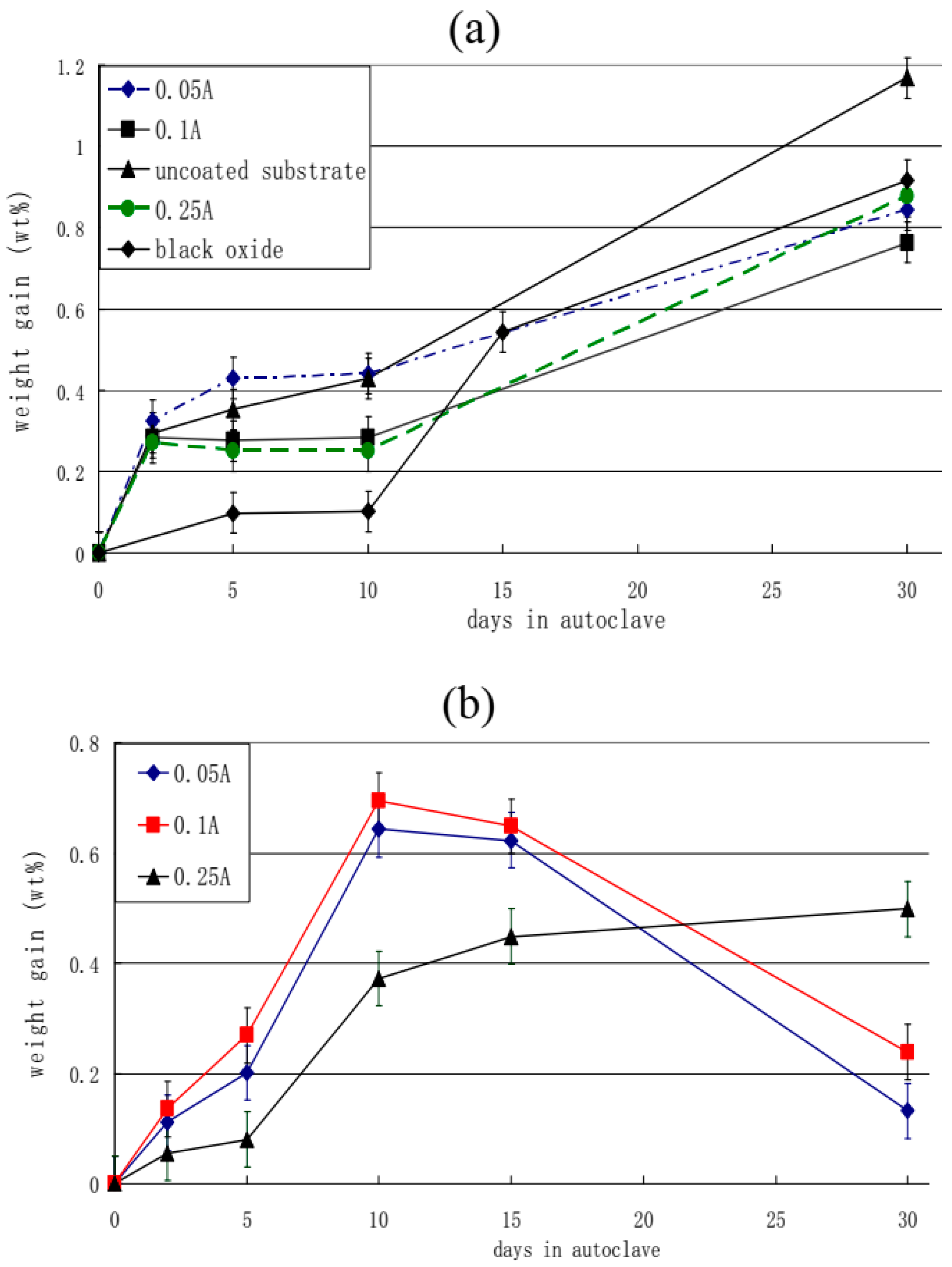
- PEM coatings obtained in a 10:1 Na2SiO3+KOH electrolyte exhibit much better corrosion resistance and lower weight gain than the Zr-2.5Nb substrates after 30 days in an autoclave (exposure at 300 °C, high pressure 10 MPa in 0.05 mol/L LiOH solution). The above-mentioned black oxide coating, having a very low weight gain value in the first 10 days, by 30 days demonstrates a final weight gain very close to that of the PEM samples made at an electrolyte component ratio of 10:1.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesnevsky, L.N.; Lyakhovetsky, M.A.; Tyurin, V.N. Microarc Oxidation of zirconium alloys applying to perspective engine and power plant. Electron. J. «Trudy MAI» 2011, 43. Available online: http://trudymai.ru/published.php?ID=24765 (accessed on 22 February 2023).
- Borisov, A.M.; Vostrikov, V.G.; Romanovsky, E.A.; Tkachenko, N.V.; Vinogradov, A.V.; Krit, B.L.; Savushkina, S.V.; Polyansky, M.N. The study of ceramic-like oxide coatings on zirconium produced by plasma treatment in electrolytes. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2013, 7, 366–370. [Google Scholar] [CrossRef]
- Krivenkov, A.O. Methods of Producing Composite Materials Based on Titanium and Zirconium by Microarc Oxidation. Ph.D. Thesis, Penza State University, Penza, Russia, 2006; p. 21. [Google Scholar]
- Miyazaki, S.; Kim, H.; Sato, Y. Super Elastic Zirconium Alloy for Biological Use, Medical Instrument and Glasses. U.S. Patent 9758846 B2, C22C14/00, 12 September 2017. [Google Scholar]
- Hsu, H.-C.; Wu, S.-C.; Sung, Y.-C.; Ho, W.-F. The structure and mechanical properties of as-cast Zr–Ti alloys. J. Alloys Compd. 2009, 488, 279–283. [Google Scholar] [CrossRef]
- Wu, R.; Yi, Q.; Lei, S.; Dai, Y.; Lin, J. Design of Ti-Zr-Ta Alloys with Low Elastic Modulus Reinforced by Spinodal Decomposition. Coatings 2022, 12, 756. [Google Scholar] [CrossRef]
- Legostaeva, E.V.; Eroshenko, Y.; Sharkeev, Y.P.; Lyamina, G.V.; Kozyreva, V.S.; Smirnov, A.I.; Bataev, V.A. Influence of structural state of titanium and zirconium and calcium-phosphate coating on their surface on behavior in corrosive medium. Met. Work. Mater. Sci. 2012, 3, 75–79. [Google Scholar]
- Ashcheulov, P.; Skoda, R.; Skarohlid, J.; Taylor, A.; Fendrych, F.; Kratochvílová, I. Layer Protecting the Surface of Zirconium Used in Nuclear Reactors. Recent Patents Nanotechnol. 2016, 10, 59–65. [Google Scholar] [CrossRef]
- Why Is Zirconium Used in Nuclear Reactors? 2019. Available online: https://www.refractorymetal.org/why-is-zirconium-used-in-nuclear-reactors/ (accessed on 22 February 2023).
- Apelfeld, A.V.; Krit, B.L.; Ludin, V.B.; Morozova, N.V.; Ryzhikov, I.A.; Savva, V.V.; Somov, O.V. Nanostructured Ceramic-Polymer Coatings for Face Seals. Mater. Sci. Forum. Mater. Sci. Nanotechnol. 2016, 863, 75–81. [Google Scholar] [CrossRef]
- Epelfeld, A.V.; Belkin, P.N.; Borisov, A.M.; Vasin, V.A.; Krit, B.L.; Lyudin, V.B.; Somov, O.V.; Sorokin VASuminov, I.V.; Frantskevich, V.P. Modern Technologies of Surface Modification of Materials and Coatings. The monograph in three volumes—M.; SPb.: Renome, Moscow; St. Petersburg, Russia, 2017; p. 1568. [Google Scholar]
- Krasnova, E.V.; Saushkin, B.P. Additive shaping of metal and alloy products by an electron beam: Direct supply of energy and material in the melting zone. Addit. Technol. 2021, 2, 44–57. [Google Scholar]
- Apelfeld, A.V.; Borisov, A.M.; Krit, B.L.; Ludin, V.B.; Polyansky, M.N.; Romanovsky, E.A.; Savushkina, S.V.; Suminov, I.V.; Tkachenko, N.V.; Vinogradov, A.V.; et al. The study of plasma electrolytic oxidation coatings on Zr and Zr-1% Nb alloy at thermal cycling. Surf. Coat. Technol. 2015, 269, 279–285. [Google Scholar] [CrossRef]
- Borisova, A.L.; Tunik, A.Y.; Adeeva, L.I.; Grishchenko, A.V.; Tsymbalistaya, T.V.; Kolomytsev, M.V. Thermal-barrier multilayer plasma coatings ZrO2-NiCrAlY. Paton Weld. J. 2010, 10, 22–28. [Google Scholar]
- Devoino, O.G.; Okovity, V.V. Plasma thermal barrier coatings based on zirconium dioxide with high thermal stability. Sci. Tech. 2015, 1, 35–39. Available online: https://sat.bntu.by/jour/article/view/795 (accessed on 22 February 2023). (In Russia).
- Il’iushchenko, A.F.; Ivashko, V.S.; Okovity, V.A.; Sobolevsk, S.B. Thermal Barrier Coatings on ZrO2-Basis; Remika: Minsk, Belarus, 1998; 128p. (In Russian) [Google Scholar]
- Valyukhov, S.G.; Stognei, O.V.; Filatov, M.S. The Influence of Magnetron Sputtering Conditions on the Structure of Heat Resistant ZrO2 Nanostructured Coatings. BMSTU J. Mech. Eng. 2015, 668, 97–105. [Google Scholar] [CrossRef]
- Kablov, E.N.; Muboiadzhian, S.A. Heat-resistant and heat-protective coatings for turbine blades of high-pressure turbine engine. Aviat. Mater. Technol. 2012, 60–70. Available online: https://journal.viam.ru/ru/system/files/uploads/pdf/2012/2012_S_6_1.pdf (accessed on 22 February 2023).
- Betsofen, S.Y.; Borisov, A.M.; Vladimirov, B.V.; Vostrikov, V.G.; Romanovsky, E.A.; Savushkina, S.V.; Sorokin, V.A.; Tkachenko, N.V.; Frantsevich, V.P.; Epelfeld, A.V. Production of nanocomposite ceramic coatings on zirconium alloy by microarc oxidation. Bull. High. Educ. Inst. Powder Metall. Funct. Coat. 2012, 2, 45–48. (In Russia) [Google Scholar]
- Bespalova, O.V.; Borisov, A.M.; Vostrikov, V.G.; Ivanova, S.V.; Romanovsky, E.A.; Tkachenko, N.V. Application of proton NBS spectrometry for studying the surface layer of zirconium alloys. Phys. Chem. Mater. Treat. 2011, 1, 45–50. [Google Scholar]
- Fattah-Alhosseini, A.; Chaharmahali, R.; Keshavarz, M.K.; Babaei, K. Surface characterization of bioceramic coatings on Zr and its alloys using plasma electrolytic oxidation (PEO): A review. Surf. Interfaces 2021, 25, 101283. [Google Scholar] [CrossRef]
- Velumani, S.; Regmi, G.; Lee, M.; Castaneda, H.; Kuttolamadom, M.; Qian, X.; Kassiba, A. Engineered Zr/Zn/Ti oxide nanocomposite coatings for multifunctionality. Appl. Surf. Sci. 2021, 563, 150353. [Google Scholar] [CrossRef]
- Nartita, R.; Ionita, D.; Demetrescu, I. Sustainable Coatings on Metallic Alloys as a Nowadays Challenge. Sustainability 2021, 13, 10217. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Li, S.; Liu, L.; Zhang, D.; Niinomi, M. Low-cost surface modification of a biomedical Zr-2.5Nb alloy fabricated by electron beam melting. J. Mater. Sci. Technol. 2023, 143, 178–188. [Google Scholar] [CrossRef]
- Aubakirova, V.; Farrakhov, R.; Astanin, V.; Sharipov, A.; Gorbatkov, M.; Parfenov, E. Plasma Electrolytic Oxidation of Zr-1%Nb Alloy: Effect of Sodium Silicate and Boric Acid Addition to Calcium Acetate-Based Electrolyte. Materials 2022, 15, 2003. [Google Scholar] [CrossRef]
- Grigoriev, S.N. Technologies of Coatings and Surface Hardening: Industrial Applications. Coatings 2023, 13, 511. [Google Scholar] [CrossRef]
- Suminov, I.V.; Apelfeld, A.V.; Lyudin, V.B.; Krit, B.L.; Borisov, A.M. Microarc Oxidation, Monography; Ekomet: Moscow, Russia, 2005; 368p. [Google Scholar]
- Suminov, I.V.; Belkin, P.N.; Apelfeld, A.V.; Lyudin, V.B.; Krit, B.L.; Borisov, A.M. Plasma-Electrolytic Surface Modification of Metals and Alloys; Monography; Tekhnosfera: Moscow, Russia, 2011; Volume 2, 512p. [Google Scholar]
- Gordienko, P.S.; Dostalov, V.A.; Efimenko, A.V. Microarc Oxidation of Metals and Alloys; FEFU: Vladivostok, Russia, 2013; 521p. [Google Scholar]
- Rakoch, A.G.; Dub, A.V.; Gladkova, A.A. Anodization of Light Alloys under Various Electric Modes; Plasma-Electrolytic Nanotechnology: Moscow, Russia, 2012; 496p. [Google Scholar]
- Arun, S.; Sooraj, P.; Hariprasad, S.; Arunnellaiappan, T.; Rameshbabu, N. Fabrication of superhydrophobic coating on PEO treated zirconium samples and its corrosion resistance. Mater. Today Proc. 2019, 27, 2056–2060. [Google Scholar] [CrossRef]
- Cengiz, S.; Uzunoglu, A.; Huang, S.M.; Stanciu, L.; Tarakci, M.; Gencer, Y. An in-vitro study: The effect of surface properties on bioactivity of the oxide layer fabricated on Zr substrate by PEO. Surf. Interfaces 2021, 22, 100884. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R.; Radić, N.; Grbić, B. Zirconia films formed by plasma electrolytic oxidation: Photoluminescent and photocatalytic properties. Opt. Mater. 2015, 40, 20–25. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadic, N.B.; Vasilić, R. Down- and up-conversion photoluminescence of ZrO2: Ho3+ and ZrO2:Ho3+/Yb3+ coatings formed by plasma electrolytic oxidation. J. Alloys Compd. 2019, 785, 1222–1232. [Google Scholar] [CrossRef]
- Ćirić, A.; Stojadinović, S. Upconversion photoluminescence properties of ZrO2: Ln3+/Yb3+ (Ln = Er, Ho, Tm) films formed by plasma electrolytic oxidation. In Upconversion Nanophosphors, Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 103–118. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Lukiyanchuk, I.V.; Shchitovskaya, E.V.; Golushko, A.D.; Kondrikov, N.B. Plasma Electrolytic Formation and Photoelectrochemical Properties of Zr- and/or Ce-Containing Oxide Layers on Titanium. Russ. J. Inorg. Chem. 2022, 67, 1460–1464. [Google Scholar] [CrossRef]
- Gurushantha, K.; Renuka, L.; Anantharaju, K.; Vidya, Y.; Nagaswarupa, H.; Prashantha, S.; Nagabhushana, H. Photocatalytic and Photoluminescence studies of ZrO2/ZnO nanocomposite for LED and Waste water treatment applications. Mater. Today Proc. 2017, 4, 11747–11755. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Banach, M. Photocatalytic properties of zirconium oxide–zinc oxide nanoparticles synthesised using microwave irradiation. Appl. Nanosci. 2019, 10, 941–954. [Google Scholar] [CrossRef]
- Lapitskaya, V.A.; Kuznetsova, T.A.; Chizhik, S.A.; Komarov, A.I.; Frolov, Y.I.; Romanyuk, A.S. Study of the Crack Resistance of Microarc Oxidation Coatings after Laser Doping with Zirconium Oxide. Tech. Phys. 2019, 64, 1609–1614. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Andreev, N.; Bublikov, J.; Kutina, N. Investigation of the properties of the Cr,Mo-(Cr,Mo,Zr,Nb)N-(Cr,Mo,Zr,Nb,Al)N multilayer composite multicomponent coating with nanostructured wear-resistant layer. Wear 2020, 468–469, 203597. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Hamdy, K.; Volosova, M.A.; Okunkova, A.A.; Fedorov, S.V. Electrical discharge machining of oxide and nitride ceramics: A review. Mater. Des. 2021, 209, 109965. [Google Scholar] [CrossRef]
- Platt, P.; Polatidis, E.; Frankel, P.; Klaus, M.; Gass, M.; Howells, R.; Preuss, M. A study into stress relaxation in oxides formed on zirconium alloys. J. Nucl. Mater. 2015, 456, 415–425. [Google Scholar] [CrossRef]
- Azhazha, Y.M.; Y’yugov, P.N.; Lavrinenko, S.D.; Lindt, C.A.; Mukhachev, A.P.; Pilipenko, N.N. Zirconium and Alloys: Production Processes, Fields of Application. Review; NSC KFTI: Kharkov, Ukraine, 1998; 89p. [Google Scholar]
- Rakov, E.G. Zirconium. In Chemical Encyclopedia; Zefirov, N.S., Ed.; Big Russian Encyclopedia: Moscow, Russia, 1998; Volume 5, p. 384. [Google Scholar]
- Blank, V.D.; Estrin, E.I. Phase Transformations in Solids at High Pressure; Fizmatlit: Moscow, Russia, 2011; p. 405. [Google Scholar]
- Shatalov, V.; Shtokal, A.; Rykov, E.; Dobrosovestnov, K.; Bazhenova, O.; Rozhkova, T. Zirconium Micro-Arc Oxidation as a Method for Producing Heat Insulation Elements in Spacecraft. Sci. Educ. Bauman MSTU 2014, 14, 162–178. [Google Scholar] [CrossRef]
- Zirconium, Properties of the Atom, Chemical and Physical Properties. Available online: https://chemicalstudy.ru/tsirkoniy-svoystva-atoma-himicheskie-i-fizicheskie-svoystva/#fizicheskie-svojstva (accessed on 22 February 2023).
- Gunderov, D.; Stotskiy, A.; Lebedev, Y.; Mukaeva, V. Influence of HPT and Accumulative High-Pressure Torsion on the Structure and Hv of a Zirconium Alloy. Metals 2021, 11, 573. [Google Scholar] [CrossRef]
- Valiev, R.; Zhilyaev, A.; Langdon, T. Bulk Nanostructured Materials: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; 456p. [Google Scholar]
- Wang, D.; Wu, H.; Wu, R.; Wang, Y.; Zhang, J.; Betsofen, S.; Krit, B.; Hou, L.; Nodir, T. The transformation of LPSO type in Mg-4Y-2Er-2Zn-0.6Zr and its response to the mechanical properties and damping capacities. J. Magnes. Alloy. 2020, 8, 793–798. [Google Scholar] [CrossRef]
- Baker, L., Jr.; Just, L.C. Experimental and Theoretical Studies of the Zirconium-Water Reaction; ANL-6548; AEC Research and Development Report: Washington, DC, USA, 1962. [Google Scholar] [CrossRef]
- Kalin, B.A. Physical Materials Science; Structural materials for nuclear technology; MEPhI: Moscow, Russia, 2012; Volume 6, 733p. [Google Scholar]
- Shyti, G.; Rosalbino, F.; Macciò, D.; Scarabelli, L.; Quarto, R.; Giannoni, P. A Comparative Evaluation between New Ternary Zirconium Alloys as Alternative Metals for Orthopedic and Dental Prosthetic Devices. Int. J. Artif. Organs 2014, 37, 149–164. [Google Scholar] [CrossRef]
- Cengiz, S.; Gencer, Y. The characterization of the oxide based coating synthesized on pure zirconium by plasma electrolytic oxidation. Surf. Coatings Technol. 2014, 242, 132–140. [Google Scholar] [CrossRef]
- Sarkisov, E.S.; Chebotarev, N.T.; Nevzorova, A.A.; Zverkov, A.I. Oxidation of zirconium at high temperatures and the structure of primary oxide films. At. Energy 1958, 5, 550–553. [Google Scholar] [CrossRef]
- Tretyakov, Y.D. (Ed.) Inorganic Chemistry; Academy Publishing Center: Moscow, Russia, 2007; Volume 3, 352p. [Google Scholar]
- Vafin, S.M.; Hwang, V.I. Ceramics based on zirconium dioxide. Dent. Pract. 2011, 1, 20–27. [Google Scholar]
- Milyavsky, V.V.; Savinykh, A.S.; Akopov, F.A.; Borovkova, L.B.; Borodin, T.I.; Vagliano, G.E.; Ziborov, V.S.; Lukin, E.S.; Popova, N.A. Ceramics based on partially stabilized zirconium dioxide: Synthesis, structure and properties under dynamic loading. Thermophys. High Temp. 2011, 49, 707–712. [Google Scholar]
- Zirconium Dioxide, Aluminum Oxide and Their Compounds. 2020. Available online: http://www.virial.ru/materials/95/ (accessed on 22 February 2023).
- Stritzker, B.; Meyer, J.D. Enhanced superconductivity in zirconium after H(D) implantation. Eur. Phys. J. B 1980, 38, 77–81. [Google Scholar] [CrossRef]
- Kanaki, A.V.; Buyakova, S.P.; Kulkov, S.N. Effect of annealing on structure and phase transformations in ZrO2—MgO ceramic powders. Perspekt. Mater. 2016, 1, 49–56. [Google Scholar] [CrossRef]
- Gabelkov, S.V.; Tarasov, R.V.; Poltavtsev, N.S.; Logvinkov, D.S.; Mironova, A.G. Phase transformations at crystallization of amorphous zirconia with formation of nanostructure. Quest. At. Sci. Tech. 2004, 3, 116–120. [Google Scholar]
- Zavodinsky, V.G.; Chibisov, A.N. On the stability of cubic zirconium dioxide and stoichiometric nanoparticles of zirconium dioxide. Solid State Phys. 2006, 2, 343–347. [Google Scholar]
- Noguchi, T.; Ōkubo, T.; Yonemochi, O. Reactions in the System ZrO2-SrO. J. Am. Ceram. Soc. 1969, 4, 178–181. [Google Scholar] [CrossRef]
- Scott, H.G. Phase relationships in the zirconia-yttria system. J. Mater. Sci. 1975, 10, 1527–1535. [Google Scholar] [CrossRef]
- Gorelov, V.P. High-temperature phase transitions in ZrO2. Solid State Phys. 2019, 7, 1346–1351. [Google Scholar] [CrossRef]
- Pintilei, G.; Crismaru, V.; Abrudeanu, M.; Munteanu, C.; Luca, D.; Istrate, B. The influence of ZrO2/20%Y2O3 and Al2O3 deposited coatings to the behavior of an aluminum alloy subjected to mechanical shock. Appl. Surf. Sci. 2015, 352, 169–177. [Google Scholar] [CrossRef]
- Sánchez-Hernández, Z.; Domínguez-Crespo, M.; Torres-Huerta, A.; Onofre-Bustamante, E.; Adame, J.A.; Dorantes-Rosales, H. Improvement of adhesion and barrier properties of biomedical stainless steel by deposition of YSZ coatings using RF magnetron sputtering. Mater. Charact. 2014, 91, 50–57. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Zhukareva, O.V.; Fukalova, E.V.; Kovalev, A.F. Mechanism of the influence of fluoride on the oxidation of zirconium and its alloys with niobium in the air in a wide temperature range. Non-Ferr. Met. 1996, 6, 56–59. [Google Scholar]
- Rakoch, A.G.; Shkuro, V.G.; Zamalin, E.J.; Zhukareva, O.V.; Fukalova, E.V. The process of high-temperature oxidation of the materials in the presence of activators and passivating. Phys. Chem. Mater. Treat. 1996, 3, 113–120. [Google Scholar]
- Kostikov, V.I.; Nechaev, Y.u.S.; Kulga, G.Y. Influence of hydrogen fluoride activators on the formation of refractory compounds. J. Perspekt. Mater. 2000, 6, 70–74. [Google Scholar]
- Kostikov, V.I.; Nechaev, Y.S.; Kulga, G.Y. Influence of hydrogen fluoride activators on the formation of protective diffusion coatings. Proc. Russ. Acad. Sci. 2001, 377, 38–39. [Google Scholar]
- Rakoch, A.G.; Bautin, V.A.; Bardin, I.V.; Kovalev, V.L. Mechanism and kinetic features of microdug oxidation of magnesium alloy ML5 in electrolytes containing NH4F. Corros. Mater. Protection. 2007, 9, 7–13. [Google Scholar]
- Heine, V.; Cohen, M.; Weir, D. The Pseudopotential Theory; Mir: Moscow, Russia, 1973; 557p. [Google Scholar]
- Tskhai, V.A.; Gel’d, P.V. Screening Me-Me Bonds and Its Effect on the Physicochemical Properties of Carbides, Nitrides, and Oxides of Transition Metals with the Structure of the NaCl Type, Preprint; Ural’sk Scientific Center, Academy of Sciences of the USSR: Sverdlovsk, Russia, 1984; 58p. (In Russian) [Google Scholar]
- Rakoch, A.G.; Delyan, V.I.; Vasiliev VYu Kazakevich, A.V. Corrosion and Protection of Metals; MISiS: Moscow, Russia, 1994; 57p. (In Russian) [Google Scholar]
- Chen, Y.; Nie, X.; Northwood, D.O. Plasma Electrolytic Oxidation (PEO) Coatings on a Zirconium Alloy for Improved Wear and Corrosion Resistance. In Tribology and Design; WIT Press: Billerica, MA, USA, 2010; Volume 66, pp. 183–194. [Google Scholar] [CrossRef]
- Chen, Y. Plasma Electrolytic Oxidation (PEO) Coatings on a Zirconium Alloy for Improved Wear and Corrosion Resistance. Master’s Thesis, University of Windsor, Windsor, ON, Canada, 2010; 179p. Available online: https://scholar.uwindsor.ca/etd/179 (accessed on 1 January 2023).
- Snizhko, L.O.; Yerokhin, A.L. Anodic processes in plasma electrolytic oxidation of aluminum in alkaline solutions. Electrochim. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Farrakhov, R.G.; Parfenov, E.V.; Mukaeva, V.R.; Gorbatkov, M.; Tarasov, P.V.; Fatcullin, A.R.; Rameshbabu, N.; Ravisankar, B. Effect of Electrolyte Composition on Protective Properties of the PEO Coating on Zr–1Nb Zirconium Alloy. Elektron. Obrab. Mater. 2019, 55, 23–30. (In Russian) [Google Scholar] [CrossRef]
- Wang, X.; Wei, K.; Guan, H.; Xu, C.; Xue, W.; Zhang, Y.; Wang, R. High temperature oxidation of Zr 1Nb alloy with plasma electrolytic oxidation coating in 900–1200 °C steam environment. Surf. Coat. Technol. 2020, 407, 126768. [Google Scholar] [CrossRef]
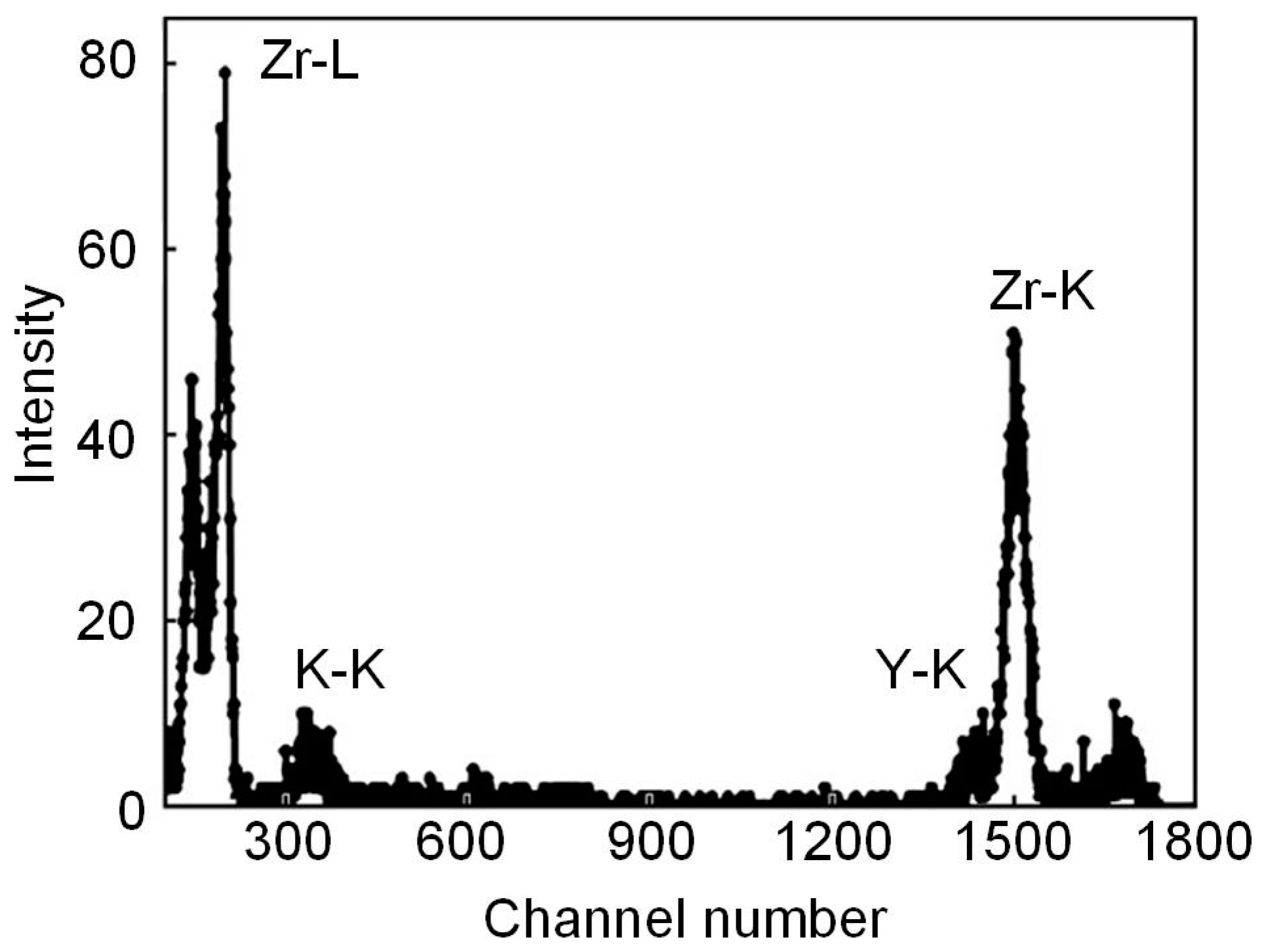
- Avilkina, V.S.; Borisov, A.M.; Vladimirov, B.V.; Petukhov, V.P.; Chernykh, P.N. Measurement of the elemental composition of carbon and composite ceramic materials by PIXE and RBS methods. Fizika i Himiya Obrabotki Mater. 2011, 1, 51–54. (In Russian) [Google Scholar] [CrossRef]
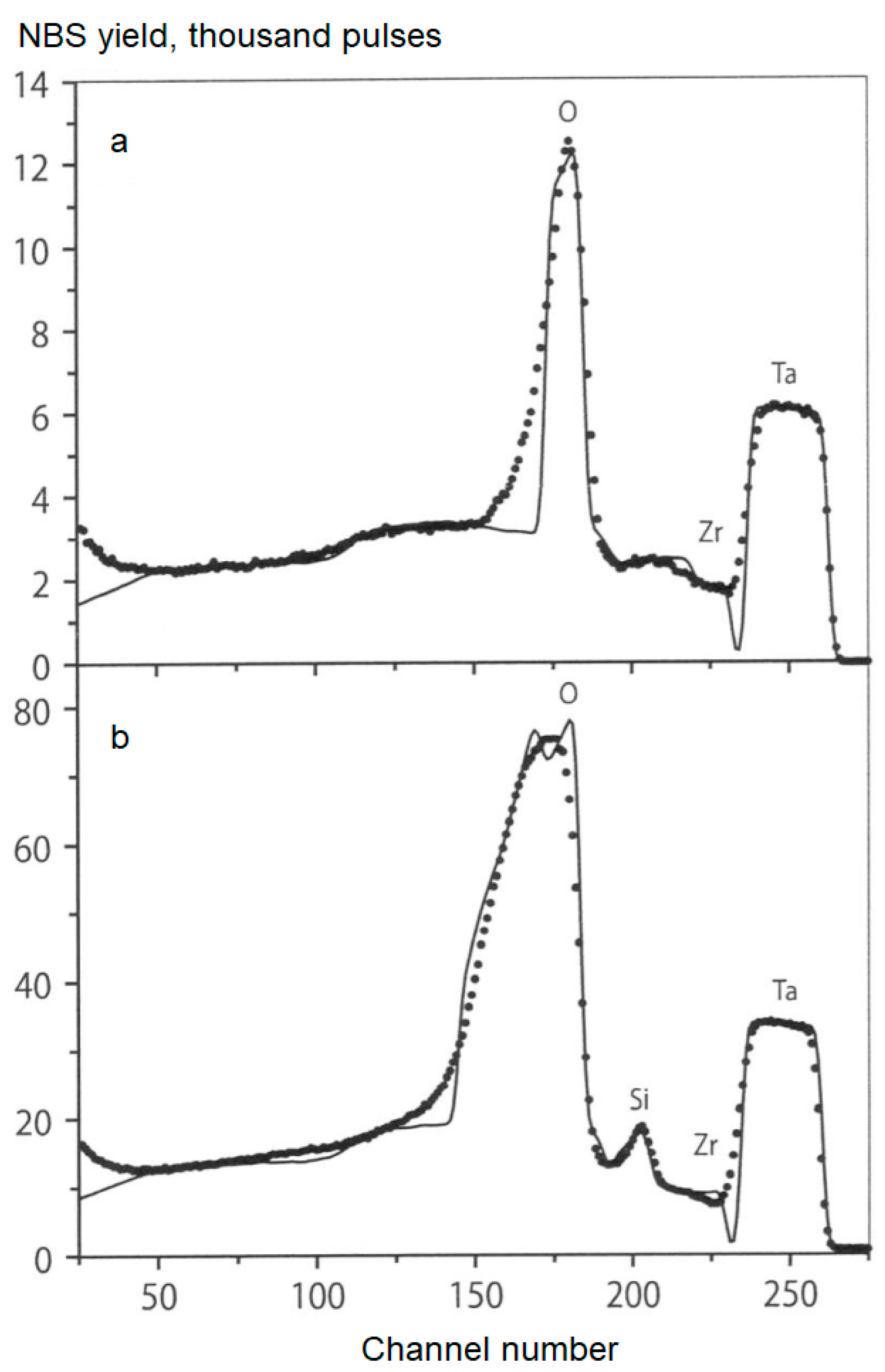
- Romanovsky, E.A.; Serkov, M.V.; Borisov, A.M.; Michurina, V.P.; Smirnova, O.A.; Suminov, I.V.; Apelfeld, A.V. Application of nuclear backscattering spectrometry of 5–8 MeV protons for the study of protective oxide coatings. Appl. Phys. 2006, 4, 85–88. [Google Scholar]
- Cengiz, S.; Yazici, M.; Gencer, Y.; Tarakci, M. Characterization of Coating Formed on Pure Zirconium by MAO in Yttrium Acetate Tetrahydrate Containing Electrolyte. Acta Phys. Pol. A 2015, 127, 1320–1325. [Google Scholar] [CrossRef]
- Dyblenko, Y.M.; Smyslov, A.M.; Smyslova, M.K.; Tamindarov, D.R.; Dyblenko, M.Y.; Mingazhev, A.D.; Pavlinich, S.P. A Method of Forming a Heat-Shielding Coating on Parts of Gas Turbines from Nickel and Cobalt Alloys. Patent RU 2479666 C1 (C23C 14/16, F01D 5/28), 20 April 2013. Bul. No. 11. [Google Scholar]
- Pichkhidze, S.Y.; Koshuro, V.A.; Nechaev, G.G. A Method of Forming a Ceramic Coating Based on Zirconium Dioxide on a Titanium Alloy Article. Patent RU 2607390 C2 (C23C 28/04, C23C 4/10, C25D 11/02), 1 December 2017. Bul. No. 1. [Google Scholar]
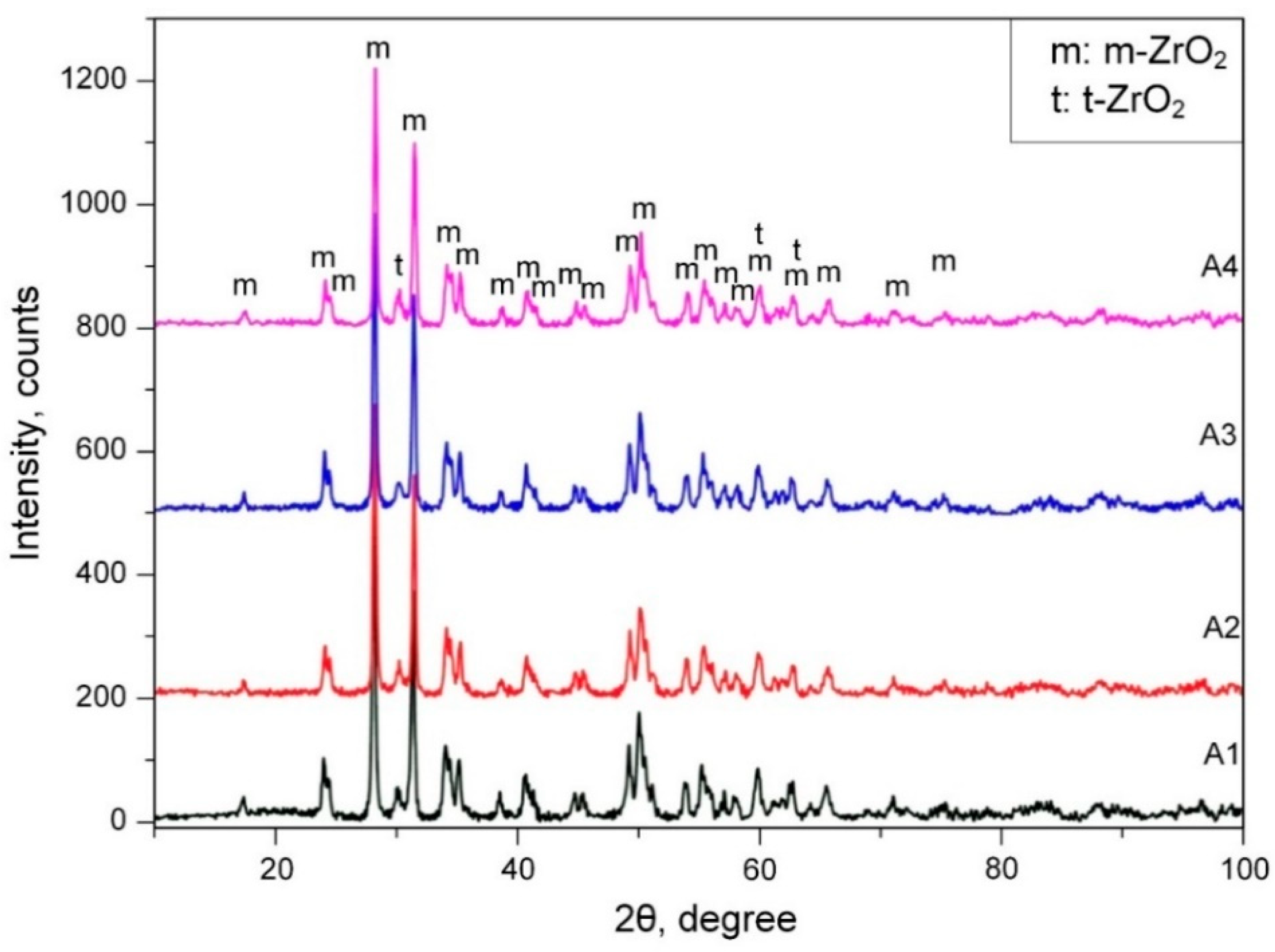
- Apelfeld, A.; Ashmarin, A.; Borisov, A.; Vinogradov, A.; Savushkina, S.; Shmytkova, E. Formation of zirconia tetragonal phase by plasma electrolytic oxidation of zirconium alloy in electrolyte comprising additives of yttria nanopowder. Surf. Coat. Technol. 2017, 328, 513–517. [Google Scholar] [CrossRef]









| Material | Strength Indicator | |||||
|---|---|---|---|---|---|---|
| 20 °C | 400 °C | |||||
| σ0.2, MPa | σB, MPa | δ, % | σ0.2, MPa | σB, MPa | δ, % | |
| Zr (iodide) | 220 | 80 | 45 | 110 | 40 | 60 |
| Zr (electrolytic) | 855 | 230 | 36 | 155 | 65 | 52 |
| Circaloy-2 | 310 | 480 | 22 | 70 | 170 | 36 |
| Zr-1% Nb | 200 | 350 | 30 | 90 | 180 | 38 |
| Zr-2.5% Nb | 280 | 450 | 25 | 180 | 270 | 22 |
| E-125 | 446 | 456 | 31 | 238 | 272 | 34.6 |
| Density, g/cm3 | 5.79–6.1 |
| Flexural strength, MPa | 700–2500 |
| Young’s modulus, GPa | 200–210 |
| Crack resistance K1C, MPa∙m1/2 | 8–10 |
| Vickers hardness, HV0.1, GPa | 12–13 |
| Thermal expansion coefficient, K−1∙106 | 10.0–11.0 |
| Coefficient of thermal conductivity, W/(m∙K) | 2–3 |
| Electrical conductivity, S∙m−1 | 2.1–2.4 |
| Composition (Na2SiO3 + KOH) | Sample No. | Current Density (A/cm2) | Treatment Time (min) |
|---|---|---|---|
| Group A Electrolyte I, pH = 11–12 (8 g/L + 0.8 g/L) | S1 | 0.05 | 4 |
| S2 | 0.1 | 2 | |
| S3 | 0.25 | 0.8 | |
| Group B Electrolyte II, pH = 13–14 (8 g/L + 8 g/L) | S4 | 0.05 | 4 |
| S5 | 0.1 | 2 | |
| S6 | 0.25 | 0.8 |
| Samples | icorr (mA/cm2) | Ecorr (V) | Rp (Ωcm2) |
|---|---|---|---|
| Zr-2.5Nb substrate | 1.02 × 10−3 | −0.35 | 1.22 × 104 |
| Black oxide coating | 1.12 × 10−5 | −0.28 | 3.57 × 106 |
| S1 (0.05 A/cm2, 8:0.8) | 6.31 × 10−5 | −0.25 | 1.22 × 106 |
| S2 (0.1 A/cm2, 8:0.8) | 5.71 × 10−5 | −0.27 | 1.39 × 106 |
| S3 (0.25 A/cm2, 8:0.8) | 1.58 × 10−5 | −0.22 | 1.58 × 106 |
| S4 (0.05 A/cm2, 8:8) | 7.08 × 10−5 | −0.25 | 5.72 × 105 |
| S5 (0.1 A/cm2, 8:8) | 1.58 × 10−4 | −0.21 | 2.68 × 105 |
| S6 (0.25 A/cm2, 8:8) | 2.09 × 10−4 | −0.28 | 2.06 × 105 |
| Rotating Mode | Zr-2.5Nb | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|
| dry/2N/ v = 0.1 m/s | 50 m | 1000 m | 970 m (failed) | 1000 m | 1000 m | 1000 m | 910 m (failed) |
| Sample | U, V | Electrolyte Composition | τ, min | h, µm | Pg, % | d, µm | λCM, W/m·K | λcoat, W/m·K | M·104, g/cm2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 350 | 10% H3PO4 | 30 | 9 | 35.43 | 4.08 | 1.9666 | 0.086 | 3.176 |
| 2 | 300 | 10% H3PO4 | 60 | 15 | 31.75 | 6.05 | 1.6639 | 0.134 | 3.414 |
| 3 | 220 | 14 g/L Na3PO4 | 33 | 20 | 31.05 | 8.09 | 1.8278 | 0.175 | 1.985 |
| 10% H3PO4 | 120 |
| Electrolyte | Critical Load, N |
|---|---|
| APS | 5.23 ± 0.57 |
| AP | 4.63 ± 0.56 |
| AS | 3.97 ± 0.57 |
| Electrolyte | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| Sodium silicate (g/L) | 12 | 12 | 12 | 12 |
| Yttrium acetate tetrahydrate (g/L) | 0 | 1 | 2 | 4 |
| Concentration of Y2O3 in Electrolyte, g/L | Duration of PEM Treatment, min | Coating Thickness, μm | t-ZrO2/(m-ZrO2+t-ZrO2), % |
|---|---|---|---|
| 4 | 30 | 30 | 74 |
| 4 | 60 | 80 | 86 |
| 6 | 30 | 100 | 100 |
| 6 | 60 | 120 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krit, B.L.; Apelfeld, A.V.; Borisov, A.M.; Morozova, N.V.; Rakoch, A.G.; Suminov, I.V.; Grigoriev, S.N. Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review. Materials 2023, 16, 5543. https://doi.org/10.3390/ma16165543
Krit BL, Apelfeld AV, Borisov AM, Morozova NV, Rakoch AG, Suminov IV, Grigoriev SN. Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review. Materials. 2023; 16(16):5543. https://doi.org/10.3390/ma16165543
Chicago/Turabian StyleKrit, Boris L., Andrey V. Apelfeld, Anatoly M. Borisov, Natalia V. Morozova, Alexander G. Rakoch, Igor V. Suminov, and Sergey N. Grigoriev. 2023. "Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review" Materials 16, no. 16: 5543. https://doi.org/10.3390/ma16165543
APA StyleKrit, B. L., Apelfeld, A. V., Borisov, A. M., Morozova, N. V., Rakoch, A. G., Suminov, I. V., & Grigoriev, S. N. (2023). Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review. Materials, 16(16), 5543. https://doi.org/10.3390/ma16165543








