Effect of Aluminosilicates’ Particle Size Distribution on the Microstructural and Mechanical Properties of Metakaolinite-Based Geopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aluminosilicates with Different Particle Size Distribution
2.3. Preparation of Geopolymers
2.4. Analytical and Testing Methods
3. Results and Discussion
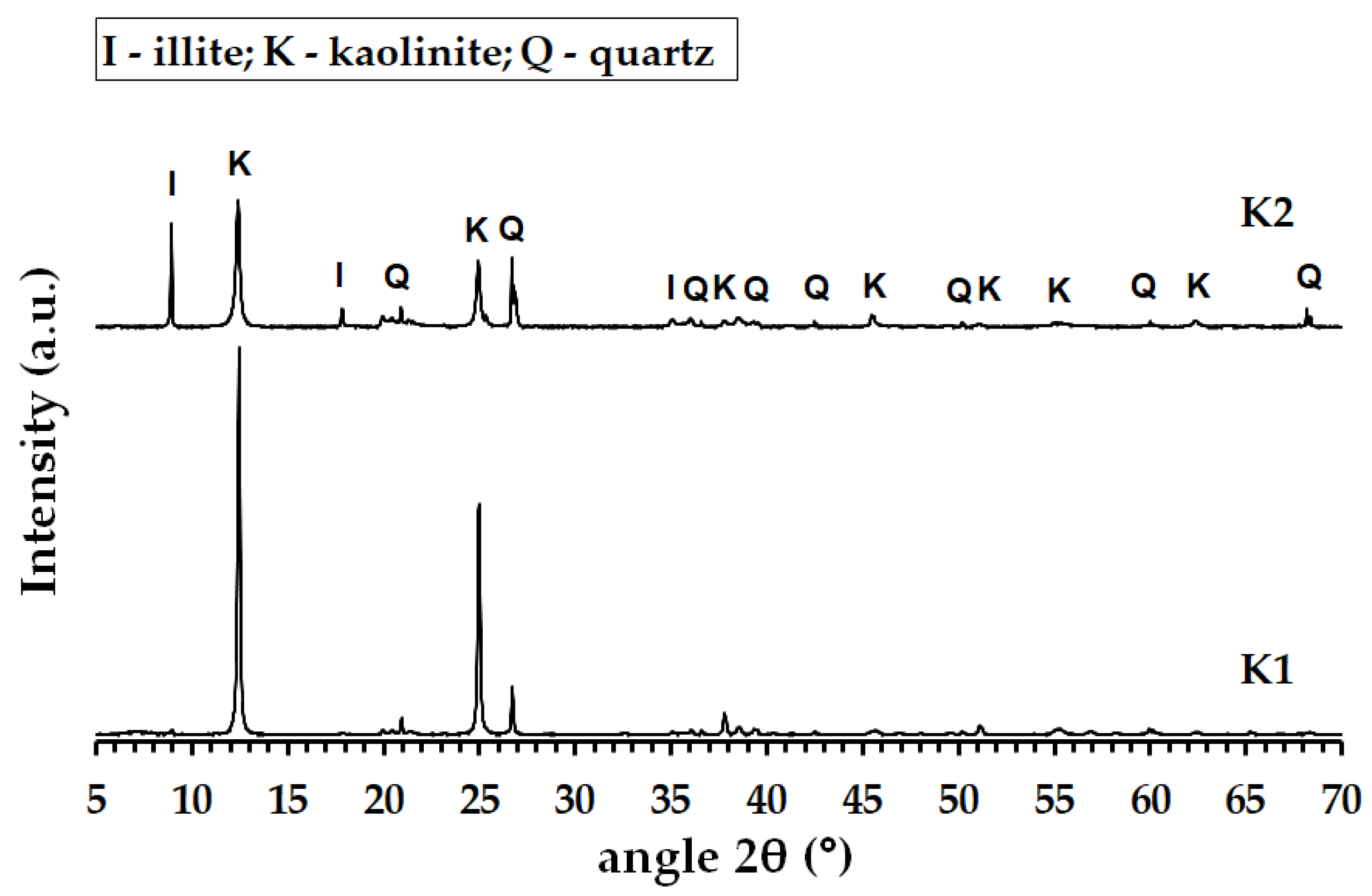
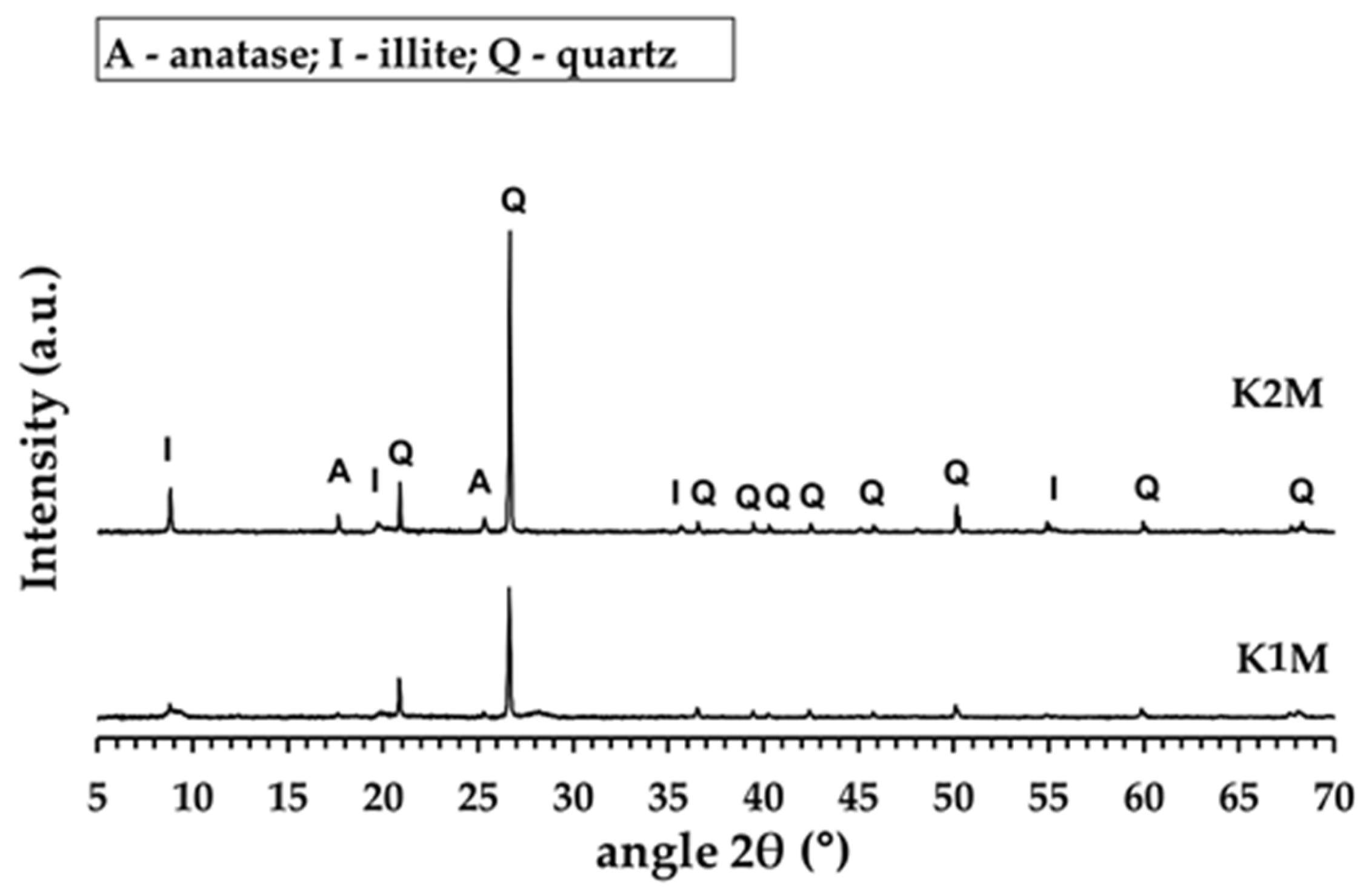
3.1. Properties of Calcined and Milled Aluminosilicates
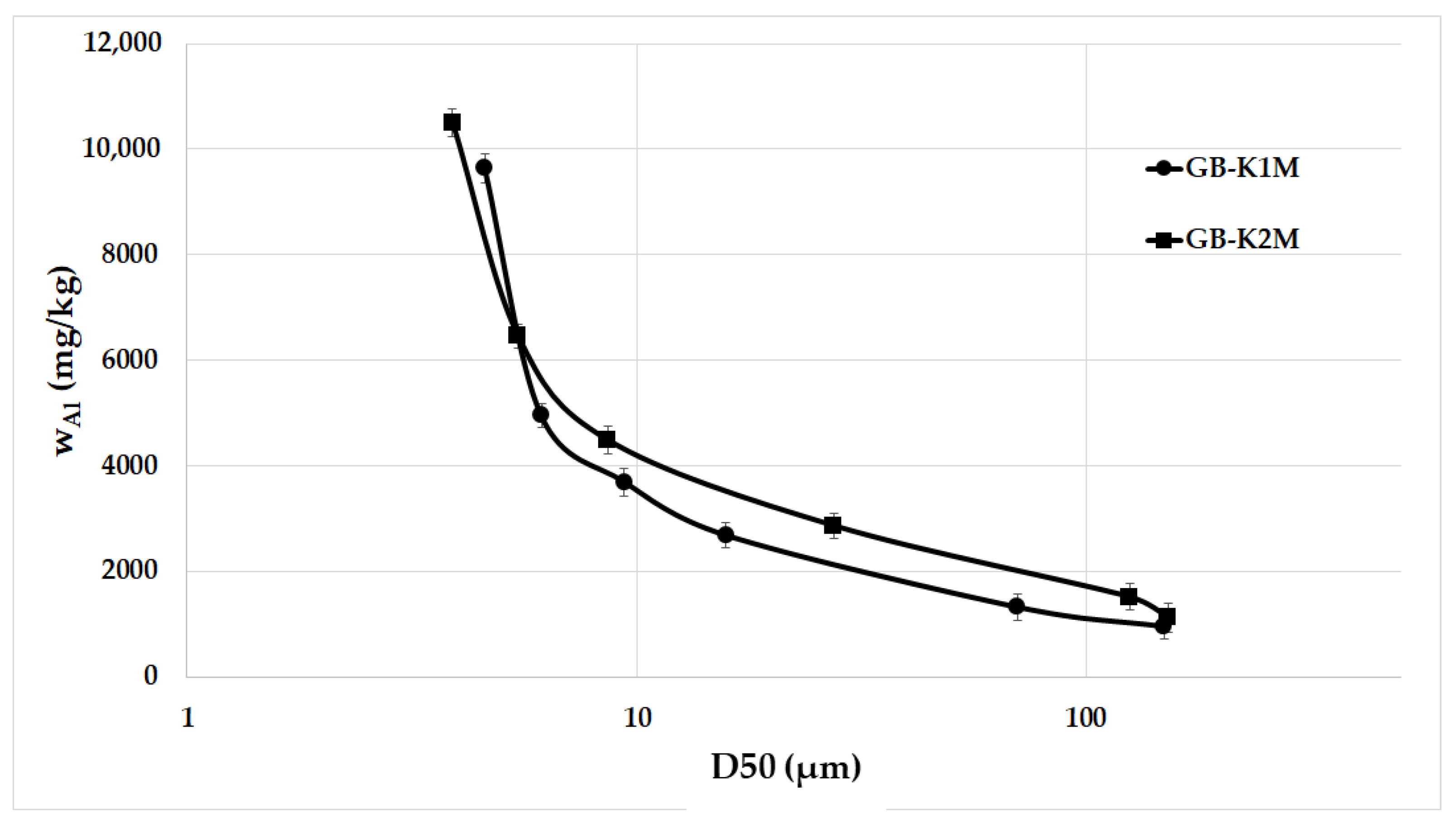
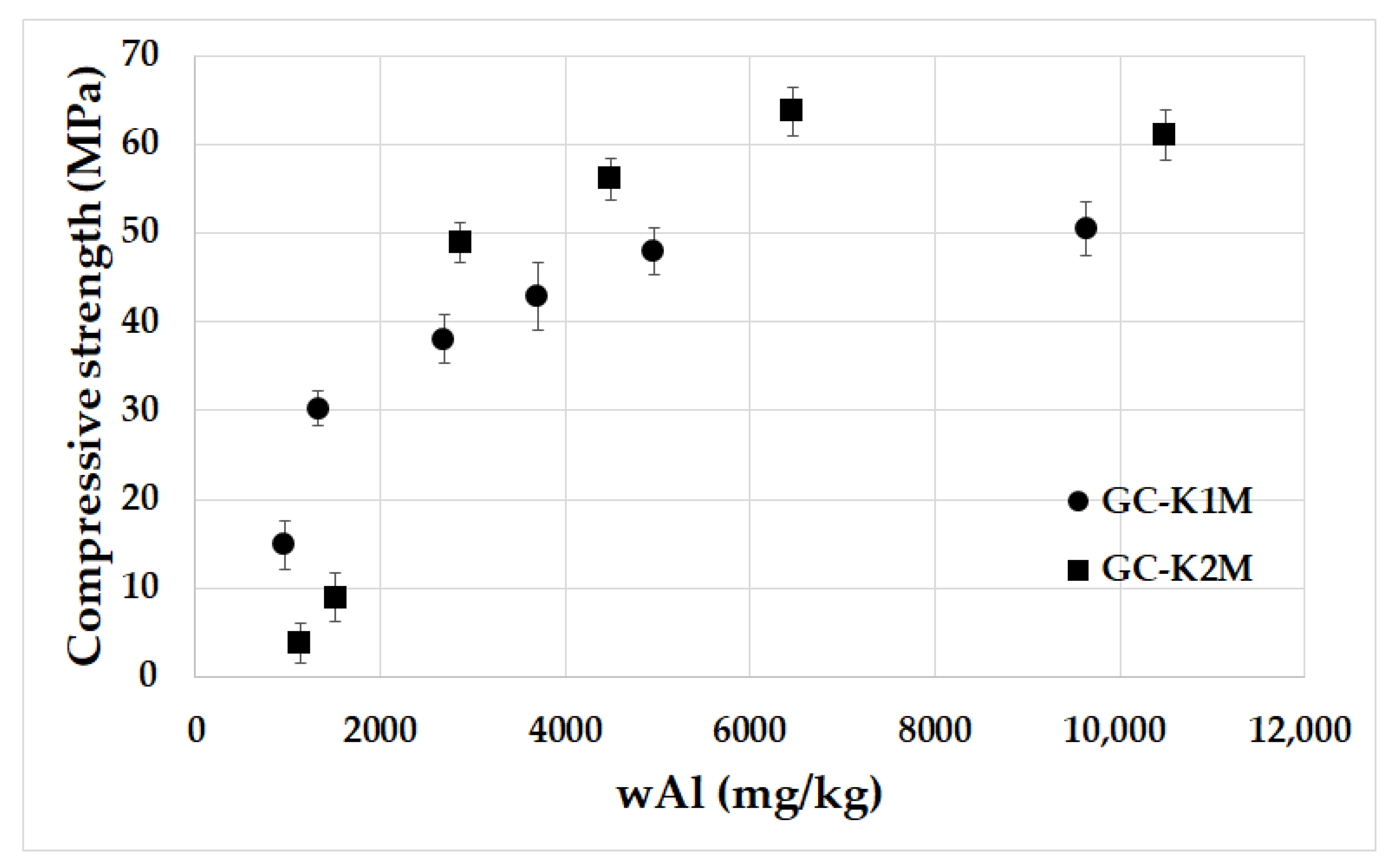
3.2. Leachability Test
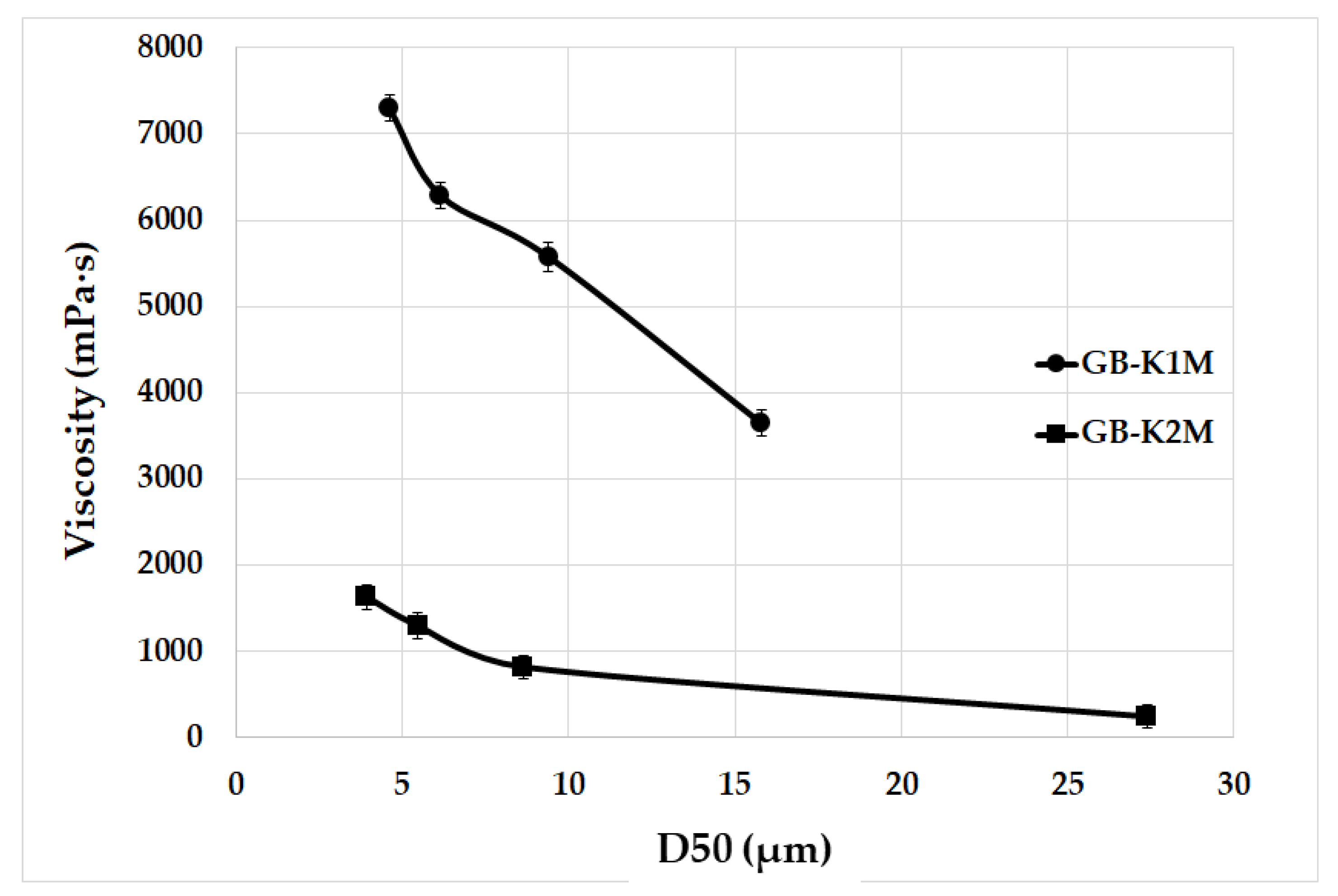
3.3. Rheological Properties
3.4. Determination of Setting Time
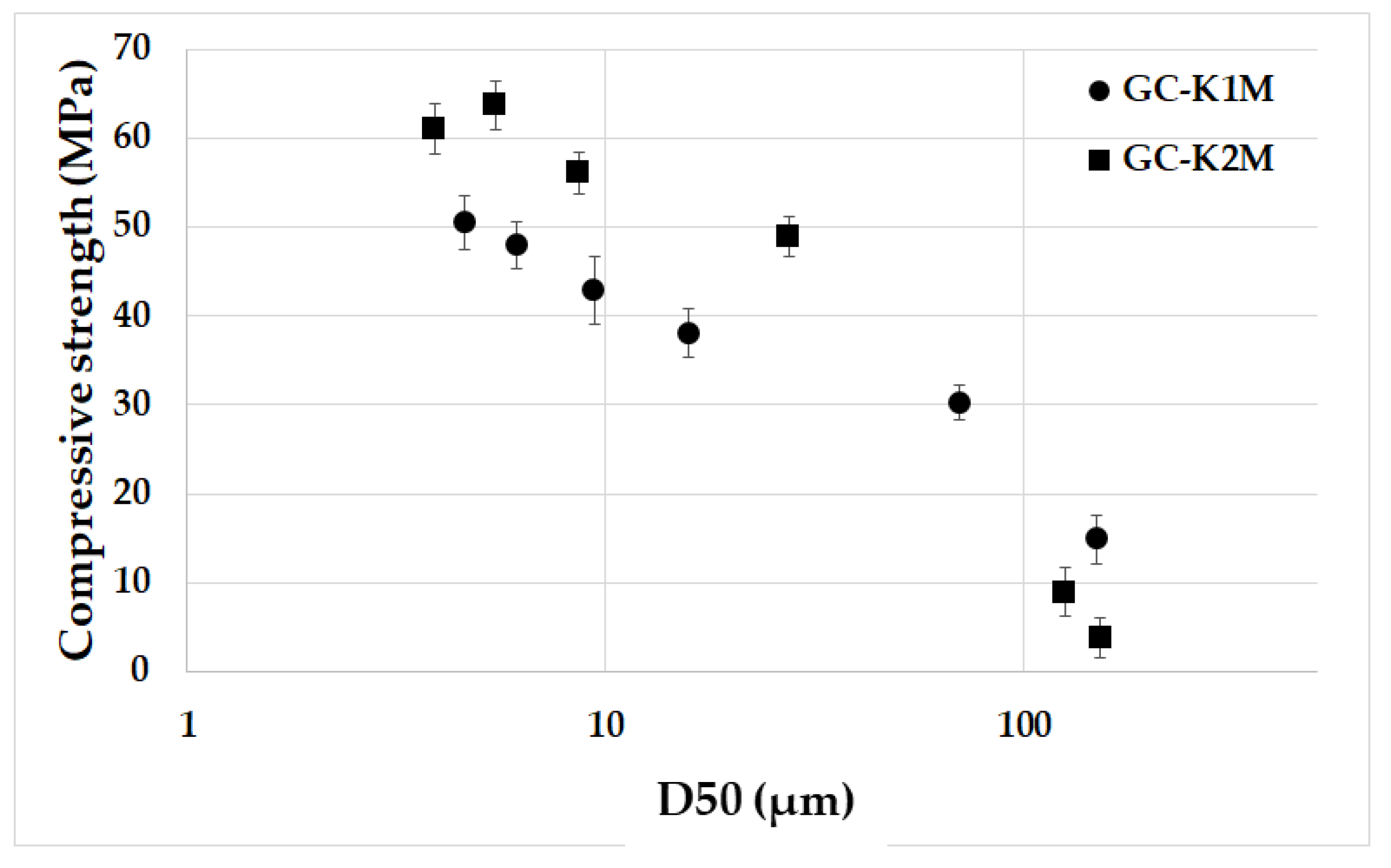
3.5. Mechanical Properties
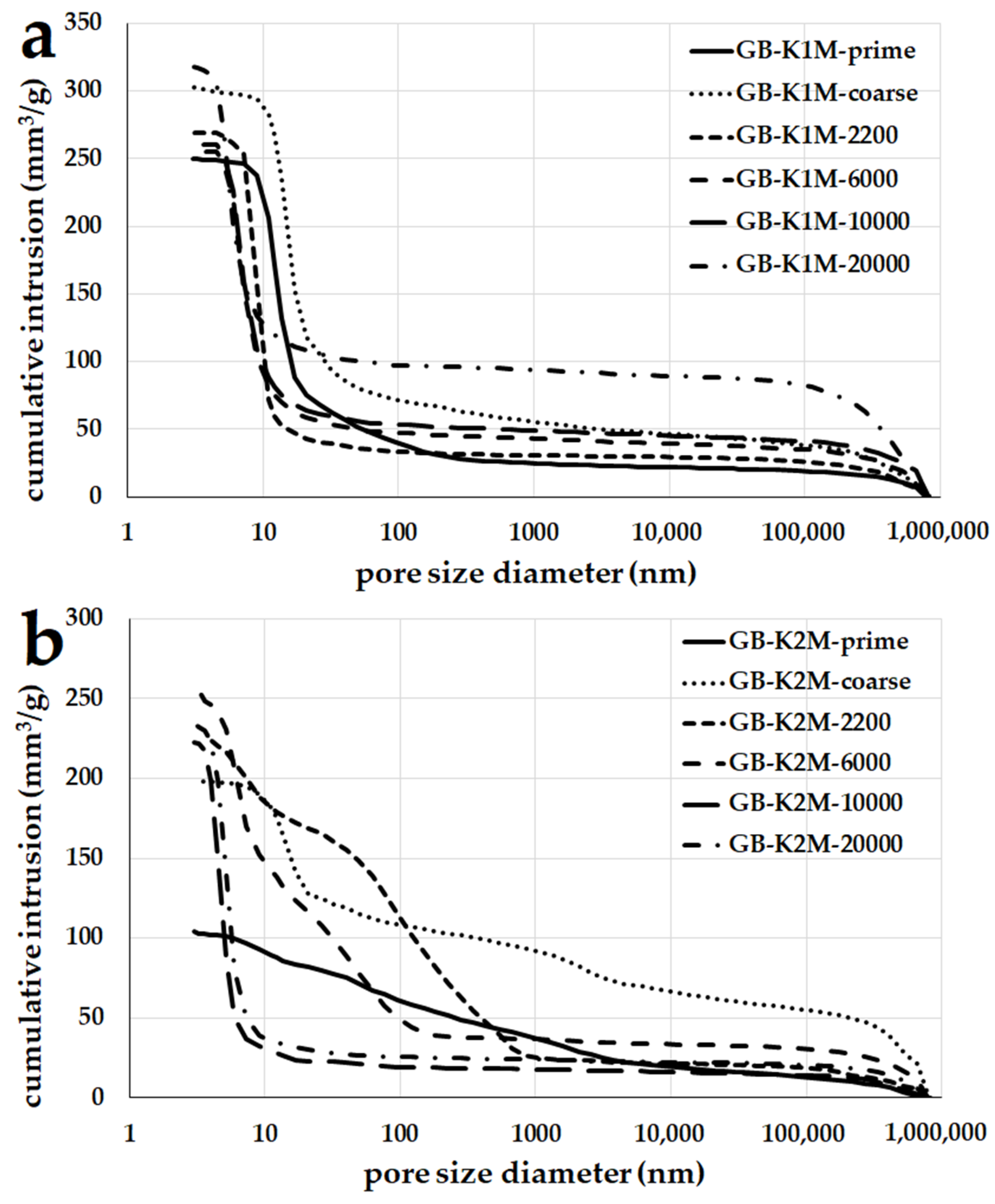
3.6. Porosity
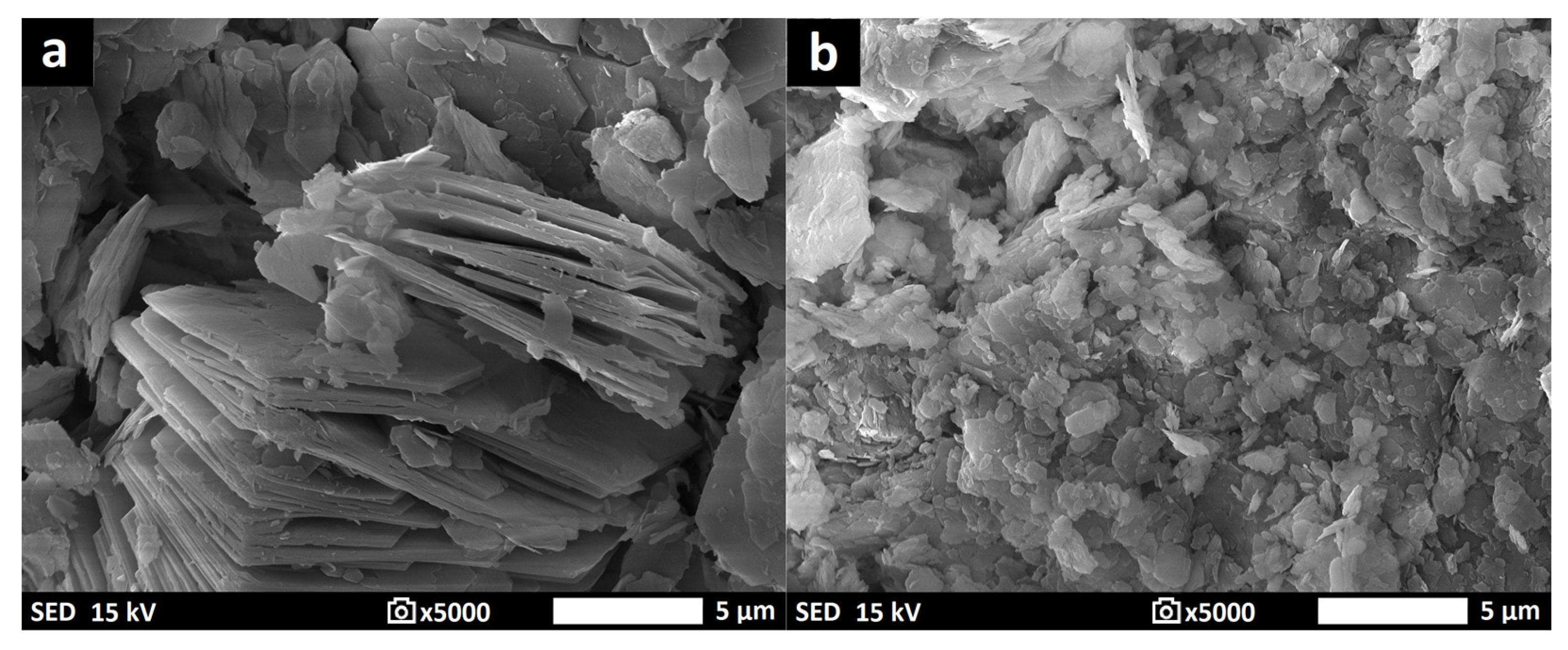
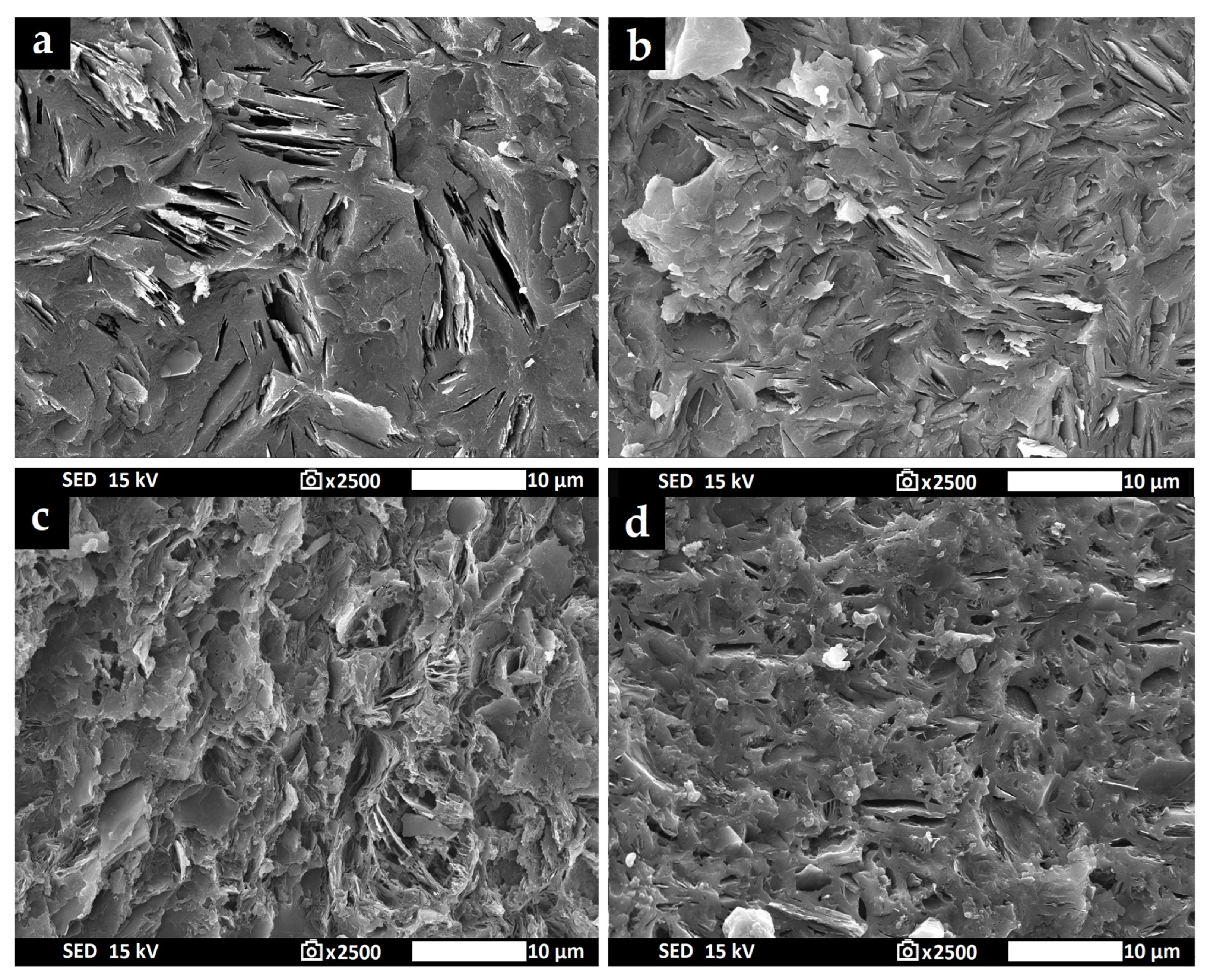
3.7. Morphology
4. Conclusions
- -
- The Al leaching rate increased significantly as the average particle size decreased, leading to a decrease in bulk density;
- -
- The dynamic viscosity of fresh geopolymer binders decreased as the mean particle size increased. Geopolymer binders prepared from calcined kaolin had much lower dynamic viscosity compared to binders prepared from calcined kaolinitic claystones;
- -
- Smaller particle sizes resulted in a shorter initial setting time for the hardening process of geopolymer binders;
- -
- The influence of particle size on the mechanical properties of geopolymer composites was observed up to a mean particle size of 10 µm. Beyond that size, mechanical properties decreased as the mean particle size increased;
- -
- Geopolymer binders prepared from calcined kaolin had mesopores up to 10 nm, while binders from calcined kaolinitic claystone with larger particle sizes contained pores ranging from 100 to 1000 nm. Average pore diameter decreased as the average particle size decreased;
- -
- The studied geopolymer binders, with different particle size distributions, showed no significant differences in their morphological structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Chemistry and Applications; Institut Géopolymère: Saint-Quentin, France, 2008; p. 285. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2013, 47, 11–25. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Huiskes, D.M.A.; Keulen, A.; Yu, Q.L.; Brouwers, H.J.H. Design and performance evaluation of ultra-lightweight geopolymer concrete. Mater. Des. 2016, 89, 516–526. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Hanzlíček, T.; Steinerová, M.; Straka, P.; Perná, I.; Siegl, P.; Švarcová, T. Reinforcement of the terracotta sculpture by geopolymer composite. Mater. Des. 2009, 30, 3229–3234. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, T.; Deng, L.; Li, Y.; Guo, H.; Zhou, J.; Li, L.; Peng, Y. Ion-adsorption type rare earth tailings for preparation of alkali-based geopolymer with capacity for heavy metals immobilization. Cem. Concr. Compos. 2022, 134, 104768. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Li, Z.; Wang, C. Pb2+ and Cr3+ immobilization efficiency and mechanism in red-mud-based geopolymer grouts. Chemosphere 2023, 321, 138129. [Google Scholar] [CrossRef]
- Supamathanon, N.; Boonserm, K.; Lisnund, S.; Chanlek, N.; Rungtaweevoranit, B.; Khemthong, P.; Wittayakun, J.; Osakoo, N. Development of CaO supported on modified geopolymer catalyst for transesterification of soybean oil to biodiesel. Mater. Today Commun. 2021, 29, 102822. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, T.; Wan, Q.; Zheng, D. Immobilization of vanadium and nickel in spent fluid catalytic cracking (SFCC) catalysts-based geopolymer. J. Clean. Prod. 2022, 332, 130112. [Google Scholar] [CrossRef]
- Li, P.; Yang, T.; Ma, P.; Fei, X.; Li, F.; Ye, J.; Zhuang, P. Luminous and bonding performance of self-luminescent cementitious coatings based on white cement and geopolymer. Constr. Build. Mater. 2023, 362, 129814. [Google Scholar] [CrossRef]
- Yang, N.; Das, C.S.; Xue, X.; Li, W.; Dai, J.-G. Geopolymer coating modified with reduced graphene oxide for improving steel corrosion resistance. Constr. Build. Mater. 2022, 342, 127942. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. 3D concrete printing of eco-friendly geopolymer containing brick waste. Cem. Concr. Compos. 2023, 138, 104943. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, M. 3D printing geopolymers: A review. Cem. Concr. Compos. 2022, 128, 104455. [Google Scholar] [CrossRef]
- Haincova, E.; Hajkova, P.; Kohout, J. Prepregs for Temperature Resistant Composites. Materials 2019, 12, 4012. [Google Scholar] [CrossRef]
- Haincova, E.; Hajkova, P. Effect of Boric Acid Content in Aluminosilicate Matrix on Mechanical Properties of Carbon Prepreg Composites. Materials 2020, 13, 5409. [Google Scholar] [CrossRef]
- Kohoutova, E.; Hajkova, P.; Kohout, J.; Soukup, A. Effect of Potassium Phosphate Content in Aluminosilicate Matrix on Mechanical Properties of Carbon Prepreg Composites. Materials 2021, 15, 61. [Google Scholar] [CrossRef]
- Kohout, J.; Koutník, P.; Bezucha, P.; Kwoczynski, Z. Leachability of the metakaolinite-rich materials in different alkaline solutions. Mater. Today Commun. 2019, 21, 100669. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2011, 47, 2782–2797. [Google Scholar] [CrossRef]
- Gonçalves, D.K.C.; Lana, S.L.B.; Sales, R.B.C.; Aguilar, M.T.P. Study of metakaolins with different amorphities and particle sizes activated by KOH and K2SiO3. Case Stud. Constr. Mater. 2022, 16, e00778. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J. Effect of the Alkali Metal Activator on the Properties of Fly Ash-Based Geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspars. Colloids Surf. A Physicochem. Eng. Asp. 2003, 216, 27–44. [Google Scholar] [CrossRef]
- da Silva Rocha, T.; Dias, D.P.; França, F.C.; de Salles Guerra, R.R.; de Oliveira Marques, L.R.d.C. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly Ash-Based geopolymer concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Xiong, L.; Wan, Z.; Zhang, Y.; Wang, F.; Wang, J.; Kang, Y. Fly Ash Particle Size Effect on Pore Structure and Strength of Fly Ash Foamed Geopolymer. Adv. Polym. Technol. 2019, 2019, 1098027. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Chinje Melo, U.F.; Delplancke, M.-P.; Rahier, H. Influence of the activating solution composition on the stability and thermo-mechanical properties of inorganic polymers (geopolymers) from volcanic ash. Constr. Build. Mater. 2013, 48, 278–286. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Melo, U.F.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Rieger, D.; Kovářík, T.; Říha, J.; Medlín, R.; Novotný, P.; Bělský, P.; Kadlec, J.; Holba, P. Effect of thermal treatment on reactivity and mechanical properties of alkali activated shale–slag binder. Constr. Build. Mater. 2015, 83, 26–33. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Tashima, M.M. 18—Reuse of aluminosilicate industrial waste materials in the production of alkali-activated concrete binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Oxford, UK, 2015; pp. 487–518. [Google Scholar]
- Thang, N.H.; Nhung, L.T.; Quyen, P.V.T.H.; Phong, D.T.; Khe, D.T.; Van Phuc, N. Development of heat resistant geopolymer-based materials from red mud and rice husk ash. AIP Conf. Proc. 2018, 1954, 040005. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Huynh, T.-P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Kohout, J.; Koutnik, P.; Hajkova, P.; Kohoutova, E.; Soukup, A. Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers 2021, 13, 3754. [Google Scholar] [CrossRef]
- Lapeyre, J.; Ma, H.; Kumar, A. Effect of particle size distribution of metakaolin on hydration kinetics of tricalcium silicate. J. Am. Ceram. Soc. 2019, 102, 5976–5988. [Google Scholar] [CrossRef]
- Rovnanik, P.; Safrankova, K. Thermal Behaviour of Metakaolin/Fly Ash Geopolymers with Chamotte Aggregate. Materials 2016, 9, 535. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Kuenzel, C.; Neville, T.P.; Donatello, S.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83–84, 308–314. [Google Scholar] [CrossRef]
- Kohout, J.; Koutnik, P.; Hajkova, P.; Kohoutova, E.; Soukup, A. Effect of Different Types of Aluminosilicates on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers 2022, 14, 4838. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Rowles, M.R.; O’Connor, B.H. Chemical and Structural Microanalysis of Aluminosilicate Geopolymers Synthesized by Sodium Silicate Activation of Metakaolinite. J. Am. Ceram. Soc. 2009, 92, 2354–2361. [Google Scholar] [CrossRef]
- Yan, D.; Xie, L.; Qian, X.; Ruan, S.; Zeng, Q. Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials 2020, 13, 2973. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Kayali, O. Effect of initial water content and curing moisture conditions on the development of fly ash-based geopolymers in heat and ambient temperature. Constr. Build. Mater. 2014, 67, 20–28. [Google Scholar] [CrossRef]
- Koutník, P.; Soukup, A.; Bezucha, P.; Šafář, J.; Kohout, J. Low viscosity metakaolinite based geopolymer binders. Constr. Build. Mater. 2020, 230, 116978. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Aredes, F.G.M.; Campos, T.M.B.; Machado, J.P.B.; Sakane, K.K.; Thim, G.P.; Brunelli, D.D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Matrix design of strain hardening fiber reinforced engineered geopolymer composite. Compos. Part B Eng. 2016, 89, 253–265. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Liu, C.; Gao, Y. Feasibility study of red mud for geopolymer preparation: Effect of particle size fraction. J. Mater. Cycles Waste Manag. 2020, 22, 1328–1338. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, J.; Zhang, C.; Chen, J.; Liu, C. Effect of particle size and thermal activation on the coal gangue based geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Sevim, Ö.; Demir, İ. Optimization of fly ash particle size distribution for cementitious systems with high compactness. Constr. Build. Mater. 2019, 195, 104–114. [Google Scholar] [CrossRef]
- Escalera, E.; Antti, M.L.; Odén, M. Thermal treatment and phase formation in kaolinite and illite based clays from tropical regions of Bolivia. IOP Conf. Ser. Mater. Sci. Eng. 2012, 31, 012017. [Google Scholar] [CrossRef]
- Boháč, M.; Novotný, R.; Frajkorová, F.; Yadav, R.S.; Opravil, T.; Palou, M. Properties of Cement Pastes with Different Particle Size Fractions of Metakaolin. Int. J. Mater. Metall. Eng. 2015, 9, 301–305. [Google Scholar]
- Koutnik, P. Comparison of Kaolin and Kaolinitic Claystones as Raw Materials for Preparing Meta-Kaolinite-Based Geopolymers. J. Ceram.-Silik. 2019, 63, 110–123. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The mechanism of geopolymer gel formation investigated through seeded nucleation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 97–105. [Google Scholar] [CrossRef]
- Kuenzel, C.; Li, L.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of sand on the mechanical properties of metakaolin geopolymers. Constr. Build. Mater. 2014, 66, 442–446. [Google Scholar] [CrossRef]
- Pelisser, F.; Guerrino, E.L.; Menger, M.; Michel, M.D.; Labrincha, J.A. Micromechanical characterization of metakaolin-based geopolymers. Constr. Build. Mater. 2013, 49, 547–553. [Google Scholar] [CrossRef]
- Latella, B.A.; Perera, D.S.; Durce, D.; Mehrtens, E.G.; Davis, J. Mechanical properties of metakaolin-based geopolymers with molar ratios of Si/Al ≈ 2 and Na/Al ≈ 1. J. Mater. Sci. 2008, 43, 2693–2699. [Google Scholar] [CrossRef]






| Material | Material Composition (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a LOI | H2O | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | ZrO2 | SO3 | Cr2O3 | |
| K1 | 12.28 | - | 34.7 | 50.7 | 0.58 | 0.13 | 0.45 | - | 0.74 | 0.37 | 0.01 | 0.01 | 0.04 | 0.01 |
| K2 | 11.88 | - | 38.1 | 46.6 | 0.67 | 0.16 | 0.13 | - | 0.75 | 1.31 | 0.05 | 0.03 | 0.28 | 0.02 |
| Potassium silicate | - | 62.18 | 0.04 | 25.2 | 0.75 | - | - | 0.25 | 12.4 | - | - | - | - | - |
| Material | Specific Gravity | BET Surface Area | Pore Volume | Average Pore Size |
|---|---|---|---|---|
| (m2/g) | V (mm3/g) | R (nm) | ||
| K1 | 2630 | 14.8 | 314 | 106.6 |
| K2 | 2 640 | 11.1 | 58 | 19.7 |
| Material | a LOI | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | ZnO | K2O | TiO2 | P2O5 | ZrO2 | SO3 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1M | 0.35 | 38.8 | 58.1 | 0.74 | 0.17 | 0.52 | 0.01 | 0.84 | 0.48 | 0.01 | 0.01 | 0.04 | 0.01 |
| K2M | 0.35 | 41.8 | 53.9 | 0.86 | 0.17 | 0.11 | 0.01 | 0.89 | 1.55 | 0.06 | 0.03 | 0.24 | 0.03 |
| Material | Specific Gravity | BET Surface Area | Bulk Density | Particle Size | ||
|---|---|---|---|---|---|---|
| (m2/g) | (kg/m3) | D10 (µm) | D50 (µm) | D90 (µm) | ||
| K1M-coarse | 2650 | 11.6 | 741 | 18.3 | 149 | 523 |
| K1M-prime | 2660 | 12.1 | 557 | 5.64 | 70.3 | 318 |
| K1M-2200 | 2810 | 12.5 | 393 | 4.14 | 15.8 | 111 |
| K1M-6000 | 2710 | 12.8 | 277 | 3.49 | 9.40 | 27.4 |
| K1M-10000 | 2610 | 13.1 | 246 | 2.92 | 6.14 | 13.2 |
| K1M-20000 | 2670 | 13.3 | 219 | 2.30 | 4.60 | 9.74 |
| K2M-coarse | 2620 | 10.3 | 1276 | 31.2 | 152 | 551 |
| K2M-prime | 2610 | 10.8 | 1170 | 6.66 | 125 | 221 |
| K2M-2200 | 2850 | 11.6 | 690 | 3.97 | 27.4 | 79.3 |
| K2M-6000 | 2810 | 12.0 | 488 | 2.88 | 8.62 | 21.2 |
| K2M-10000 | 2990 | 13.7 | 400 | 2.40 | 5.45 | 12.6 |
| K2M-20000 | 2950 | 14.7 | 326 | 1.93 | 3.90 | 9.29 |
| Measurement Conditions | Sample | Setting Time (min) | ||
|---|---|---|---|---|
| Intial | Final | Real | ||
| 25 °C, 95% humidity | GB-K1M-coarse | 470 | 640 | 170 |
| GB-K1M- prime | 507 | 572 | 65 | |
| GB-K1M-2200 | 491 | 526 | 35 | |
| GB-K1M-6000 | 464 | 504 | 40 | |
| GB-K1M-10000 | 404 | 454 | 50 | |
| GB-K1M-20000 | 390 | 436 | 46 | |
| GB-K2M-coarse | 290 | 320 | 30 | |
| GB-K2M-prime | 290 | 315 | 25 | |
| GB-K2M-2200 | 330 | 420 | 90 | |
| GB-K2M-6000 | 325 | 375 | 50 | |
| GB-K2M-10000 | 222 | 266 | 44 | |
| GB-K2M-20000 | 220 | 255 | 35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohout, J.; Koutník, P.; Hájková, P.; Kohoutová, E.; Soukup, A.; Vakili, M. Effect of Aluminosilicates’ Particle Size Distribution on the Microstructural and Mechanical Properties of Metakaolinite-Based Geopolymers. Materials 2023, 16, 5008. https://doi.org/10.3390/ma16145008
Kohout J, Koutník P, Hájková P, Kohoutová E, Soukup A, Vakili M. Effect of Aluminosilicates’ Particle Size Distribution on the Microstructural and Mechanical Properties of Metakaolinite-Based Geopolymers. Materials. 2023; 16(14):5008. https://doi.org/10.3390/ma16145008
Chicago/Turabian StyleKohout, Jan, Petr Koutník, Pavlína Hájková, Eliška Kohoutová, Aleš Soukup, and Mohammadtaghi Vakili. 2023. "Effect of Aluminosilicates’ Particle Size Distribution on the Microstructural and Mechanical Properties of Metakaolinite-Based Geopolymers" Materials 16, no. 14: 5008. https://doi.org/10.3390/ma16145008
APA StyleKohout, J., Koutník, P., Hájková, P., Kohoutová, E., Soukup, A., & Vakili, M. (2023). Effect of Aluminosilicates’ Particle Size Distribution on the Microstructural and Mechanical Properties of Metakaolinite-Based Geopolymers. Materials, 16(14), 5008. https://doi.org/10.3390/ma16145008






