Fusion of Ni Plating on CP-Titanium by Electron Beam Single-Track Scanning: Toward a New Approach for Fabricating TiNi Self-Healing Shape Memory Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results
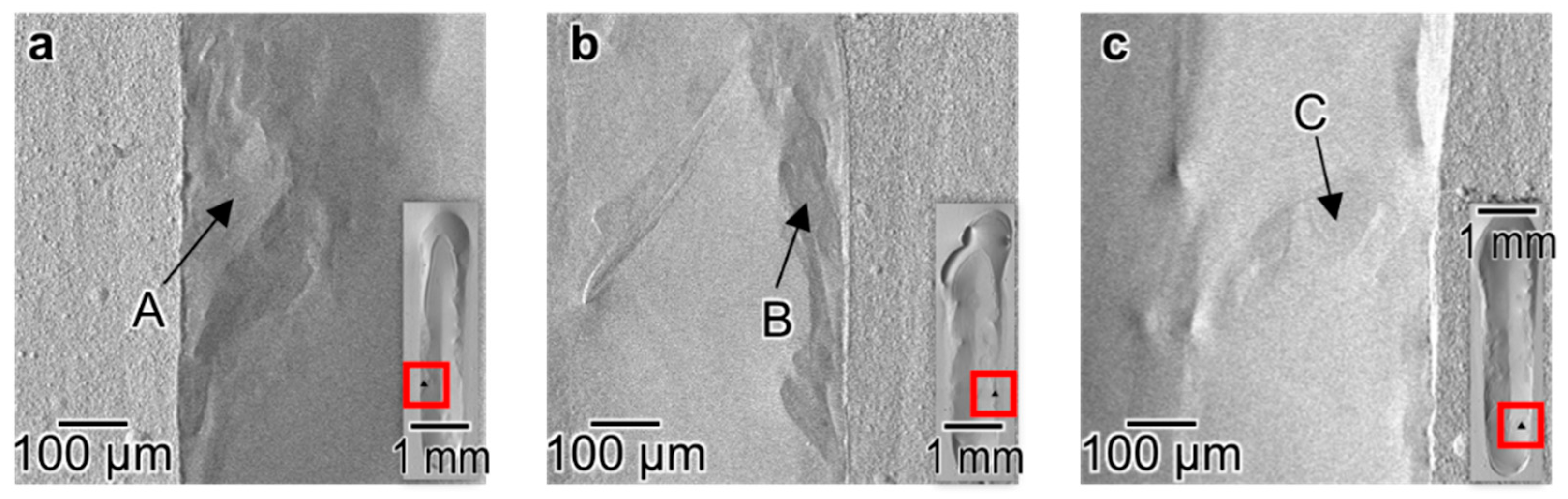
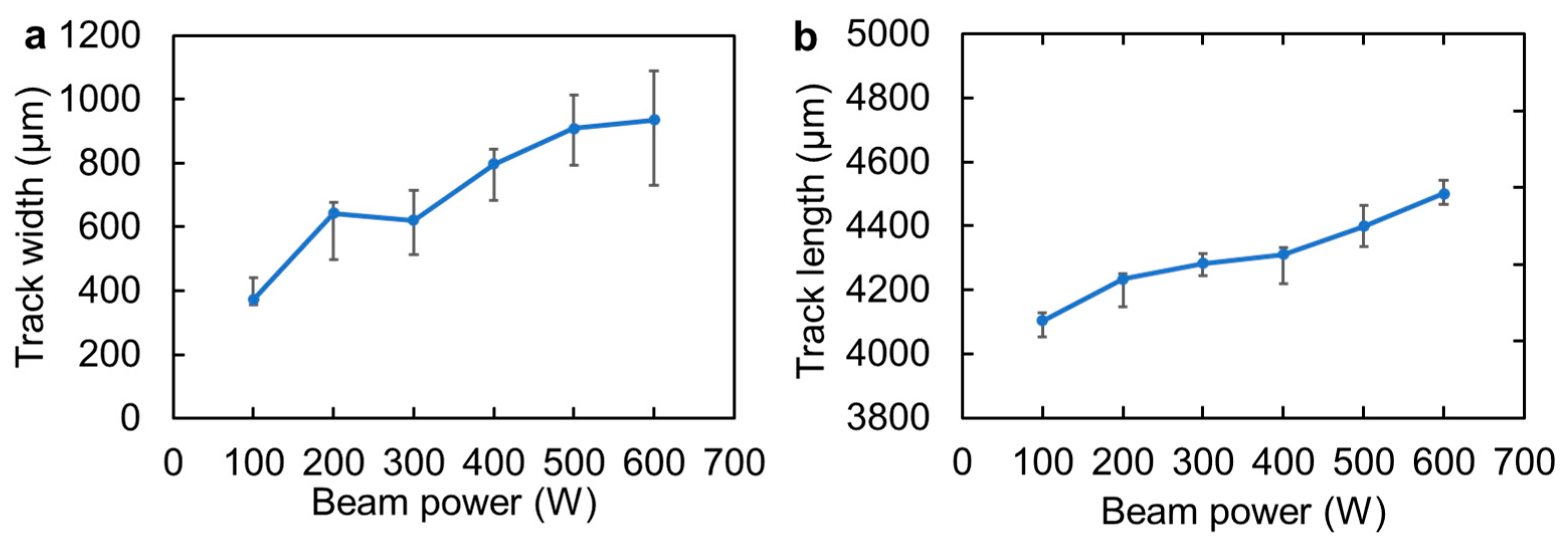
3.1. Surface Morphology of the Melt Track
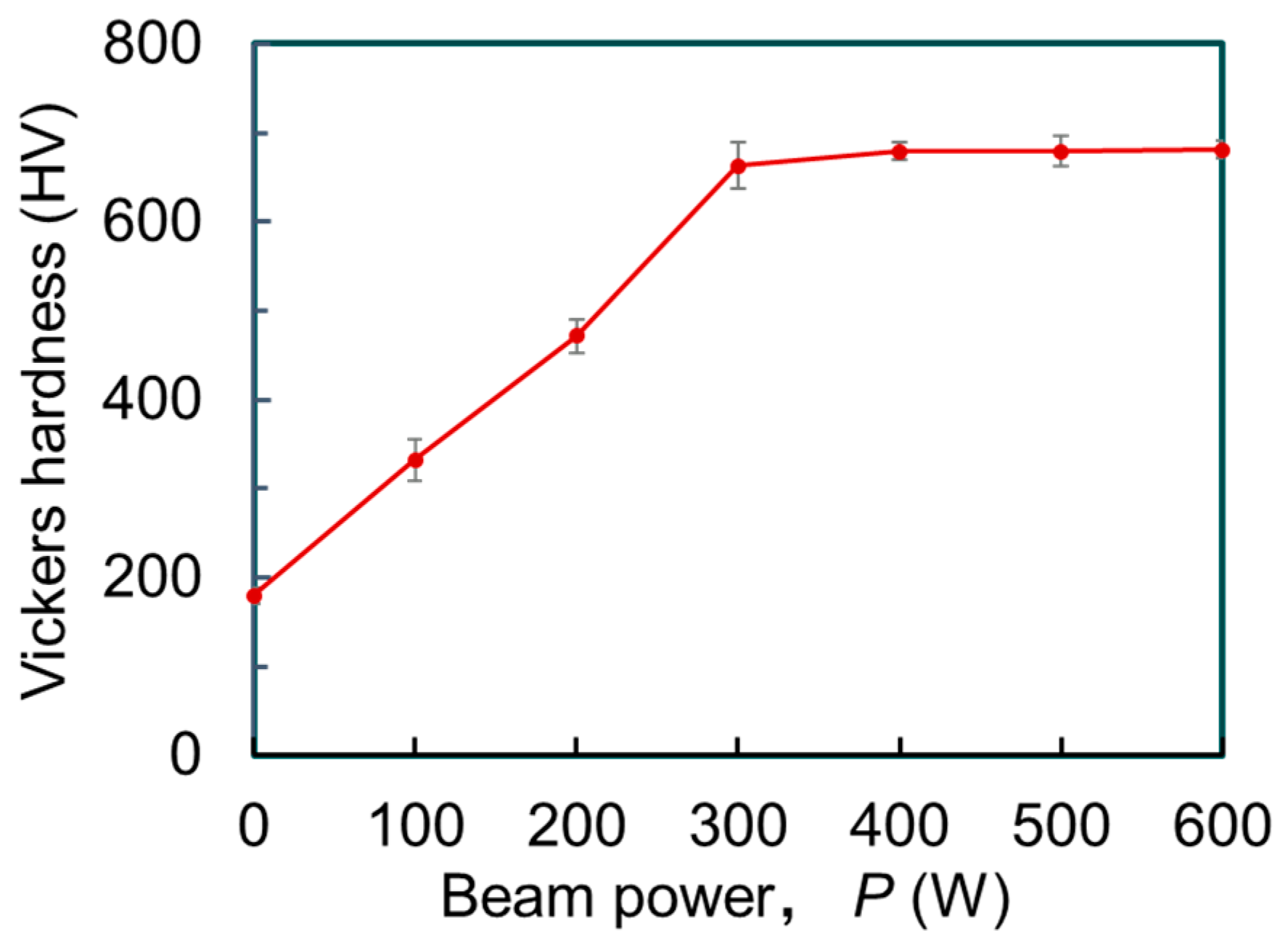
3.2. Cross-Sectional Observation of Melt Track
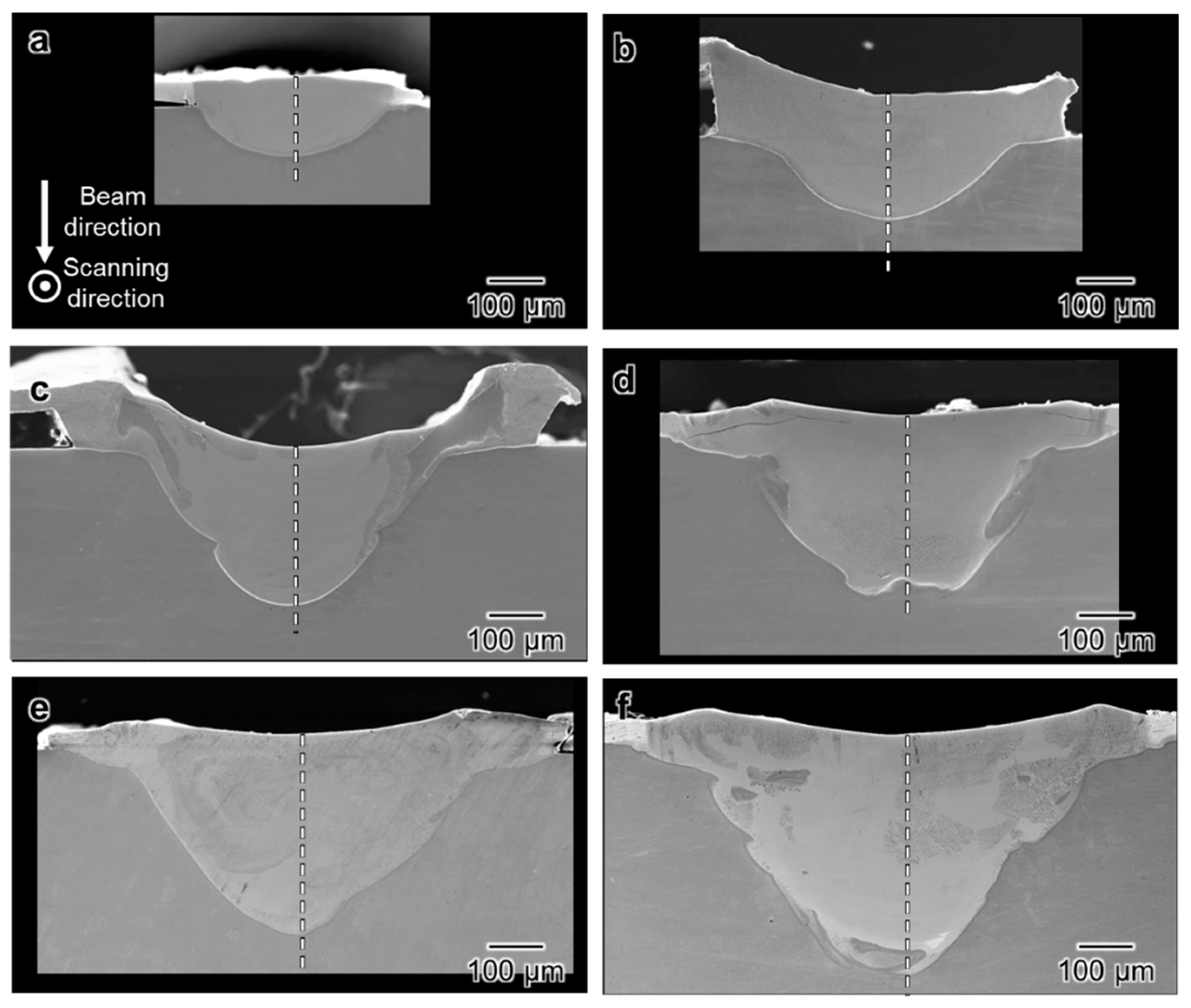

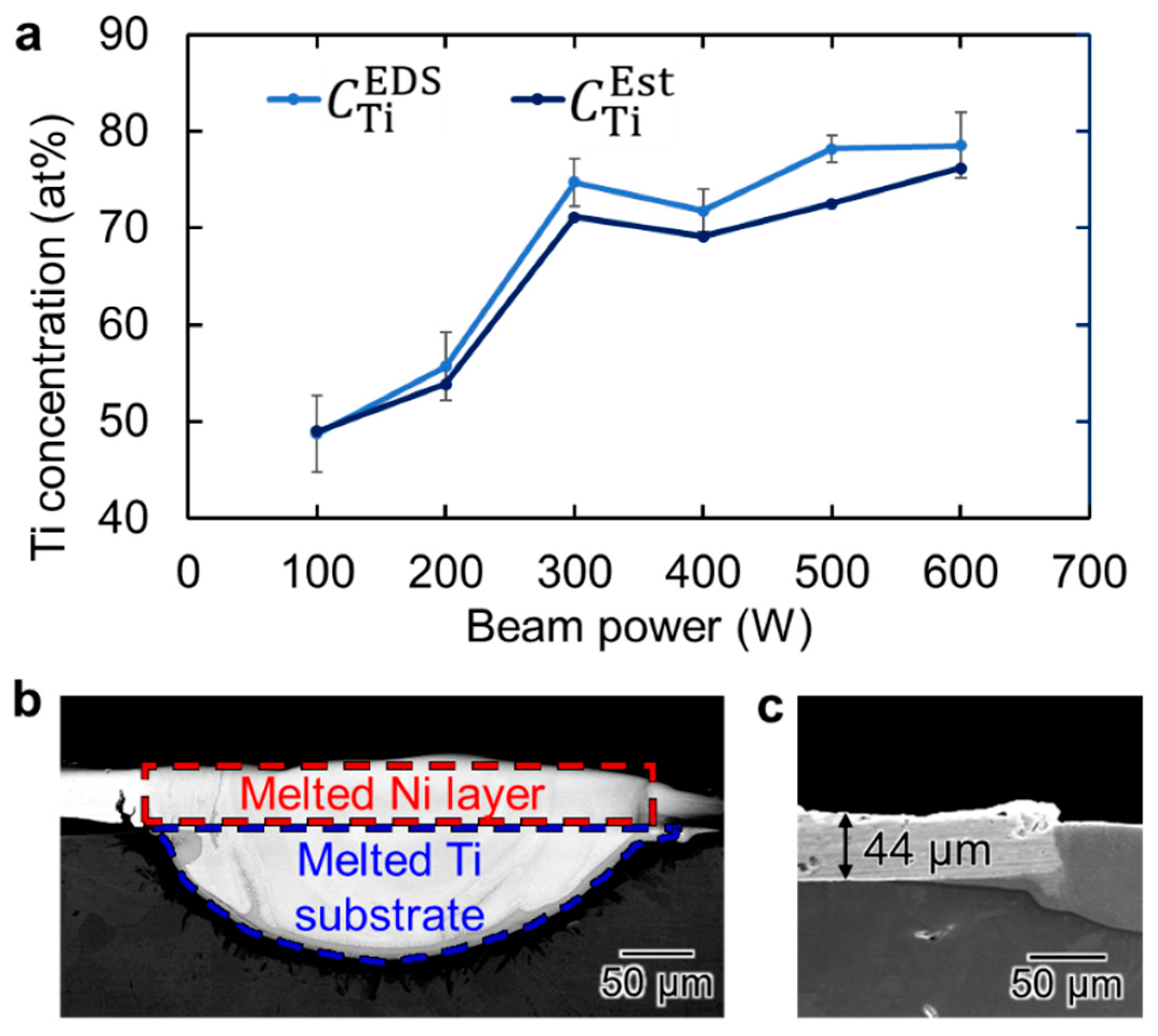
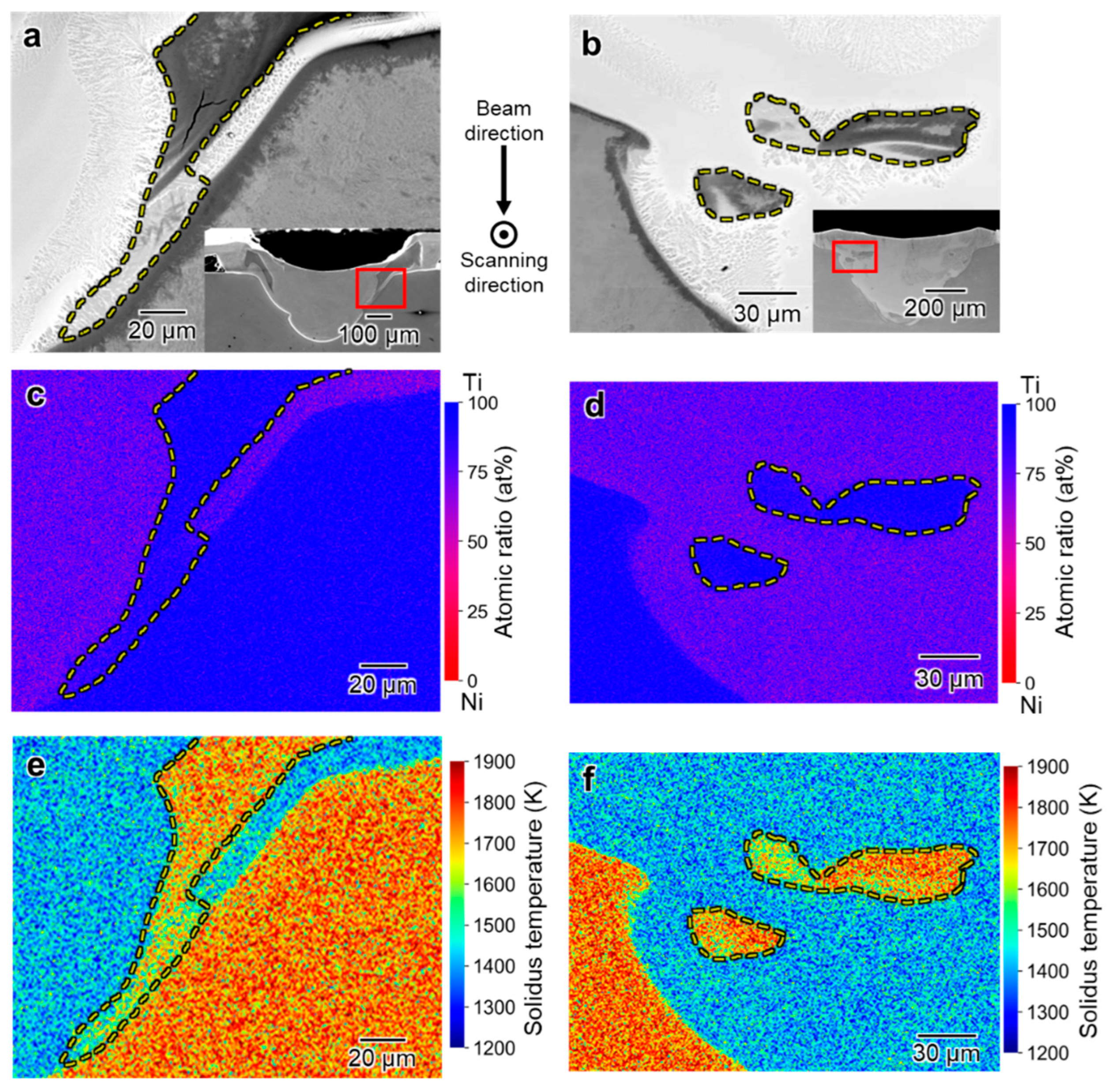
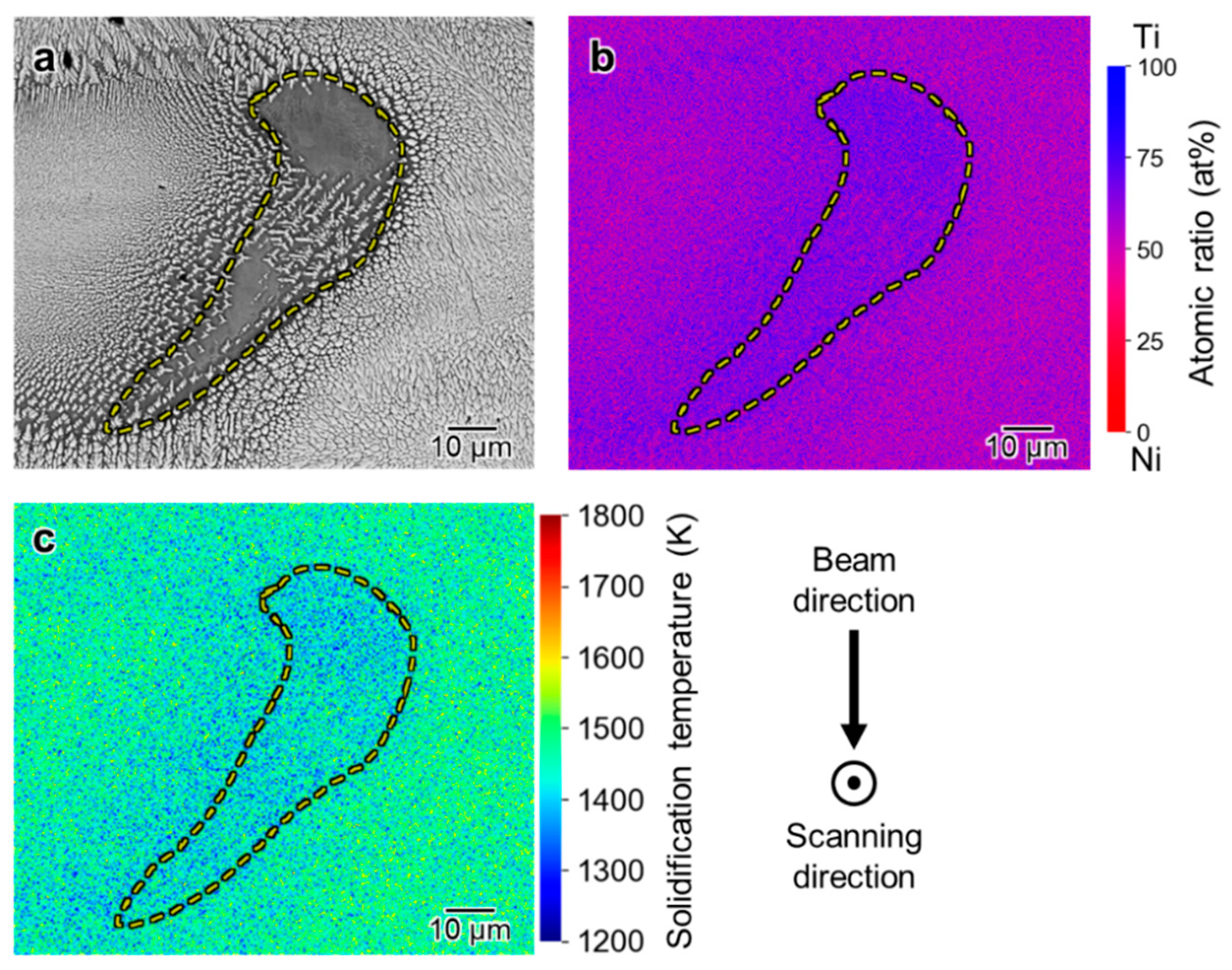
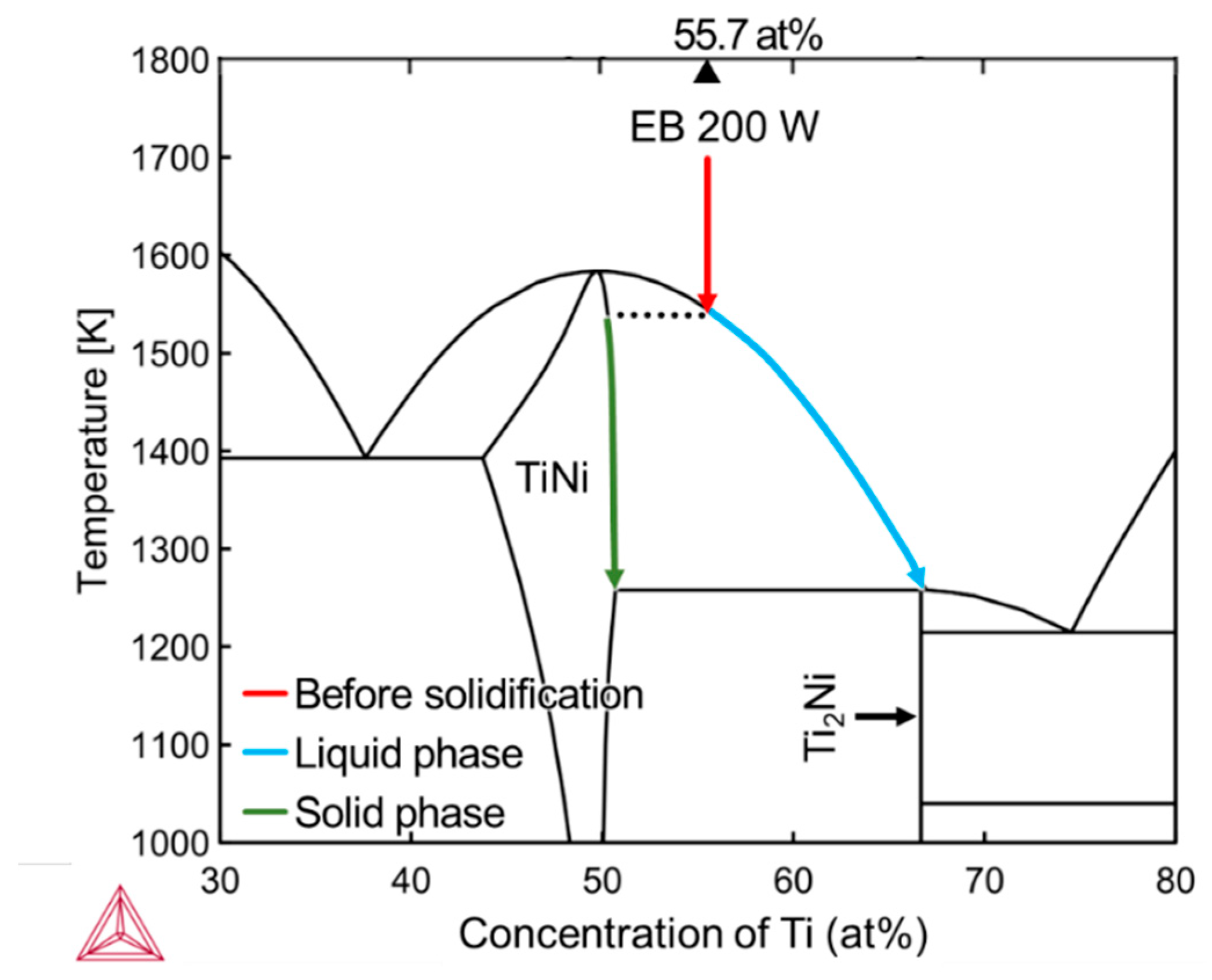
3.3. Spatial Elemental Distributions in the Melt Regions

4. Discussion
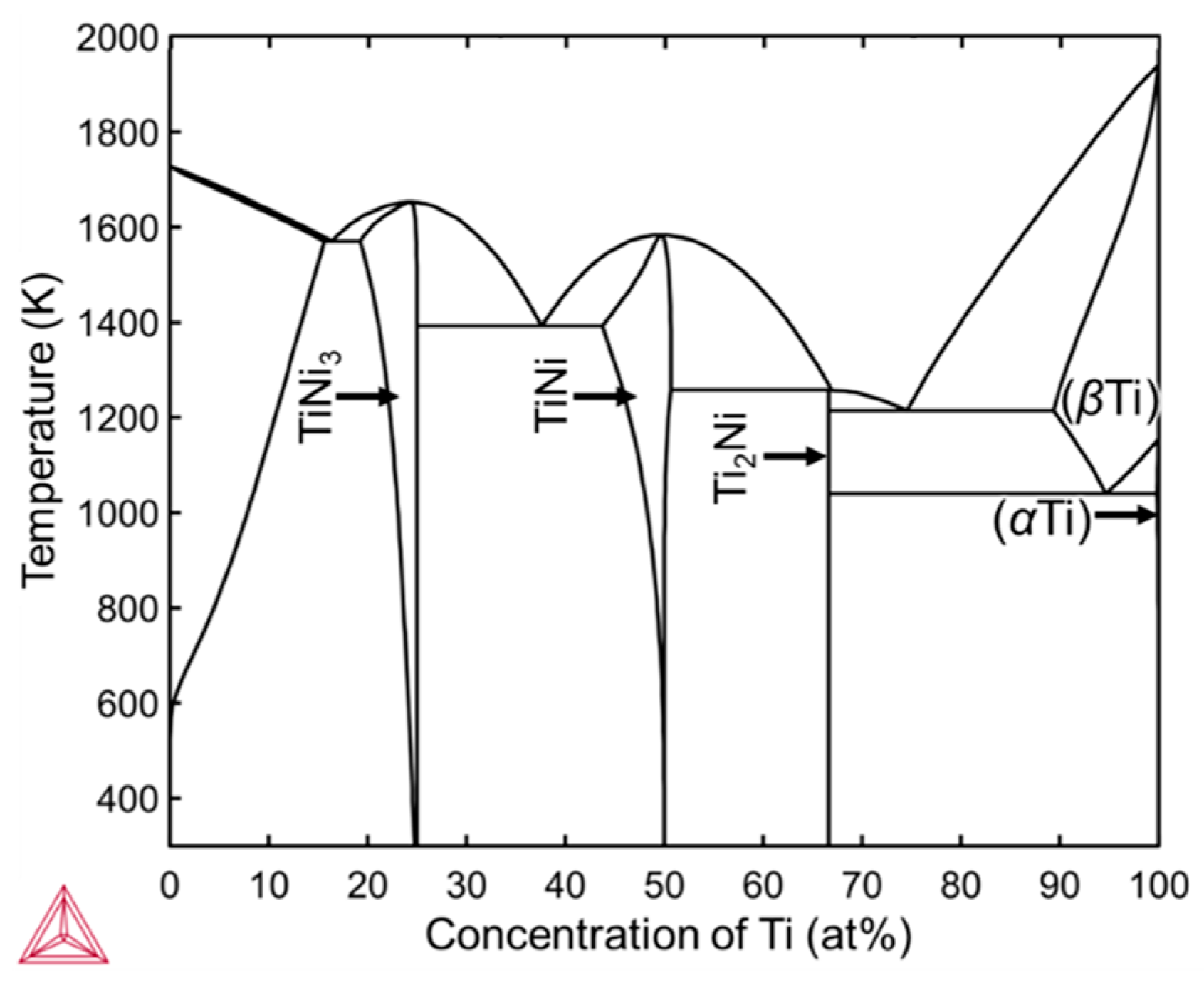
4.1. Inhomogeneous Formation of Ti2Ni Phase under Low Beam Power Conditions
4.2. Strategy for Fabrication of TiNi Coating Based on the Results of This Study
5. Conclusions
- EB scanning of CP-Ti substrates plated with Ni yields a dense and porosity-free microstructure within the formed melt track, exhibiting superior uniformity when compared to thermal spray coatings. The depth of the melt track is influenced by the intensity of the EB and can reach a range from 100 μm to 300 μm.
- Under optimal experimental conditions for single-track scanning, the melt track attains an elemental composition close to Ni-50 at.% Ti, which represents the stoichiometric chemical composition of the TiNi alloy. In this study, the optimal condition is an Ni-plating thickness of 44 μm, EB scanning speed of 100 mm s−1, and EB power of 100 W.
- There is a good homogeneity of the elemental composition in the melt track under optimal scanning experimental conditions. This can be attributed to the intense local heating caused by EB irradiation, resulting in a significant temperature gradient at the free surface. Consequently, the enhanced Marangoni convection vigorously stirs the liquid within the melt pool.
- EB Multi-Track Scanning emerges as a highly efficient technique for fabricating TiNi Self-Healing Shape Memory Coatings through the fusion of an Ni plate onto CP-Ti. This method holds the potential in achieving the desired outcome.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially Pure Titanium (Cp-Ti) versus Titanium Alloy (Ti6Al4V) Materials as Bone Anchored Implants—Is One Truly Better than the Other? Mater. Sci. Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef]
- Boyer, R.R. An Overview on the Use of Titanium in the Aerospace Industry. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Jorge, J.R.P.; Barão, V.A.; Delben, J.A.; Faverani, L.P.; Queiroz, T.P.; Assunção, W.G. Titanium in Dentistry: Historical Development, State of the Art and Future Perspectives. J. Indian Prosthodont. Soc. 2013, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gorynin, I.V. Titanium Alloys for Marine Application. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Kumar, A.; Mahto, M.K.; Rana, V.S.; Vashista, M.; Yusufzai, M.Z.K. Investigation of Microstructure Evolution and Mechanical Properties of Gas Tungsten Arc Welded Dissimilar Titanium (CP-Ti/Ti–6Al–4V) Alloys. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2022, 09544089221126431. [Google Scholar] [CrossRef]
- Welsch, G.; Boyer, R.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1993; ISBN 978-0-87170-481-8. [Google Scholar]
- Qin, Y.; Geng, L.; Ni, D. Dry Sliding Wear Behavior of Extruded Titanium Matrix Composite Reinforced by in Situ TiB Whisker and TiC Particle. J. Mater. Sci. 2011, 46, 4980–4985. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.G.; Chaubey, A.K.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Comparison of Wear Properties of Commercially Pure Titanium Prepared by Selective Laser Melting and Casting Processes. Mater. Lett. 2015, 142, 38–41. [Google Scholar] [CrossRef]
- Hou, L.; Grummon, D.S. Transformational Superelasticity in Sputtered Titanium-Nickel Thin Films. Scr. Metall. Et Mater. 1995, 33, 989–995. [Google Scholar] [CrossRef]
- Ni, W.; Cheng, Y.-T.; Grummon, D.S. Recovery of Microindents in a Nickel–Titanium Shape-Memory Alloy: A “Self-Healing” Effect. Appl. Phys. Lett. 2002, 80, 3310–3312. [Google Scholar] [CrossRef]
- Liu, R.; Li, D.Y.; Xie, Y.S.; Llewellyn, R.; Hawthorne, H.M. Indentation Behavior of Pseudoelastic TiNi Alloy. Scr. Mater. 1999, 41, 691–696. [Google Scholar] [CrossRef]
- Hiraga, H.; Inoue, T.; Shimura, H.; Matsunawa, A. Cavitation Erosion Mechanism of NiTi Coatings Made by Laser Plasma Hybrid Spraying. Wear 1999, 231, 272–278. [Google Scholar] [CrossRef]
- Swain, B.; Mantry, S.; Mohapatra, S.S.; Mishra, S.C.; Behera, A. Investigation of Tribological Behavior of Plasma Sprayed NiTi Coating for Aerospace Application. J. Therm. Spray Tech. 2022, 31, 2342–2369. [Google Scholar] [CrossRef]
- Jardine, A.P.; Field, Y.; Herman, H. Shape Memory Effect in Vacuum Plasma Sprayed NiTi. J. Mater. Sci. Lett. 1991, 10, 943–945. [Google Scholar] [CrossRef]
- Bram, M.; Ahmad-Khanlou, A.; Buchkremer, H.P.; Stöver, D. Vacuum Plasma Spraying of NiTi Protection Layers. Mater. Lett. 2002, 57, 647–651. [Google Scholar] [CrossRef]
- Cinca, N.; Isalgué, A.; Fernández, J.; Guilemany, J.M. Structure Characterization and Wear Performance of NiTi Thermal Sprayed Coatings. Smart Mater. Struct. 2010, 19, 085011. [Google Scholar] [CrossRef]
- Tria, S.; Elkedim, O.; Li, W.Y.; Liao, H. Ball Milled Ni–Ti Powder Deposited by Cold Spraying. J. Alloys Compd. 2009, 483, 334–336. [Google Scholar] [CrossRef]
- Tria, S.; Elkedim, O.; Hamzaoui, R.; Guo, X.; Bernard, F.; Millot, N.; Rapaud, O. Deposition and Characterization of Cold Sprayed Nanocrystalline NiTi. Powder Technol. 2011, 210, 181–188. [Google Scholar] [CrossRef]
- Mauer, G.; Rauwald, K.-H.; Sohn, Y.J.; Weirich, T.E. Cold Gas Spraying of Nickel-Titanium Coatings for Protection Against Cavitation. J. Therm. Spray Tech. 2021, 30, 131–144. [Google Scholar] [CrossRef]
- Cicek, H.; Efeoglu, I.; Totik, Y.; Ezirmik, K.V.; Arslan, E. A Low Temperature In-Situ Crystalline TiNi Shape Memory Thin Film Deposited by Magnetron Sputtering. Surf. Coat. Technol. 2015, 284, 90–93. [Google Scholar] [CrossRef]
- Fu, Y.; Du, H.; Zhang, S. Adhesion and Interfacial Structure of Magnetron Sputtered TiNi Films on Si/SiO2 Substrate. Thin Solid Film. 2003, 444, 85–90. [Google Scholar] [CrossRef]
- ASM Handbook Committee. Metals Handbook, 9th ed.; American Society for Metals: Detroit, MI, USA, 1978. [Google Scholar]
- Li, C.; Liu, Z.Y.; Fang, X.Y.; Guo, Y.B. Residual Stress in Metal Additive Manufacturing. Procedia CIRP 2018, 71, 348–353. [Google Scholar] [CrossRef]
- Takase, A.; Ishimoto, T.; Suganuma, R.; Nakano, T. Surface Residual Stress and Phase Stability in Unstable β-Type Ti–15Mo–5Zr–3Al Alloy Manufactured by Laser and Electron Beam Powder Bed Fusion Technologies. Addit. Manuf. 2021, 47, 102257. [Google Scholar] [CrossRef]
- Miyata, Y.; Okugawa, M.; Koizumi, Y.; Nakano, T. Inverse Columnar-Equiaxed Transition (CET) in 304 and 316L Stainless Steels Melt by Electron Beam for Additive Manufacturing (AM). Crystals 2021, 11, 856. [Google Scholar] [CrossRef]
- Zech, N.; Landolt, D. The Influence of Boric Acid and Sulfate Ions on the Hydrogen Formation in Ni-Fe Plating Electrolytes. Electrochim. Acta 2000, 45, 3461–3471. [Google Scholar] [CrossRef]
- Hoffmann, H.; Paulisch-Rinke, M.C.; Gernhard, M.; Jännsch, Y.; Timm, J.; Brandmeir, C.; Lechner, S.; Marschall, R.; Moos, R.; Manke, I.; et al. Multi-Scale Morphology Characterization of Hierarchically Porous Silver Foam Electrodes for Electrochemical CO2 Reduction. Commun. Chem. 2023, 6, 50. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zou, B.; Gu, L.; Huang, W.; Cao, X. Preparation and Corrosion Resistance of MAO/Ni–P Composite Coat on Mg Alloy. Appl. Surf. Sci. 2013, 277, 272–280. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Nolan, A.M.; Mo, Y. Interfacial Atomistic Mechanisms of Lithium Metal Stripping and Plating in Solid-State Batteries. Adv. Mater. 2021, 33, 2008081. [Google Scholar] [CrossRef]
- Thermo-Calc Software AB. TCBIN: Thermodynamic Database for Binary Systems. Version 1.1. Database. 2022. Available online: https://thermocalc.com/products/databases/tcbin/ (accessed on 20 June 2023).
- Kang, N.; Coddet, P.; Dembinski, L.; Liao, H.; Coddet, C. Microstructure and Strength Analysis of Eutectic Al-Si Alloy in-Situ Manufactured Using Selective Laser Melting from Elemental Powder Mixture. J. Alloys Compd. 2017, 691, 316–322. [Google Scholar] [CrossRef]
- Ahsan, M.d.R.U.; Cheepu, M.; Ashiri, R.; Kim, T.-H.; Jeong, C.; Park, Y.-D. Mechanisms of Weld Pool Flow and Slag Formation Location in Cold Metal Transfer (CMT) Gas Metal Arc Welding (GMAW). Weld. World 2017, 61, 1275–1285. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, L.; Long, W.; Zhong, S.; Zhang, L. Influence of Doping Ti Particles on Intermetallic Compounds Growth at Sn58Bi/Cu Interface during Solid–Liquid Diffusion. J. Mater. Sci. Mater. Electron. 2021, 32, 3341–3351. [Google Scholar] [CrossRef]
- Okugawa, M.; Ohigashi, Y.; Furishiro, Y.; Koizumi, Y.; Nakano, T. Equiaxed Grain Formation by Intrinsic Heterogeneous Nucleation via Rapid Heating and Cooling in Additive Manufacturing of Aluminum-Silicon Hypoeutectic Alloy. J. Alloys Compd. 2022, 919, 165812. [Google Scholar] [CrossRef]
- Okugawa, M.; Furushiro, Y.; Koizumi, Y. Effect of Rapid Heating and Cooling Conditions on Microstructure Formation in Powder Bed Fusion of Al-Si Hypoeutectic Alloy: A Phase-Field Study. Materials 2022, 15, 6092. [Google Scholar] [CrossRef]
- Rubacha, K.; Godlewska, E.; Zawadzka, K.; Dąbrowa, J. Formation of Silicide Layers on a Ti-46Al-8Ta Alloy in Pack Cementation and Diffusion Couple Experiments. Surf. Coat. Technol. 2022, 429, 127860. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, B.; Wang, Z.; Liu, Y.; Wang, L.; Zhang, Y.; Li, S. Successive Phase and Morphology Evolution of Galvannealed Coating in Hot Stamping and Diffusion Modeling of α-Fe(Zn)/Steel System Considering the Effect of Zn Concentration. Surf. Coat. Technol. 2019, 380, 125036. [Google Scholar] [CrossRef]
- Pan, C.; Fang, C.; Shi, J.; Ma, K.; Yang, H.; He, P. Synthesis and Properties of Ti-Al Coating by High-Frequency Induction Heated Combustion. J. Alloys Compd. 2023, 939, 168739. [Google Scholar] [CrossRef]
- Koizumi, Y.; Okugawa, M. Digital Twin Science of Metal Powder Bed Fusion Additive Manufacturing: A Selective Review of Simulations for Integrated Computational Materials Engineering and Science. ISIJ Int. 2022, 62, 2183–2196. [Google Scholar] [CrossRef]
- Sato, K.; Takagi, S.; Ichikawa, S.; Ishimoto, T.; Nakano, T. Microstructure and Solute Segregation around the Melt-Pool Boundary of Orientation-Controlled 316L Austenitic Stainless Steel Produced by Laser Powder Bed Fusion. Materials 2023, 16, 218. [Google Scholar] [CrossRef]
- Okugawa, M.; Izumikawa, D.; Koizumi, Y. Simulations of Non-Equilibrium and Equilibrium Segregation in Nickel-Based Superalloy Using Modified Scheil-Gulliver and Phase-Field Methods. Mater. Trans. 2020, 61, 2072–2078. [Google Scholar] [CrossRef]
- Liu, Y.; Nose, K.; Okugawa, M.; Koizumi, Y.; Nakano, T. Fabrication and Process Monitoring of 316L Stainless Steel by Laser Powder Bed Fusion with Μ-Helix Scanning Strategy and Narrow Scanning Line Intervals. Mater. Trans. 2023, 64, 1135–1142. [Google Scholar] [CrossRef]

| Name | Value | |
|---|---|---|
| Composition | NiCl2·6H2O | 0.10–0.13 mol/L |
| Ni(SO3NH2)2 | 1.70–1.79 mol/L | |
| H3BO3 | 0.49–0.73 mol/L | |
| Parameters | Temperature | 50–60 °C |
| Cathodic current density | 2.5–3.0 A/dm2 | |
| pH | 3.0–5.0 |
| Selected Area | Composition (at.%) | |
|---|---|---|
| Ti | Ni | |
| A | 99.80 | 0.20 |
| B | 100.00 | 0 |
| C | 98.22 | 1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Okugawa, M.; Konishi, H.; Liu, Y.; Koizumi, Y.; Nakano, T. Fusion of Ni Plating on CP-Titanium by Electron Beam Single-Track Scanning: Toward a New Approach for Fabricating TiNi Self-Healing Shape Memory Coating. Materials 2023, 16, 5449. https://doi.org/10.3390/ma16155449
Wang L, Okugawa M, Konishi H, Liu Y, Koizumi Y, Nakano T. Fusion of Ni Plating on CP-Titanium by Electron Beam Single-Track Scanning: Toward a New Approach for Fabricating TiNi Self-Healing Shape Memory Coating. Materials. 2023; 16(15):5449. https://doi.org/10.3390/ma16155449
Chicago/Turabian StyleWang, Lei, Masayuki Okugawa, Hirokazu Konishi, Yuheng Liu, Yuichiro Koizumi, and Takayoshi Nakano. 2023. "Fusion of Ni Plating on CP-Titanium by Electron Beam Single-Track Scanning: Toward a New Approach for Fabricating TiNi Self-Healing Shape Memory Coating" Materials 16, no. 15: 5449. https://doi.org/10.3390/ma16155449
APA StyleWang, L., Okugawa, M., Konishi, H., Liu, Y., Koizumi, Y., & Nakano, T. (2023). Fusion of Ni Plating on CP-Titanium by Electron Beam Single-Track Scanning: Toward a New Approach for Fabricating TiNi Self-Healing Shape Memory Coating. Materials, 16(15), 5449. https://doi.org/10.3390/ma16155449








