Porous Rod-like NiTiO3-BiOBr Heterojunctions with Highly Improved Visible-Light Photocatalytic Performance
Abstract
:1. Introduction
2. Experiments
2.1. Synthesis of NiTiO3
2.2. Synthesis of NiTiO3-BiOBr Composites
2.3. Characterization
2.4. Photocatalytic Activity Experiments
3. Results and Discussion
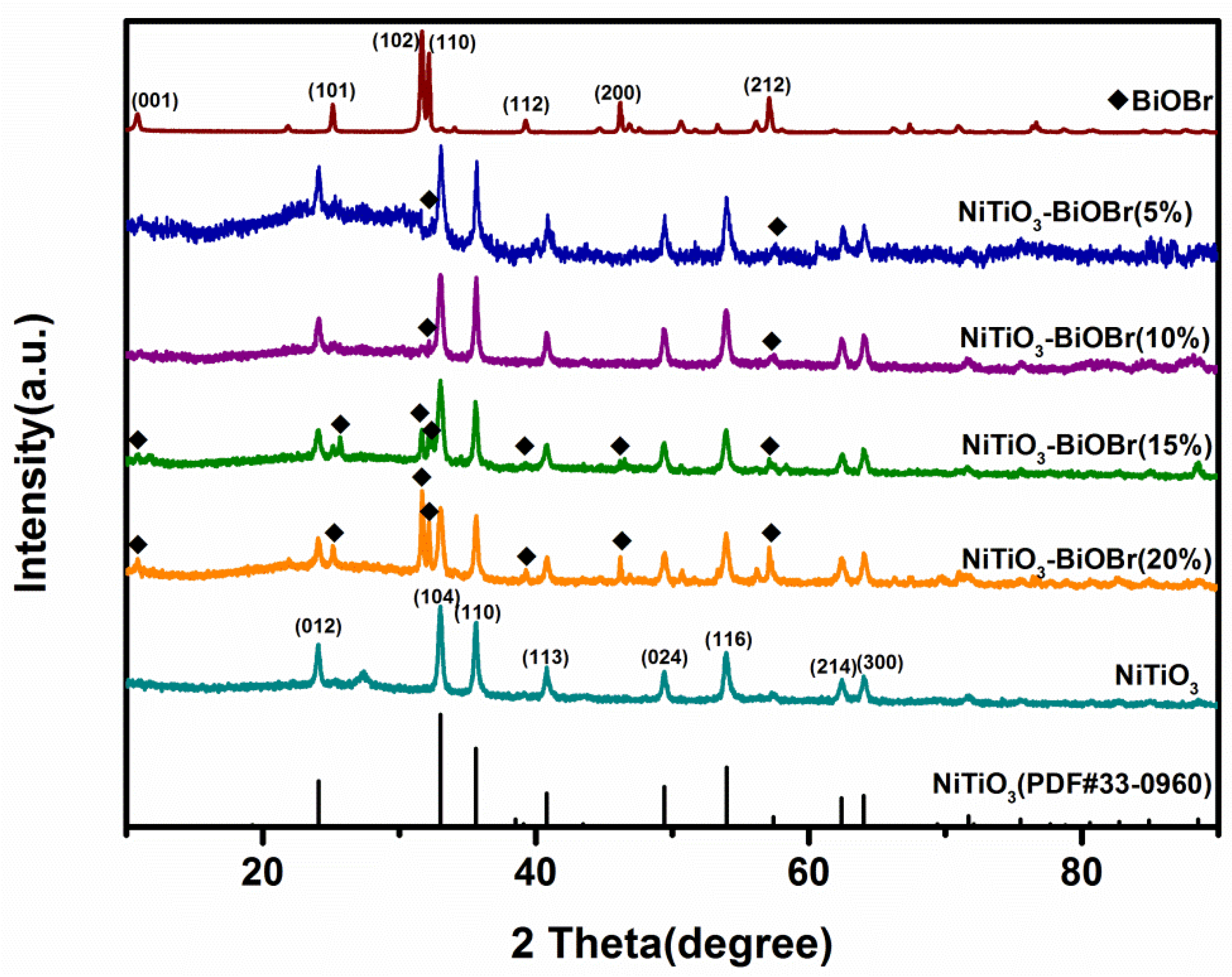
3.1. XRD Analysis
3.2. SEM and TEM Analysis
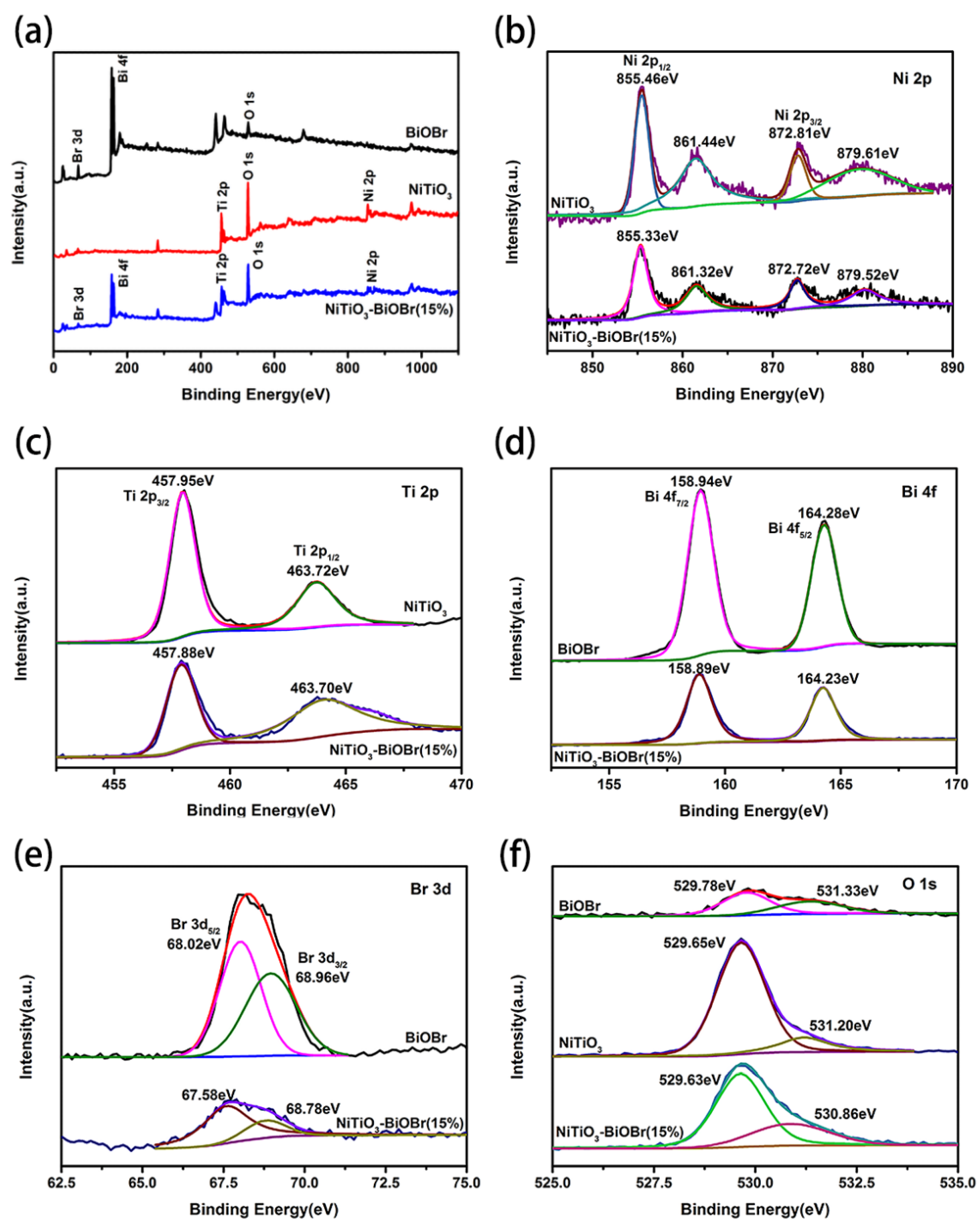
3.3. XPS Analysis
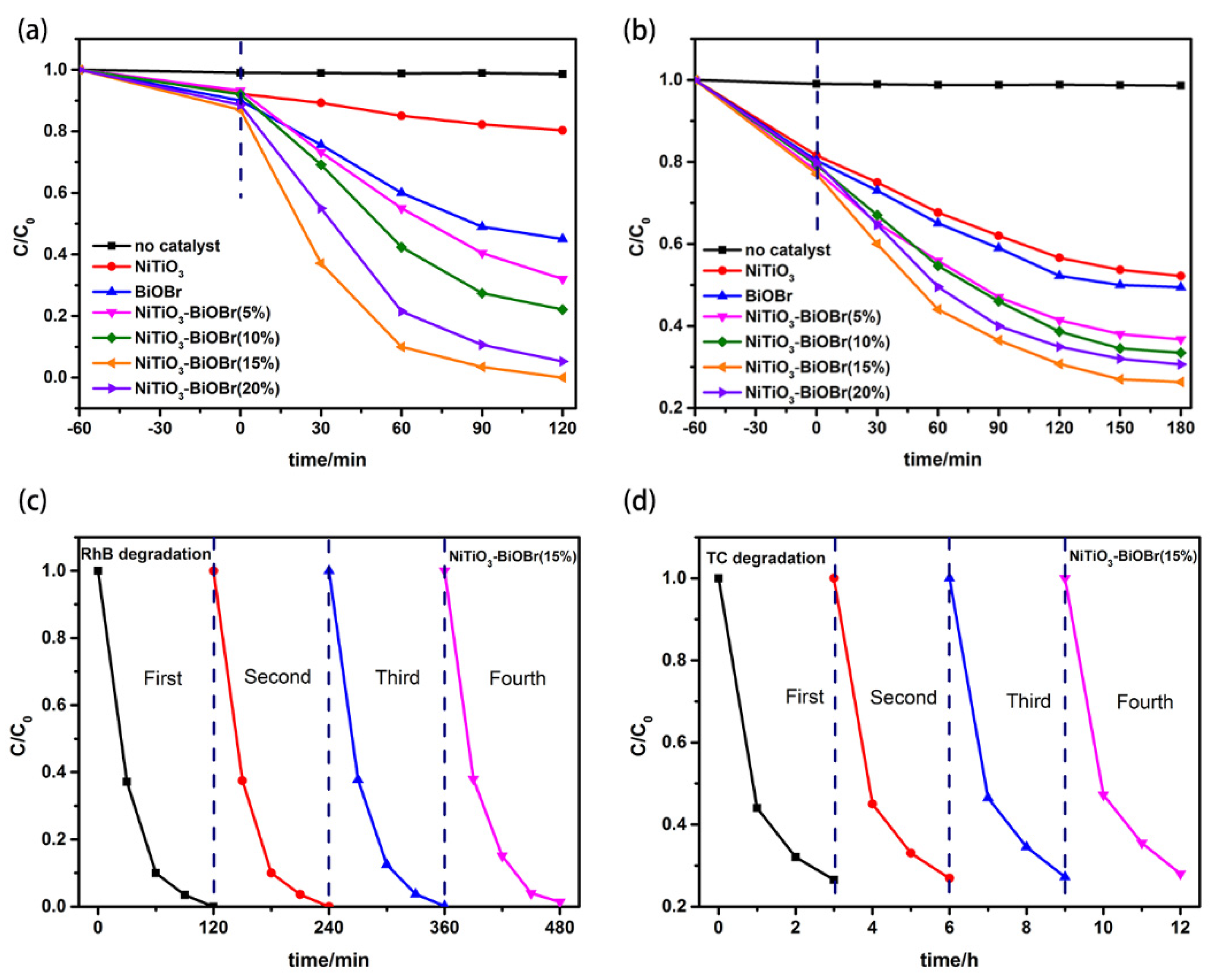
3.4. Photocatalytic Properties
3.5. Photocatalytic Mechanism Discussion
3.5.1. UV-Vis DRS Analysis
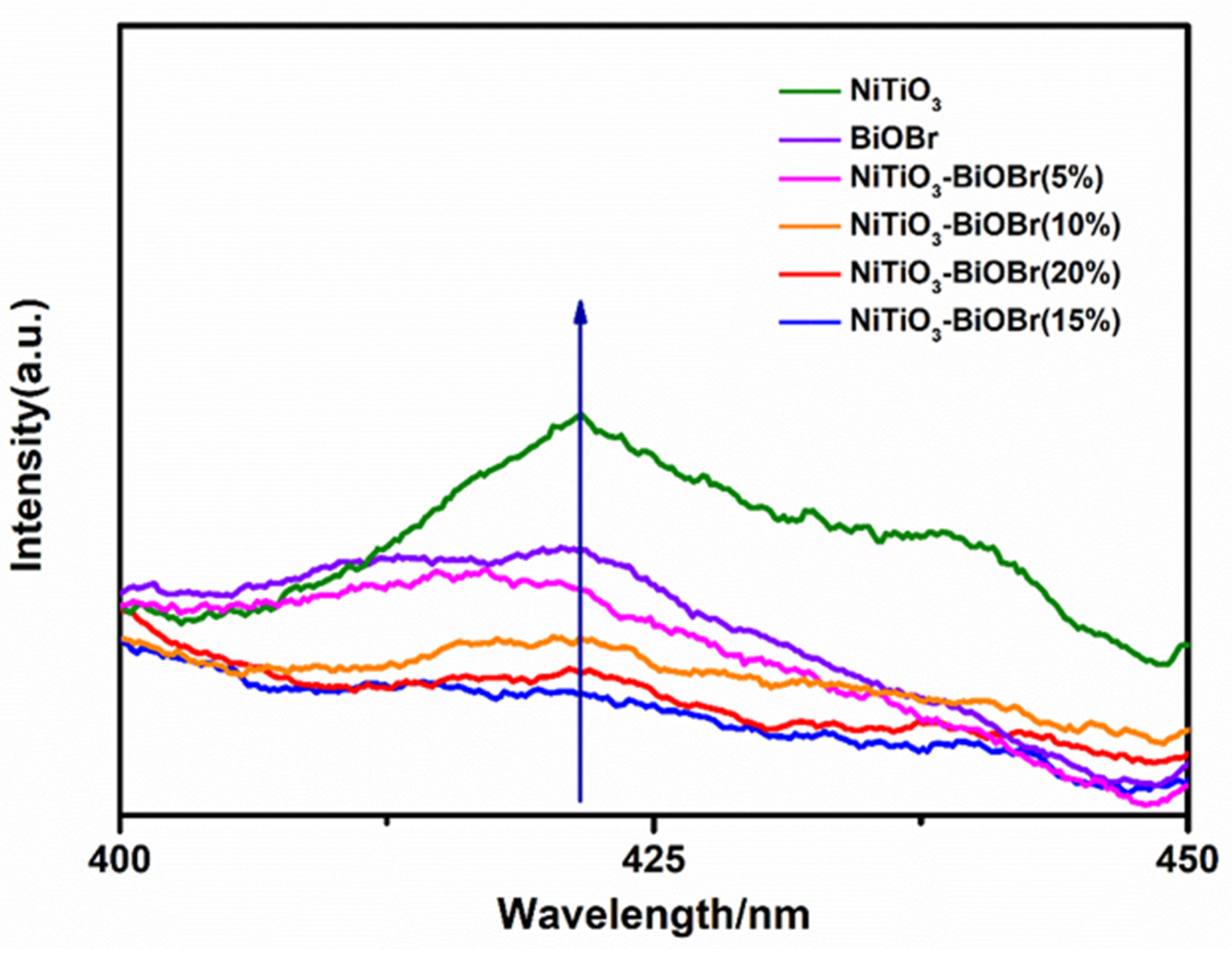
3.5.2. PL Emission Spectra
3.5.3. Free Radical Capture Tests
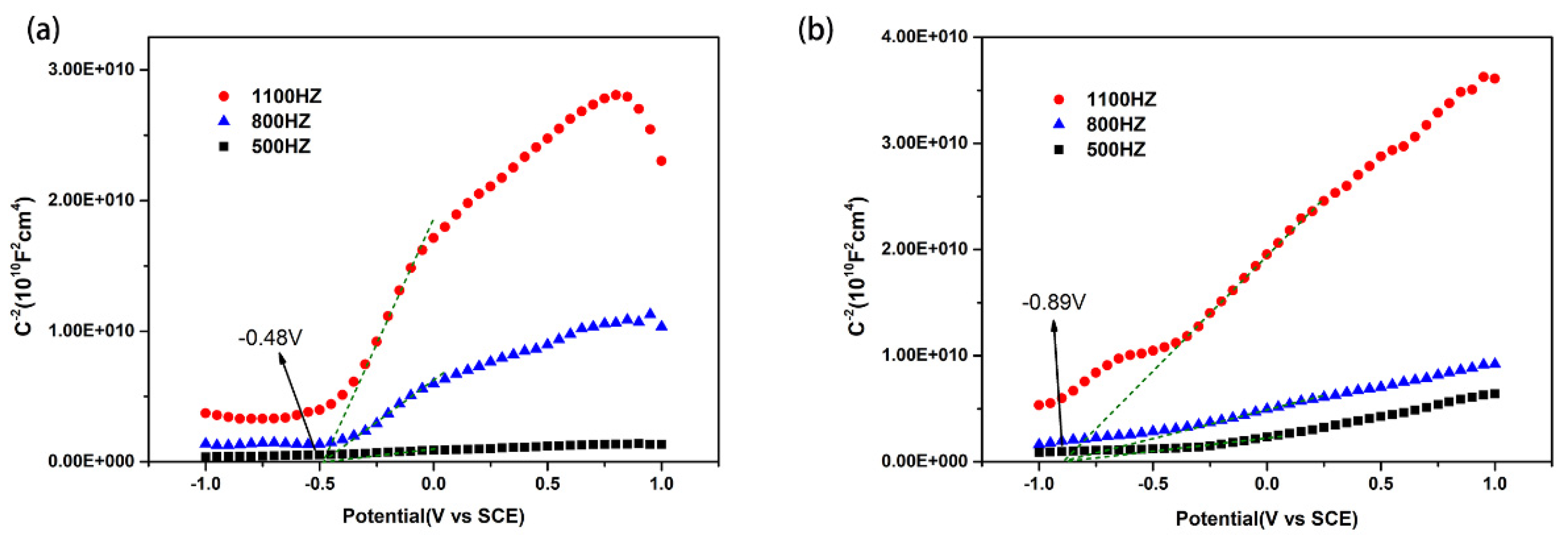
3.5.4. Band Position Determination
3.6. Photocatalytic Mechanism of NiTiO3-BiOBr Photocatalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, K.Y.; Zhou, H.L.; Li, X.Y.; Ma, X.H.; Zhang, D.H.; Li, M.C. The novel 2-dimensional Bi2MoO6-Bi2O3-Ag3PO4 ternary photocatalystwith n-n-p heterojunction for enhanced degradation performance. J. Alloys Compd. 2022, 913, 165119. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.; Salama, T.; Falaras, P. Silver decorated TiO2/g-C3N4 bifunctional nanocomposites for photocatalytic elimination of water pollutants under UV and artificial solar light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Fu, K.; Pan, Y.; Ding, C.; Shi, J.; Deng, H. Photocatalytic degradation of naproxen by Bi2MoO6/g-C3N4 heterojunction photocatalyst under visible light: Mechanisms, degradation pathway, and DFT calculation. J. Photochem. Photobiol. A Chem. 2021, 412, 113235. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhao, W.L.; Zhai, Z.C.; Ren, K.X.; Wang, T.Y.; Guan, H.; Shi, H.F. 2D/2D Bi2MoO6/g-C3N4 S-scheme heterojunction photocatalyst with enhanced visible-light activity by Au loading. J. Mater. Sci. Technol. 2020, 56, 216–226. [Google Scholar] [CrossRef]
- Falara, P.; Ibrahim, I.; Zourou, A.; Sygellou, L.; Sanchez, D.E.; Romanos, G.; Givalou, L.; Antoniadou, M.; Arfanis, M.; Han, C.; et al. Bi-functional photocatalytic heterostructures combining titania thin films with carbon quantum dots (C-QDs/TiO2) for effective elimination of water pollutants. Environ. Sci. Pollut. Res. Int. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Wang, G.R.; Wang, Y.B.; Jin, Z.L. Phosphating 2D CoAl LDH anchored on 3D self-assembled NiTiO3 hollow rods for efficient hydrogen evolution. Catal. Sci. Technol. 2020, 10, 2931–2947. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.H.; Han, C.H.; Zhou, X.J.; Li, X.W.; Liang, Y.; Liu, S.; Shao, C.L.; Liu, Y.C. Ternary NiTiO3@g-C3N4-Au nanofibers with a synergistic Z-scheme core@shell interface and dispersive Schottky contact surface for enhanced solar photocatalytic activity. Mater. Chem. Front. 2021, 5, 2730–2741. [Google Scholar] [CrossRef]
- Trang, N.T.T.; Khang, D.M.; Dung, D.D.; Trung, N.N.; Phuong, N.T.; Bac, L.H. Synthesis of Ilmenite NiTiO3 Rods and Effect of pH on Rhodamine B Textile Dye Degradation under LED Visible-Light Irradiation. J. Electeon. Mater. 2021, 50, 7188–7197. [Google Scholar] [CrossRef]
- Absalan, Y.; Bratchikova, I.; Kovalchukova, O. Accurate investigation to determine the best conditions for using NiTiO3 for bromophenol blue degradation in the environment under UV–vis light based on concentration reduction and to compare it with TiO2. Environ. Nanotechnol. Monit. Manag. 2017, 8, 244–253. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.Z.; Wang, H.; Chen, X.H.; Wu, Z.B.; Jiang, L.B.; Xiong, W.P.; Zhang, Y.X.; Zeng, G.M. One-step calcination method for synthesis of mesoporous g-C3N4/NiTiO3 heterostructure photocatalyst with improved visible light photoactivity. RSC Adv. 2015, 5, 95643–95648. [Google Scholar] [CrossRef]
- Peng, D.X.; Wang, Y.T.; Shi, H.F.; Jiang, W.; Jin, T.; Jin, Z.H.; Chen, Z. Fabrication of novel Cu2WS4/NiTiO3 heterostructures for efficient visible-light photocatalytic hydrogen evolution and pollutant degradation. J. Colloid Interface Sci. 2022, 613, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, G.R.; Gong, H.M.; Jin, Z.L. Phosphated 2D MoS2 nanosheets and 3D NiTiO3 nanorods for efficient photocatalytic hydrogen evolution. ChemCatChem 2020, 12, 5492–5503. [Google Scholar] [CrossRef]
- Li, Z.Z.; Zhang, H.G.; Wang, L.; Meng, X.C.; Shi, J.J.; Qi, C.X.; Zhang, Z.S.; Feng, L.J.; Li, C.H. 2D/2D BiOBr/Ti3C2 heterojunction with dual applications in both waterdetoxification and water splitting. J. Photochem. Photobiol. A Chem. 2020, 386, 112099. [Google Scholar] [CrossRef]
- Li, X.B.; Xiong, J.; Gao, X.M.; Ma, J.; Chen, Z.; Kang, B.B.; Liu, J.Y.; Li, H.; Feng, Z.J.; Huang, J.T. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.L.; Liu, X.T.; Li, H.D.; Ren, C.J.; Li, X.Y.; Li, W.J.; Lian, Z.Q.; Zhang, M. Engineering design of hierarchical g-C3N4@Bi/BiOBr ternary heterojunction with Z-scheme system for efficient visible-light photocatalytic performance. J. Alloys Compd. 2019, 798, 741–749. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, H.B.; Muhammad, Y.; Fu, T.; Tang, R.; Tong, Z.F.; Wang, Y. Fabrication of n-n isotype BiOBr-Bi2WO6 heterojunctions by inserting Bi2WO6 nanosheets onto BiOBr microsphere for the superior photocatalytic degradation of Ciprofloxacin and tetracycline. Sep. Purif. Technol. 2021, 274, 118992–119005. [Google Scholar] [CrossRef]
- Hao, L.; Ju, P.; Zhang, Y.; Sun, C.J.; Dou, K.P.; Liao, D.K.; Zhai, X.F.; Lu, Z.X. Novel plate-on-plate hollow structured BiOBr/Bi2MoO6 p-n heterojunctions: In-situ chemical etching preparation and highly improved photocatalytic antibacterial activity. Sep. Purif. Technol. 2022, 298, 121666–121682. [Google Scholar] [CrossRef]
- Fu, S.; Yuan, W.; Liu, X.M.; Yan, Y.H.; Liu, H.P.; Li, L.; Zhao, F.Y.; Zhou, J.G. A novel 0D/2D WS2/BiOBr heterostructure with rich oxygen vacancies for enhanced broad-spectrum photocatalytic performance. J. Colloid Interface Sci. 2020, 569, 150–163. [Google Scholar] [CrossRef]
- Yan, Q.S.; Guo, Z.Y.; Wang, P.Y.; Cheng, Y.N.; Wu, C.Y.; Zuo, H.R. Facile construction of 0D/2D In2O3/Bi2WO6 Z-scheme heterojunction with enhanced photocatalytic activity for antibiotics removal. J. Alloys Compd. 2023, 937, 168362. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.Y.; Du, S.C.; Meng, X.Y.; Tian, G.H.; Pan, K.; Wang, G.F.; Fu, H.G. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471–16476. [Google Scholar] [CrossRef]
- Kim, S.R.; Jo, W.K. Application of a photostable silver-assisted Z-scheme NiTiO3 nanorod/g-C3N4 nanocomposite for efficient hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 801–808. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Xiong, X.Y.; Ding, S.P.; Liu, X.F.; Jiang, Q.Q.; Hu, J.C. In-situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures. Appl. Catal. B Environ. 2018, 220, 570–580. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, B.J.; Chen, M.; Zhang, H.; Peng, J.B.; Ding, L.; Wang, W.F. Simple synthesis of BiOAc/BiOBr heterojunction composites for the efficient photocatalytic removal of organic pollutants. Sep. Purif. Technol. 2021, 261, 118286. [Google Scholar] [CrossRef]
- Qu, X.F.; Liu, M.H.; Zhang, W.X.; Sun, Z.; Meng, W.; Shi, L.; Du, F.L. A facile route to construct NiTiO3/Bi4NbO8Cl heterostructures for enhanced photocatalytic water purification. J. Mater. Sci. 2020, 55, 9330–9342. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen-Huy, C.; Shin, E.W. NiTiO3/reduced graphene oxide materials synthesized by a two-step microwave-assisted method. Mater. Lett. 2016, 184, 38–42. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Hafeez, H.Y.; Veluswamy, P.; Ganesh, V.; Khan, A.; Ikeda, H.; Neppolian, B. Enhanced photocatalytic degradation and hydrogen production activity of in situ grown TiO2 coupled NiTiO3 nanocomposites. Appl. Surf. Sci. 2018, 449, 790–798. [Google Scholar] [CrossRef]
- Jin, Y.M.; Shen, X.F.; Liu, Z.X.; Wang, Z.J.; Zhu, B.; Xu, P.F.; Luo, L.; Zhang, L.S. Synthesis of NiTiO3-Bi2MoO6 core-shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. New J. Chem. 2018, 42, 411–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Murugananthan, M.; Zhang, Y.R. Development of novel α-Fe2O3/NiTiO3 heterojunction nanofibers material with enhanced visible-light photocatalytic performance. J. Alloys Compd. 2015, 630, 110–116. [Google Scholar] [CrossRef]
- Zarazúa-Morín, M.E.; Galindo-Luna, A.S.; Gallegos-Sánchez, V.J.; Zermeño-Resendiz, B.B.; Torres-Martínez, L.M. Novel hydrothermal-assisted microwave synthesis of NiTiO3/ZnO and sonophotocatalytic effect for degradation of rhodamine B. Top. Catal. 2022, 65, 1182–1190. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Peng, J.W.; Feng, X.; Ding, Z.X.; Li, Z.H. Wide spectrum responsive CdS/NiTiO3/CoS with superior photocatalytic performance for hydrogen evolution. Catal. Sci. Technol. 2017, 7, 2524–2530. [Google Scholar] [CrossRef]
- Wang, Z.L.; Huo, Y.; Zhang, J.F.; Lu, C.; Dai, K.; Liang, C.H.; Zhu, G.P. Facile preparationof two-dimensional Bi2MoO6@Ag2MoO4 core-shell composite with enhancedvisible light photocatalytic activity. J. Alloys Compd. 2017, 729, 100–108. [Google Scholar] [CrossRef]
- Hu, T.P.; Yang, Y.; Dai, K.; Zhang, J.F.; Liang, C.H. A novel Z-scheme Bi2MoO6/BiOBr photocatalyst for enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2018, 456, 473–481. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, G.R.; Gong, H.M.; Jin, Z.L. Hollow Nanorods and Amorphous Co9S8 Quantum Dots Construct S-Scheme Heterojunction for Efficient Hydrogen Evolution. J. Phys. Chem. C 2021, 125, 648–659. [Google Scholar] [CrossRef]
- Li, B.F.; Wang, W.J.; Zhao, J.W.; Wang, Z.Y.; Su, B.; Hou, Y.D.; Ding, Z.X.; Ong, W.J.; Wang, S.B. All-solid-state direct Z-scheme NiTiO3/Cd0.5Zn0.5S heterostructures for photocatalytic hydrogen evolution with visible light. J. Mater. Chem. A 2021, 9, 10270–10276. [Google Scholar] [CrossRef]
- Pham, T.T.; Shin, E.W. Inhibition of charge recombination of NiTiO3 photocatalyst by the combination of Mo-doped impurity state and Z-scheme charge transfer. Appl. Surf. Sci. 2020, 501, 143992. [Google Scholar] [CrossRef]
- Tang, C.N.; Liu, E.Z.; Fan, J.; Hu, X.Y.; Ma, Y.N.; Wan, J. Graphitic-C3N4-hybridized Ag3PO4 tetrahedron with reactive{111} facets to enhance the visible-light photocatalytic activity. RSC Adv. 2015, 5, 91979–91987. [Google Scholar] [CrossRef]






| Photocatalysts | C0/mg·L−1 | Time/min | Dosage of Catalyst/g·L−1 | Efficiency/% | Refs. |
|---|---|---|---|---|---|
| NiTiO3-Bi4NbO8Cl | 5 | 90 | 1.0 | ~50 | [24] |
| NiTiO3-GO | 10 | 90 | 0.2 | ~90 | [25] |
| NiTiO3-TiO2 | - | 90 | 1.0 | ~62 | [26] |
| NiTiO3-Bi2MoO6 | 10 | 90 | 0.6 | ~92 | [27] |
| NiTiO3-α-Fe2O3 | 3 | 90 | 0.1 | ~75 | [28] |
| NiTiO3-ZnO | 5 ppm | 120 | 0.5 | 95 | [29] |
| NiTiO3-BiOBr | 20 | 90 | 1.0 | 96.6 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Li, M.; Zhou, H.; Ma, X.; Li, W. Porous Rod-like NiTiO3-BiOBr Heterojunctions with Highly Improved Visible-Light Photocatalytic Performance. Materials 2023, 16, 5033. https://doi.org/10.3390/ma16145033
Sun K, Li M, Zhou H, Ma X, Li W. Porous Rod-like NiTiO3-BiOBr Heterojunctions with Highly Improved Visible-Light Photocatalytic Performance. Materials. 2023; 16(14):5033. https://doi.org/10.3390/ma16145033
Chicago/Turabian StyleSun, Kaiyue, Mengchao Li, Hualei Zhou, Xiaohui Ma, and Wenjun Li. 2023. "Porous Rod-like NiTiO3-BiOBr Heterojunctions with Highly Improved Visible-Light Photocatalytic Performance" Materials 16, no. 14: 5033. https://doi.org/10.3390/ma16145033
APA StyleSun, K., Li, M., Zhou, H., Ma, X., & Li, W. (2023). Porous Rod-like NiTiO3-BiOBr Heterojunctions with Highly Improved Visible-Light Photocatalytic Performance. Materials, 16(14), 5033. https://doi.org/10.3390/ma16145033





