Extra-Articular Distal Humerus Plate 3D Model Creation by Using the Method of Anatomical Features
Abstract
1. Introduction
The Proposed Solution for Creating a 3D Model of the Plate
2. Materials and Methods
- 3D Geometrical Models: These models, which have been used in Computer-Aided Design (CAD) for many years, encompass standard polygonal, surface, and volume models. CAD software (CATIA V5) package was employed to create these models by applying conventional CAD technical features.
- Predictive (Parametric) Models: By utilizing morphometric and other readable parameters obtained from medical imaging techniques, MAF can adjust these models to match the geometry and morphology of a specific patient’s bone. These models are based on input data acquired from medical imaging methods such as CT or MRI.
2.1. Creation of PPI LCP Models
- The Curve Based Method (CBM) that utilized commonly used geometric elements (spline segments) as parametrized geometry. The bone model was then employed as a deformable template model through the application of Free-Form Deformation (FFD) techniques.
- The Subdivision Surface-Based Method (SDBM) and Regions of Interest (ROI) were employed to define a control mesh, acting as the parameterized grid. By adjusting the vertices and normals of the control mesh polygons, the SubD surface was capable of conforming to the underlying bone template model.
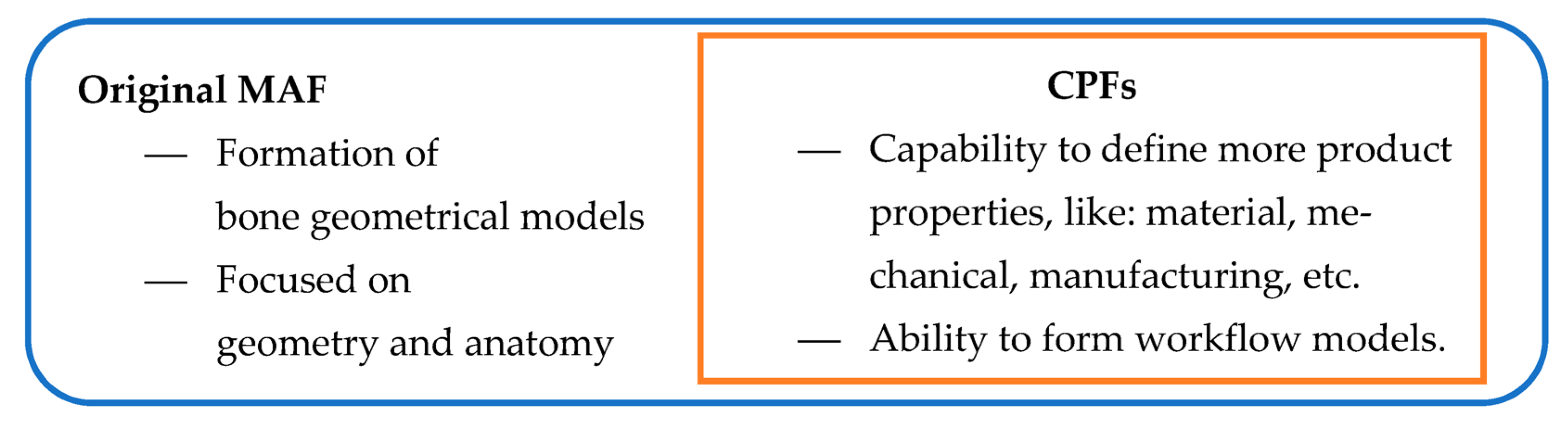
- The CPF creation method—The produced FM model is based on the additional product characteristics/properties, not just geometrical and anatomical. The additional properties are defined as parameters, including shape modification, geometry and topology optimization, material selection, and manufacturing process definition. To describe and define additional properties, different descriptive elements can be used. For example, if we need to impose some limitations to plate manufacturing, a simple programming script can be added to the programming routines. This programming script can be used as a control mechanism.
- (a)
- The material model, closely related to the manufacturing model, enables the definition of adequate material for plate production using additive manufacturing.
- (b)
- The production capability reflects the possibility of plate model manufacturing. In this study, additive manufacturing was chosen as the primary methodology for producing PPI. Additional manufacturing technologies, such as cutting or forging, will be considered in future work.
- (c)
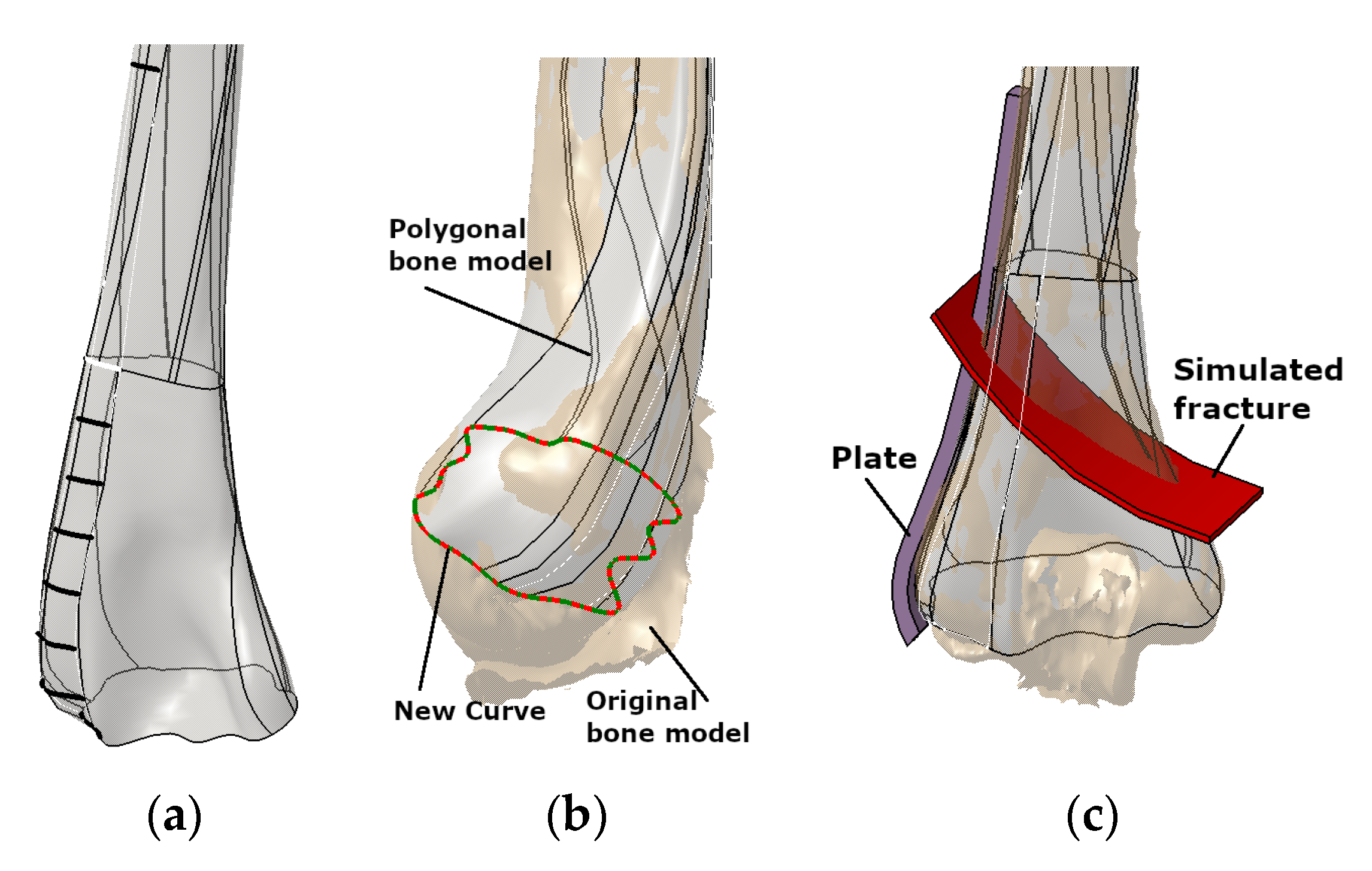
- The improved geometrical model includes additional ROI points to enable better capability for shape modification. The original models [1] were accurate enough, but extra ROI points were selected to improve model capability for geometrical/shape adaptation to the specific bone. This FM feature is very useful in pre-operative surgery simulation [6,7].
- (d)
- The formation of the workflow system for the application of PPI in clinical or other cases (e.g., in medical education). This workflow model is presented for the optimized parametric model application.
2.2. The Improved Curve Based and Subdivision Surface-Based Methodologies
2.3. CPF Methodology for the Creation of Additional Models and Process Workflow
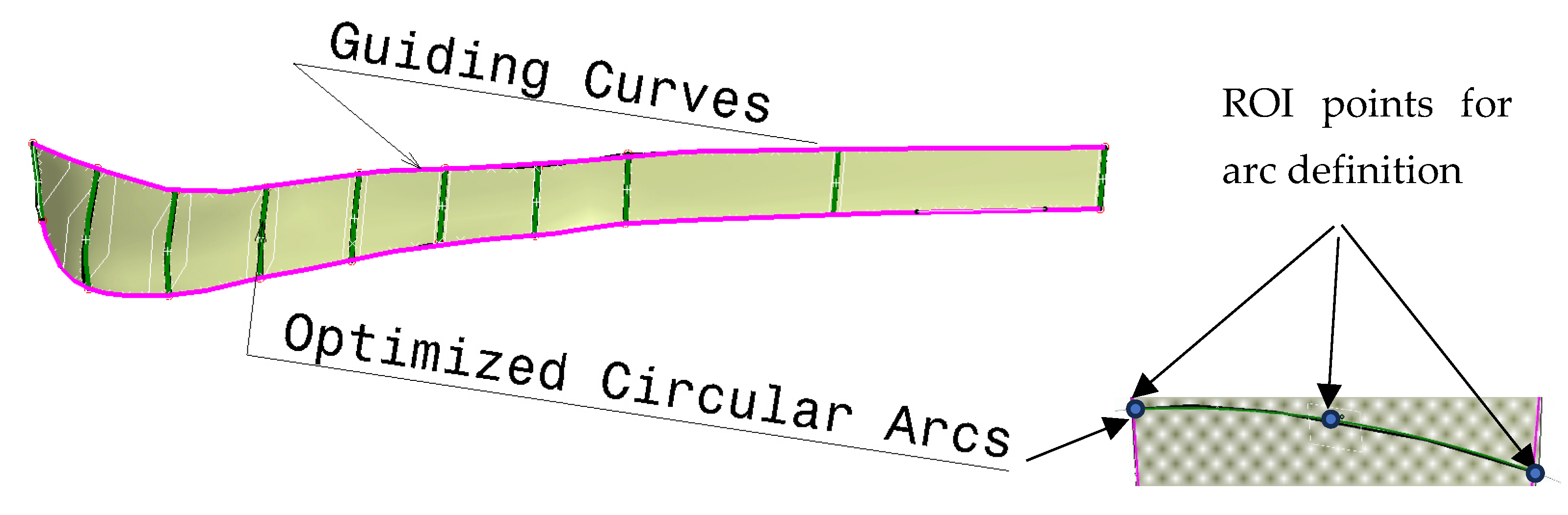
- Creation of the optimization curves that can be easily adapted. Circular arcs are used as the simple geometrical element defined by three points: Left max, Right max, and Middle point of the corresponding spline curves (used in first two methods). According to the previous testing, nine cross-sectional curves provide the plate model with enough accuracy, so nine circular arcs are defined. These three points are specific ROIs defined on each spline, as shown in Figure 5.
- The definition of the left and right boundary guiding curves going through left and right maximum points on each spline curve, as shown in Figure 5.
- Definition of the angles of the circular arcs. An important parameter for the easier control of the plate width in cases when boundary-guiding curve application cannot produce a manifold model, or medical images do not provide enough data.
- Definition of the circular arc center distance to the bone anatomical axis as parameters [7], as shown in Figure 6. This is necessary for cases where there is only one X-ray image available. By using these distances and arc angles, a less accurate plate model can be created, but it is still usable for additional processing.
- Formation of the matrix that includes ROI points for each parametric curve (circular arc) and for the left and right guiding curve (1)—OptROI.
- Formation of the vector of circular arc angles for each section (1)—Optangles
- Formation of the vector of arc middle point distances to the anatomical axes (1)—Optdist
- Definition of additional or supporting distances for better calculation of plate geometry. Supporting distances are measured for the left and right circular arc point, and they are defined as normal distances from these points to the anatomical axes in the AP (Anterior–Posterior) plane (1)—OptSdistl/r (d1l/r means d1l (left) and d1r (right) distances to points—these distances form two vectors).
- Parameter that defines the thickness of the plate solid model. This parameter is defined according to the physician specifications, and usually the value is 2 mm, but it can be set up as required.

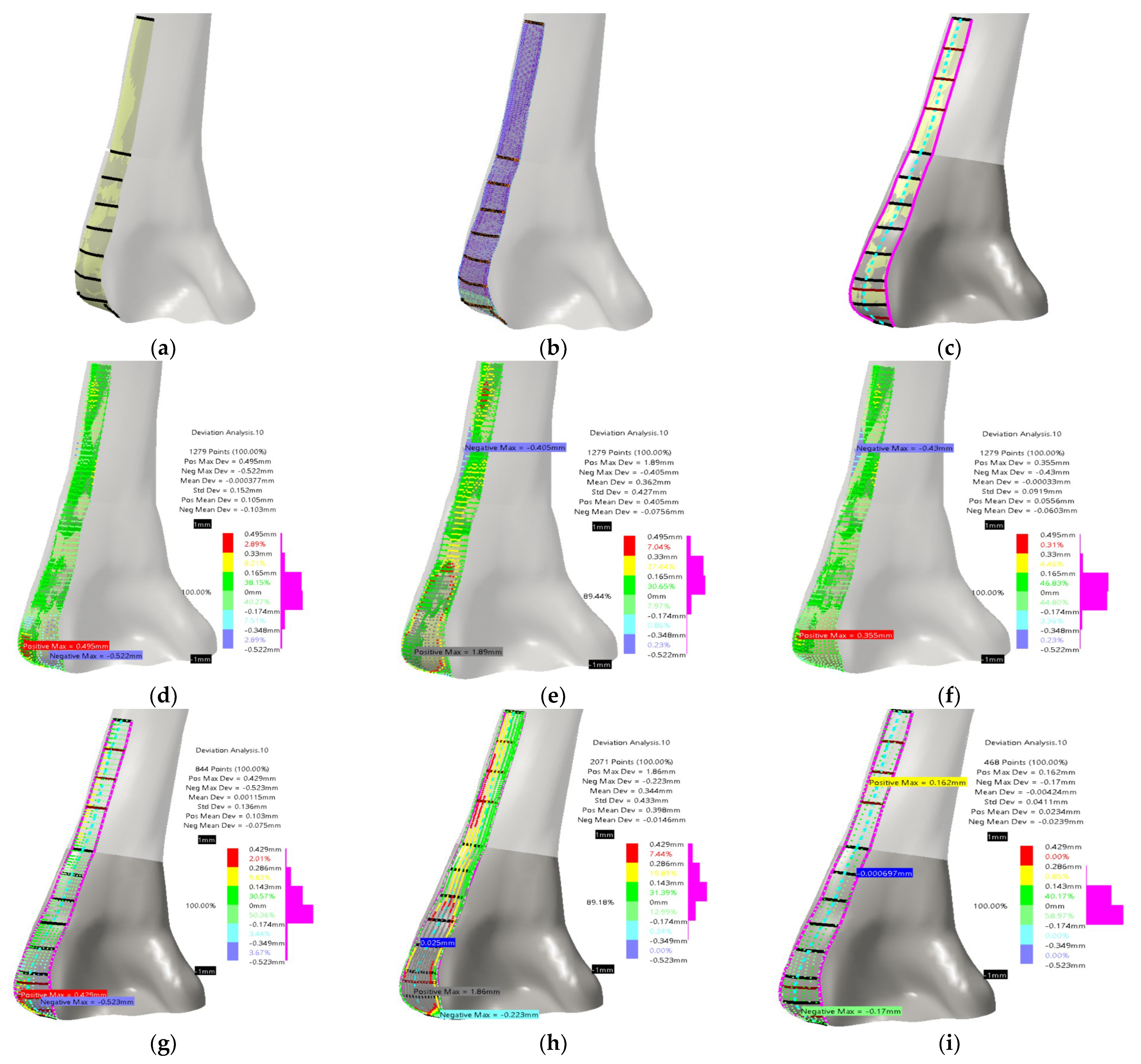
- The defined PPI model was adapted (scaled) to fit the dimensions of the scanned bone.
- Instead of etalon, the FFD bone model with known dimensions was scaled to the X-ray image in the AP (Anterior–Posterior) plane. For the initial testing, this is quite satisfactory.
- The ROI points were collected, and distances were calculated.
- The angles for circular arcs were applied to define curves lengths.
- The personalized plate surface model was created.
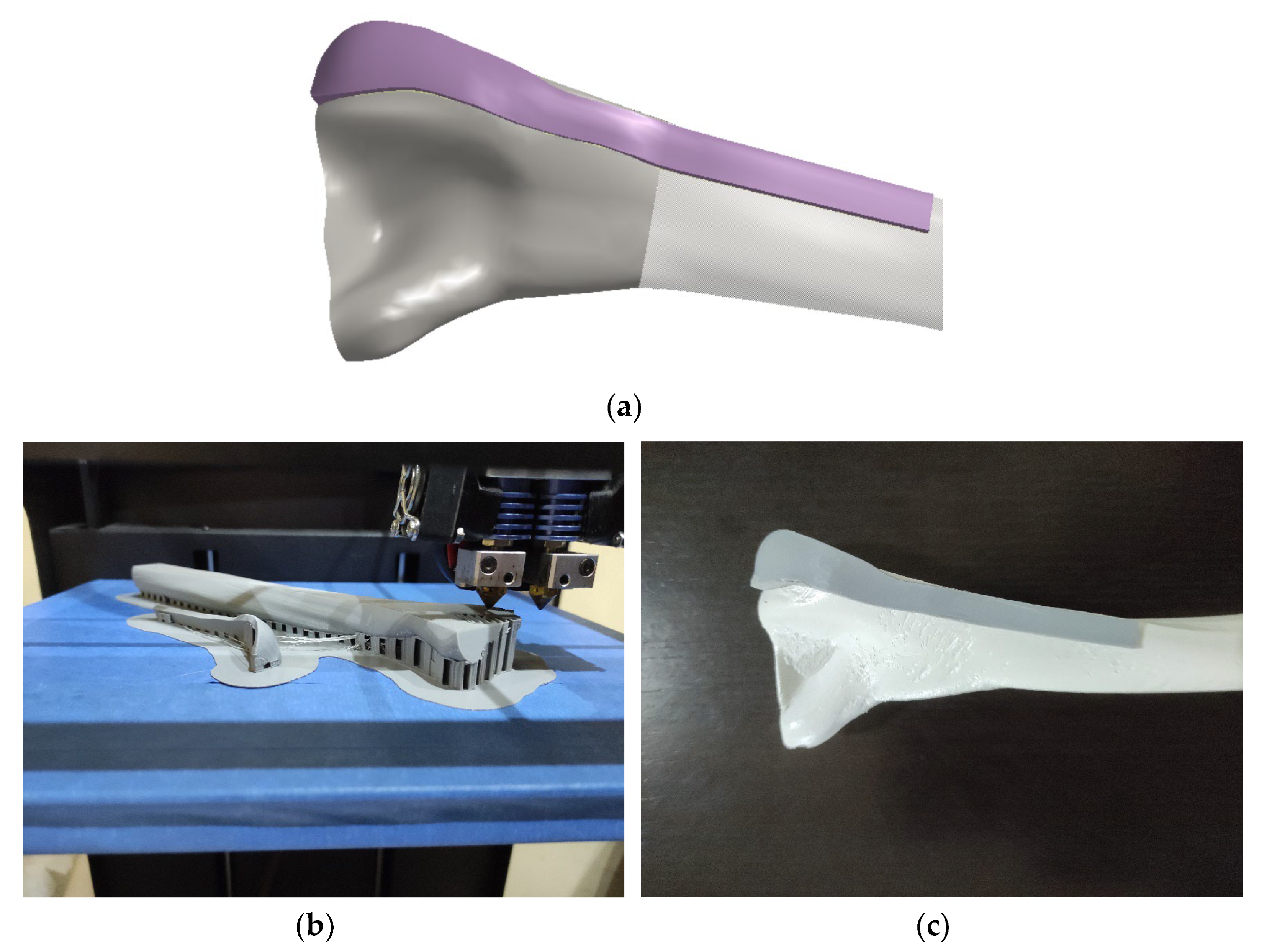
- A thickness of 2 mm was added and plate model was created, as shown in Figure 7a.
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitković, N.; Trajanović, M.; Aranđelović, J.; Păcurar, R.; Borzan, C. Contact Surface Model Parameterization of the Ex-tra-Articular Distal Humerus Plate. In Advances in Manufacturing III. MANUFACTURING 2022; Gorski, F., Rychlik, M., Păcurar, R., Eds.; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Hadeed, A.; Werntz, R.L.; Varacallo, M. External Fixation Principles and Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547694/ (accessed on 15 June 2023).
- Lotzien, S.; Hoberg, C.; Rausch, V.; Rosteius, T.; Schildhauer, T.; Gessmann, J. Open reduction and internal fixation of humeral midshaft fractures: Anterior versus posterior plate fixation. BMC Musculoskelet. Disord. 2019, 20, 527. [Google Scholar] [CrossRef] [PubMed]
- Amer, K.M.; Kurland, A.M.; Smith, B.; Abdo, Z.; Amer, R.; Vosbikian, M.M.; Ahmed, I.H. Intramedullary Nailing Versus Plate Fixation for Humeral Shaft Fractures: A Systematic Review and Meta-Analysis. Arch. Bone Jt. Surg. 2022, 10, 661–667. [Google Scholar] [CrossRef]
- Musuvathy, S.; Azernikov, S.; Fang, T. Semi-automatic customization of internal fracture fixation plates. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Boston, MA, USA, 30 August–3 September 2011; pp. 595–598. [Google Scholar] [CrossRef]
- Vitkovic, N.; Radovic, L.; Trajanovic, M.; Manic, M. 3d point cloud model of human bio form created by the application of geometric morphometrics and method of anatomical features: Human tibia example. Filomat 2019, 33, 1217–1225. [Google Scholar] [CrossRef]
- Rashid, M.M.; Husain, K.N.; Vitković, N.; Manić, M.; Trajanović, M.; Mitković, M.B.; Mitković, M.M. Geometrical Model Creation Methods for Human Humerus Bone and Modified Cloverleaf Plate. J. Sci. Ind. Res. 2017, 76, 631–639. [Google Scholar]
- Filippi, S.; Motyl, B.; Bandera, C. Analysis of existing methods for 3D modelling of femurs starting from two orthogonal images and development of a script for a commercial software package. Comput. Methods Programs Biomed. 2008, 89, 76–82. [Google Scholar] [CrossRef]
- Chenna, Y.N.D.; Ghassemi, P.; Pfefer, T.J.; Casamento, J.; Wang, Q. Free-Form Deformation Approach for Registration of Visible and Infrared Facial Images in Fever Screening. Sensors 2018, 18, 125. [Google Scholar] [CrossRef]
- Nolte, D.; Xie, S.; Bull, A.M.J. 3D shape reconstruction of the femur from planar X-ray images using statistical shape and appearance models. Biomed. Eng. Online 2023, 22, 30. [Google Scholar] [CrossRef]
- Nolte, D.; Ko, S.-T.; Bull, A.M.; Kedgley, A.E. Reconstruction of the lower limb bones from digitised anatomical landmarks using statistical shape modelling. Gait Posture 2020, 77, 269–275. [Google Scholar] [CrossRef]
- Čavojská, J.; Petrasch, J.; Mattern, D.; Lehmann, N.J.; Voisard, A.; Böttcher, P. Estimating and abstracting the 3D structure of feline bones using neural networks on X-ray (2D) images. Commun. Biol. 2020, 3, 337. [Google Scholar] [CrossRef]
- Shiode, R.; Kabashima, M.; Hiasa, Y.; Oka, K.; Murase, T.; Sato, Y.; Otake, Y. 2D–3D reconstruction of distal forearm bone from actual X-ray images of the wrist using convolutional neural networks. Sci. Rep. 2021, 11, 15249. [Google Scholar] [CrossRef]
- Khalid, M.U.; Saeed, K.M.; Javed, M.B.; Akhtar, M.; Gillani, S.F.U.H.S. Comparison of locking compression plate and dynamic compression plate with cancellous bone graft in treating non-union of humeral shaft fractures. Ann. King Edw. Med. Univ. Lahore Pak. 2019, 25, 910–915. [Google Scholar]
- Null, N. LCP Versus LISS in the Treatment of Open and Closed Distal Femur Fractures: Does it Make a Difference? J. Orthop. Trauma 2016, 30, e212–e216. [Google Scholar] [CrossRef]
- Koch, D.W.; Johnson, J.W.; Smith, Q.E.; Brekhus, C.; Gadomski, B.C.; Palmer, R.H.; Easley, J.T.; Nelson, B.B. Biomechanical evaluation of interlocking nail and locking compression plating for stabilization of ovine critical-sized segmental tibia defects. Ann. Transl. Med. 2023, 11, 258. [Google Scholar] [CrossRef]
- Zha, Y.; Hua, K.; Huan, Y.; Chen, C.; Sun, W.; Ji, S.; Xiao, D.; Gong, M.; Jiang, X. Biomechanical comparison of three internal fixation configurations for low transcondylar fractures of the distal humerus. Injury 2022, 54, 362–369. [Google Scholar] [CrossRef]
- Ziegler, P.; Maier, S.; Stöckle, U.; Gühring, M.; Stuby, F.M. The Treatment of Proximal Humerus Fracture Using Internal Fixation with Fixed-angle Plates. Dtsch. Ärzteblatt Int. 2019, 116, 757–763. [Google Scholar] [CrossRef]
- Rashid, M.; Husain, K.; Vitković, N.; Manić, M.; Petrović, S. Towards patient specific plate implants for the human long bones: A distal humerus example. Facta Univ. Series: Mech. Eng. 2018, 16, 347–357. [Google Scholar] [CrossRef]
- Walsh, S.; Reindl, R.; Harvey, E.; Berry, G.; Beckman, L.; Steffen, T. Biomechanical comparison of a unique locking plate versus a standard plate for internal fixation of proximal humerus fractures in a cadaveric model. Clin. Biomech. 2006, 21, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Omid, R.; Trasolini, N.A.; Stone, M.A.; Namdari, S. Principles of Locking Plate Fixation of Proximal Humerus Fractures. J. Am. Acad. Orthop. Surg. 2021, 29, e523–e535. [Google Scholar] [CrossRef]
- Lim, J.; Yoon, T.; Choi, Y.; Lee, H.; Chun, Y. Biomechanical evaluation of a modified proximal humeral locking plate application for distal extra-articular diaphyseal humeral fractures. J. Orthop. Res. 2020, 39, 1877–1883. [Google Scholar] [CrossRef]
- Bégué, T. Articular fractures of the distal humerus. Orthop. Traumatol. Surg. Res. 2014, 100, S55–S63. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Bhashyam, R.A. Concise Review on Surgical Fixation of Distal Humerus Fractures. Orthop. J. Harv. Med. Sch. 2020, 21, 68–72. [Google Scholar]
- AO Foundation. Plate fixation and Open Reduction. Available online: https://www.aofoundation.org/ (accessed on 1 July 2023).
- Scolaro, J.A.; Hsu, J.E.; Svach, D.J.; Mehta, S. Plate selection for fixation of extra-articular distal humerus fractures: A biomechanical comparison of three different implants. Injury 2014, 45, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Pilliar, R.M. Metallic Biomaterials. In Biomedical Materials; Narayan, R., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Jahan, A.; Edwards, K.L. A state-of-the-art survey on the influence of normalization techniques in ranking: Improving the materials selection process in engineering design. Mater. Des. 2015, 65, 335–342. [Google Scholar] [CrossRef]
- Nomura, N. Artificial organs: Recent progress in metals and ceramics. J. Artif. Organs 2010, 13, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Vitković, N.; Korunović, N.; Aranđelović, J.; Miltenović, A.; Perić, M. Remodeling of complex surface patches by using the method of characteristic features—The ski shoe heel lip example. Innov. Mech. Eng. 2022, 1, 96–105. [Google Scholar]
- Osiris, C.J.; Trajanovic, M. Personalized Orthopedics; Springer: Cham, Switzerland, 2023; 553p, ISBN 978-3-030-98278-2. [Google Scholar] [CrossRef]
- AO Surgical Reference. Available online: https://surgeryreference.aofoundation.org/orthopedic-trauma/adult-trauma/distal-humerus/extraarticular-simple-oblique/orif-lag-screw-with-neutralization-plate (accessed on 1 July 2023).
- Scolaro, J.A.; Voleti, P.; Makani, A.; Namdari, S.; Mirza, A.; Mehta, S. Surgical fixation of extra-articular distal humerus fractures with a posterolateral plate through a triceps-reflecting technique. J. Shoulder Elb. Surg. 2013, 23, 251–257. [Google Scholar] [CrossRef]
- Maszybrocka, J.; Dworak, M.; Nowakowska, G.; Osak, P.; Łosiewicz, B. The Influence of the Gradient Infill of PLA Samples Produced with the FDM Technique on Their Mechanical Properties. Materials 2022, 15, 1304. [Google Scholar] [CrossRef]
- Păcurar, R.; Berce, P.; Petrilak, A.; Nemeş, O.; Borzan, C.Ş.M.; Harničárová, M.; Păcurar, A. Selective Laser Sintering of PA 2200 for Hip Implant Applications: Finite Element Analysis, Process Optimization, Morphological and Mechanical Characterization. Materials 2021, 14, 4240. [Google Scholar] [CrossRef]
- Ghods, S.; Schur, R.; Montelione, A.; Schleusener, R.; Arola, D.D.; Ramulu, M. Importance of Build Design Parameters to the Fatigue Strength of Ti6Al4V in Electron Beam Melting Additive Manufacturing. Materials 2022, 15, 5617. [Google Scholar] [CrossRef]
- Bassis, M.; Ron, T.; Leon, A.; Kotliar, A.; Kotliar, R.; Shirizly, A.; Aghion, E. The Influence of Intralayer Porosity and Phase Transition on Corrosion Fatigue of Additively Manufactured 316L Stainless Steel Obtained by Direct Energy Deposition Process. Materials 2022, 15, 5481. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitković, N.; Stojković, J.R.; Korunović, N.; Teuţan, E.; Pleşa, A.; Ianoşi-Andreeva-Dimitrova, A.; Górski, F.; Păcurar, R. Extra-Articular Distal Humerus Plate 3D Model Creation by Using the Method of Anatomical Features. Materials 2023, 16, 5409. https://doi.org/10.3390/ma16155409
Vitković N, Stojković JR, Korunović N, Teuţan E, Pleşa A, Ianoşi-Andreeva-Dimitrova A, Górski F, Păcurar R. Extra-Articular Distal Humerus Plate 3D Model Creation by Using the Method of Anatomical Features. Materials. 2023; 16(15):5409. https://doi.org/10.3390/ma16155409
Chicago/Turabian StyleVitković, Nikola, Jelena R. Stojković, Nikola Korunović, Emil Teuţan, Alin Pleşa, Alexandru Ianoşi-Andreeva-Dimitrova, Filip Górski, and Răzvan Păcurar. 2023. "Extra-Articular Distal Humerus Plate 3D Model Creation by Using the Method of Anatomical Features" Materials 16, no. 15: 5409. https://doi.org/10.3390/ma16155409
APA StyleVitković, N., Stojković, J. R., Korunović, N., Teuţan, E., Pleşa, A., Ianoşi-Andreeva-Dimitrova, A., Górski, F., & Păcurar, R. (2023). Extra-Articular Distal Humerus Plate 3D Model Creation by Using the Method of Anatomical Features. Materials, 16(15), 5409. https://doi.org/10.3390/ma16155409








