The Intraoperative Use of Defensive Antibacterial Coating (DAC®) in the Form of a Gel to Prevent Peri-Implant Infections in Orthopaedic Surgery: A Clinical Narrative Review
Abstract
1. Introduction
1.1. Pathogenesis of PJI and OAI
1.2. Strategies to Prevent PJI and OAI
1.3. Local Carriers and Coating Systems
2. Materials and Methods
2.1. DAC® Characteristics
2.2. Study Selection and Data Extraction
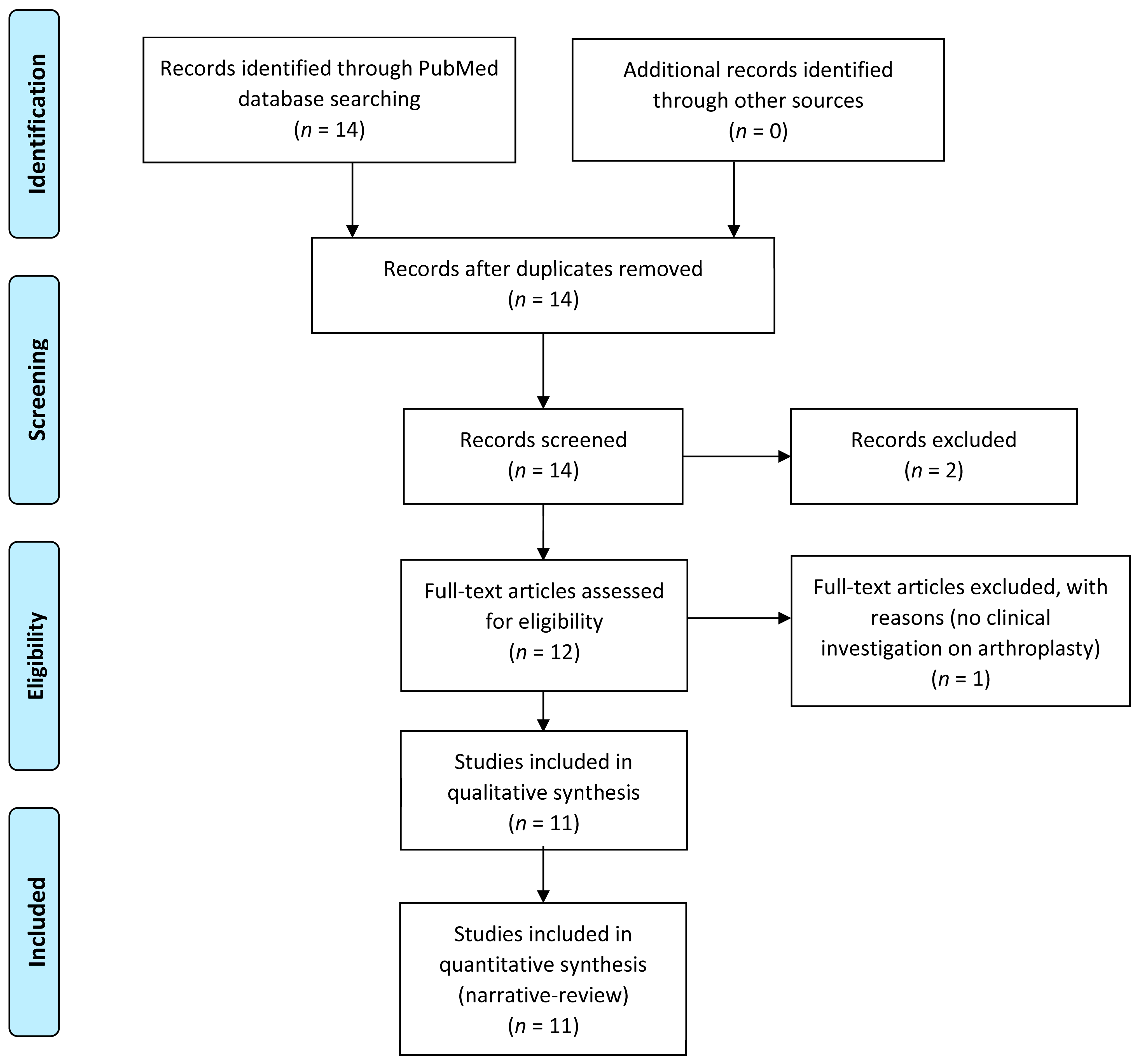
3. Results
4. Discussion
5. Conclusions and Future Perspectives
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, A.J.; Blom, A.W.; Whitehouse, M.R.; Gooberman-Hill, R. Deep prosthetic joint infection: A qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open 2015, 5, e009495. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; de Steiger, R. How to manage treatment failure in prosthetic joint infection. Clin. Microbiol. Infect. 2020, 26, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.; Hovding, P.; Tveit, S.M.; Graff, J.B.; Lutro, O.; Schrama, J.C.; Wik, T.S.; Skramm, I.; Westberg, M.; Fenstad, A.M.; et al. Increasing but levelling out risk of revision due to infection after total hip arthroplasty: A study on 108,854 primary THAs in the Norwegian Arthroplasty Register from 2005 to 2019. Acta Orthop. 2021, 92, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Huotari, K.; Peltola, M.; Jamsen, E. The incidence of late prosthetic joint infections: A registry-based study of 112,708 primary hip and knee replacements. Acta Orthop. 2015, 86, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.C.; Liu, W.Y.; Goosen, J.H.M.; Rijnen, W.H.C.; van Steenbergen, L.N.; van der Weegen, W.; Regional Prosthetic Joint Infection Working Group. Mismatch in Capture of Periprosthetic Joint Infections Between the Dutch Arthroplasty Register (LROI) and a Detailed Regional Periprosthetic Joint Infection Registry. J. Arthroplast. 2022, 37, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, Z.P.; Davis, J.S.; Lorimer, M.; de Steiger, R.N.; Graves, S.E.; Yates, P.; Manning, L. The accuracy of reporting of periprosthetic joint infection to the Australian Orthopaedic Association National Joint Replacement Registry. Bone Jt. Open 2022, 3, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Alamanda, V.K.; Springer, B.D. Perioperative and Modifiable Risk Factors for Periprosthetic Joint Infections (PJI) and Recommended Guidelines. Curr. Rev. Musculoskelet. Med. 2018, 11, 325–331. [Google Scholar] [CrossRef]
- Eka, A.; Chen, A.F. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann. Transl. Med. 2015, 3, 233. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; Team, I. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef]
- Boelch, S.P.; Jakuscheit, A.; Doerries, S.; Fraissler, L.; Hoberg, M.; Arnholdt, J.; Rudert, M. Periprosthetic infection is the major indication for TKA revision-experiences from a university referral arthroplasty center. BMC Musculoskelet. Disord. 2018, 19, 395. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Troelsen, A.; Thomsen, R.W.; Soballe, K. Chronic infections in hip arthroplasties: Comparing risk of reinfection following one-stage and two-stage revision: A systematic review and meta-analysis. Clin. Epidemiol. 2012, 4, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Werner, B.C.; Bolarinwa, S.A.; Browne, J.A. Removal of an Infected Total Hip Arthroplasty: Risk Factors for Repeat Debridement, Long-term Spacer Retention, and Mortality. J. Arthroplast. 2017, 32, 2519–2522. [Google Scholar] [CrossRef] [PubMed]
- Okafor, C.; Hodgkinson, B.; Nghiem, S.; Vertullo, C.; Byrnes, J. Cost of septic and aseptic revision total knee arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 706. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Onsea, J.; Neutjens, E.; Steffens, E.; Schuermans, A.; McNally, M.; Nijs, S. Prevention of fracture-related infection: A multidisciplinary care package. Int. Orthop. 2017, 41, 2457–2469. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37 (Suppl. S2), S59–S66. [Google Scholar] [CrossRef]
- Ma, Q.; Aierxiding, A.; Wang, G.; Wang, C.; Yu, L.; Shen, Z. Incidence and risk factors for deep surgical site infection after open reduction and internal fixation of closed tibial plateau fractures in adults. Int. Wound J. 2018, 15, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, H.; Smeets, B.; Metsemakers, W.J.; Spitz, A.C.; Nijs, S. Economics of open tibial fractures: The pivotal role of length-of-stay and infection. Health Econ. Rev. 2017, 7, 32. [Google Scholar] [CrossRef]
- Olesen, U.K.; Pedersen, N.J.; Eckardt, H.; Lykke-Meyer, L.; Bonde, C.T.; Singh, U.M.; McNally, M. The cost of infection in severe open tibial fractures treated with a free flap. Int. Orthop. 2017, 41, 1049–1055. [Google Scholar] [CrossRef]
- Shao, J.; Chang, H.; Zhu, Y.; Chen, W.; Zheng, Z.; Zhang, H.; Zhang, Y. Incidence and risk factors for surgical site infection after open reduction and internal fixation of tibial plateau fracture: A systematic review and meta-analysis. Int. J. Surg. 2017, 41, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, H.; Yin, B.; Li, J.; Zhu, Y.; Zhang, Y. Risk factors for surgical site infection following operative treatment of ankle fractures: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Chang, C.H.; Lin, Y.C.; Lee, S.H.; Hsieh, P.H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Zhao, K.; Zhang, J.; Meng, H.; Jin, Z.; Ma, J.; Zhang, Y. Incidence and risks for surgical site infection after closed tibial plateau fractures in adults treated by open reduction and internal fixation: A prospective study. J. Orthop. Surg. Res. 2020, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. S1), i37–i40. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95-B, 1450–1452. [Google Scholar] [CrossRef]
- Willenegger, H.; Roth, B. [Treatment tactics and late results in early infection following osteosynthesis]. Unfallchirurgie 1986, 12, 241–246. [Google Scholar] [CrossRef]
- Azboy, I.; Bedair, H.; Demirtas, A.; Ford, E., Jr.; Gahramanov, A.; Klement, M.R.; Ploegmakers, J.; Schwarz, E.; Turkmen, I. General Assembly, Prevention, Risk Mitigation, General Factors: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S55–S59. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Mont, M.A.; Callaghan, J.J. Introduction: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S1–S2. [Google Scholar] [CrossRef]
- Chan, V.W.; Chan, P.K.; Fu, H.; Cheung, M.H.; Cheung, A.; Yan, C.H.; Chiu, K.Y. Preoperative optimization to prevent periprosthetic joint infection in at-risk patients. J. Orthop. Surg. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
- Rezapoor, M.; Alvand, A.; Jacek, E.; Paziuk, T.; Maltenfort, M.G.; Parvizi, J. Operating Room Traffic Increases Aerosolized Particles and Compromises the Air Quality: A Simulated Study. J. Arthroplast. 2018, 33, 851–855. [Google Scholar] [CrossRef]
- Birgand, G.; Toupet, G.; Rukly, S.; Antoniotti, G.; Deschamps, M.N.; Lepelletier, D.; Pornet, C.; Stern, J.B.; Vandamme, Y.M.; van der Mee-Marquet, N.; et al. Air contamination for predicting wound contamination in clean surgery: A large multicenter study. Am. J. Infect. Control 2015, 43, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Hooper, G.J.; Rothwell, A.G.; Frampton, C.; Wyatt, M.C. Does the use of laminar flow and space suits reduce early deep infection after total hip and knee replacement?: The ten-year results of the New Zealand Joint Registry. J. Bone Jt. Surg. Br. 2011, 93, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Berrios-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Aboltins, C.A.; Berdal, J.E.; Casas, F.; Corona, P.S.; Cuellar, D.; Ferrari, M.C.; Hendershot, E.; Huang, W.; Kuo, F.C.; Malkani, A.; et al. Hip and Knee Section, Prevention, Antimicrobials (Systemic): Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S279–S288. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Forte, S.A.; Docter, S.; Bryant, D.; Sheth, N.P.; Chen, A.F. Perioperative Antibiotic Prophylaxis in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. Am. 2019, 101, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Naresh-Babu, J.; Arun-Kumar, V. Do Prophylactic Antibiotics Reach the Operative Site Adequately?: A Quantitative Analysis of Serum and Wound Concentrations of Systemic and Local Prophylactic Antibiotics in Spine Surgery. Spine 2020, 45, E196–E202. [Google Scholar] [CrossRef]
- Roy, M.E.; Peppers, M.P.; Whiteside, L.A.; Lazear, R.M. Vancomycin concentration in synovial fluid: Direct injection into the knee vs. intravenous infusion. J. Arthroplast. 2014, 29, 564–568. [Google Scholar] [CrossRef]
- Salgado, C.D.; Dash, S.; Cantey, J.R.; Marculescu, C.E. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin. Orthop. Relat. Res. 2007, 461, 48–53. [Google Scholar] [CrossRef]
- Valour, F.; Bouaziz, A.; Karsenty, J.; Ader, F.; Lustig, S.; Laurent, F.; Chidiac, C.; Ferry, T.; Lyon, B.J.I.s.g. Determinants of methicillin-susceptible Staphylococcus aureus native bone and joint infection treatment failure: A retrospective cohort study. BMC Infect. Dis. 2014, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; Bingham, J.S.; Clarke, H.D.; Spangehl, M.J.; Young, S.W. Intraosseous Regional Administration of Antibiotic Prophylaxis in Total Knee Arthroplasty. JBJS Essent. Surg. Technol. 2020, 10, e20. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, B.; McEwen, P.; Wilkinson, M.; Hazratwala, K.; Hellman, J.; Khan, H.; McLean, A.; Panwar, Y.; Doma, K.; Grant, A. Intraosseous Regional Prophylactic Antibiotics Decrease the Risk of Prosthetic Joint Infection in Primary TKA: A Multicenter Study. Clin. Orthop. Relat. Res. 2021, 479, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Xie, J.; Huang, Z.; Huang, Q.; Cao, G.; Pei, F. Efficacy and safety of intrawound vancomycin in primary hip and knee arthroplasty. Bone Jt. Res. 2020, 9, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Han, K.; Cong, X.; Cai, J.; Tong, D.; Han, D.; Wang, Y.; Yu, B. The use of calcium sulfate impregnated with vancomycin in the treatment of open fractures of long bones: A preliminary study. Orthopedics 2010, 33, 152–157. [Google Scholar] [CrossRef]
- Craig, J.; Fuchs, T.; Jenks, M.; Fleetwood, K.; Franz, D.; Iff, J.; Raschke, M. Systematic review and meta-analysis of the additional benefit of local prophylactic antibiotic therapy for infection rates in open tibia fractures treated with intramedullary nailing. Int. Orthop. 2014, 38, 1025–1030. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef]
- Overstreet, D.; McLaren, A.; Calara, F.; Vernon, B.; McLemore, R. Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin. Orthop. Relat. Res. 2015, 473, 337–347. [Google Scholar] [CrossRef]
- Zahid, M.; Lodhi, M.; Afzal, A.; Rehan, Z.A.; Mehmood, M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Gasik, M.; Van Mellaert, L.; Pierron, D.; Braem, A.; Hofmans, D.; De Waelheyns, E.; Anne, J.; Harmand, M.F.; Vleugels, J. Reduction of biofilm infection risks and promotion of osteointegration for optimized surfaces of titanium implants. Adv. Healthc. Mater. 2012, 1, 117–127. [Google Scholar] [CrossRef]
- Harris, L.G.; Richards, R.G. Staphylococci and implant surfaces: A review. Injury 2006, 37 (Suppl. S2), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Pitarresi, G.; Palumbo, F.S.; Calascibetta, F.; Fiorica, C.; Di Stefano, M.; Giammona, G. Medicated hydrogels of hyaluronic acid derivatives for use in orthopedic field. Int. J. Pharm. 2013, 449, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Sartori, M.; Contartese, D.; Giavaresi, G.; Aldini, N.N.; Fini, M. Use of Antibiotic Loaded Biomaterials for the Management of Bone Prosthesis Infections: Rationale and Limits. Curr. Med. Chem. 2019, 26, 3150–3174. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hansch, G.M.; Stuyck, J.; Gawlitta, D.; Romano, C.L. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Meani, E.; Sartori, M.; Ferrari, A.; Bellini, D.; Sacchetta, A.C.; Meraner, J.; Sambri, A.; Vocale, C.; Sambri, V.; et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int. Orthop. 2014, 38, 1505–1512. [Google Scholar] [CrossRef]
- Romano, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef]
- Kizilkonca, E.; Torlak, E.; Erim, F.B. Preparation and characterization of antibacterial nano cerium oxide/chitosan/hydroxyethylcellulose/polyethylene glycol composite films. Int. J. Biol. Macromol. 2021, 177, 351–359. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tabaran, F.; Magerusan, L.; Todor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Stephen Inbaraj, B.; Chen, B.H. An overview on recent in vivo biological application of cerium oxide nanoparticles. Asian J. Pharm. Sci. 2020, 15, 558–575. [Google Scholar] [CrossRef]
- Jung, S.W.; Oh, S.H.; Lee, I.S.; Byun, J.H.; Lee, J.H. In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis. Tissue Eng. Regen. Med. 2019, 16, 479–490. [Google Scholar] [CrossRef]
- Valverde, A.; Perez-Alvarez, L.; Ruiz-Rubio, L.; Pacha Olivenza, M.A.; Garcia Blanco, M.B.; Diaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.A.; Dragoo, J.L.; Samimi, B.; Bruckner, D.A.; Bernard, G.W.; Hedrick, M.; Benhaim, P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem. Biophys. Res. Commun. 2004, 321, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic effects of hyaluronic acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romano, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef]
- Capuano, N.; Logoluso, N.; Gallazzi, E.; Drago, L.; Romano, C.L. One-stage exchange with antibacterial hydrogel coated implants provides similar results to two-stage revision, without the coating, for the treatment of peri-prosthetic infection. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3362–3367. [Google Scholar] [CrossRef]
- Zagra, L.; Gallazzi, E.; Romano, D.; Scarponi, S.; Romano, C. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: Results of a comparative series. Int. Orthop. 2019, 43, 111–115. [Google Scholar] [CrossRef]
- De Meo, D.; Calogero, V.; Are, L.; Cavallo, A.U.; Persiani, P.; Villani, C. Antibiotic-Loaded Hydrogel Coating to Reduce Early Postsurgical Infections in Aseptic Hip Revision Surgery: A Retrospective, Matched Case-Control Study. Microorganisms 2020, 8, 571. [Google Scholar] [CrossRef]
- Franceschini, M.; Sandiford, N.A.; Cerbone, V.; Araujo, L.C.T.; Kendoff, D. Defensive antibacterial coating in revision total hip arthroplasty: New concept and early experience. Hip. Int. 2020, 30, 7–11. [Google Scholar] [CrossRef]
- Zoccali, C.; Scoccianti, G.; Biagini, R.; Daolio, P.A.; Giardina, F.L.; Campanacci, D.A. Antibacterial hydrogel coating in joint mega-prosthesis: Results of a comparative series. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1647–1655. [Google Scholar] [CrossRef]
- Corona, P.S.; Altayo, M.; Amat, C.; Vicente, M.; Velez, R. Reconstruction of infected post-traumatic bone defects of the distal femur with the Compress(®) implant. Preliminary results of a staged non-biological strategy. Injury 2021, 52, 606–615. [Google Scholar] [CrossRef]
- Parbonetti, G.; Puglisi, A.; La Maida, E.; Rizzo, B.; Granata, R. Antibiotic-Loaded Hydrogel Coating for the Prevention of Local Infection after Vertebral Surgery: A Retrospective Cohort Analysis. Surg. Technol. Int. 2021, 39, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Legnani, C. High rate of infection eradication following cementless one-stage revision hip arthroplasty with an antibacterial hydrogel coating. Int. J. Artif. Organs 2022, 45, 113–117. [Google Scholar] [CrossRef]
- De Meo, D.; Cera, G.; Pica, R.; Perfetti, F.; Martini, P.; Perciballi, B.; Ceccarelli, G.; Persiani, P.; Villani, C. Antibiotic-Loaded Coatings to Reduce Fracture-Related Infections: Retrospective Case Series of Patients with Increased Infectious Risk. Antibiotics 2023, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- Allison Md, M.B.A.F.D.; Huang, M.D.E.; Ahlmann, M.D.E.; Carney, M.D.S.; Wang, P.A.C.L.; Menendez, M.D.F.L. Peri-Prosthetic Infection in the Orthopedic Tumor Patient. Reconstr. Rev. 2014, 4. [Google Scholar] [CrossRef]
- Kapoor, S.K.; Thiyam, R. Management of infection following reconstruction in bone tumors. J. Clin. Orthop. Trauma 2015, 6, 244–251. [Google Scholar] [CrossRef] [PubMed]
- McClelland, S., 3rd; Takemoto, R.C.; Lonner, B.S.; Andres, T.M.; Park, J.J.; Ricart-Hoffiz, P.A.; Bendo, J.A.; Goldstein, J.A.; Spivak, J.M.; Errico, T.J. Analysis of Postoperative Thoracolumbar Spine Infections in a Prospective Randomized Controlled Trial Using the Centers for Disease Control Surgical Site Infection Criteria. Int. J. Spine Surg. 2016, 10, 14. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L., Jr. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef]
- Getzlaf, M.A.; Lewallen, E.A.; Kremers, H.M.; Jones, D.L.; Bonin, C.A.; Dudakovic, A.; Thaler, R.; Cohen, R.C.; Lewallen, D.G.; van Wijnen, A.J. Multi-disciplinary antimicrobial strategies for improving orthopaedic implants to prevent prosthetic joint infections in hip and knee. J. Orthop. Res. 2016, 34, 177–186. [Google Scholar] [CrossRef]
- Kallala, R.; Harris, W.E.; Ibrahim, M.; Dipane, M.; McPherson, E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: Safety profile and complication rates. Bone Jt. Res. 2018, 7, 570–579. [Google Scholar] [CrossRef] [PubMed]
- ter Boo, G.J.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Kheir, M.M.; Meneghini, R.M. Do Antibiotic-Impregnated Intramedullary Dowels Assist in Eradicating Infection in Total Knee Arthroplasty? J. Arthroplast. 2020, 35, S50–S52. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.C.; Foxall-Smith, M.; Roberton, A.; Beswick, A.; Kieser, D.C.; Whitehouse, M.R. The use of silver coating in hip megaprostheses: A systematic review. Hip. Int. 2019, 29, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Douglas, S.P.; Wu, G.; MacRobert, A.J.; Allan, E.; Knapp, C.E.; Parkin, I.P. A rugged, self-sterilizing antimicrobial copper coating on ultra-high molecular weight polyethylene: A preliminary study on the feasibility of an antimicrobial prosthetic joint material. J. Mater. Chem. B 2019, 7, 3310–3318. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, G.; Frenos, F.; Beltrami, G.; Campanacci, D.A.; Capanna, R. Levels of silver ions in body fluids and clinical results in silver-coated megaprostheses after tumour, trauma or failed arthroplasty. Injury 2016, 47 (Suppl. S4), S11–S16. [Google Scholar] [CrossRef]
- Sille, I.E.; Pissinis, D.E.; Fagali, N.S.; Ghilini, F.; Urrutia, M.N.; Schilardi, P.L. Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery. Pathogens 2023, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Wouthuyzen-Bakker, M.; Lowik, C.A.M.; Knobben, B.A.S.; Zijlstra, W.P.; Ploegmakers, J.J.W.; Mithoe, G.; Al Moujahid, A.; Kampinga, G.A.; Jutte, P.C. Use of gentamicin-impregnated beads or sponges in the treatment of early acute periprosthetic joint infection: A propensity score analysis. J. Antimicrob. Chemother. 2018, 73, 3454–3459. [Google Scholar] [CrossRef]
- Trentinaglia, M.T.; Van Der Straeten, C.; Morelli, I.; Logoluso, N.; Drago, L.; Romano, C.L. Economic Evaluation of Antibacterial Coatings on Healthcare Costs in First Year Following Total Joint Arthroplasty. J. Arthroplast. 2018, 33, 1656–1662. [Google Scholar] [CrossRef]

| Authors | Year | Study Design | Type of Surgery | Sample Size | Mean Age | Average Follow–Up (Months) | ||
|---|---|---|---|---|---|---|---|---|
| DAC® gel + Ab | Non DAC® | DAC® gel + Ab | Non DAC® | |||||
| Romanò et al. [56] | 2016 | RCT | Hip, knee arthroplasty—primary and revision | 189 | 184 | 71 ± 10.6 | 69 ± 12.6 | 14.5 ± 5.5 |
| Malizos et al. [64] | 2017 | RCT | Trauma | 126 | 127 | 62.5 ± 21.2 | 58.6 ± 17.6 | 18.1 ± 4.5 |
| Capuano et al. [65] | 2018 | PCCS | Septic hip arthroplasty revision (one-stage) | 22 | 22 | 71.3 ± 13.6 | 71.9 ± 8.3 | 29.3 ± 5.0 |
| Zagra et al. [66] | 2019 | RCCS | Septic hip arthroplasty revision (two-stage) uncemented | 27 | 27 | 63.9 ± 11.7 | 64.8 ± 10.1 | 32.4 ± 7.2 |
| De Meo et al. [67] | 2020 | RCCS | Aseptic hip arthroplasty revision | 17 | 17 | 74.9 ± 11.5 | 75.9 ± 9.6 | 12.4 ± 5.7 |
| Franceschini et al. [68] | 2020 | PCS | Septic hip arthroplasty revision (two-stage) uncemented | 28 | - | NR | - | 24 ± 4.0 |
| Zoccali et al. [69] | 2021 | RCOS | Arthroplasty, mega-prosthesis implant | 43 | 43 | 45.6 ± 21.3 | 47.4 ± 19.5 | 24.2 ± 11.5 |
| Corona et al. [70] | 2021 | RCS | Infected bone reconstruction | 10 | - | 52.4 ± 11.1 | - | 27.0 (range 12–50) |
| Parbonetti et al. [71] | 2021 | RCS | Vertebral surgery, treatment of degenerative spinal disorders | 73 | 61.6 ± 10.6 | - | 12 | |
| Pellegrini et al. [72] | 2022 | RCS | Septic hip arthroplasty revision (one-stage) uncemented | 10 | - | 69.4 ± 8.3 | - | 37.2 (range 24–60) |
| De Meo et al. [73] | 2023 | ROS | Trauma | 27 | - | 63.0 ± 24.84 | - | 34.41 ± 9.46 |
| Authors | Year | Intraoperative Treatment with DAC® Antibiotic-Loaded Gel and Related n. of Patients | DAC® Volume (mL) Used Mean ± SD | Main Achievements | |
|---|---|---|---|---|---|
| Type of Antibiotic | n. of Patients | ||||
| Romanò et al. [56] | 2016 | DAC® + vancomycin 5% | 100 | 8.3 ± 2.7 | Significant reduction in PJI in the DAC® antibiotic-loaded group vs. control. Eleven early surgical site infections were observed in the control group and only one in the treatment group (6% vs. 0.6%; p = 0.003). No local or systemic side effects related to the DAC® hydrogel coating were observed, and no detectable interference with implant osseointegration was observed. |
| DAC® + gentamicin 3.2% | 70 | ||||
| DAC® + vancomycin 2% + meropenem 2% | 15 | ||||
| DAC® + other associations | 4 | ||||
| Malizos et al. [64] | 2017 | DAC® + gentamicin | 78 | 5.7 ± 3.0 | Significant reduction in PJI in the DAC® antibiotic-loaded group vs. control. Six surgical site infections (4.6%) were observed in the control group compared to none in the treated group (p < 0.03). Wound healing, clinical scores, laboratory tests, and radiographic findings did not show any significant difference between the two groups. No local or systemic side-effects related to the DAC® hydrogel product were observed, and no detectable interference with bone healing was noted. |
| DAC® + vancomycin | 46 | ||||
| DAC® + vancomycin + meropenem | 2 | ||||
| Capuano et al. [65] | 2018 | DAC® + vancomycin 5% | 14 | 10.2 ± 1.3 | Two patients (9.1%) in the DAC® group showed an infection recurrence, in comparison to three patients (13.6%) in the two-stage group. No significant differences were observed comparing the two groups. Clinical scores were similar between groups, while the average hospital stay and antibiotic treatment duration were significantly reduced after one-stage treatment with DAC®, compared to two-stage (18.9 ± 2.9 vs. 35.8 ± 3.4 and 23.5 ± 3.3 versus 53.7 ± 5.6 days, respectively). |
| DAC® + vancomycin + meropenem | 8 | ||||
| Zagra et al. [66] | 2019 | DAC® + vancomycin 5% | 17 | 10.2 ± 1.3 | No evidence of infection, implant loosening, or adverse events was observed in the DAC®-treated group, compared to four cases of infection recurrence in the control group. |
| DAC® + teicoplanin 2.5% and ceftazidime 2.5% | 1 | ||||
| DAC + vancomycin and rifampicin | 1 | ||||
| DAC + vancomycin and meropenem | 7 | ||||
| De Meo et al. [67] | 2020 | DAC® + vancomycin 2.5% | 13 | NR | No PJIs were reported in the DAC®-treated group, whereas six cases were observed in the matching control group (p < 0.0001). No significant differences were reported with regard to prosthetic osseointegration and functional results, nor were there side effects in the DAC® treatment group. |
| DAC® + vancomycin 2.5% and gentamicin 2% | 4 | ||||
| Franceschini et al. [68] | 2020 | NR | 28 | 10 | Authors have found two early failures/re-infections after the 2-staged protocol. Both occurred during the first 3 weeks after implantation. Patients underwent revision with implant removal and re-implantation during the same procedure. The remaining 26 patients did not show clinical, laboratory signs of reinfection after the last follow-up. No loosening or failure of uncemented implants was recorded. |
| Zoccali C et al. [69] | 2021 | DAC® + gentamicin 3% | 23 | 9.4 ± 6.5 | No evidence of infection or adverse events was observed in the DAC®-treated group, compared to six cases of post-surgical infection in the control group (p = 0.028). |
| DAC® + vancomycin 5% | 9 | ||||
| DAC + other associations | 11 | ||||
| Corona et al. [70] | 2021 | DAC® + vancomycin and gentamicin (concentration NR) | 10 | NR | Distal femur implants were used in a two-stage strategy, together with DAC®. At follow-up, limb salvage was achieved in all patients in this series of limb-threatening infected femoral injuries. No patient (10/10) presented signs of recurrence of the infection at the end of the follow-up. |
| Parbonetti et al. [71] | 2021 | DAC® + gentamicin 5% | 73 | 10.0 | At 12 months of follow-up, no infection was recorded in the overall population. None of the patients reported significant pain or functional limitations after surgery. Post-surgically, computed tomography scans confirmed the correct positioning of the instruments. |
| Pellegrini et al. [72] | 2022 | DAC® + vancomycin 5% and gentamicin 5% | 10 | 5.0 | None of the patients had clinical or radiographic symptoms of recurrent infection. A follow-up examination showed significant improvements in all variables [range of motion, Harris Hip Score (HHS), visual analogue scale (VAS) pain score] compared to pre-operative values (p < 0.05). Through radiographs, complete osseointegration of the implant was observed without progressive radiolucent lines or change in the position of the implant. |
| De Meo et al. [73] | 2023 | DAC® + vancomycin 2.5% | 27 | 7.5 ± 3.5 | Only one case (2.94%) showed the onset of FRI at 5 months after surgery. Local antibiotic prophylaxis by coating resulted in a reduction in the incidence of FRI, as compared to the estimated preoperative risk. The use of the DAC® antibiotic-loaded gel allows for the choice of antibiotic. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pressato, D.; Battista, A.; Govoni, M.; Vivarelli, L.; Dallari, D.; Pellegrini, A. The Intraoperative Use of Defensive Antibacterial Coating (DAC®) in the Form of a Gel to Prevent Peri-Implant Infections in Orthopaedic Surgery: A Clinical Narrative Review. Materials 2023, 16, 5304. https://doi.org/10.3390/ma16155304
Pressato D, Battista A, Govoni M, Vivarelli L, Dallari D, Pellegrini A. The Intraoperative Use of Defensive Antibacterial Coating (DAC®) in the Form of a Gel to Prevent Peri-Implant Infections in Orthopaedic Surgery: A Clinical Narrative Review. Materials. 2023; 16(15):5304. https://doi.org/10.3390/ma16155304
Chicago/Turabian StylePressato, Daniele, Angela Battista, Marco Govoni, Leonardo Vivarelli, Dante Dallari, and Antonio Pellegrini. 2023. "The Intraoperative Use of Defensive Antibacterial Coating (DAC®) in the Form of a Gel to Prevent Peri-Implant Infections in Orthopaedic Surgery: A Clinical Narrative Review" Materials 16, no. 15: 5304. https://doi.org/10.3390/ma16155304
APA StylePressato, D., Battista, A., Govoni, M., Vivarelli, L., Dallari, D., & Pellegrini, A. (2023). The Intraoperative Use of Defensive Antibacterial Coating (DAC®) in the Form of a Gel to Prevent Peri-Implant Infections in Orthopaedic Surgery: A Clinical Narrative Review. Materials, 16(15), 5304. https://doi.org/10.3390/ma16155304









