Development of Al/Mg Bimetal Processed by Ultrasonic Vibration-Assisted Compound Casting: Effects of Ultrasonic Vibration Treatment Duration Time
Abstract
1. Introduction
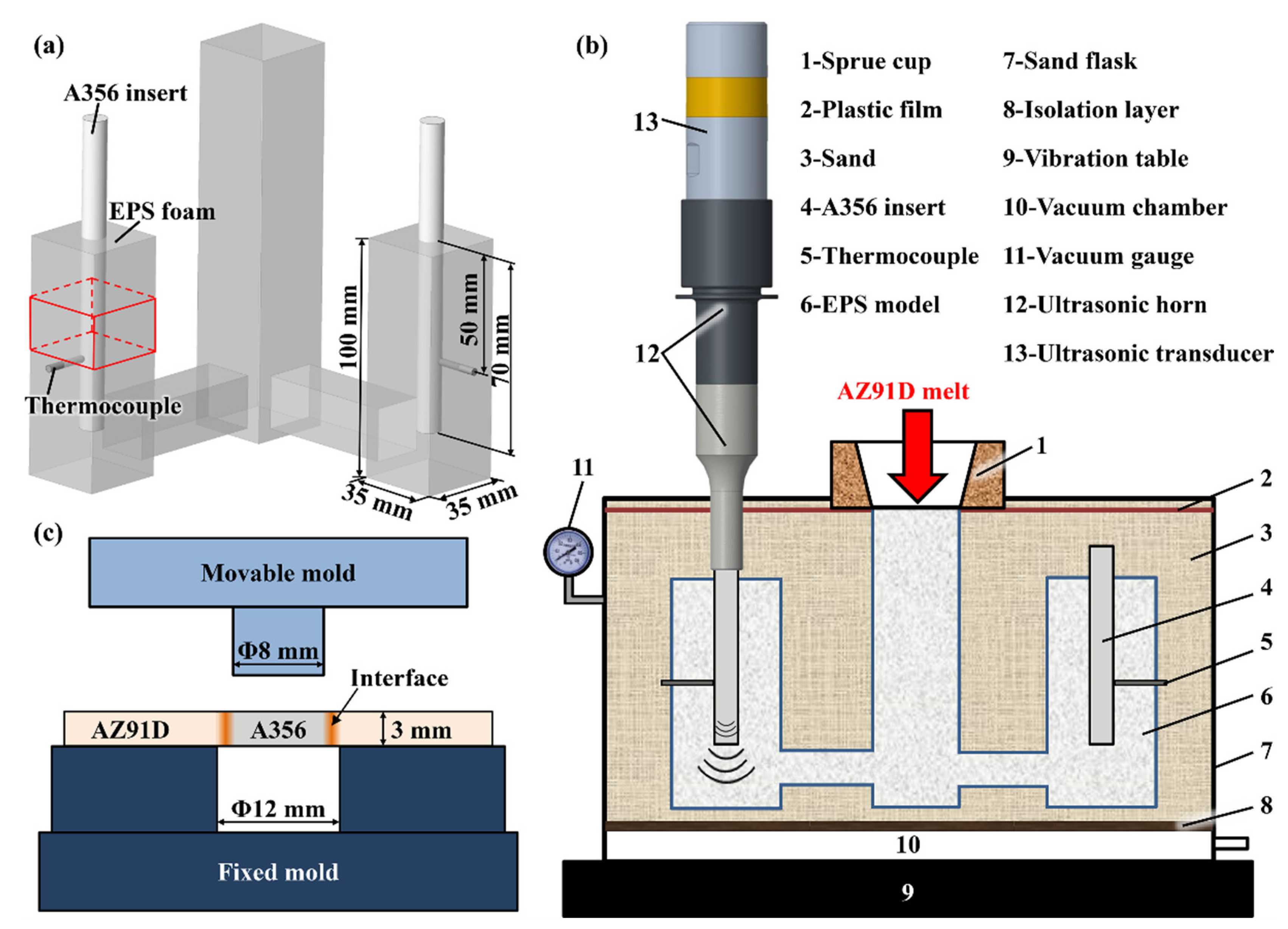
2. Material and Method
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Mechanical Properties
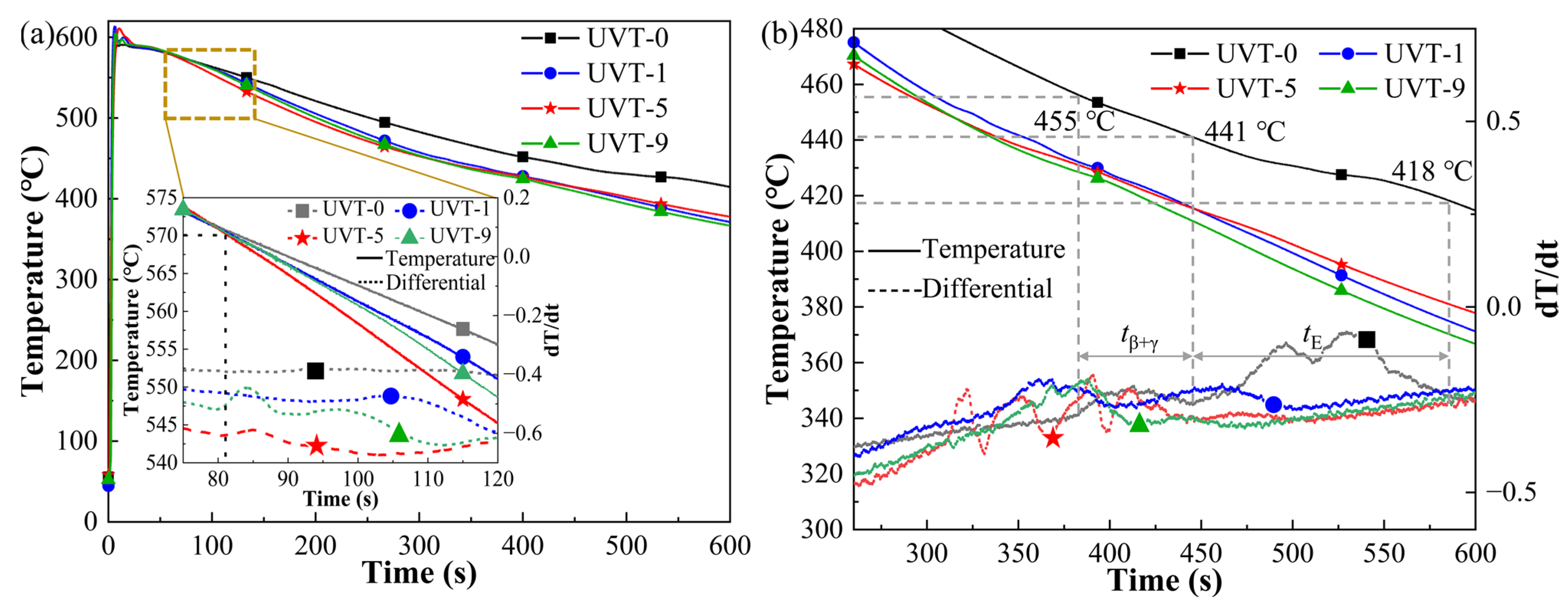
3. Results and Discussion
3.1. Macrostructure and Microstructure Evolution
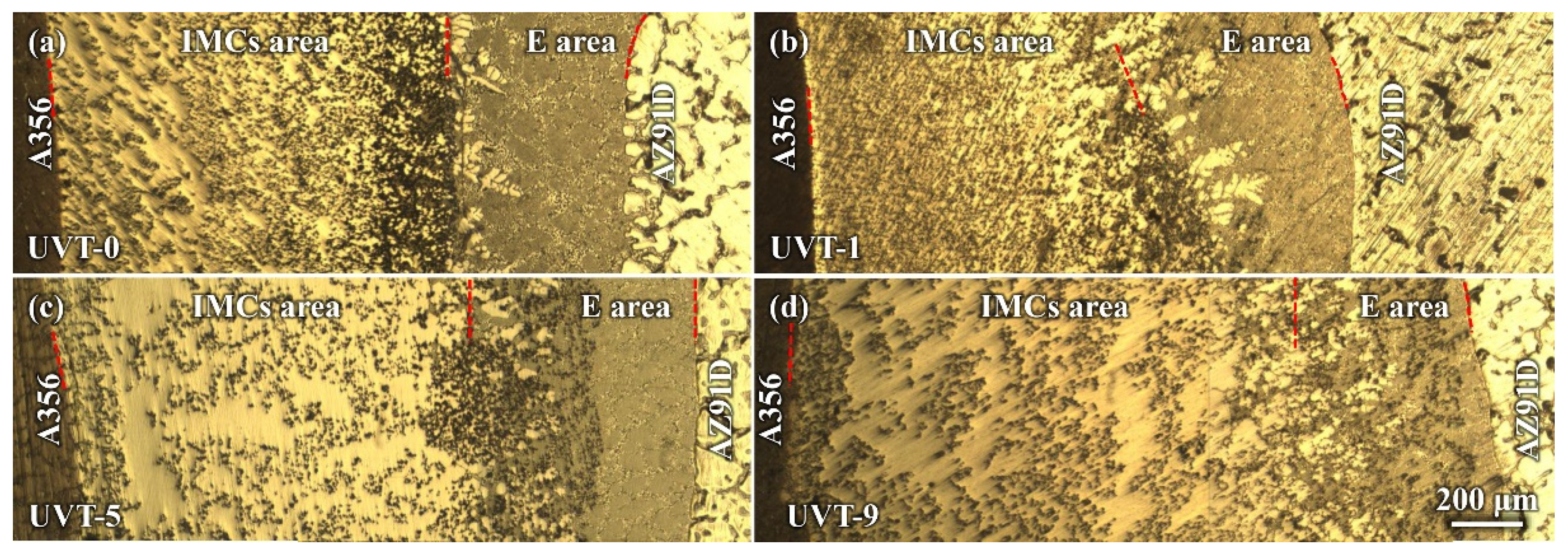
3.1.1. Macrostructure of the Al/Mg Interface
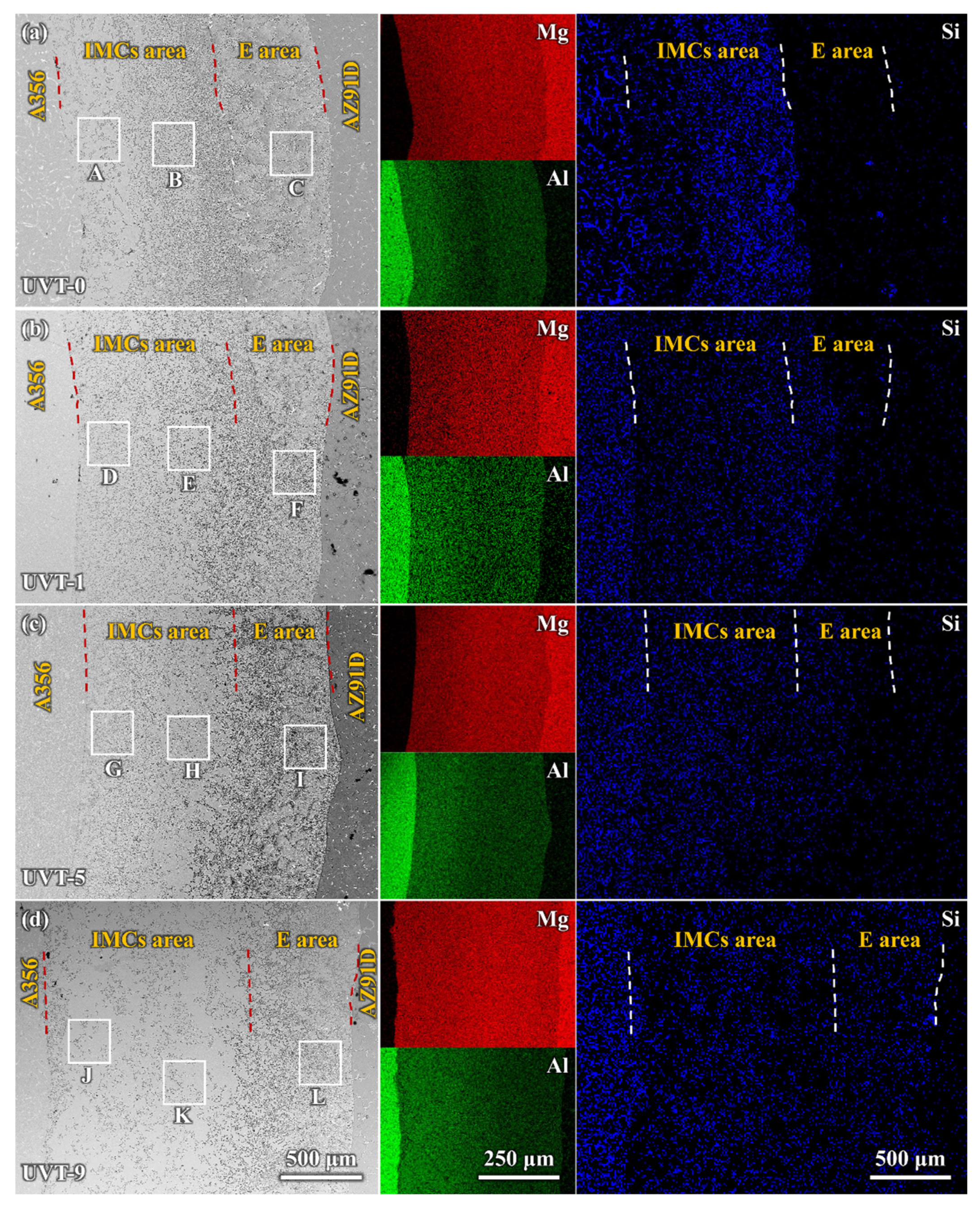
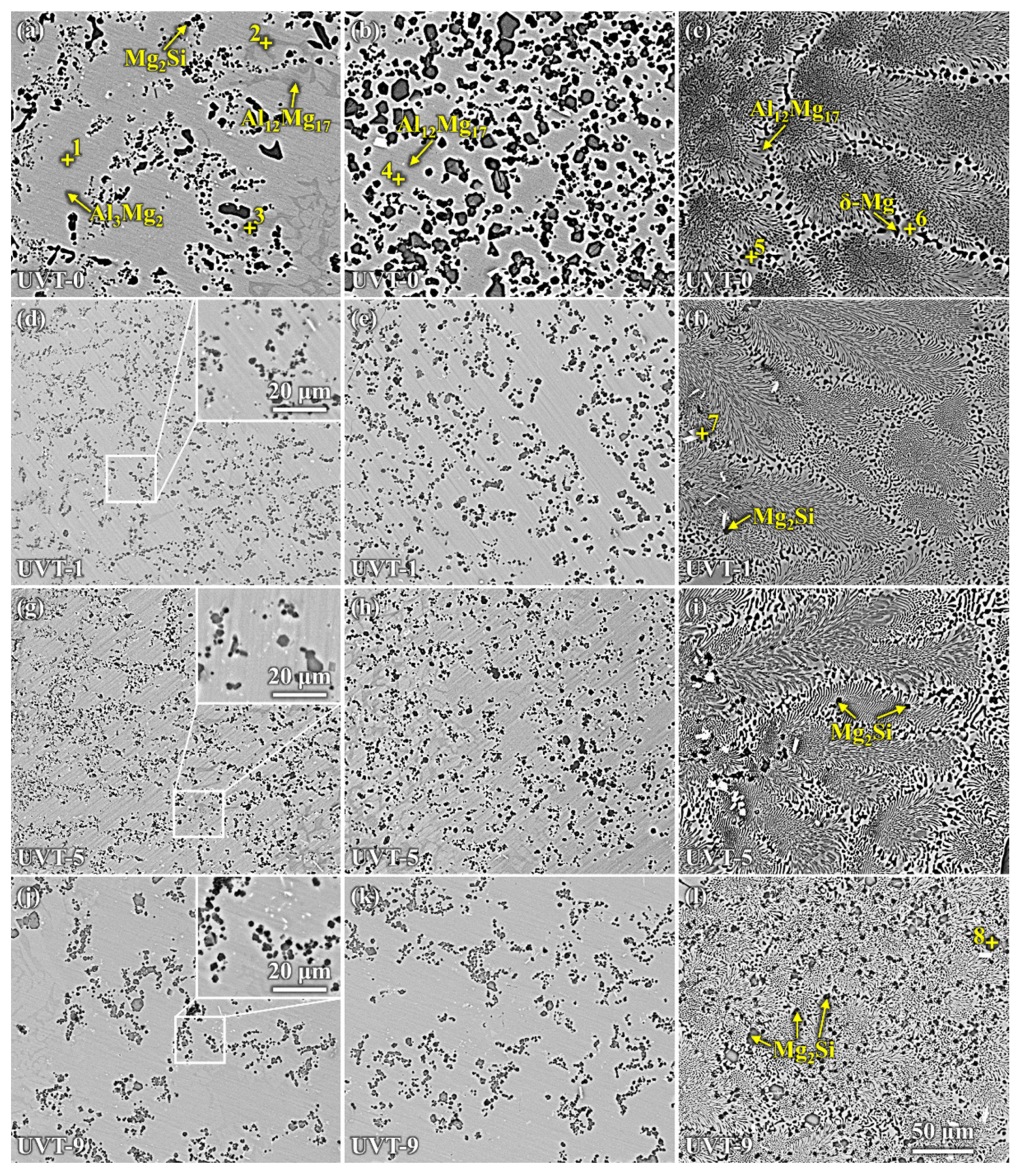
3.1.2. Microstructure of the Al/Mg Interface
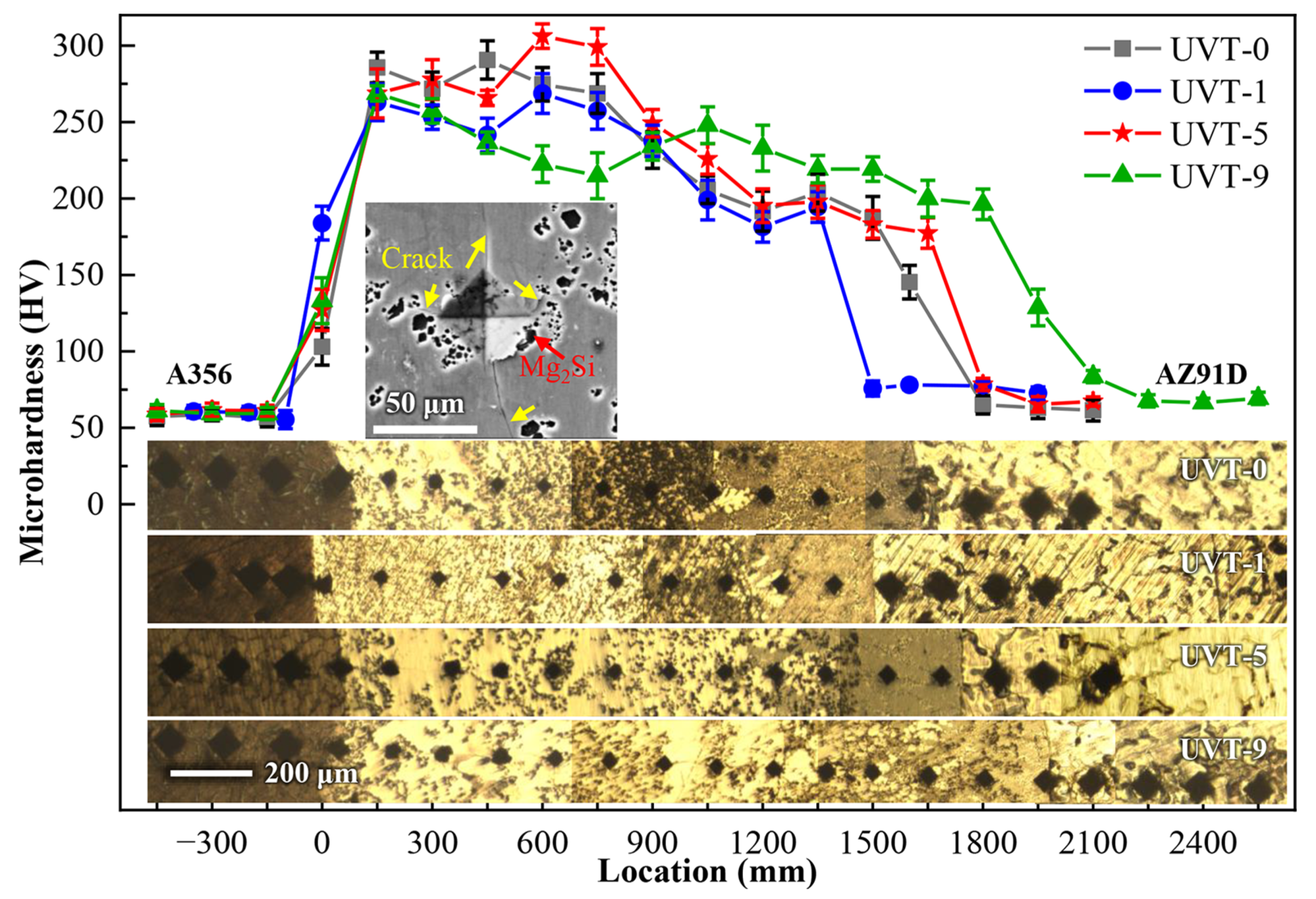
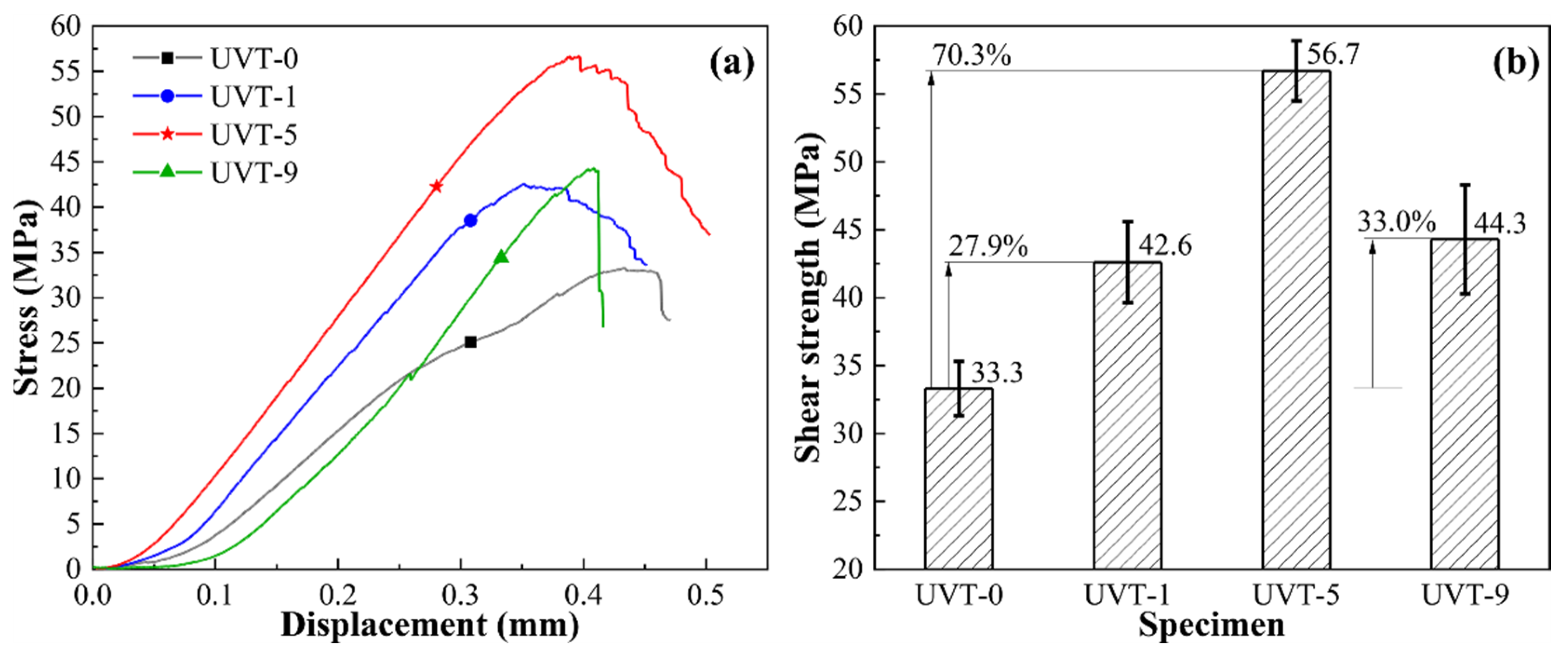
3.2. Mechanical Properties
3.2.1. Microhardness
3.2.2. Shear Strength
4. Conclusions
- The Al/Mg bimetallic interface consisted of IMC area (β-Al3Mg2 + γ-Al12Mg17 + Mg2Si) and E area (δ-Mg + γ-Al12Mg17 + Mg2Si); the application of UVT did not change the composition of phases at the interface. However, the distribution of the phases, especially Mg2Si particles, became more uniform. With a UVT duration of 1 s, the thickness of the Al/Mg interface layer was almost unchanged, compared with the UVT-0 specimen. However, the thickness of the Al/Mg interface layer and IMC area exhibited an increasing tendency with the increase of UVT duration.
- Si mainly gathered in the IMC area of the UVT-0 specimen, but it could diffuse to the E area with UVT. Si and Mg2Si particles were more homogeneously dispersed in the IMC area and E area with the increase of UVT duration, which could be attributed to the removal of the oxide film. Additionally, the Mg2Si particles were refined by UVT via its acoustic cavitation and streaming flow effects, and the shape of them was changed to polygon-shaped from worm-like, with sizes of no more than 5 μm.
- The IMCs exhibited the highest microhardness among the A356, AZ91D and eutectics. The microhardness of the Al/Mg bimetallic interface was not obviously changed with UVT. However, the cracks at the edges of the Vickers indentation were suppressed by the refined Mg2Si particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.J.; Kim, S.H.; Lee, J.U.; Choi, J.O.; Kim, H.S.; Kim, Y.M.; Kim, Y.; Park, S.H. Effects of cold pre-forging on microstructure and tensile properties of extruded AZ80 alloy. Mater. Sci. Eng. A 2017, 708, 405–410. [Google Scholar] [CrossRef]
- Li, Z.; Yi, D.; Tan, C.; Wang, B. Investigation of the stress corrosion cracking behavior in annealed 5083 aluminum alloy sheets with different texture types. J. Alloys Compd. 2020, 817, 152690. [Google Scholar] [CrossRef]
- Mei, X.M.; Mei, Q.S.; Li, J.Y.; Li, C.L.; Wan, L.; Chen, F.; Chen, Z.H.; Xu, T.; Wang, Y.C.; Tan, Y.Y. Solid-state alloying of Al-Mg alloys by accumulative roll-bonding: Microstructure and properties. J. Mater. Sci. Technol. 2022, 125, 238–251. [Google Scholar] [CrossRef]
- Griffiths, S.; Croteau, J.R.; Rossell, M.D.; Erni, R.; De Luca, A.; Vo, N.Q.; Dunand, D.C.; Leinenbach, C. Coarsening- and creep resistance of precipitation-strengthened Al–Mg–Zr alloys processed by selective laser melting. Acta Mater. 2020, 188, 192–202. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Wu, R.; Wang, Y.; Zhang, J.; Betsofen, S.; Krit, B.; Hou, L.; Nodir, T. The transformation of LPSO type in Mg-4Y-2Er-2Zn-0.6Zr and its response to the mechanical properties and damping capacities. J. Magnes. Alloy 2020, 8, 793–798. [Google Scholar] [CrossRef]
- Li, R.X.; Li, R.D.; Zhao, Y.H.; He, L.Z.; Li, C.X.; Guan, H.R.; Hu, Z.Q. Age-hardening behavior of cast Al–Si base alloy. Mater. Lett. 2004, 58, 2096–2101. [Google Scholar] [CrossRef]
- Förster, W.; Binotsch, C.; Awiszus, B. Process Chain for the Production of a Bimetal Component from Mg with a Complete Al Cladding. Metals 2018, 8, 97. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Luo, A.A. A novel aluminum surface treatment for improved bonding in magnesium/aluminum bimetallic castings. Scripta Mater. 2014, 86, 52–55. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Z.; Hu, C.; Li, B.; Mo, T.; Liu, Q. Effects of annealing on the interfacial structures and mechanical properties of hot roll bonded Al/Mg clad sheets. Mater. Sci. Eng. A 2020, 792, 139673. [Google Scholar] [CrossRef]
- Luo, C.; Liang, W.; Chen, Z.; Zhang, J.; Chi, C.; Yang, F. Effect of high temperature annealing and subsequent hot rolling on microstructural evolution at the bond-interface of Al/Mg/Al alloy laminated composites. Mater. Charact. 2013, 84, 34–40. [Google Scholar] [CrossRef]
- Dorbane, A.; Mansoor, B.; Ayoub, G.; Shunmugasamy, V.C.; Imad, A. Mechanical, microstructural and fracture properties of dissimilar welds produced by friction stir welding of AZ31B and Al6061. Mater. Sci. Eng. A 2016, 651, 720–733. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, C.; Shi, L.; Su, H. Evolution of microstructures and intermetallic compounds at bonding interface in friction stir welding of dissimilar Al/Mg alloys with/without ultrasonic assistance. J. Mater. Sci. Technol. 2023, 139, 31–46. [Google Scholar] [CrossRef]
- Sheng, K.; Lu, L.; Xiang, Y.; Ma, M.; Wu, Z. Crack behavior in Mg/Al alloy thin sheet during hot compound extrusion. J. Magnes. Alloy 2019, 7, 717–724. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Zhao, G.; Zhang, C.; Sun, L. Achieving three-layered Al/Mg/Al sheet via combining porthole die co-extrusion and hot forging. J. Magnes. Alloy 2020, 8, 654–666. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Microstructure evolution, mechanical properties and fracture behavior of Al-xSi/AZ91D bimetallic composites prepared by a compound casting. J. Magnes. Alloy 2022, 10, 1075–1085. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, R.; Klarner, A.D.; Luo, A.A.; Chen, Y. Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding. J. Magnes. Alloy 2020, 8, 578–586. [Google Scholar] [CrossRef]
- Bae, J.H.; Prasada Rao, A.K.; Kim, K.H.; Kim, N.J. Cladding of Mg alloy with Al by twin-roll casting. Scr. Mater. 2011, 64, 836–839. [Google Scholar] [CrossRef]
- Griffiths, W.D.; Ainsworth, M.J. Instability of the Liquid Metal–Pattern Interface in the Lost Foam Casting of Aluminum Alloys. Metall. Mater. Trans. A 2016, 47, 3137–3149. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Li, G.; Zhu, J.; Wang, J.; Jie, G.; Fan, Z. Effect of vibration on interfacial microstructure and mechanical properties of Mg/Al bimetal prepared by a novel compound casting. J. Magnes. Alloy 2022, 10, 2296–2309. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, G.; Wang, Y.; Xiao, Y.; Shen, Q.; Zhang, L. Effect of Al thin film and Ni foil interlayer on diffusion bonded Mg–Al dissimilar joints. J. Alloys Compd. 2013, 556, 139–142. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Improving mechanical properties of AZ91D magnesium/A356 aluminum bimetal prepared by compound casting via a high velocity oxygen fuel sprayed Ni coating. J. Magnes. Alloy 2022, 10, 1075–1085. [Google Scholar] [CrossRef]
- Lv, X.; Liu, L.; Song, G. Laser welding of AZ31Mg alloy with 6061Al alloy via Ti interlayer based on two kinds of bonding mechanisms. J. Manuf. Process. 2022, 83, 678–684. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhang, Y.Q.; He, J.S.; Li, P. Interfacial microstructure in joining of arc sprayed Al-Zn coating to AZ91D by solid-liquid compound casting. Surf. Coat. Technol. 2016, 307, 301–307. [Google Scholar] [CrossRef]
- Sui, D.; Han, Q. Ultrasound-assisted cast-on method: Obtaining high-quality metallurgical bonds between a bare steel insert and A354 aluminum alloy within a composite casting. J. Mater. Process. Technol. 2023, 311, 117783. [Google Scholar] [CrossRef]
- Eskin, D.G.; Tzanakis, I.; Wang, F.; Lebon, G.S.B.; Subroto, T.; Pericleous, K.; Mi, J. Fundamental studies of ultrasonic melt processing. Ultrason. Sonochem. 2019, 52, 455–467. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Mi, J.; Wang, C.; Koe, B.; King, A.; Reinhard, C.; Connolley, T. A synchrotron X-radiography study of the fragmentation and refinement of primary intermetallic particles in an Al-35 Cu alloy induced by ultrasonic melt processing. Acta Mater. 2017, 141, 142–153. [Google Scholar] [CrossRef]
- Lebon, G.S.B.; Salloum-Abou-Jaoude, G.; Eskin, D.; Tzanakis, I.; Pericleous, K.; Jarry, P. Numerical modelling of acoustic streaming during the ultrasonic melt treatment of direct-chill (DC) casting. Ultrason. Sonochem. 2019, 54, 171–182. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, S.; Wei, M.; Peng, L.; Wu, Y.; Liu, X. Research progress on solidification structure of alloys by synchrotron X-ray radiography: A review. J. Magnes. Alloy 2020, 8, 396–413. [Google Scholar] [CrossRef]
- Wang, B.; Tan, D.; Lee, T.L.; Khong, J.C.; Wang, F.; Eskin, D.; Connolley, T.; Fezzaa, K.; Mi, J. Ultrafast synchrotron X-ray imaging studies of microstructure fragmentation in solidification under ultrasound. Acta Mater. 2018, 144, 505–515. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Koe, B.; Schlepütz, C.M.; Irvine, S.; Mi, J. Synchrotron X-ray imaging and ultrafast tomography in situ study of the fragmentation and growth dynamics of dendritic microstructures in solidification under ultrasound. Acta Mater. 2021, 209, 116796. [Google Scholar] [CrossRef]
- Han, Q. Ultrasonic Processing of Materials. Metall. Mater. Trans. B 2015, 46, 1603–1614. [Google Scholar] [CrossRef]
- Suslick, K.S. The Chemical Effects of Ultrasound. Sci. Am. 1989, 260, 80–86. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Chen, J.; Guo, B.; Wang, W.; Jing, Y.; Liu, Y. Effect of ultrasonic micro-forging treatment on microstructure and mechanical properties of GH3039 superalloy processed by directed energy deposition. J. Mater. Sci. Technol. 2021, 70, 185–196. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Connolley, T.; Wang, C.; Koe, B.; King, A.; Reinhard, C.; Mi, J. In-situ synchrotron X-ray radiography observation of primary Al2Cu intermetallic growth on fragments of aluminium oxide film. Mater. Lett. 2018, 213, 303–305. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Wang, J.; Li, G.; Zhang, Z.; Fan, Z. Development of high strength Mg/Al bimetal by a novel ultrasonic vibration aided compound casting process. J. Mater. Process Technol. 2022, 300, 117441. [Google Scholar] [CrossRef]
- Guo, R.; Zeng, D.; Li, F. Promoting of metallurgical bonding by ultrasonic insert process in steel–aluminum bimetallic castings. High Temp. Mater. Processes 2022, 41, 289–295. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Z.; Sun, Z.; Zhang, B.; Rao, W. Ultrasonic-assisted liquid–solid composite method to fabricate bimetallic aluminum alloy. Mater. Lett. 2022, 314, 131894. [Google Scholar] [CrossRef]
- Nie, X.Y.; Liu, J.C.; Li, H.X.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. An investigation on bonding mechanism and mechanical properties of Al/Ti compound materials prepared by insert moulding. Mater. Des. 2014, 63, 142–150. [Google Scholar] [CrossRef]
- Emami, S.M.; Divandari, M.; Arabi, H.; Hajjari, E. Effect of Melt-to-Solid Insert Volume Ratio on Mg/Al Dissimilar Metals Bonding. J. Mater. Eng. Perform. 2012, 22, 123–130. [Google Scholar] [CrossRef]
- Jamalpour, M.; Alizadeh, R. Effects of heat treatment and Y addition on the microstructure and mechanical properties of as-cast Mg–Si alloys. Mater. Sci. Eng. A 2022, 859, 144209. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Z.; Li, X.; Zhang, L.; Li, R.; Jiang, R.; Dong, F. The cavitation erosion of ultrasonic sonotrode during large-scale metallic casting: Experiment and simulation. Ultrason. Sonochem. 2018, 43, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Qian, M.; Davis, B.; Wilks, T.; StJohn, D.H. Potency of high-intensity ultrasonic treatment for grain refinement of magnesium alloys. Scr. Mater. 2008, 59, 19–22. [Google Scholar] [CrossRef]
- Haq, I.U.; Shin, J.S.; Lee, Z.H. Computer-Aided Cooling Curve Analysis of A356 Aluminum Alloy. Met. Mater. Int. 2004, 10, 89–96. [Google Scholar]
- Dong, F.; Li, X.; Zhang, L.; Ma, L.; Li, R. Cavitation erosion mechanism of titanium alloy radiation rods in aluminum melt. Ultrason. Sonochem. 2016, 31, 150–156. [Google Scholar] [CrossRef]
- Lee, K.S.; Yoon, D.H.; Kim, H.K.; Kwon, Y.N.; Lee, Y.S. Effect of annealing on the interface microstructure and mechanical properties of a STS–Al–Mg 3-ply clad sheet. Mater. Sci. Eng. A 2012, 556, 319–330. [Google Scholar] [CrossRef]




| Mg | Al | Si | Ti | Mn | Fe | Zn | |
|---|---|---|---|---|---|---|---|
| A356 | 0.96 | Bal. | 8.12 | 0.17 | 0.28 | 0.17 | - |
| AZ91D | Bal. | 9.08 | - | - | 0.41 | - | 1.08 |
| Point No. | Element Compositions (at.%) | Possible Phase | |||
|---|---|---|---|---|---|
| Mg | Al | Si | Mn | ||
| 1 | 41.23 | 58.77 | - | - | Al3Mg2 |
| 2 | 53.96 | 46.04 | - | - | Al12Mg17 |
| 3 | 59.39 | - | 40.61 | - | Mg2Si |
| 4 | 57.22 | 42.78 | - | - | Al12Mg17 |
| 5 | 63.36 | 36.64 | - | - | Al12Mg17 |
| 6 | 80.55 | 19.45 | - | - | δ-Mg |
| 7 | 5.35 | 72.23 | - | 22.42 | Al11Mn4 |
| 8 | 65.63 | - | 34.37 | - | Mg2Si |
| Point No. | Element Compositions (at.%) | Possible Phase | ||
|---|---|---|---|---|
| Mg | Al | Si | ||
| 1 | 35.44 | 64.36 | - | Al3Mg2 |
| 2 | 47.81 | 30.38 | 21.81 | Mg2Si |
| 3 | 60.06 | 39.94 | - | Al12Mg17 |
| 4 | 42.29 | 57.71 | - | Al3Mg2 |
| 5 | 48.24 | 40.03 | 11.73 | Mg2Si |
| 6 | 62.63 | 37.37 | - | Al12Mg17 |
| 7 | 51.67 | 24.99 | 23.34 | Mg2Si |
| 8 | 41.47 | 58.53 | - | Al3Mg2 |
| 9 | 40.11 | 51.80 | 8.09 | Mg2Si |
| 10 | 85.77 | 14.23 | - | δ-Mg |
| 11 | 61.54 | 38.46 | - | Al12Mg17 |
| 12 | 47.65 | 24.96 | 27.38 | Mg2Si |
| 13 | 36.43 | 63.57 | - | Al3Mg2 |
| 14 | 40.40 | 46.49 | 13.11 | Mg2Si |
| 15 | 56.90 | 10.61 | 32.49 | Mg2Si |
| 16 | 54.20 | 45.80 | - | Al12Mg17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Guan, F.; Xu, Y.; Zhang, Z.; Fan, Z.; Jiang, W. Development of Al/Mg Bimetal Processed by Ultrasonic Vibration-Assisted Compound Casting: Effects of Ultrasonic Vibration Treatment Duration Time. Materials 2023, 16, 5009. https://doi.org/10.3390/ma16145009
Li Q, Guan F, Xu Y, Zhang Z, Fan Z, Jiang W. Development of Al/Mg Bimetal Processed by Ultrasonic Vibration-Assisted Compound Casting: Effects of Ultrasonic Vibration Treatment Duration Time. Materials. 2023; 16(14):5009. https://doi.org/10.3390/ma16145009
Chicago/Turabian StyleLi, Qingqing, Feng Guan, Yuancai Xu, Zheng Zhang, Zitian Fan, and Wenming Jiang. 2023. "Development of Al/Mg Bimetal Processed by Ultrasonic Vibration-Assisted Compound Casting: Effects of Ultrasonic Vibration Treatment Duration Time" Materials 16, no. 14: 5009. https://doi.org/10.3390/ma16145009
APA StyleLi, Q., Guan, F., Xu, Y., Zhang, Z., Fan, Z., & Jiang, W. (2023). Development of Al/Mg Bimetal Processed by Ultrasonic Vibration-Assisted Compound Casting: Effects of Ultrasonic Vibration Treatment Duration Time. Materials, 16(14), 5009. https://doi.org/10.3390/ma16145009






