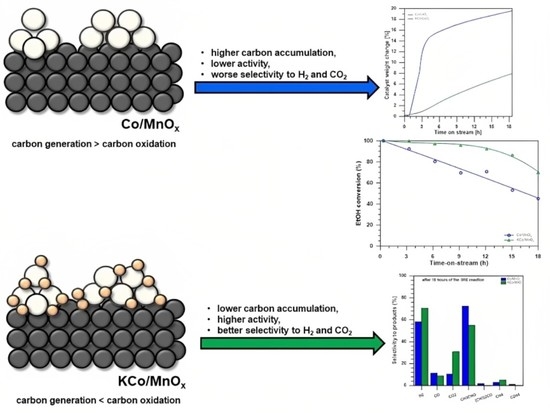
Effect of Potassium Doping on the Structural and Catalytic Properties of Co/MnOx Catalyst in the Steam Reforming of Ethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Catalysts Characterization
2.4. SRE Experimental Tests
3. Results and Discussion
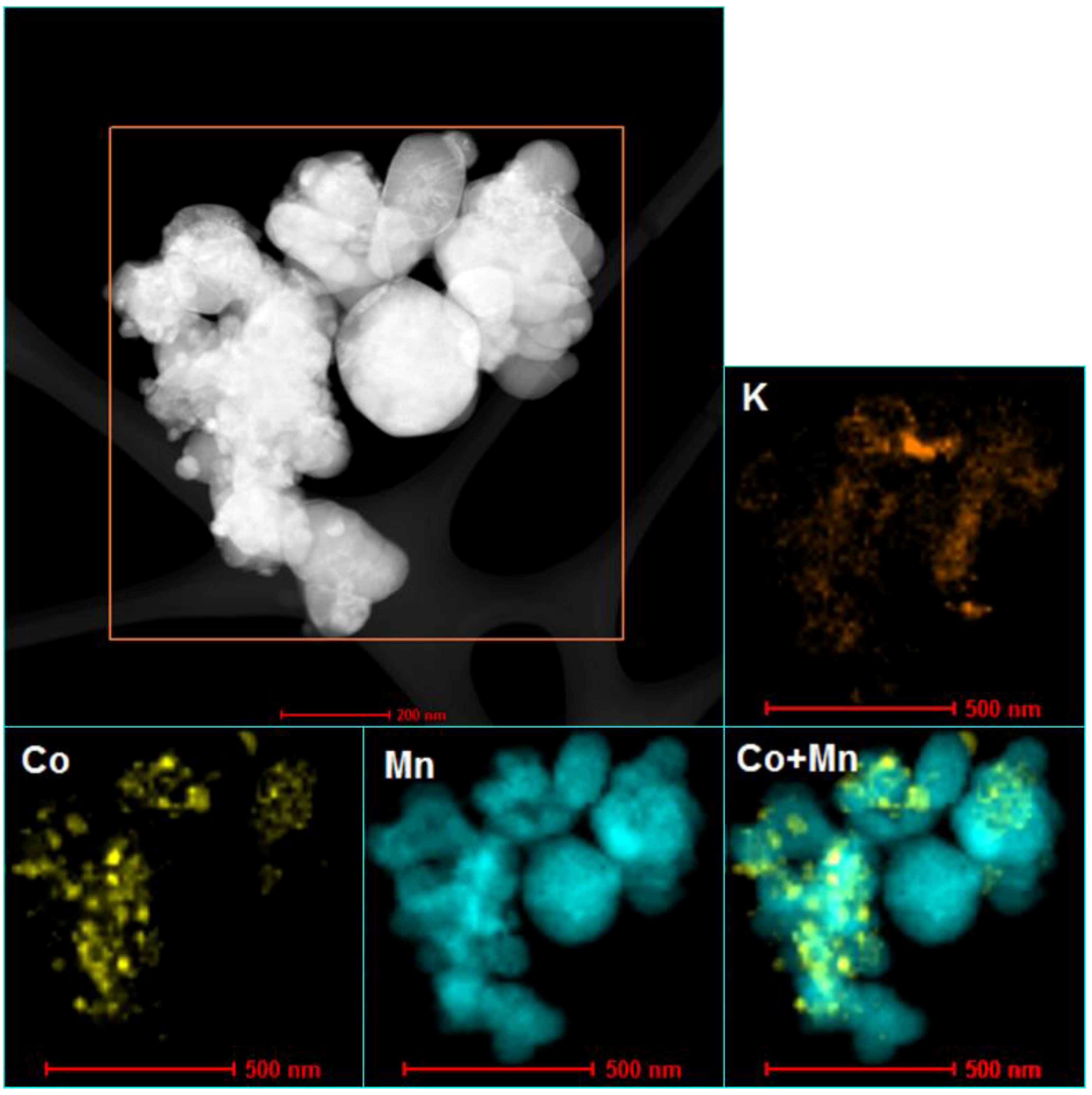
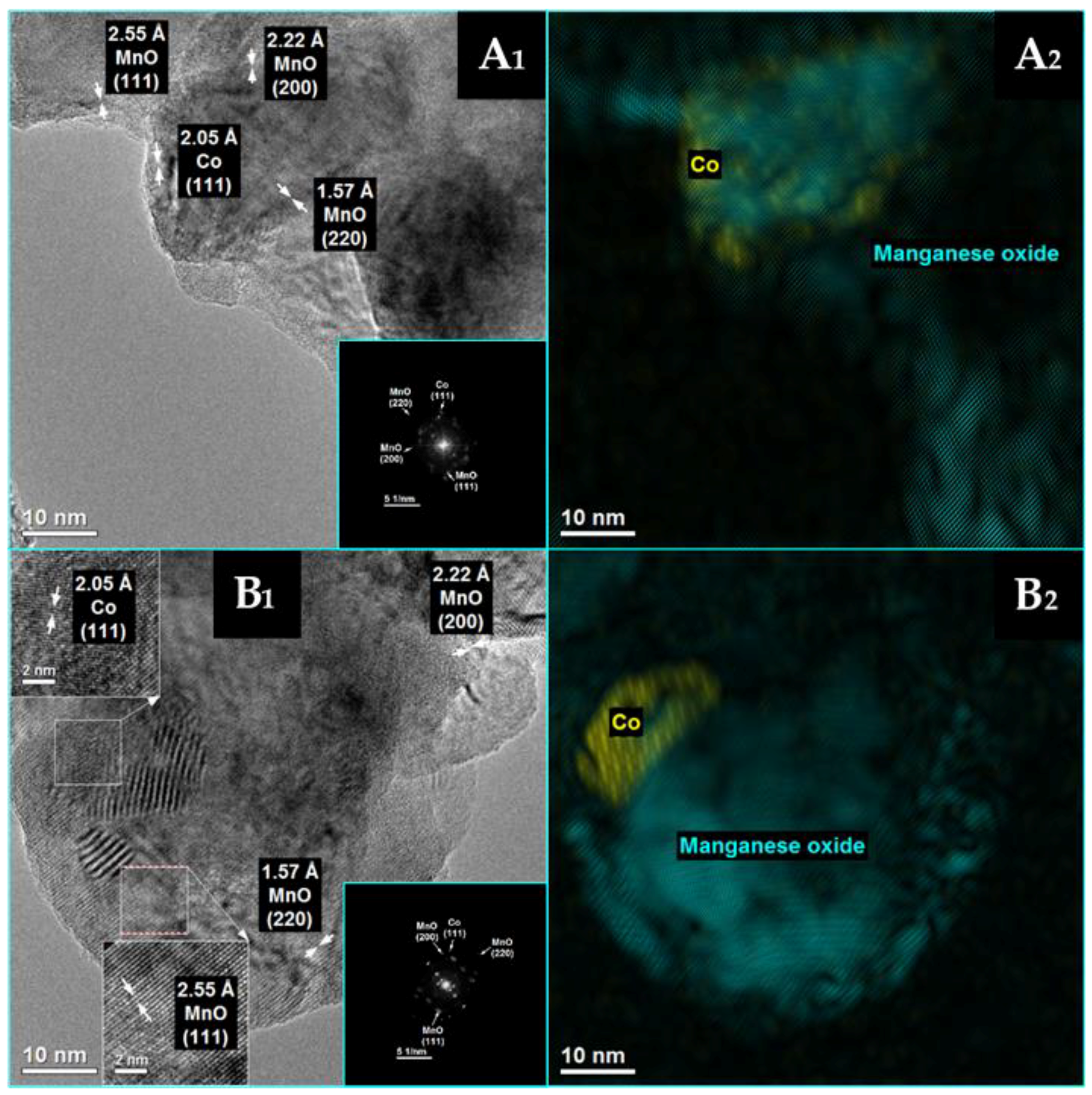
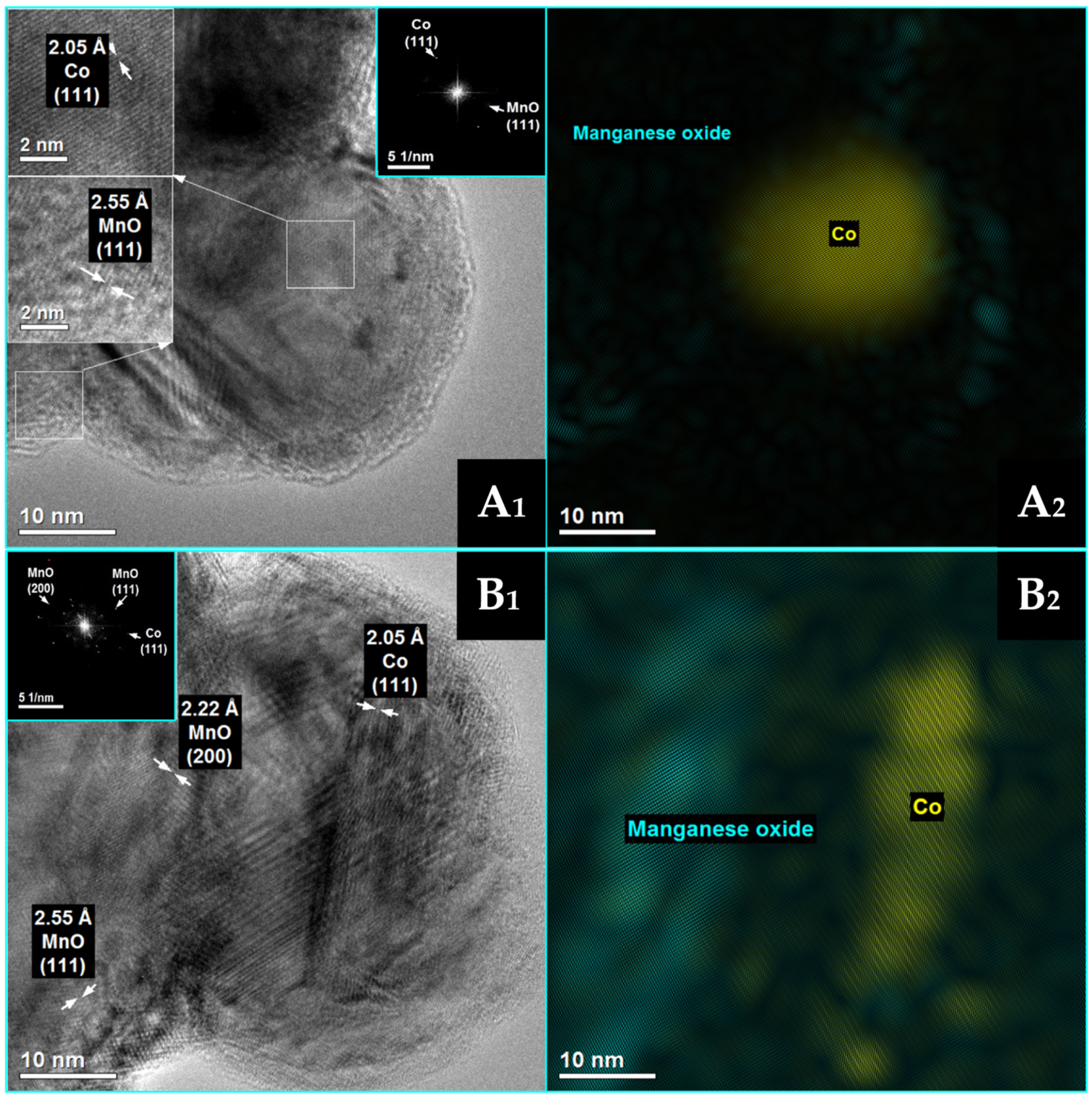
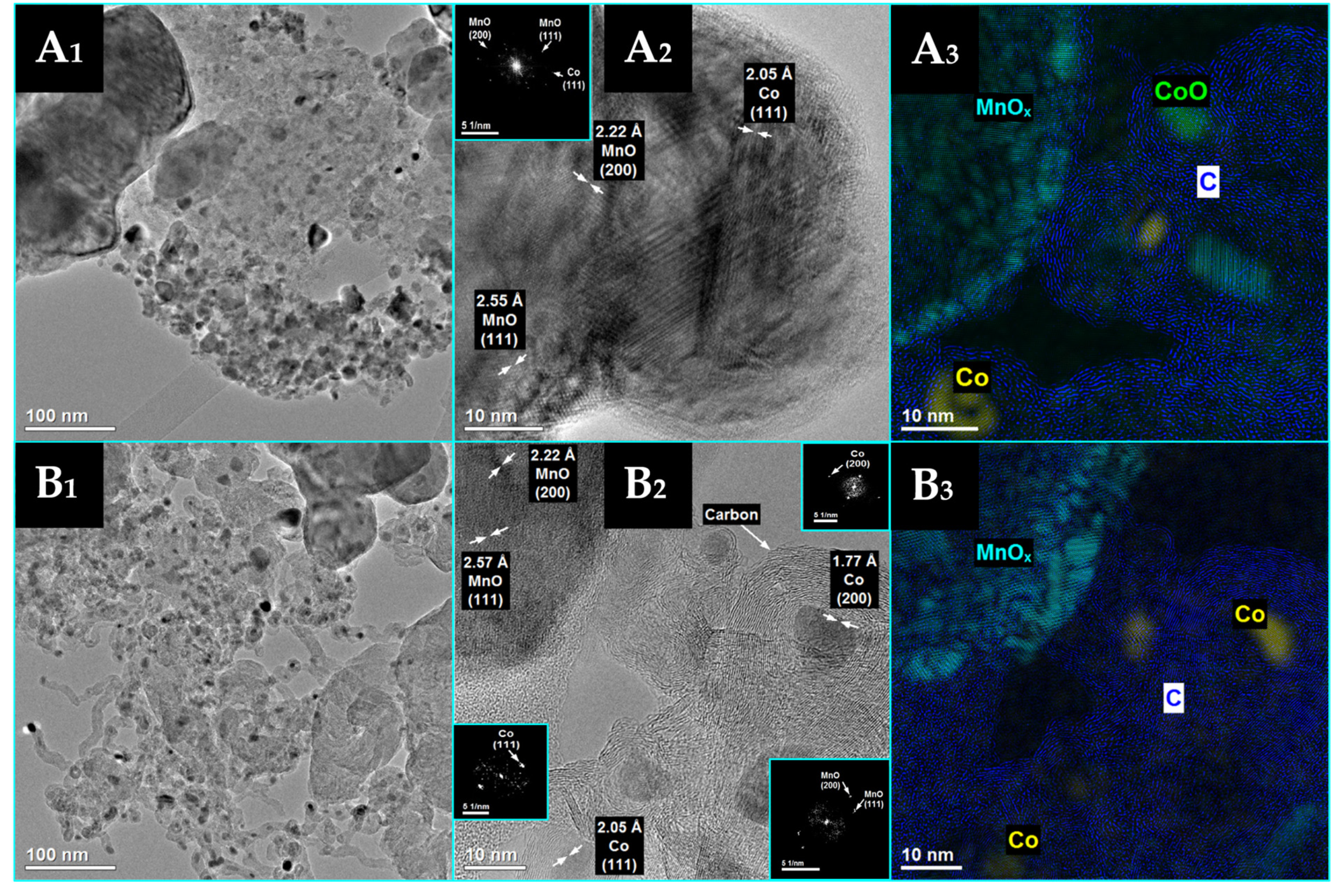
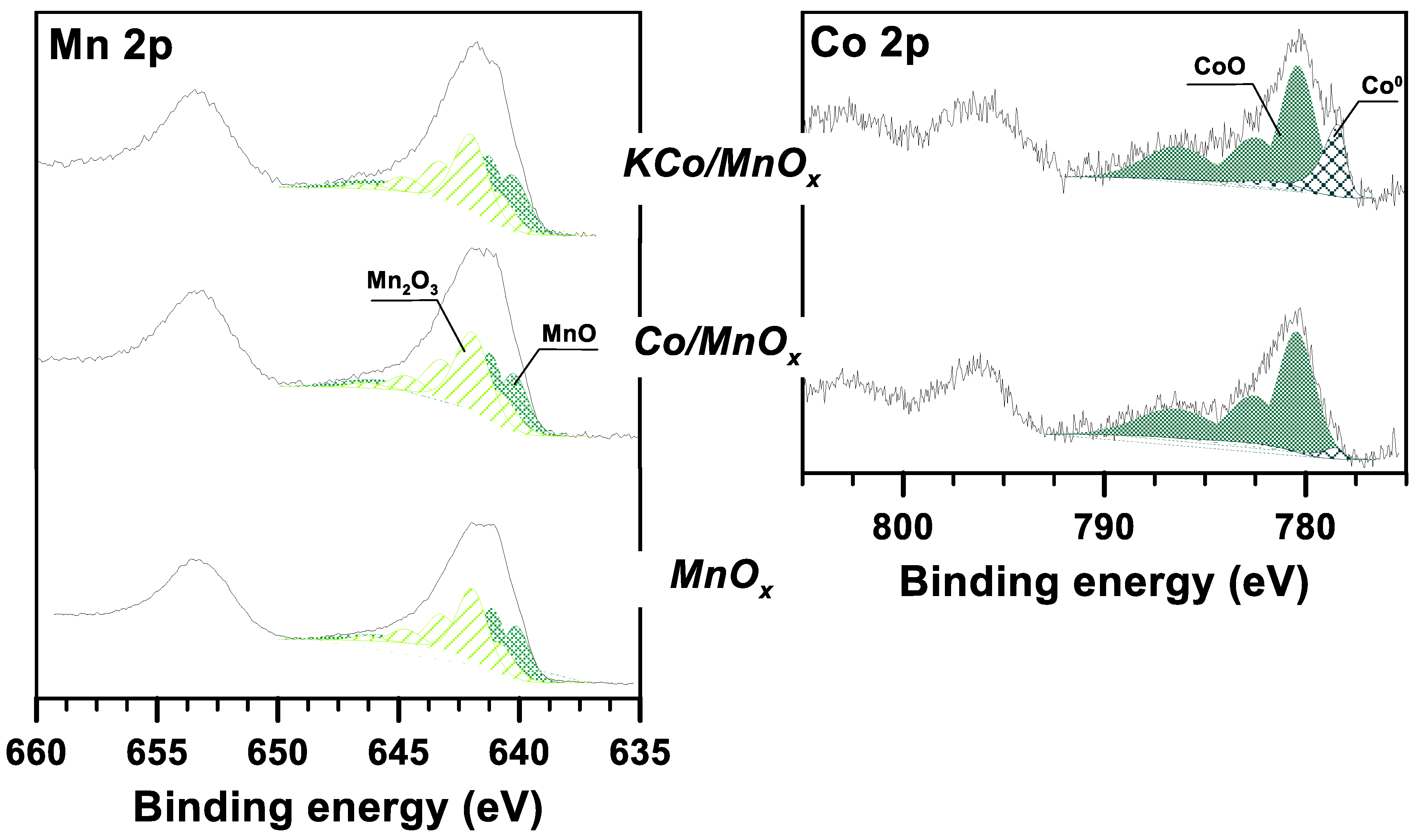
3.1. The Effect of Potassium Doping on the Catalysts’ Physicochemical Properties
3.2. The Effect of Potassium Doping on the Performance of the Catalysts in the Ethanol Steam Reforming Reaction
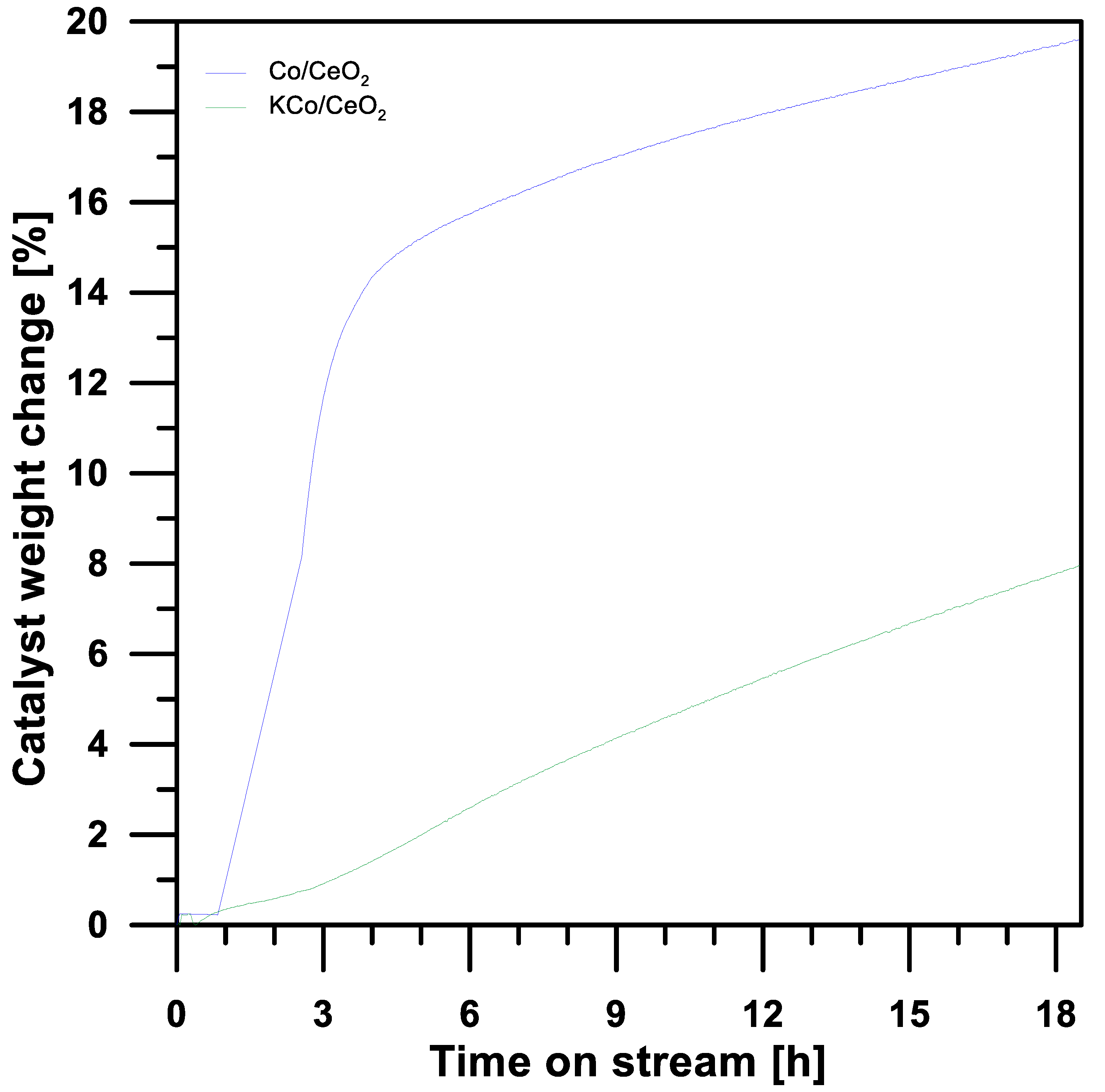
3.3. The Effect of Potassium Doping on Prevention of the Cobalt-Based Catalyst Deactivation under SRE Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sohrabi, S.; Irankhah, A. Synthesis, characterization, and catalytic activity of Ni/CeMnO2 catalysts promoted by copper, cobalt, potassium and iron for ethanol steam reforming. Int. J. Hydrogen Energy 2021, 46, 12846–12856. [Google Scholar] [CrossRef]
- Yu, S.-W.; Huang, H.-H.; Tang, C.-W.; Wang, C.-B. The effect of accessible oxygen over Co3O4-CeO2 catalysts on the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 20700–20711. [Google Scholar] [CrossRef]
- Liu, Z.; Duchoň, T.; Wang, H.; Peterson, E.W.; Zhou, Y.; Luo, S.; Zhou, Y.; Matolín, V.; Stacchiola, D.J.; Rodriguez, J.A.; et al. Mechanistic insight of ethanol steam reforming over Ni-CeOx(111): The importance of hydroxyl groups for suppressing coke formation. J. Phys. Chem C 2015, 119, 18248–18256. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, L. A comparative DFT study of ethanol steam reforming over Co(1 0 0) and CoO(1 0 0) surfaces: Molecular reaction mechanism. Comput. Theor. Chem. 2022, 1217, 113926. [Google Scholar] [CrossRef]
- Idriss, H. Ethanol reactions over the surfaces of noble metal/cerium oxide catalysts. Platin. Met. Rev. 2004, 48, 105–115. [Google Scholar] [CrossRef]
- Passos, A.R.; Martins, L.; Pulcinelli, S.H.; Santilli, C.V.; Briois, V. Effect of the balance between Co(II) and Co(0) oxidation states on the catalytic activity of cobalt catalysts for Ethanol Steam Reforming. Catal. Today 2014, 229, 88–94. [Google Scholar] [CrossRef]
- Liu, X.; Murata, K.; Inaba, M. Steam Reforming of Bio-Ethanol to Produce Hydrogen over Co/CeO2 Catalysts Derived from Ce1−xCoxO2−y Precursors. Catalysts 2016, 6, 26. [Google Scholar] [CrossRef]
- You, W.; Zhang, Q.; Jia, H.; Ta, N.; Sheng, X.; Yang, X.; Wang, Y.; Shen, W.; Goldbach, A. Insights into the state of ceria during ethanol steam reforming over Ir/CeO2. Catal. Sci. Technol. 2023, 13, 558–572. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ruocco, C.; Ciambelli, P.; Iaquaniello, G. Low temperature-ethanol steam reforming over Ni-based catalysts supported on CeO2. J. Power Technol. 2015, 95, 54–66. [Google Scholar]
- Chiou, J.Y.Z.; Wang, W.-Y.; Yang, S.-Y.; Lai, C.-L.; Huang, H.-H.; Wang, C.-B. Ethanol Steam Reforming to Produce Hydrogen Over Co/ZnO and PtCo/ZnO Catalysts. Catal. Lett. 2013, 143, 501–507. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, H.; Davidson, S.D.; Sun, J.; Wang, Y. Effect of ZnO facet on ethanol steam reforming over Co/ZnO. Catal. Commun. 2016, 73, 93–97. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Chica, A. Catalysts based on Co-Birnessite and Co-Todorokite for the efficient production of hydrogen by ethanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 16859–16865. [Google Scholar] [CrossRef]
- Kwak, B.S.; Lee, G.; Park, S.-M.; Kang, M. Effect of MnOx in the catalytic stabilization of Co2MnO4 spinel during the ethanol steam reforming reaction. Appl. Catal. A Gen. 2015, 503, 165–175. [Google Scholar] [CrossRef]
- Fuertes, A.; Da Costa-Serra, J.F.; Chica, A. New catalysts based on Ni-Birnessite and Ni-Todorokite for the efficient production of hydrogen by bioethanol steam reforming. Energy Procedia 2012, 29, 181–191. [Google Scholar] [CrossRef][Green Version]
- Gac, W.; Greluk, M.; Słowik, G.; Turczyniak-Surdacka, S. Structural and surface changes of cobalt modified manganese oxide during activation and ethanol steam reforming reaction. Appl. Surf. Sci. 2018, 440, 1047–1062. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Słowik, M.; Turczyniak-Surdacka, S.; Grzybek, G.; Góra-Marek, K.; Kotarba, A. Effect of Potassium Promoter on the Performance of Nickel-Based Catalysts Supported on MnOx in Steam Reforming of Ethanol. Catalysts 2022, 12, 600. [Google Scholar] [CrossRef]
- Grzybek, G.; Góra-Marek, K.; Tarach, K.; Pyra, K.; Patulski, P.; Greluk, M.; Słowik, G.; Kotarba, A. Tuning the properties of the cobalt-zeolite nanocomposite catalyst by potassium: Switching between dehydration and dehydrogenation of ethanol. J. Catal. 2022, 407, 364–380. [Google Scholar] [CrossRef]
- Grzybek, G.; Greluk, M.; Tarach, K.; Pyra, K.; Słowik, G.; Rotko, M.; Góra-Marek, K. Bioethanol Steam Reforming over Cobalt-Containing USY and ZSM-5 Commercial Zeolite Catalysts. Front. Mater. 2020, 7, 597528. [Google Scholar] [CrossRef]
- Yoo, S.; Park, S.; Song, H.; Kim, D.H. Hydrogen production by the steam reforming of ethanol over K-promoted Co/Al2O3–CaO xerogel catalysts. Mol. Catal. 2020, 491, 110980. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Legutko, P.; Gryboś, J.; Indyka, P.; Leszczyński, B.; Kotarba, A.; Sojka, Z. Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4|α-Al2O3 catalyst for N2O decomposition: Crucial role of activation temperature on catalytic performance. Appl. Catal. B Environ. 2017, 205, 597–604. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Electrochemical Supercapacitance Properties of Reduced Graphene Oxide/Mn2O3:Co3O4 Nanocomposite. J. Inorg. Organomet. Polym. 2017, 27, 576–585. [Google Scholar] [CrossRef]
- Sharrouf, M.; Awad, R.; Roumié, M.; Marhaba, S. Structural, Optical and Room Temperature Magnetic Study of Mn2O3 Nanoparticles. Mater. Sci. Appl. 2015, 6, 850–859. [Google Scholar]
- Gnana Sundara Raj, B.; Asiri, A.M.; Wu, J.J.; Anandan, S. Synthesis of Mn3O4 nanoparticles via chemical precipitation approach for supercapacitor application. J. Alloys Compd. 2015, 636, 234–240. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Soleymanifard, M.; Jarosova, M.; Machek, P. Facile synthesis and characterization of Mn3O4, Co3O4, and NiO. Acta Phys. Pol. 2002, 137, 1043–1045. [Google Scholar] [CrossRef]
- Ciura, K.; Grzybek, G.; Wójcik, S.; Indyka, P.; Kotarba, A.; Sojka, S. Optimization of cesium and potassium promoter loading in alkali-doped Zn0.4Co2.6O4|Al2O3 catalysts for N2O abatement. React. Kinet. Mech. Catal. 2017, 121, 645–655. [Google Scholar] [CrossRef]
- Hou, H.; Li, J.; Lan, J.; Chen, F.; Huang, B. Porous walnut-like Mn2O3 anode derived from the waste graphene production effluent. J. Porous Mater. 2022, 29, 837–847. [Google Scholar] [CrossRef]
- Atique Ullah, A.K.M.; Fazle Kibria, A.K.M.; Akter, M.; Khan, M.N.I.; Tareq, A.R.M.; Firoz, S.H. Oxidative Degradation of Methylene Blue Using Mn3O4 Nanoparticles. Water Conserv. Sci. Eng. 2017, 1, 249–256. [Google Scholar] [CrossRef]
- Martinez de la Torre, C.; Bennewitz, M.F. Manganese oxide nanoparticle synthesis by thermal decomposition of manganese(II) acetylacetonate. J. Vis. Exp. 2020, 160, e61572. [Google Scholar]
- Grzybek, G.; Greluk, M.; Indyka, P.; Góra-Marek, K.; Legutko, P.; Słowik, G.; Turczyniak-Surdacka, S.; Rotko, M.; Sojka, Z.; Kotarba, A. Cobalt catalyst for steam reforming of ethanol–Insights into the promotional role of potassium. Int. J. Hydrogen Energy 2020, 45, 22658–22673. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Cheng, W.; Wu, F.; Lu, X.; Li, Z. Controlled synthesis of diverse manganese based catalysts for complete oxidation of toluene and carbon monoxide. Chem. Eng. J. 2014, 22, 59–67. [Google Scholar] [CrossRef]
- Shu, S.; Guo, J.; Li, J.; Fang, N.; Li, J.; Yuan, S. Effect of post-treatment on the selective catalytic reduction of NO with NH3 over Mn3O4. Mater. Chem. Phys. 2019, 237, 121845. [Google Scholar] [CrossRef]
- Gil, A.; Gandía, L.M.; Korili, S.A. Effect of the temperature of calcination on the catalytic performance of manganese- and samarium-manganese-based oxides in the complete oxidation of acetone. Appl. Catal. A Gen. 2004, 274, 229–235. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S.C. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chen, Y.-W. The mechanism of reduction of cobalt by hydrogen. Mater. Chem. Phys. 2004, 85, 171–175. [Google Scholar] [CrossRef]
- Darda, S.; Pachatouridou, E.; Lappas, A.; Iliopoulou, E. Effect of preparation method of Co-Ce catalysts on CH4 combustion. Catalysts 2019, 9, 219. [Google Scholar] [CrossRef]
- Zhong, L.; Barreau, M.; Chen, D.; Caps, V.; Haevecker, M.; Teschnerb, D.; Simonne, D.H.; Borfecchia, E.; Baaziz, W.; Šmí, B.; et al. Effect of manganese promotion on the activity and selectivity of cobalt catalysts for CO preferential oxidation. Appl. Catal. B Environ. 2021, 297, 120397. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhu, Q.; Ma, M.; Jiang, Z.; Liao, X.; He, C. Unraveling the effects of potassium incorporation routes and positions on toluene oxidation over α-MnO2 nanorods: Based on experimental and density functional theory (DFT) studies. J. Colloid Interface Sci. 2021, 598, 324–338. [Google Scholar] [CrossRef]
- Grzona, C.B.; Lick, I.D.; Rodriguez Castellón, E.; Ponzi, M.I.; Ponzi, E.N. Cobalt and KNO3 supported on alumina catalysts for diesel soot combustion. Mater. Chem. Phys. 2010, 123, 557–562. [Google Scholar] [CrossRef]
- Claude, V.; Mahy, J.G.; Lohay, T.; Tilkin, R.G.; Micheli, F.; Lambert, S.D. Sol–gel synthesis of Ni/Al2O3 catalysts for toluene reforming: Support modification with alkali, alkaline earth or rare-earth dopant (Ca, K, Mg or Ce). Surf. Interfaces 2020, 20, 100511. [Google Scholar] [CrossRef]
- Yildirim, O.A.; Arslan, H.; Sönmezoglu, S. Facile synthesis of cobalt-doped zinc oxide thin films for highly efficient visible light photocatalysts. Appl. Surf. Sci. 2016, 390, 111–121. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.V.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]



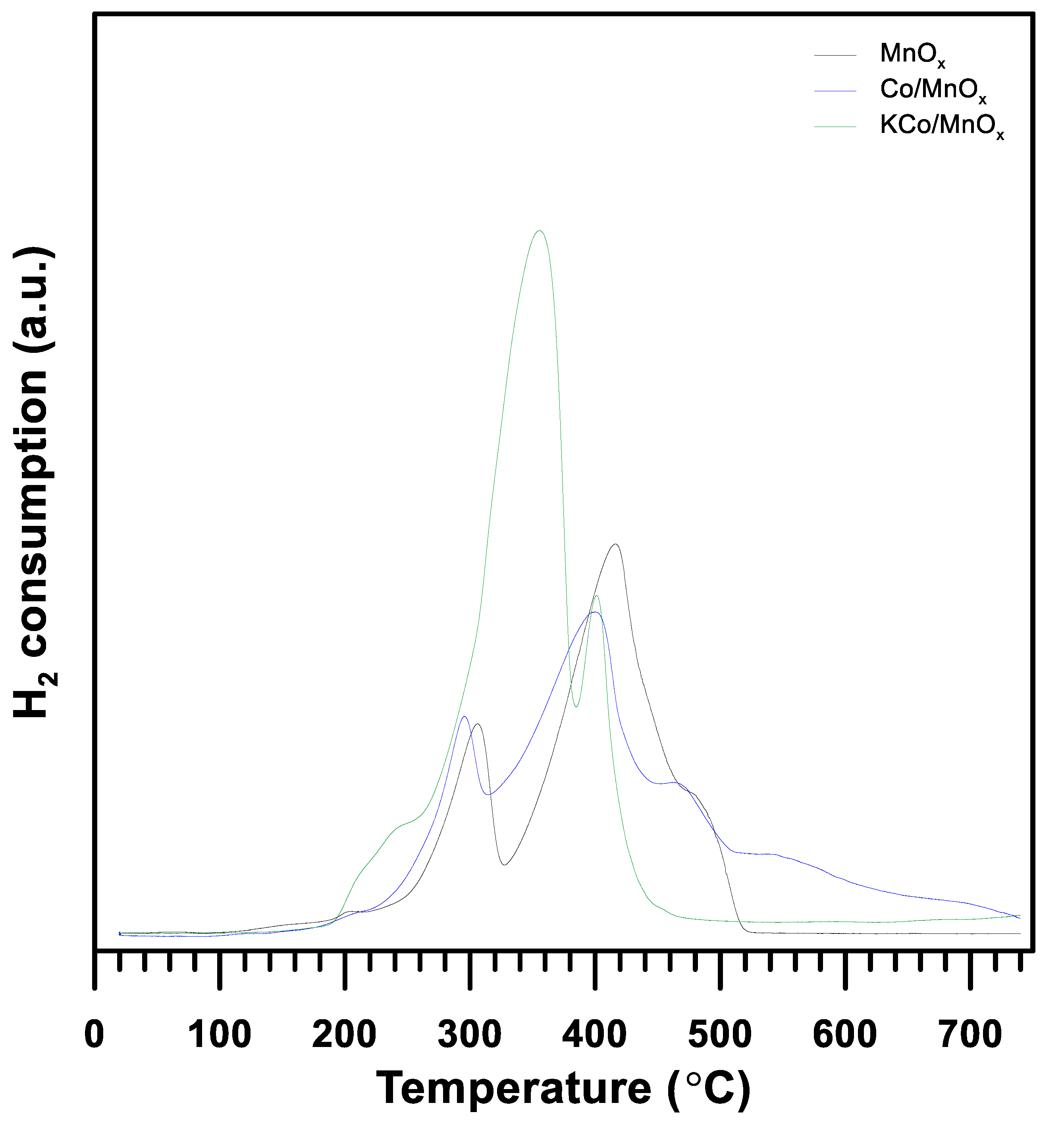
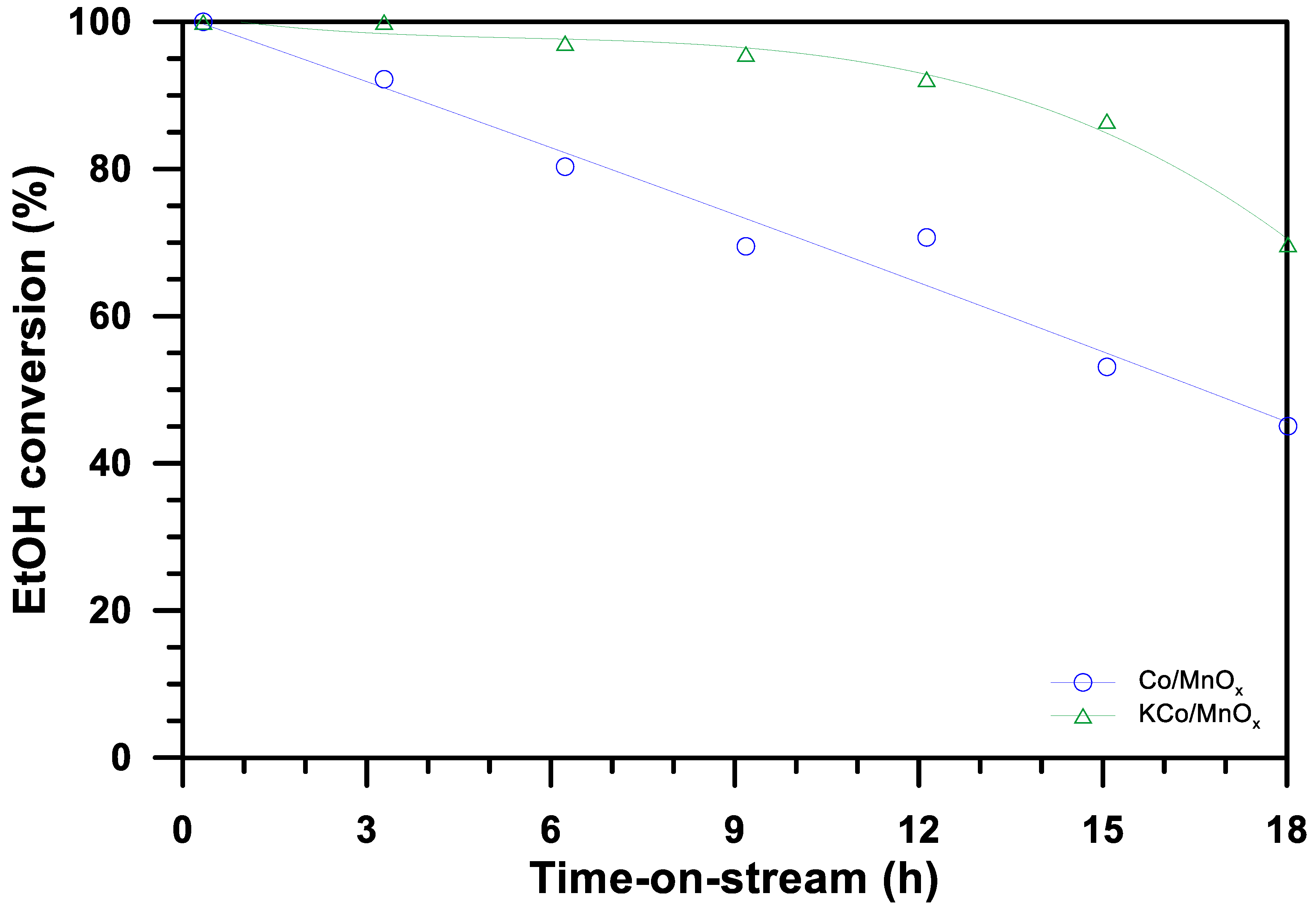
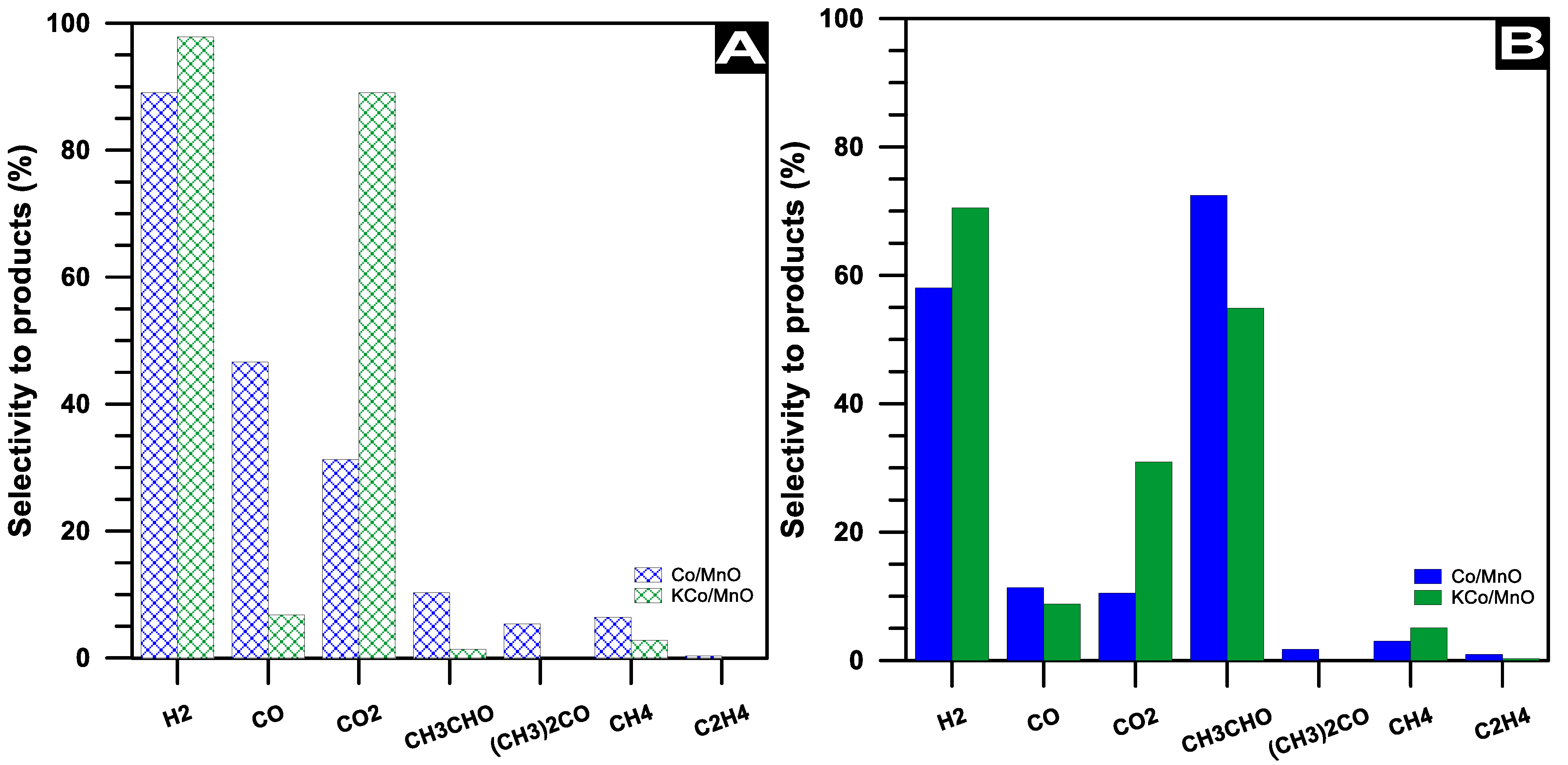
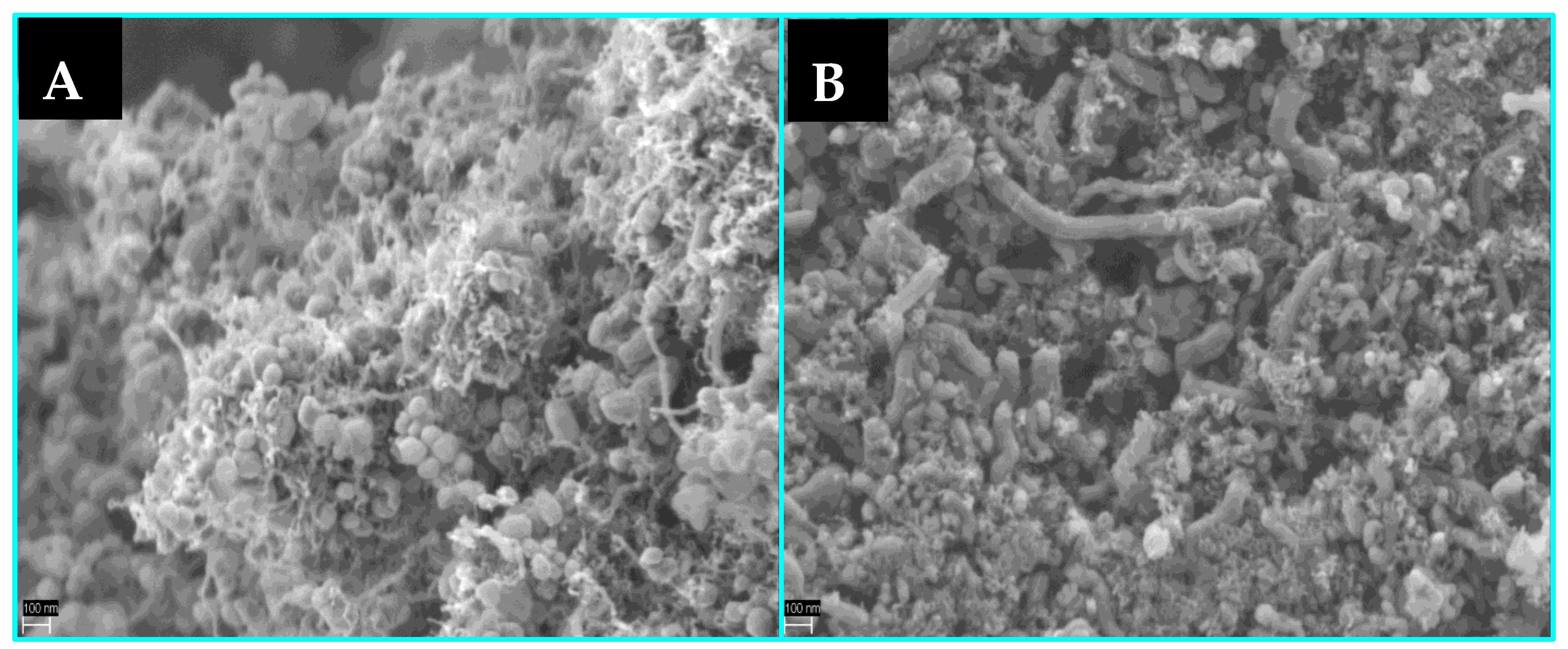
| Parameter | Co/MnOx | KCo/MnOx | |
|---|---|---|---|
| Metal content (wt%) | 9.7 | 9.7 | |
| Potassium content (wt%) | - | 1.25 | |
| BET surface area (m2/g) | 11.2 | 5.8 | |
| Co0 crystallite size (nm) 1 | By XRD | 14 | 23 |
| By TEM | 21 | 26 | |
| H2 consumption | Theoretical (mmol/gCo) | 2.18 | 2.19 |
| Experimental (mmol/g) | 5.21 | 7.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greluk, M.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S.; Grzybek, G.; Tyszczuk-Rotko, K. Effect of Potassium Doping on the Structural and Catalytic Properties of Co/MnOx Catalyst in the Steam Reforming of Ethanol. Materials 2023, 16, 5377. https://doi.org/10.3390/ma16155377
Greluk M, Rotko M, Słowik G, Turczyniak-Surdacka S, Grzybek G, Tyszczuk-Rotko K. Effect of Potassium Doping on the Structural and Catalytic Properties of Co/MnOx Catalyst in the Steam Reforming of Ethanol. Materials. 2023; 16(15):5377. https://doi.org/10.3390/ma16155377
Chicago/Turabian StyleGreluk, Magdalena, Marek Rotko, Grzegorz Słowik, Sylwia Turczyniak-Surdacka, Gabriela Grzybek, and Katarzyna Tyszczuk-Rotko. 2023. "Effect of Potassium Doping on the Structural and Catalytic Properties of Co/MnOx Catalyst in the Steam Reforming of Ethanol" Materials 16, no. 15: 5377. https://doi.org/10.3390/ma16155377
APA StyleGreluk, M., Rotko, M., Słowik, G., Turczyniak-Surdacka, S., Grzybek, G., & Tyszczuk-Rotko, K. (2023). Effect of Potassium Doping on the Structural and Catalytic Properties of Co/MnOx Catalyst in the Steam Reforming of Ethanol. Materials, 16(15), 5377. https://doi.org/10.3390/ma16155377