Comparative Analysis of Trifluoracetic Acid Pretreatment for Lignocellulosic Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Chemical Characterization
2.3. TFA Pretreatment Methodology
2.4. HPLC Analysis
2.5. Process Evaluation
2.5.1. Process Description
2.5.2. Techno-Energetic Assessment
2.5.3. Economic Assessment
3. Results
3.1. Experimental Results
3.1.1. Characterization
3.1.2. TFA Pretreatment and Sugars’ Quantification
3.2. Simulation Results
3.2.1. Technical Assessment
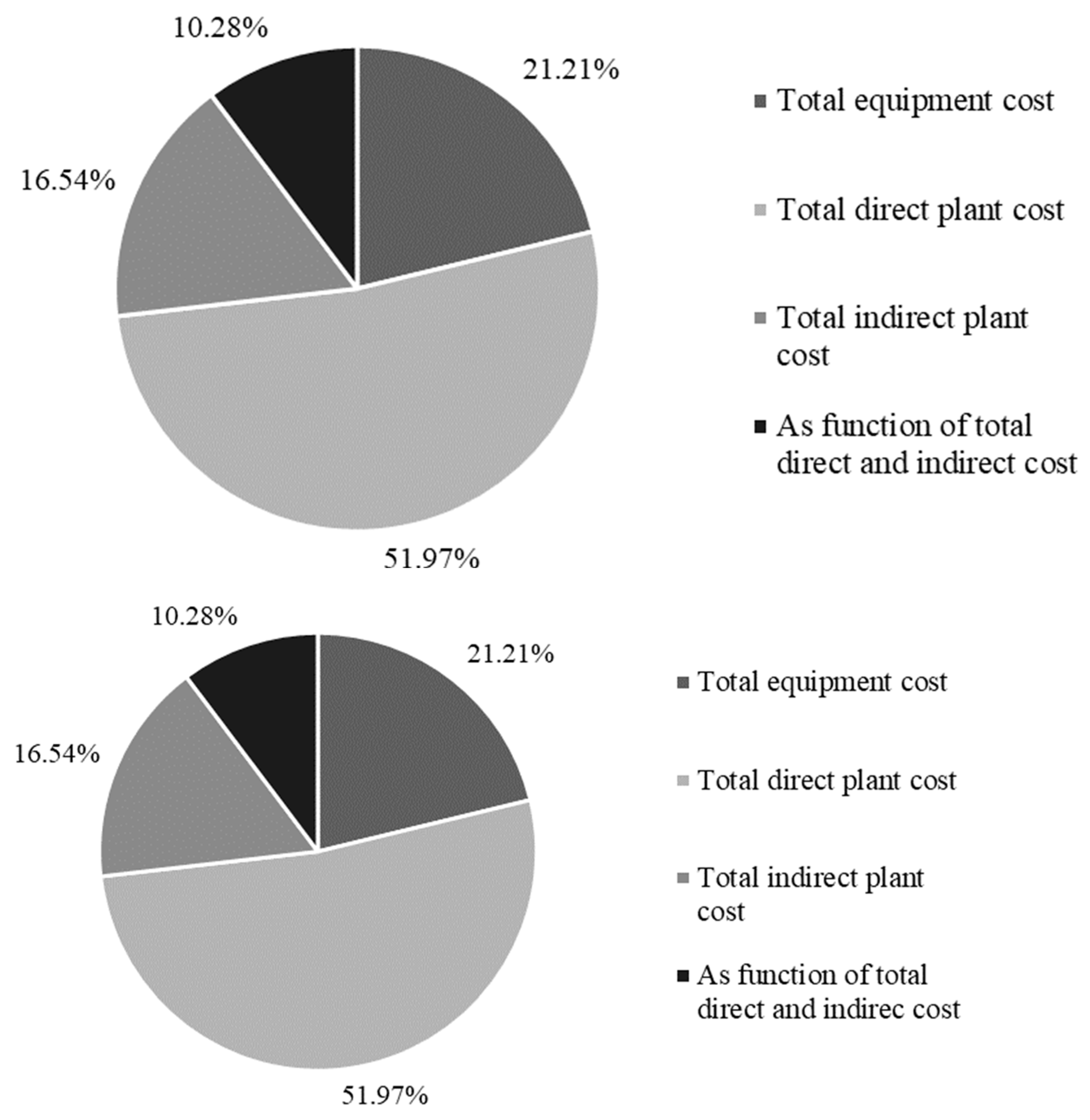
3.2.2. Economic Evaluation
4. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Calibration curve and chromatograms for sugar standards

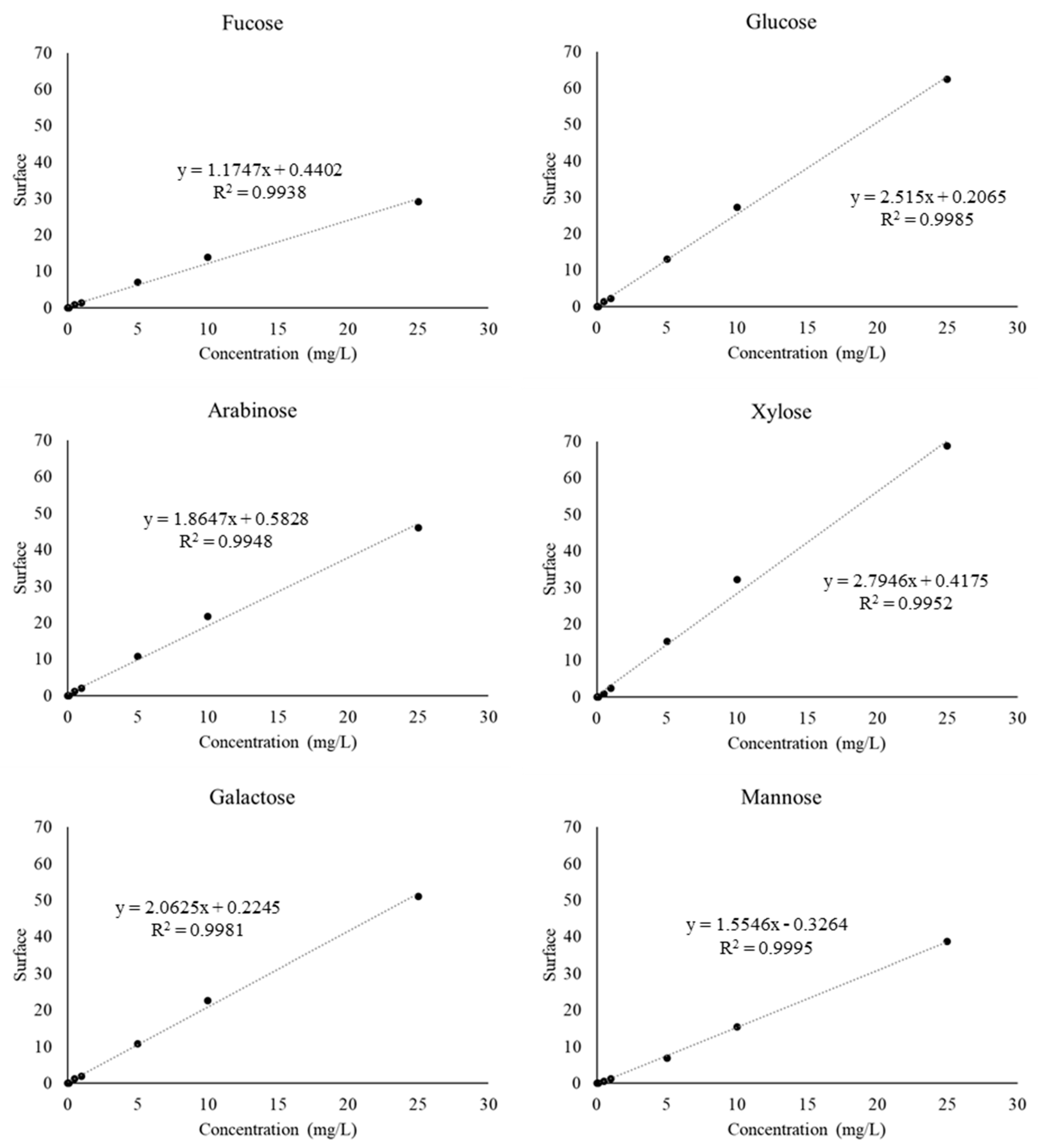
References
- Rader, R.A. Current Challenges in Bioprocesses Development. Pharm. Technol. 2018, 42, 64–65. [Google Scholar]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Redina, E.; Tkachenko, O.; Salmi, T. Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules 2022, 27, 1353. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Amezcua-Allieri, M.A.; Aburto, J. Conversion of Lignin to Heat and Power. In Chemicals or Fuels into the Transition Energy Strategy; IntechOpen: London, UK, 2017; ISBN 978-953-51-3902-7. [Google Scholar]
- Li, X.; Ding, W.; Wang, S.; Yang, L.; Yu, Q.; Xiao, C.; Chen, G.; Zhang, L.; Guan, S.; Sun, D. Three-Dimensional Sulfated Bacterial Cellulose/Gelatin Composite Scaffolds for Culturing Hepatocytes. Cyborg Bionic Syst. 2023, 4, 21. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef]
- Dong, D.; Sun, J.; Huang, F.; Gao, Q.; Wang, Y.; Li, R. Using trifluoroacetic acid to pretreat lignocellulosic biomass. Biomass- Bioenergy 2009, 33, 1719–1723. [Google Scholar] [CrossRef]
- Gardner, S.L.; Burrell, M.; Fry, S.C. Screening of Arabidopsis thaliana stems for variation in cell wall polysaccharides. Phytochemistry 2002, 60, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kang, J.; Li, Y.; Wang, Y.; Tang, X.; Jiang, Z. Carbon-Based Stimuli-Responsive Nanomaterials: Classification and Application. Cyborg Bionic Syst. 2023, 4, 22. [Google Scholar] [CrossRef]
- Marzialetti, T.; Valenzuela Olarte, M.B.; Sievers, C.; Hoskins, T.J.C.; Agrawal, P.K.; Jones, C.W. Dilute Acid Hydrolysis of Loblolly Pine: A Comprehensive Approach. Ind. Eng. Chem. Res. 2008, 47, 7131–7140. [Google Scholar] [CrossRef]
- Gütsch, J.S.; Nousiainen, T.; Sixta, H. Comparative evaluation of autohydrolysis and acid-catalyzed hydrolysis of Eucalyptus globulus wood. Bioresour. Technol. 2012, 109, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Solarte-Toro, J.C.; Chacón-Pérez, Y.; Piedrahita-Rodríguez, S.; Poveda-Giraldo, J.A.; Teixeira, J.A.; Moustakas, K.; Alzate, C.A.C. Effect of dilute sulfuric acid pretreatment on the physicochemical properties and enzymatic hydrolysis of coffee cut-stems. Energy 2020, 195, 116986. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, S.; Liu, X.; Zhang, H.; Tan, J. The prediction of elemental composition of biomass based on proximate analysis. Energy Convers. Manag. 2010, 51, 983–987. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lapierre, C.; Méchin, V.; Baumberger, S. Isolation of structurally distinct lignin–carbohydrate fractions from maize stem by sequential alkaline extractions and endoglucanase treatment. Bioresour. Technol. 2013, 133, 522–528. [Google Scholar] [CrossRef]
- Alonso-Gómez, L.; Solarte-Toro, J.C.; Bello-Pérez, L.A.; Cardona-Alzate, C.A. Performance evaluation and economic analysis of the bioethanol and flour production using rejected unripe plantain fruits (Musa paradisiaca L.) as raw material. Food Bioprod. Process. 2020, 121, 29–42. [Google Scholar] [CrossRef]
- Rueda-Duran, C.-A.; Ortiz-Sanchez, M.; Cardona-Alzate, C.A. Detailed economic assessment of polylactic acid production by using glucose platform: Sugarcane bagasse, coffee cut stems, and plantain peels as possible raw materials. Biomass- Convers. Biorefinery 2022, 12, 4419–4434. [Google Scholar] [CrossRef]
- PWC. Corporate Income Tax (CIT) Rates. Worldwide Tax Summaries. 2022. Available online: https://taxsummaries.pwc.com/quick-charts/corporate-income-tax-cit-rates#anchor-C (accessed on 5 April 2022).
- Indexmundi. Lending Interest Rate (%). 2022. Available online: https://www.indexmundi.com/facts/indicators/FR.INR.LEND/compare#country=co (accessed on 5 April 2022).
- Maxwell, C.; Cost Indices. Towering Skills. 2023. Available online: https://www.toweringskills.com/financial-analysis/cost-indices/ (accessed on 5 April 2022).
- Botero Gutiérrez, C.D. Use of Process Engineering and Life Cycle Assessment to Calculate the Environmental Impact of Butanol Production. Master’s Thesis, Universidad Nacional de Colombia sede Manizales, Manizales, Colombia, 2018. [Google Scholar]
- García-Velásquez, C.A.; Cardona, C.A. Comparison of the biochemical and thermochemical routes for bioenergy production: A techno-economic (TEA), energetic and environmental assessment. Energy 2019, 172, 232–242. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, Z.; Xu, G.; Yang, H.; Yang, J. A stepwise pretreatment of sugarcane bagasse by alkaline and hydroxymethyl reagent for bioethanol production. Ind. Crop. Prod. 2020, 145, 112136. [Google Scholar] [CrossRef]
- Wang, D.; Tian, J.; Guan, J.; Ding, Y.; Wang, M.L.; Tonnis, B.; Liu, J.; Huang, Q. Valorization of sugarcane bagasse for sugar extraction and residue as an adsorbent for pollutant removal. Front. Bioeng. Biotechnol. 2022, 10, 893941. [Google Scholar] [CrossRef]
- Ponce, J.; da Silva Andrade, J.G.; dos Santos, L.N.; Bulla, M.K.; Barros, B.C.B.; Favaro, S.L.; Hioka, N.; Caetano, W.; Batistela, V.R. Alkali pretreated sugarcane bagasse, rice husk and corn husk wastes as lignocellulosic biosorbents for dyes. Carbohydr. Polym. Technol. Appl. 2021, 2, 100061. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Castiblanco, E.A.; Cortés, F.B. Determinación de parámetros cinéticos para la pirólisis rápida de aserrín de pino pátula. Boletín Grupo Español Carbón 2015, 38, 9–11. [Google Scholar]
- Soto, N.A.; Machado, W.R.; López, D.L. Determinación de los parámetros cinéticos en la pirólisis del pino ciprés. Química Nova 2010, 33, 1500–1505. [Google Scholar] [CrossRef][Green Version]
- De Souza Moretti, M.M.; Perrone, O.M.; Nunes, C.D.C.C.; Taboga, S.; Boscolo, M.; da Silva, R.; Gomes, E. Effect of pretreatment and enzymatic hydrolysis on the physical-chemical composition and morphologic structure of sugarcane bagasse and sugarcane straw. Bioresour. Technol. 2016, 219, 773–777. [Google Scholar] [CrossRef]
- Bay, M.S.; Karimi, K.; Esfahany, M.N.; Kumar, R. Structural modification of pine and poplar wood by alkali pretreatment to improve ethanol production. Ind. Crop. Prod. 2020, 152, 112506. [Google Scholar] [CrossRef]
- Gómez, L.D.; Vanholme, R.; Bird, S.; Goeminne, G.; Trindade, L.M.; Polikarpov, I.; Simister, R.; Morreel, K.; Boerjan, W.; McQueen-Mason, S.J. Side by Side Comparison of Chemical Compounds Generated by Aqueous Pretreatments of Maize Stover, Miscanthus and Sugarcane bagasse. BioEnergy Res. 2014, 7, 1466–1480. [Google Scholar] [CrossRef]
- Berrocal, A.; Baeza, J.; Rodríguez, J.; Espinosa, M.; Freer, J. Effect of tree age on variation of Pinus radiata D. Don chemical composition. J. Chil. Chem. Soc. 2004, 49, 251–256. [Google Scholar] [CrossRef]
- Yamakawa, C.K.; D’Imperio, I.; Bonfiglio, F.; Mussatto, S.I. Valorization of Pinus taeda hemicellulosic hydrolysate for the production of value-added compounds in an ethanol biorefinery. Fuel 2022, 318, 123489. [Google Scholar] [CrossRef]
- Koekemoer, T. Lactic Acid Production from Sugarcane bagasse and Harvesting Residues. Master’s Thesis, Faculty of Engineering at Stellenbosch University, Stellenbosch, Sudáfrica, 2019. [Google Scholar]
- Hans, M.; Pellegrini, V.O.; Filgueiras, J.G.; de Azevedo, E.R.; Guimaraes, F.E.; Chandel, A.K.; Polikarpov, I.; Chadha, B.S.; Kumar, S. Optimization of Dilute Acid Pretreatment for Enhanced Release of Fermentable Sugars from Sugarcane Bagasse and Validation by Biophysical Characterization. BioEnergy Res. 2023, 16, 416–434. [Google Scholar] [CrossRef]
- Lorencini, P.; Siqueira, M.R.; Maniglia, B.C.; Tapia, D.R.; Maintinguer, S.I.; Reginatto, V. Biohydrogen Production from Liquid and Solid Fractions of Sugarcane Bagasse After Optimized Pretreatment with Hydrochloric Acid. Waste Biomass-Valorization 2016, 7, 1017–1029. [Google Scholar] [CrossRef]
- Chacha, N.; Toven, K.; Mtui, G.; Katima, J.; Mrema, G. Steam pretreatment of pine (Pinus patula) Wood residue for the production of reducing sugars. Cellul. Chem. Technol. 2011, 45, 495–501. [Google Scholar]
- Lavarack, B.P.; Griffin, G.J.; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass-Bioenergy 2002, 23, 367–380. [Google Scholar] [CrossRef]
- Banerjee, P.N.; Pranovich, A.; Dax, D.; Willför, S. Non-cellulosic heteropolysaccharides from sugarcane bagasse—Se-quential extraction with pressurized hot water and alkaline peroxide at different temperatures. Bioresour. Technol. 2014, 155, 446–450. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Liu, C.F.; Sun, R.C. Comparative study of alkali- and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr. Res. 2006, 341, 253–261. [Google Scholar] [CrossRef]
- Reyes, P.; Teixeira Mendonca, R.; Rodriguez, J.; Fardim, P.; Vega, B. Characterization of the Hemicellulosic Fraction Obtained After Pre-Hydrolysis of Pinus radiata Wood Chips with Hot-Water at Different Initial Ph. J. Chil. Chem. Soc. 2013, 58, 1614–1618. [Google Scholar] [CrossRef]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, D.M.; Queiroz, J.H.; Colodette, J.L. Hydrothermal and Acid Pretreatments Improve Ethanol Production from Lignocellulosic Biomasses. Bioresources 2017, 12, 3088–3107. [Google Scholar] [CrossRef]
- Sun, F.F.; Zhao, X.; Hong, J.; Tang, Y.; Wang, L.; Sun, H.; Li, X.; Hu, J. Industrially relevant hydrolyzability and fermentability of sugarcane bagasse improved effectively by glycerol organosolv pretreatment. Biotechnol. Biofuels 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Duque, S.H.; Cardona, C.A.; Moncada, J. Techno-Economic and Environmental Analysis of Ethanol Production from 10 Agroindustrial Residues in Colombia. Energy Fuels 2015, 29, 775–783. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. BioEnergy Res. 2012, 6, 388–401. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Xu, J.; Ou, X.; Chang, S.; Wu, M. Techno-Economic Analysis of Bioethanol Production from Lignocellulosic Biomass in China: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Energies 2015, 8, 4096–4117. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Garcia-Vallejo, M.C.; Cardona Alzate, C.A. Analysis of Single-Step Pretreatments for Lignocellulosic Platform Isolation as the Basis of Biorefinery Design. Molecules 2023, 28, 1278. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
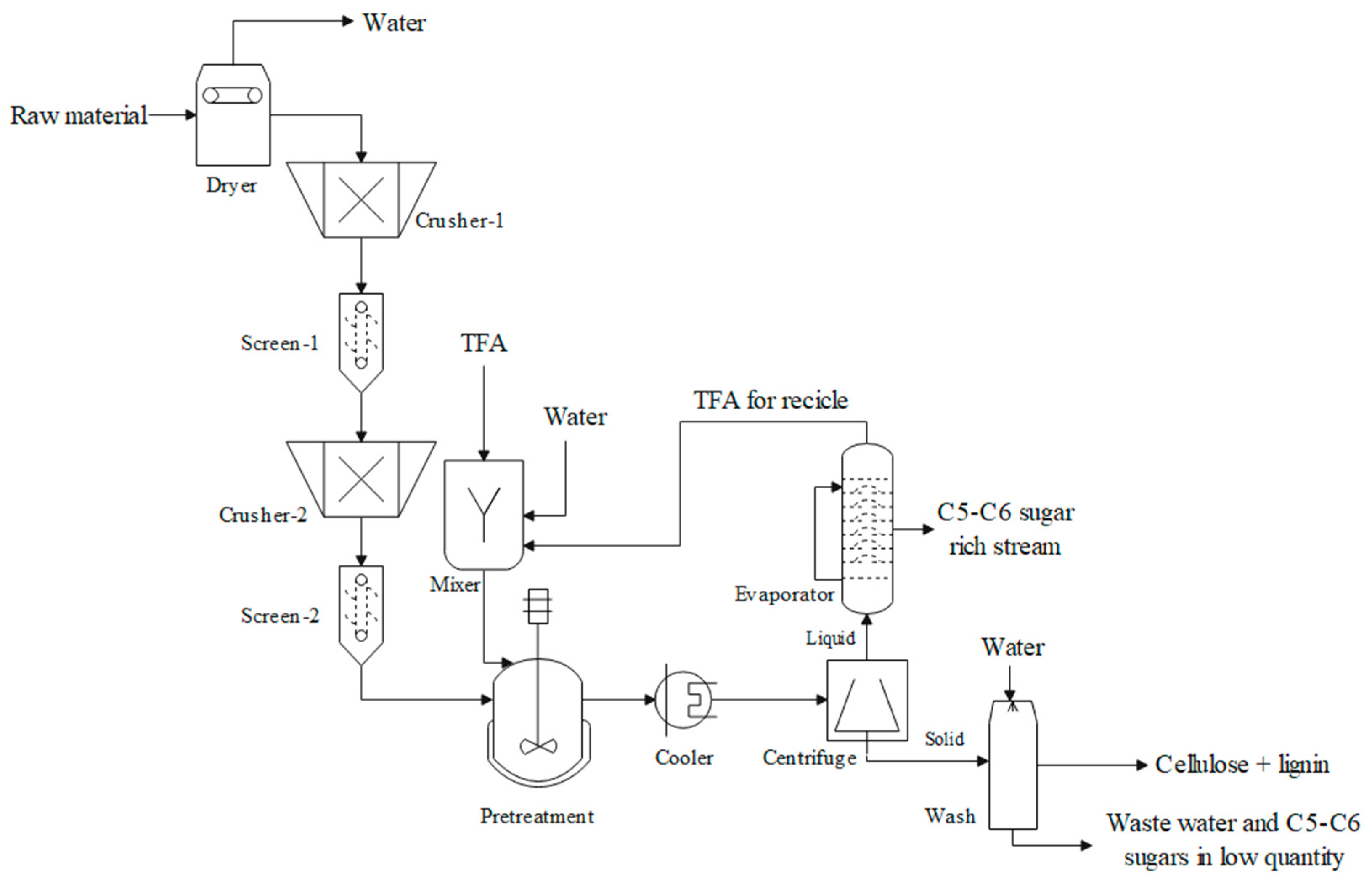

| Equipment | Description | Conditions | Model |
|---|---|---|---|
| Dryer | Moisture removal of the raw material | 1 bar, 60 °C | Dryer |
| Crusher-1 | Primary mill, gyratory, particle size reduction to 2 cm | 1 bar, 20 °C | Crusher |
| Screen-1 | Separation of particles, size of screen opening 2 cm | 1 bar, 20 °C | Screen |
| Crusher-2 | Secondary mill, gyratory, particle size reduction to 1 cm | 1 bar, 20 °C | Crusher |
| Screen-2 | Separation of particles, size of screen opening 1 cm | 1 bar, 20 °C | Screen |
| Mixer | TFA 2.5 M preparation | 1 bar, 25 °C | Mixer |
| Pretreatment reactor | Reaction of hydrolysis to produce xylose and glucose mainly. Also considering the production of furfural and 5-HMF | 2 bar, 120 °C | Heater and RStoic |
| Cooler | Reduction of the temperature to further purification the hydrolysate stream | 1 bar, 30 °C | Heater |
| Centrifuge | Separation of solid and liquid fractions | 1 bar, 30 °C | CFuge |
| Washer | Washing of the solid stream rich in cellulose and lignin fractions | 1 bar, 26 °C | SWash |
| Evaporator | Moisture and acid remotion of the liquid fraction, rich in xylose and glucose | 0.5 bar, 80 °C | Flash2 |
| Item | Value | Conditions | Description |
|---|---|---|---|
| Operators wage | 232.15 | USD/month | Minimum wage 2022. Non-skilled person. RMR = 4812.37 COP = 1 USD (6 December 2022) |
| Supervisor wage | 464.29 | USD/month | High-skilled person. RMR = 4812.37 COP = 1 USD (6 December 2022) |
| Tax rate | 33 | % | [20] |
| Interest rate | 13 | % | [21] |
| CEPCI 2022 | 816.3 | - | [22] |
| Operating time | 350 | days/year | Small-scale process |
| NCSB | 0.015 | USD/kg | [23] |
| PP | 0.127 | USD/kg | [24] |
| TFA | 0.780 | USD/kg | Cemotechnology-China (2022) |
| Processing water | 0.326 | USD/cum | [24] |
| LP steam | 7.89 | USD/ton | |
| MP steam | 8.07 | USD/ton | |
| Electricity | 0.055 | USD/kWh | |
| Cooling water | 0.042 | USD/cum |
| Sample | Volatile Matter | Ash | Fixed Carbon | C % | H % | O % | Empirical Formula | Reference |
|---|---|---|---|---|---|---|---|---|
| PP | 88.12 | 0.44 | 11.44 | 47.76 | 5.96 | 45.21 | C6H8.92O4.26 | This work |
| NCSB | 85.96 | 1.05 | 12.99 | 47.69 | 5.91 | 44.71 | C6H8.87O4.22 | This work |
| Sample | Acid | Value | Arabinose | Galactose | Glucose | Xylose | Mannose | Reference |
|---|---|---|---|---|---|---|---|---|
| PP | TFA | Content (g/g raw material) | 0.07 ± 0.01 | 0.16 ± 0.03 | 0.26 ± 0.04 | 0.26 ± 0.07 | 0.25 ± 0.06 | This work |
| PR | H2SO4 | Content (g/g raw material) | 0.02 | 0.07 | 0.37 | 0.08 | 0.09 | [33] |
| PT | H2SO4 | Concentration (g/L) | 2.96 | 7.90 | 16.93 | 4.5 | 29.82 | [34] |
| NCSB | TFA | Content (g/g raw material) | 0.17 ± 0.04 | 0.05 ± 0.01 | 0.31 ± 0.08 | 0.46 ± 0.06 | 0.01 ± 0.01 | This work |
| SCB | H2SO4 | Concentration (g/L) | 1.14 | N.R. | 2.72 | 33.24 | N.R. | [35] |
| SCB | H2SO4 | Concentration (g/L) | 7.90 | 6.44 | 4.38 | 25.57 | 9.64 | [36] |
| SCB | HCl | Concentration (mg/L) | 460 | N.R. | 20 | 40 | N.R. | [37] |
| SCB | H2SO4 | Content (mg/g raw material) | 0.39 | 0.05 | 1.32 | 0.11 | N.R. | [32] |
| Raw Material | Pretreatment | Acid Concentration | T (°C) | t (min) | Severity Factor | Reference |
|---|---|---|---|---|---|---|
| PP | TFA | 2.5 M | 120 | 120 | 2.67 | This work |
| PP | Steam explosion | N.A | 180 | 10 | 3.36 | [38] |
| PR | HCl diluted | 2 M | 120 | 30 | 2.07 | [42] |
| EU and EG | Diluted Sulfuric acid | 4.5% w/w | 175 | 15 | 3.05 | [44] |
| NCSB | TFA | 2.5 M | 120 | 120 | 2.67 | This work |
| SCB | Diluted sulfuric acid | 8% w/w | 90 | 400 | 2.31 | [39] |
| SCB | Organosolv | N.A | 220 | 120 | 5.61 | [45] |
| SCB | Acid–ultrasound–thermal treatment | 3% v/v | 80 | 60 | 1.19 | [46] |
| WS | TFA | 10% w/w | 60 | 960 | 1.80 | [10] |
| Case | Mass Indicators | Energy Indicator | |||
|---|---|---|---|---|---|
| (kg product/kg raw material) | (kg raw material/kg products) | (kg waste/kg products) | (% kg renewable feedstock/kg raw materials) | (MJ/kg) | |
| PP | 0.22 (sugar-rich stream *) | 11.71 | 10.71 | 13.23 | 207.67 |
| 0.43 (cellulose–lignin stream **) | |||||
| NCSB | 0.27 (sugar-rich stream) | 11.71 | 10.70 | 13.92 | 190.93 |
| 0.35 (cellulose–lignin stream) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedrahita-Rodríguez, S.; Baumberger, S.; Cézard, L.; Poveda-Giraldo, J.A.; Alzate-Ramírez, A.F.; Cardona Alzate, C.A. Comparative Analysis of Trifluoracetic Acid Pretreatment for Lignocellulosic Materials. Materials 2023, 16, 5502. https://doi.org/10.3390/ma16155502
Piedrahita-Rodríguez S, Baumberger S, Cézard L, Poveda-Giraldo JA, Alzate-Ramírez AF, Cardona Alzate CA. Comparative Analysis of Trifluoracetic Acid Pretreatment for Lignocellulosic Materials. Materials. 2023; 16(15):5502. https://doi.org/10.3390/ma16155502
Chicago/Turabian StylePiedrahita-Rodríguez, Sara, Stéphanie Baumberger, Laurent Cézard, Jhonny Alejandro Poveda-Giraldo, Andrés Felipe Alzate-Ramírez, and Carlos Ariel Cardona Alzate. 2023. "Comparative Analysis of Trifluoracetic Acid Pretreatment for Lignocellulosic Materials" Materials 16, no. 15: 5502. https://doi.org/10.3390/ma16155502
APA StylePiedrahita-Rodríguez, S., Baumberger, S., Cézard, L., Poveda-Giraldo, J. A., Alzate-Ramírez, A. F., & Cardona Alzate, C. A. (2023). Comparative Analysis of Trifluoracetic Acid Pretreatment for Lignocellulosic Materials. Materials, 16(15), 5502. https://doi.org/10.3390/ma16155502









