Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. PEO Process
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussions
3.1. Transient Voltage Response and Nucleation of Plasma Discharges
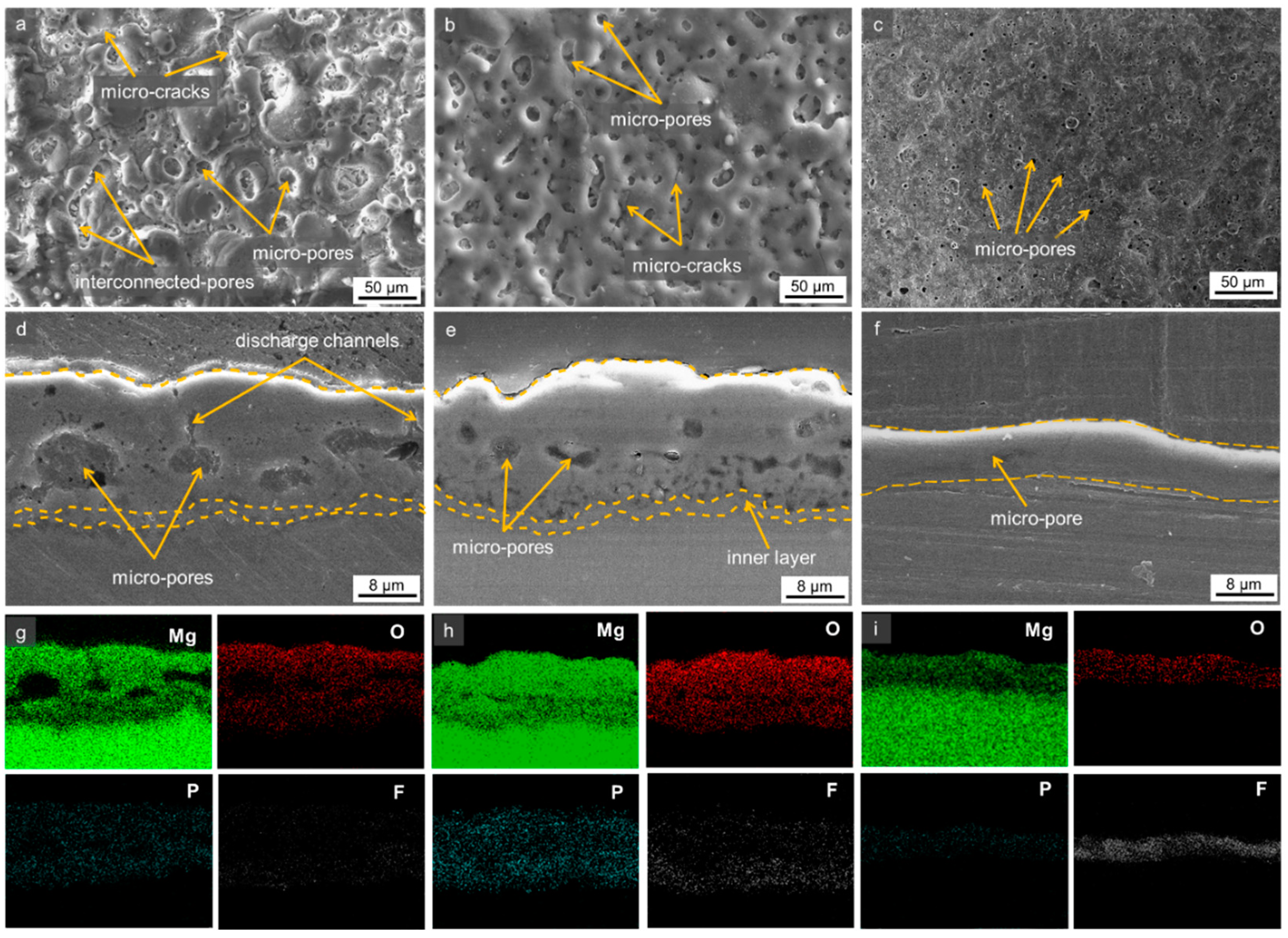
3.2. Morphologies of the Oxide Layer
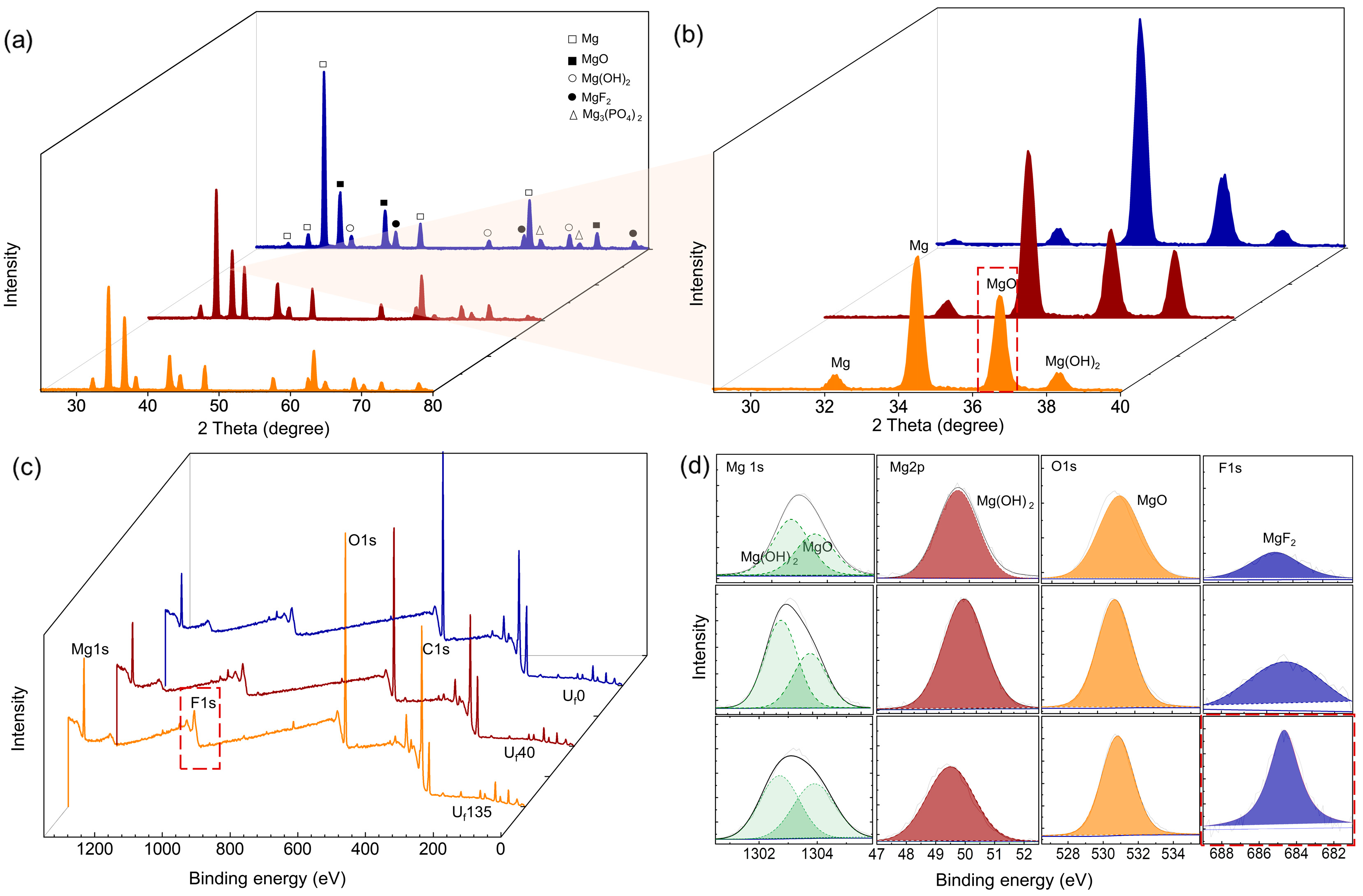
3.3. Compositional Analysis of the Oxide Layer
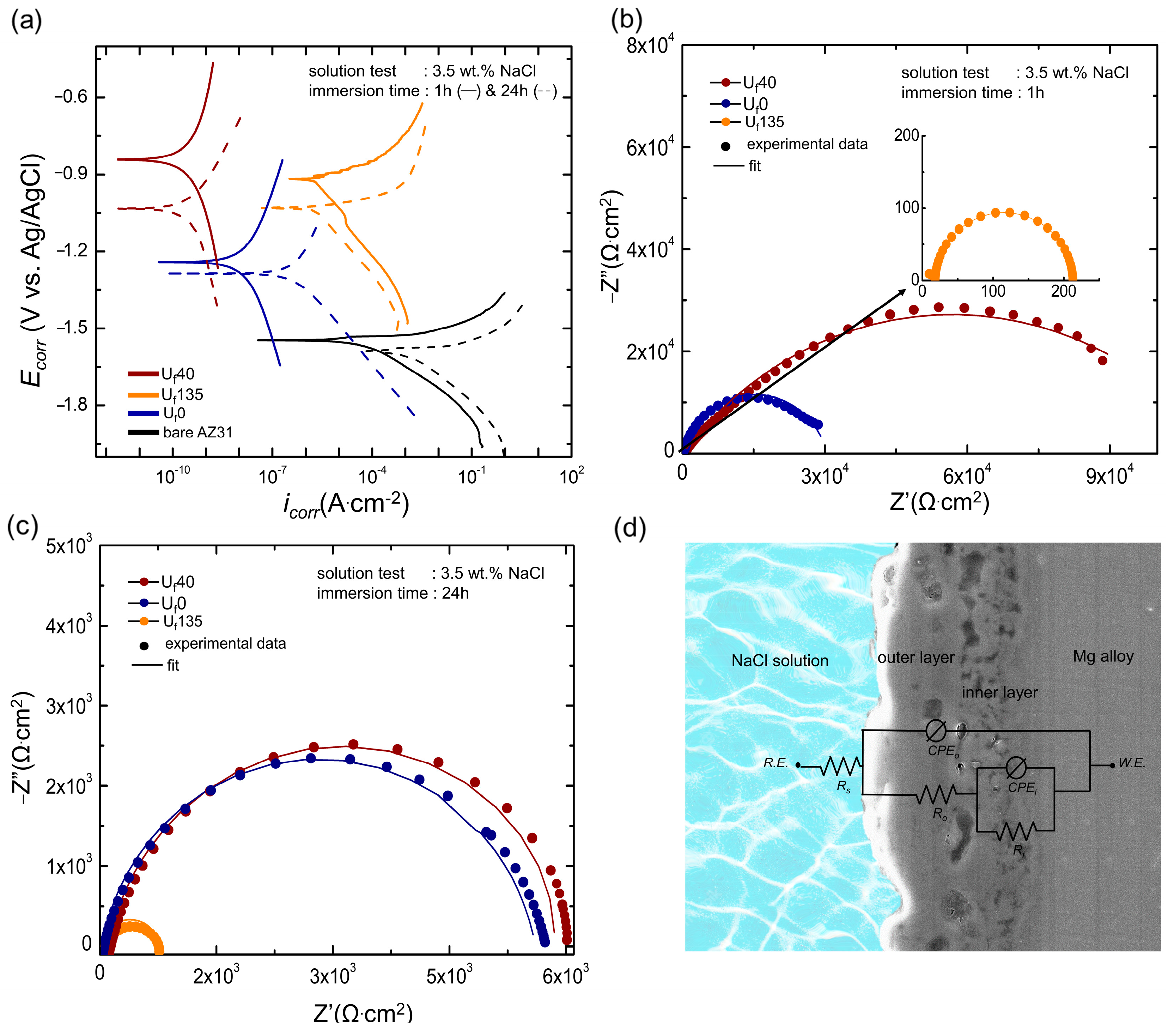
3.4. Corrosion Protection Capabilities of the Oxide Layer
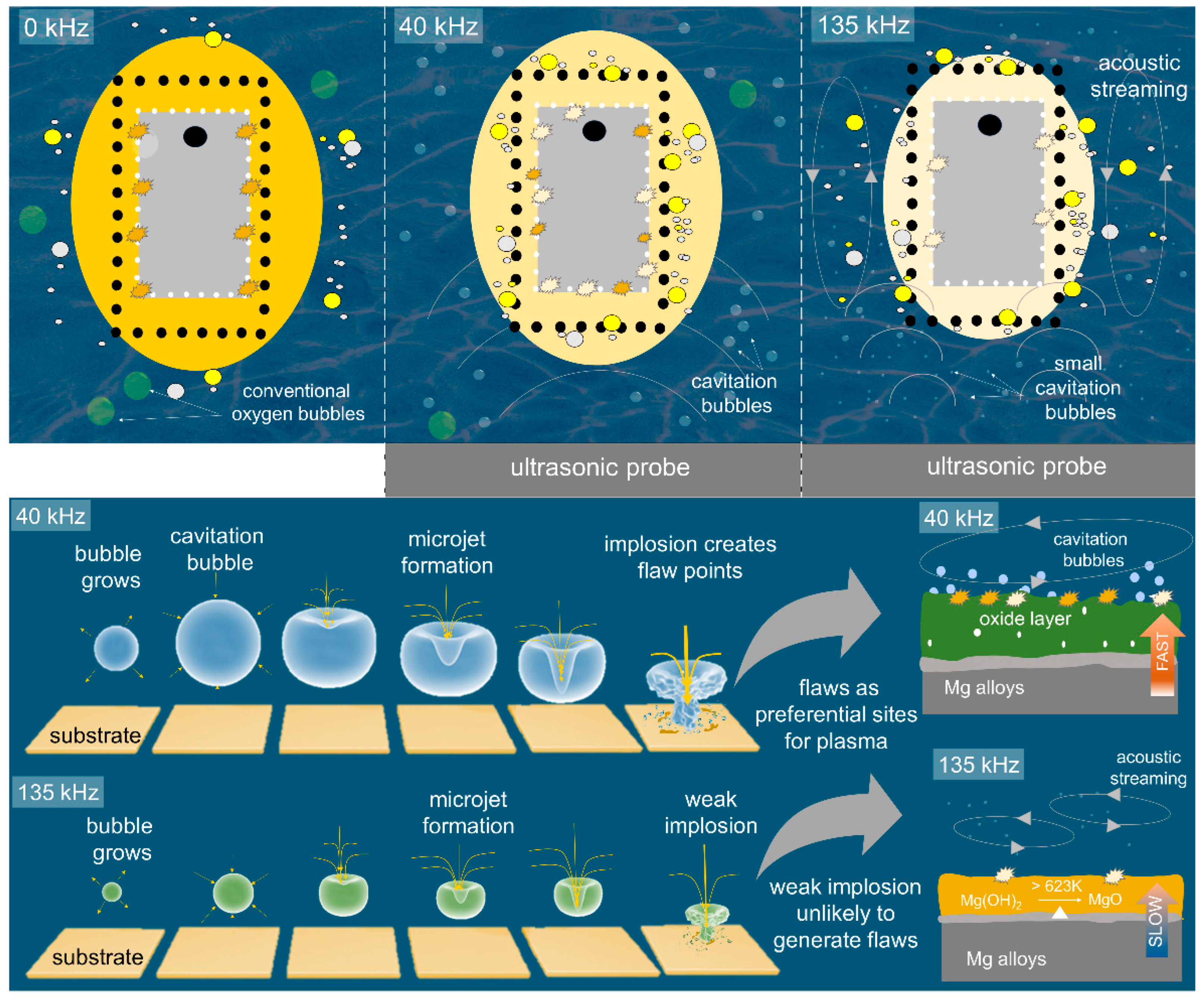
3.5. Formation Mechanism of the Oxide Layer under the Interference of Ultrasonic Wave
4. Conclusions
- Plasma swarm appeared later at a lower voltage when ultrasonic vibration was introduced to the system during PEO due to the presence of collapsing cavitation bubbles which created flaws for the initiation of dielectric breakdown.
- The density of plasma discharges increased while their average size decreased with the use of ultrasonic vibration, thanks to ultrasonic waves which created homogeneous mixing through agitation, acoustic streaming, and cavitation bubbles which led to collision and plasma softening.
- The ultrasonic vibration induced the formation of a dense oxide layer by virtue of softened plasma characteristics and homogeneous incorporation of F− ions throughout coating thickness.
- A trade-off between thickness and compactness appeared when a high ultrasonic frequency of 135 kHz was employed, which induced a decrease in corrosion resistance. While the high frequency allowed for enhanced compactness, it came at the expense of reduced thickness, which in turn had a negative impact on the material’s ability to withstand corrosion.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Microstructural Characteristics of Oxide Layers Formed on Mg—9 Wt % Al—1 Wt % Zn Alloy via Two-Step Plasma Electrolytic Oxidation. J. Alloys Compd. 2014, 615, S418–S422. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Morphological Modification and Corrosion Response of MgO and Mg3(PO4)2 Composite Formed on Magnesium Alloy. Compos. Part B Eng. 2019, 176, 107225. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent Progress in Surface Modification of Metals Coated by Plasma Electrolytic Oxidation: Principle, Structure, and Performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Incorporation of Carbon Nanotubes into Micro-Coatings Film Formed on Aluminum Alloy via Plasma Electrolytic Oxidation. Mater. Lett. 2011, 65, 2269–2273. [Google Scholar] [CrossRef]
- Fatimah, S.; Nashrah, N.; Tekin, K.; Ko, Y.G. Improving Corrosion and Photocatalytic Properties of Composite Oxide Layer Fabricated by Plasma Electrolytic Oxidation with NaAlO2. Materials 2022, 15, 7055. [Google Scholar] [CrossRef]
- Jung, Y.C.; Shin, K.R.; Ko, Y.G.; Shin, D.H. Surface Characteristics and Biological Response of Titanium Oxide Layer Formed via Micro-Arc Oxidation in K3PO4 and Na3PO4 Electrolytes. J. Alloys Compd. 2014, 586, 548–552. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. A Novel Composite System Composed of Zirconia and LDHs Film Grown on Plasma Electrolysis Coating: Toward a Stable Smart Coating. Ultrason. Sonochem. 2018, 49, 316–324. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Belov, E.A.; Imshinetskiy, I.M.; Sinebrukhov, S.L.; Gnedenkov, S.V. Features of Composite Layers Created Using an Aqueous Suspension of a Fluoropolymer. Polymers 2022, 14, 4667. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Mashtalyar, D.V.; Gnedenkov, A.S.; Filonina, V.S.; Vyaliy, I.E.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Izotov, N.V.; Sinebryukhov, S.L.; et al. Icephobic Performance of Combined Fluorine-containing Composite Layers on Al-mg-mn–Si Alloy Surface. Polymers 2021, 13, 3827. [Google Scholar] [CrossRef]
- Pezzato, L.; Colusso, E.; Cerchier, P.; Settimi, A.G.; Brunelli, K. Production and Characterization of Photocatalytic PEO Coatings Containing TiO2 Powders Recovered from Wastes. Coatings 2023, 13, 411. [Google Scholar] [CrossRef]
- Costa, J.M.; Almeida Neto, A.F. de Ultrasound-Assisted Electrodeposition and Synthesis of Alloys and Composite Materials: A Review. Ultrason. Sonochem. 2020, 68, 105193. [Google Scholar] [CrossRef] [PubMed]
- Zempoalteca, A.L.; David, Á.; Flores, A.L.; Luna, A.; Ben, A. MnPc Films Deposited by Ultrasonic Spray Pyrolysis at Low Temperatures: Optical, Morphological and Structural Properties. Materials 2023, 16, 4357. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.; Mohd Nawi, M.N.; Othuman Mydin, M.A.; Sari, M.W.; Ahmad, R.; Abdullah, M.M.A.B. The Significant Effect of Mechanical Treatment on Ceramic Coating for Biomedical Application. Materials 2022, 15, 6550. [Google Scholar] [CrossRef] [PubMed]
- Fathyunes, L.; Khalili, V. Effect of Ultrasonic Waves on the Electrochemical Deposition of Calcium Phosphate/Nano-Sized Silica Composite Coating. J. Mater. Res. Technol. 2021, 14, 2345–2356. [Google Scholar] [CrossRef]
- Kamali, M.; Costa, M.E.V.; Otero-Irurueta, G.; Capela, I. Ultrasonic Irradiation as a Green Production Route for Coupling Crystallinity and High Specific Surface Area in Iron Nanomaterials. J. Clean. Prod. 2019, 211, 185–197. [Google Scholar] [CrossRef]
- Shen, D.; He, D.; Liu, F.; Guo, C.; Cai, J.; Li, G.; Ma, H. Effects of Ultrasound on the Evolution of Plasma Electrolytic Oxidation Process on 6061Al Alloy. Ultrasonics 2014, 54, 1065–1070. [Google Scholar] [CrossRef]
- Ajiriyanto, M.K.; Anawati, A. Ultrasonication Assisted Plasma Electrolytic Oxidation Accelerated Growth of SiO2/ZrO2 Coating on Zircaloy-4. Surf. Coat. Technol. 2023, 456, 129261. [Google Scholar] [CrossRef]
- Kaseem, M.; Yang, H.W.; Ko, Y.G. Toward a Nearly Defect-Free Coating via High-Energy Plasma Sparks. Sci. Rep. 2017, 7, 2378. [Google Scholar] [CrossRef]
- Kazanski, B.; Kossenko, A.; Zinigrad, M.; Lugovskoy, A. Fluoride Ions as Modifiers of the Oxide Layer Produced by Plasma Electrolytic Oxidation on AZ91D Magnesium Alloy. Appl. Surf. Sci. 2013, 287, 461–466. [Google Scholar] [CrossRef]
- Kropman, D.; Dolgov, S.; Onufrijevs, P.; Dauksta, E. Dedicated to the Memory of Prof. M. Sheinkman Effect of Ultrasonic Treatment on the Defect Structure of the Si-SiO2 System. Solid State Phenom. 2014, 205–206, 352–357. [Google Scholar] [CrossRef]
- Li, M.; Ji, H.; Wang, C.; Bang, H.S.; Bang, H.S. Interdiffusion of Al-Ni System Enhanced by Ultrasonic Vibration at Ambient Temperature. Ultrasonics 2006, 45, 61–65. [Google Scholar] [CrossRef]
- Jalal, J.; Leong, T.S.H. Microstreaming and Its Role in Applications: A Mini-Review. Fluids 2018, 3, 93. [Google Scholar] [CrossRef]
- Dejiu, S.; Jingrui, C.; Guolong, L.; Donglei, H.; Lailei, W.; Haojie, M.; Yonghong, X.; He, C.; Yaqian, Y. Effect of Ultrasonic on Microstructure and Growth Characteristics of Micro-Arc Oxidation Ceramic Coatings on 6061 Aluminum Alloy. Vacuum 2014, 99, 143–148. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.P.; Han, D.I.; Al-Zoubi, W.; Ko, Y.G. Development of Anti-Corrosive Coating on AZ31 Mg Alloy Subjected to Plasma Electrolytic Oxidation at Sub-Zero Temperature. J. Magnes. Alloys 2022, 10, 1915–1929. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft Plasma Electrolysis with Complex Ions for Optimizing Electrochemical Performance. Sci. Rep. 2017, 7, 44458. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G. Dual Incorporation of SiO2 and ZrO2 Nanoparticles into the Oxide Layer on 6061 Al Alloy via Plasma Electrolytic Oxidation: Coating Structure and Corrosion Properties. J. Alloys Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Cao, G.; Konishi, H.; Li, X. Mechanical Properties and Microstructure of SiC-Reinforced Mg-(2,4)Al-1Si Nanocomposites Fabricated by Ultrasonic Cavitation Based Solidification Processing. Mater. Sci. Eng. A 2008, 486, 357–362. [Google Scholar] [CrossRef]
- Kerboua, K.; Merouani, S.; Hamdaoui, O.; Alghyamah, A.; Islam, M.H.; Hansen, H.E.; Pollet, B.G. How Do Dissolved Gases Affect the Sonochemical Process of Hydrogen Production? An Overview of Thermodynamic and Mechanistic Effects—On the “Hot Spot Theory”. Ultrason. Sonochem. 2021, 72, 105422. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 559. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Du, Y.; Zhang, W.; Cao, H. Correlations between the Growth Mechanism and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on AZ31B Magnesium Alloy. Int. J. Electrochem. Sci. 2019, 14, 11465–11466. [Google Scholar] [CrossRef]
- Ko, Y.G.; Namgung, S.; Shin, D.H. Correlation between KOH Concentration and Surface Properties of AZ91 Magnesium Alloy Coated by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2010, 205, 2525–2531. [Google Scholar] [CrossRef]
- Hoshikawa, T.; Yamada, M.; Kikuchi, R.; Eguchi, K. Impedance Analysis for Dye-Sensitized Solar Cells with a Three-Electrode System. J. Electroanal. Chem. 2005, 577, 339–348. [Google Scholar] [CrossRef]
- Fatimah, S.; Yang, H.W.; Kamil, M.P.; Ko, Y.G. Control of Surface Plasma Discharge Considering the Crystalline Size of Al Substrate. Appl. Surf. Sci. 2019, 477, 60–70. [Google Scholar] [CrossRef]
- Cooper, E.L.; Coury, L.A. Mass Transport in Sonovoltammetry with Evidence of Hydrodynamic Modulation from Ultrasound. J. Electrochem. Soc. 1998, 145, 1994–1999. [Google Scholar] [CrossRef]
- Dang, F.; Enomoto, N.; Hojo, J.; Enpuku, K. Sonochemical Coating of Magnetite Nanoparticles with Silica. Ultrason. Sonochem. 2010, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Atobe, M.; Yamada, N.; Fuchigami, T.; Nonaka, T. Ultrasonic Effects on Electroorganic Processes—Part 24. Rate and Saturated Amount of the Self-Assembled Adsorption of Thiolate Species on a Gold Surface. Electrochim. Acta 2003, 48, 1759–1766. [Google Scholar] [CrossRef]
- Lucas, V.S.; Burk, R.S.; Creehan, S.; Grap, M.J. Utility of High-Frequency Ultrasound: Moving Beyond the Surface. Plast. Surg. Nurse 2014, 34, 34. [Google Scholar] [CrossRef]
- Poulain, S.; Guenoun, G.; Gart, S.; Crowe, W.; Jung, S. Particle Motion Induced by Bubble Cavitation. Phys. Rev. Lett. 2015, 114, 214501. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O.; Alghyamah, A. A Comprehensive Numerical Analysis of Heat and Mass Transfer Phenomenons during Cavitation Sono-Process. Ultrason. Sonochem. 2021, 73, 105498. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yasui, K.; Zhu, T.; Ashokkumar, M. A Model for the Effect of Bulk Liquid Viscosity on Cavitation Bubble Dynamics. Phys. Chem. Chem. Phys. 2017, 19, 20635–20640. [Google Scholar] [CrossRef]
- Wu, G.R.; Wang, D.D.; Liu, X.T.; Wang, Y.; Chen, D.; Wang, M.; Shen, D. Effects of Ultrasound on the Microstructure and Corrosion Behaviour of a PEO Coating. Int. J. Electrochem. Sci. 2019, 14, 11312–11324. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Madhavan, J.; Lee, S.J.; Choi, M.Y.; Ashokkumar, M.; Pollet, B.G. Sonoelectrochemistry for Energy and Environmental Applications. Ultrason. Sonochem. 2020, 63, 104960. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, D.; Bond, L.J. Ultrasonics: Fundamentals, Technologies, and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781420020274. [Google Scholar]

| Sample | Mg (wt.%) | O (wt.%) | P (wt.%) | F (wt.%) |
|---|---|---|---|---|
| Uf0 | 23.26 ± 1.30 | 68.28 ± 1.80 | 5.41 ± 1.33 | 3.05 ± 0.53 |
| Uf40 | 22.39 ± 1.14 | 68.79 ± 1.22 | 5.42 ± 0.91 | 3.40 ± 0.61 |
| Uf135 | 21.10 ± 0.51 | 69.98 ± 0.73 | 2.74 ± 0.40 | 6.18 ± 0.22 |
| Sample | Plasma Intensity | Microdefects | Passivation Layer | ||||
|---|---|---|---|---|---|---|---|
| Pores Size (Μm) | Porosity (%) | Cracks | Thickness (Μm) | Growth Rate (Nm/S) | Percentage of F Particles | ||
| Uf0 | bright | 6.25 ± 0.72 | 19.38 | many | 15.10 ± 0.53 | 50.34 ± 0.52 | low |
| Uf40 | moderate | 4.69 ± 0.54 | 14.83 | few | 15.41 ± 0.33 | 51.30 ± 0.31 | medium |
| Uf135 | dim | 2.01 ± 0.21 | 2.05 | scarce | 7.32 ± 0.24 | 24.33 ± 0.12 | high |
| Immersion Time | Sample | icorr (A·cm2) | Ecorr (V) | βa (V/Decade) | βc (V/Decade) | Rp (Ωcm2) |
|---|---|---|---|---|---|---|
| 1 h | bare AZ31 | 1.20 × 10−5 | −1.51 | −0.05 | 0.16 | 1.36 × 103 |
| Uf0 | 2.39 × 10−8 | −1.24 | −0.43 | 0.47 | 4.06 × 106 | |
| Uf40 | 1.26 × 10−9 | −0.84 | −0.11 | 0.15 | 2.24 × 107 | |
| Uf135 | 2.80 × 10−6 | −1.00 | −0.04 | 0.19 | 5.34 × 103 | |
| 24 h | bare AZ31 | 5.25 × 10−3 | −1.54 | −0.04 | 0.12 | 2.62 × 101 |
| Uf0 | 2.48 × 10−7 | −1.29 | −0.31 | 0.16 | 1.85 × 105 | |
| Uf40 | 5.47 × 10−10 | −1.03 | −0.27 | 0.63 | 1.51 × 108 | |
| Uf135 | 2.40 × 10−5 | −1.12 | −0.02 | 0.16 | 3.75 × 102 |
| Immersion Time | Sample | Rs (Ω·cm2) | Ro (Ω·cm2) | Ri (Ω·cm2) | no | CPEo (S.sn.cm−2) | ni | CPEi (S.sn.cm−2) |
|---|---|---|---|---|---|---|---|---|
| 1 h | Uf0 | 17.32 | 3.94 × 103 | 3.35 × 104 | 0.58 | 4.17 × 10−6 | 0.87 | 3.81 × 10−6 |
| Uf40 | 19.50 | 1.35 × 105 | 1.14 × 105 | 0.56 | 1.99 × 10−8 | 0.85 | 3.84 × 10−6 | |
| Uf135 | 18.31 | 1.77 × 102 | 2.13 × 102 | 0.95 | 9.62 × 10−9 | 0.99 | 4.87 × 10−5 | |
| 24 h | Uf0 | 19.74 | 5.58 × 103 | 1.50 × 104 | 0.73 | 8.24 × 10−3 | 0.95 | 8.43 × 10−7 |
| Uf40 | 20.23 | 1.06 × 105 | 1.05 × 105 | 0.87 | 3.73 × 10−7 | 0.96 | 5.91 × 10−5 | |
| Uf135 | 22.50 | 2.03 × 102 | 5.36 × 102 | 0.92 | 1.03 × 10−5 | 0.98 | 1.70 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimah, S.; Hazmatulhaq, F.; Sheng, Y.; Suhartono, T.; Oh, J.M.; Nashrah, N.; Kang, J.-H.; Ko, Y.G. Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation. Materials 2023, 16, 5424. https://doi.org/10.3390/ma16155424
Fatimah S, Hazmatulhaq F, Sheng Y, Suhartono T, Oh JM, Nashrah N, Kang J-H, Ko YG. Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation. Materials. 2023; 16(15):5424. https://doi.org/10.3390/ma16155424
Chicago/Turabian StyleFatimah, Siti, Farah Hazmatulhaq, Yujun Sheng, Tri Suhartono, Jeong Moo Oh, Nisa Nashrah, Jee-Hyun Kang, and Young Gun Ko. 2023. "Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation" Materials 16, no. 15: 5424. https://doi.org/10.3390/ma16155424
APA StyleFatimah, S., Hazmatulhaq, F., Sheng, Y., Suhartono, T., Oh, J. M., Nashrah, N., Kang, J.-H., & Ko, Y. G. (2023). Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation. Materials, 16(15), 5424. https://doi.org/10.3390/ma16155424








