PVD for Decorative Applications: A Review
Abstract
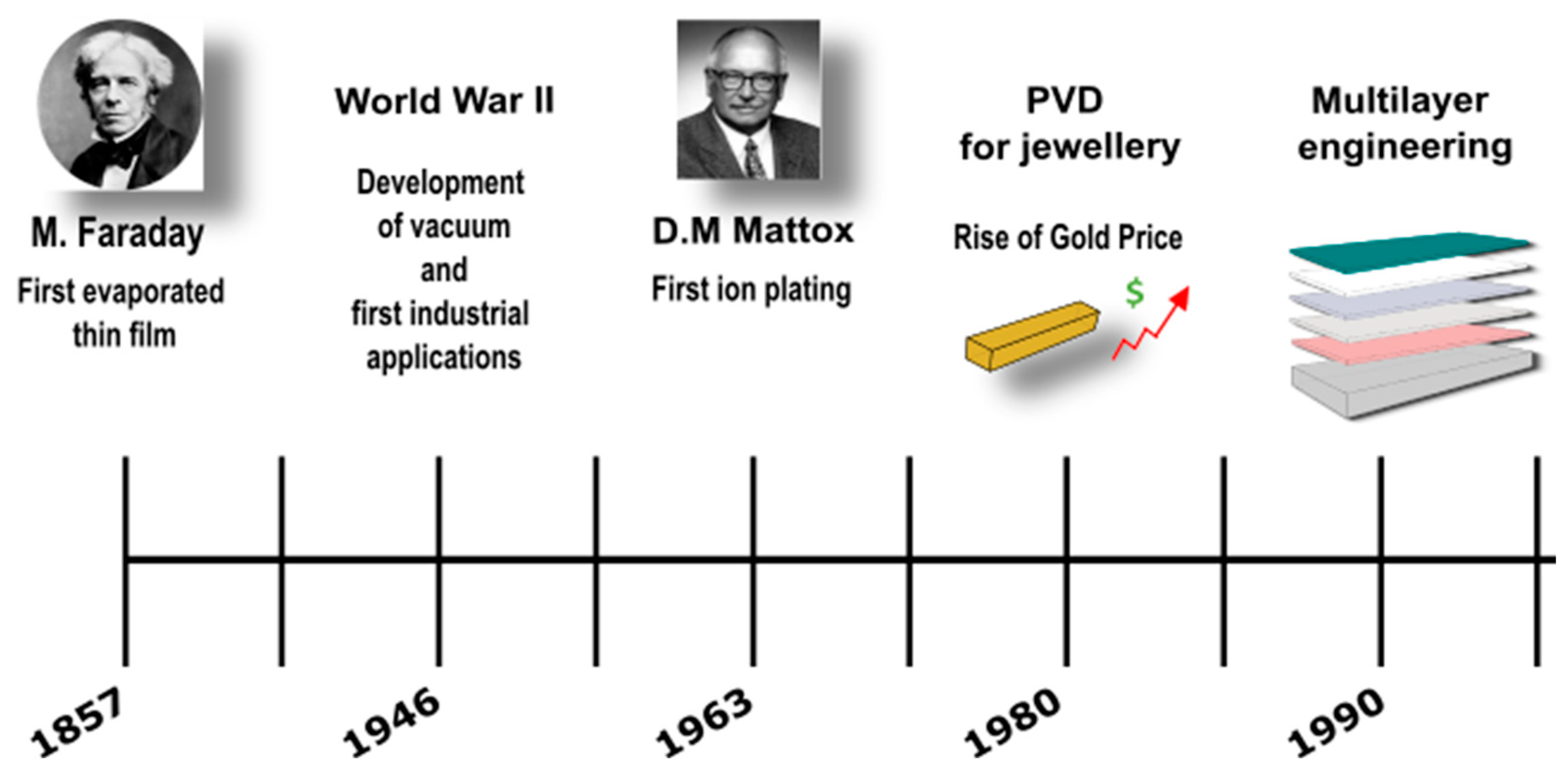
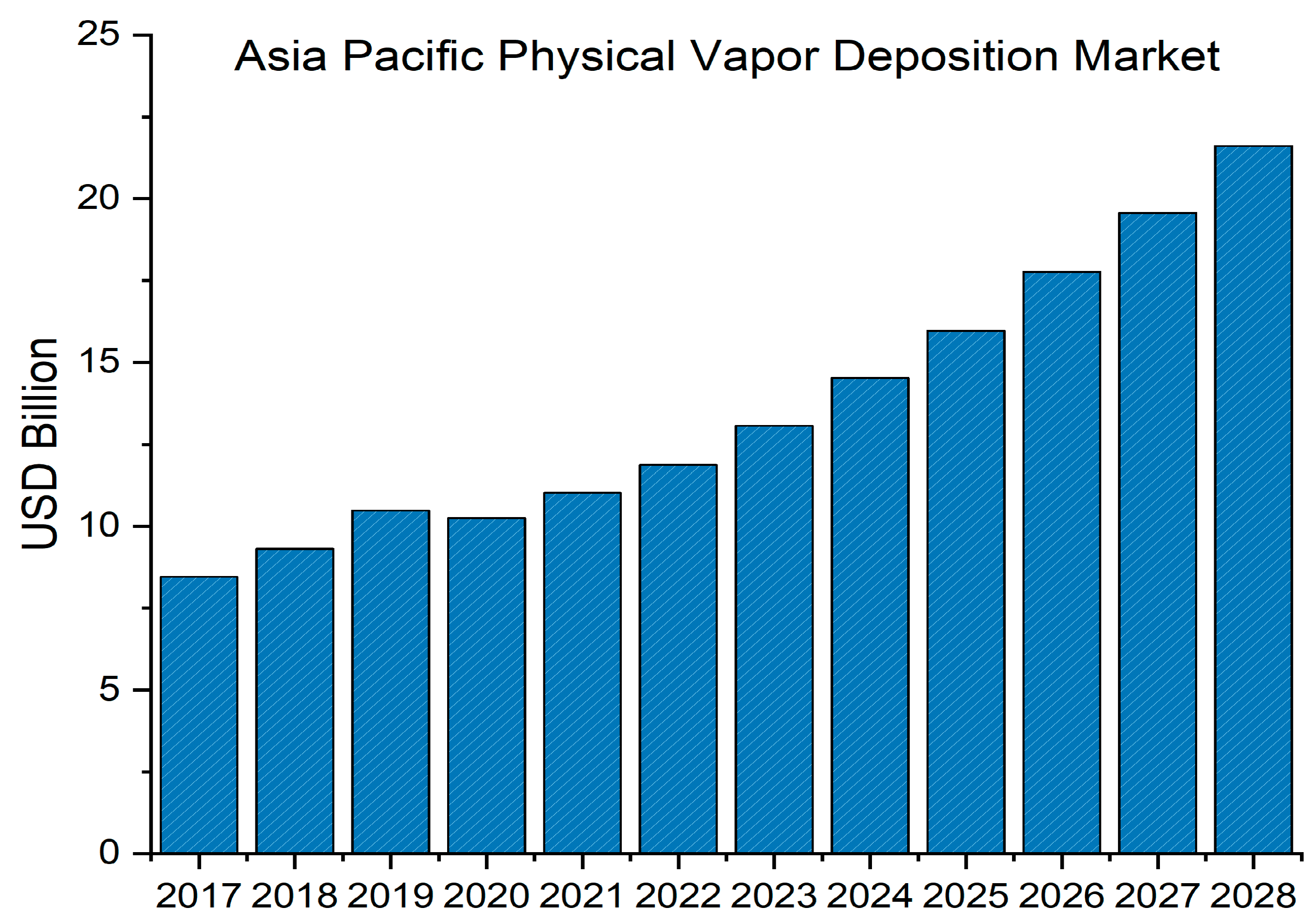
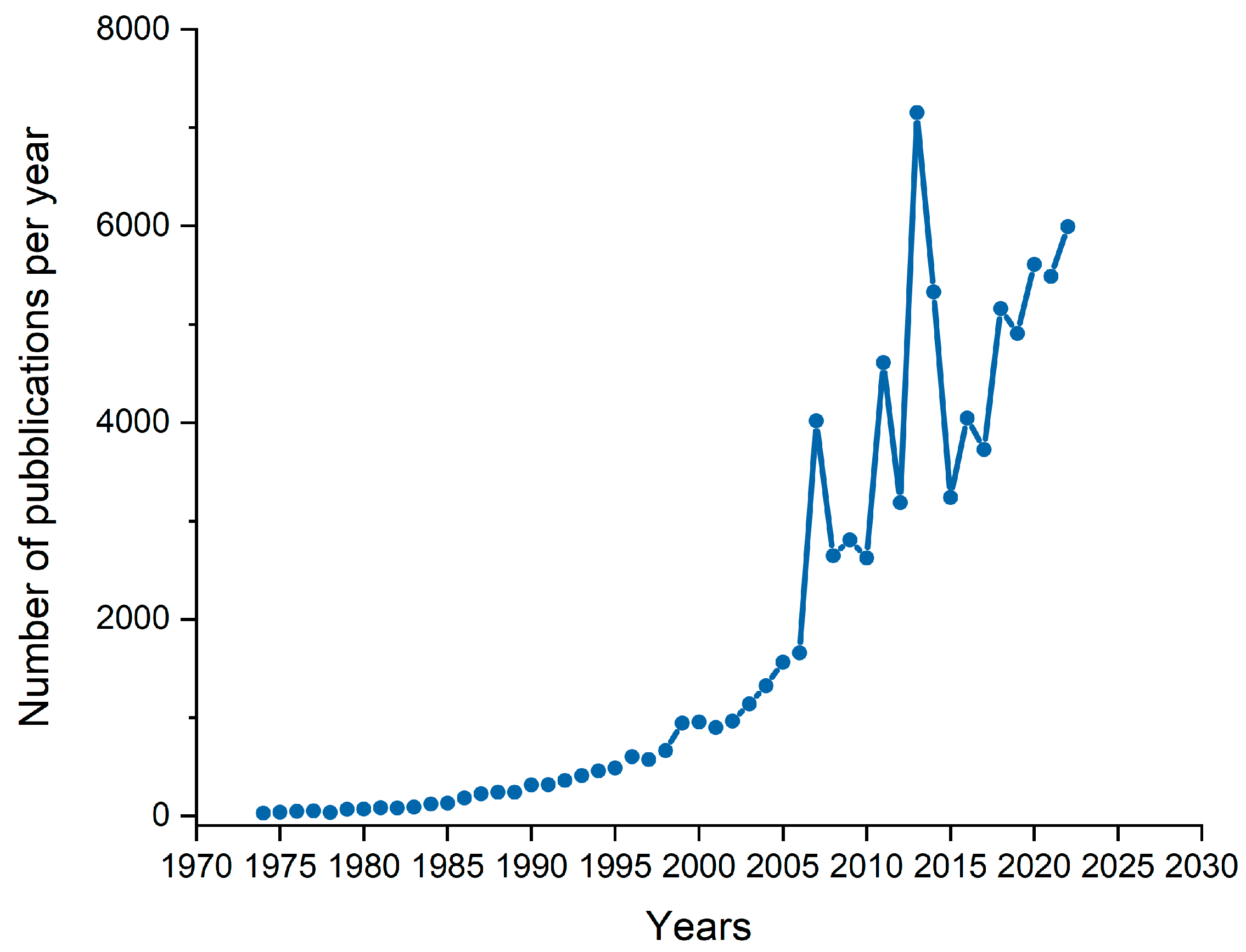
1. Introduction
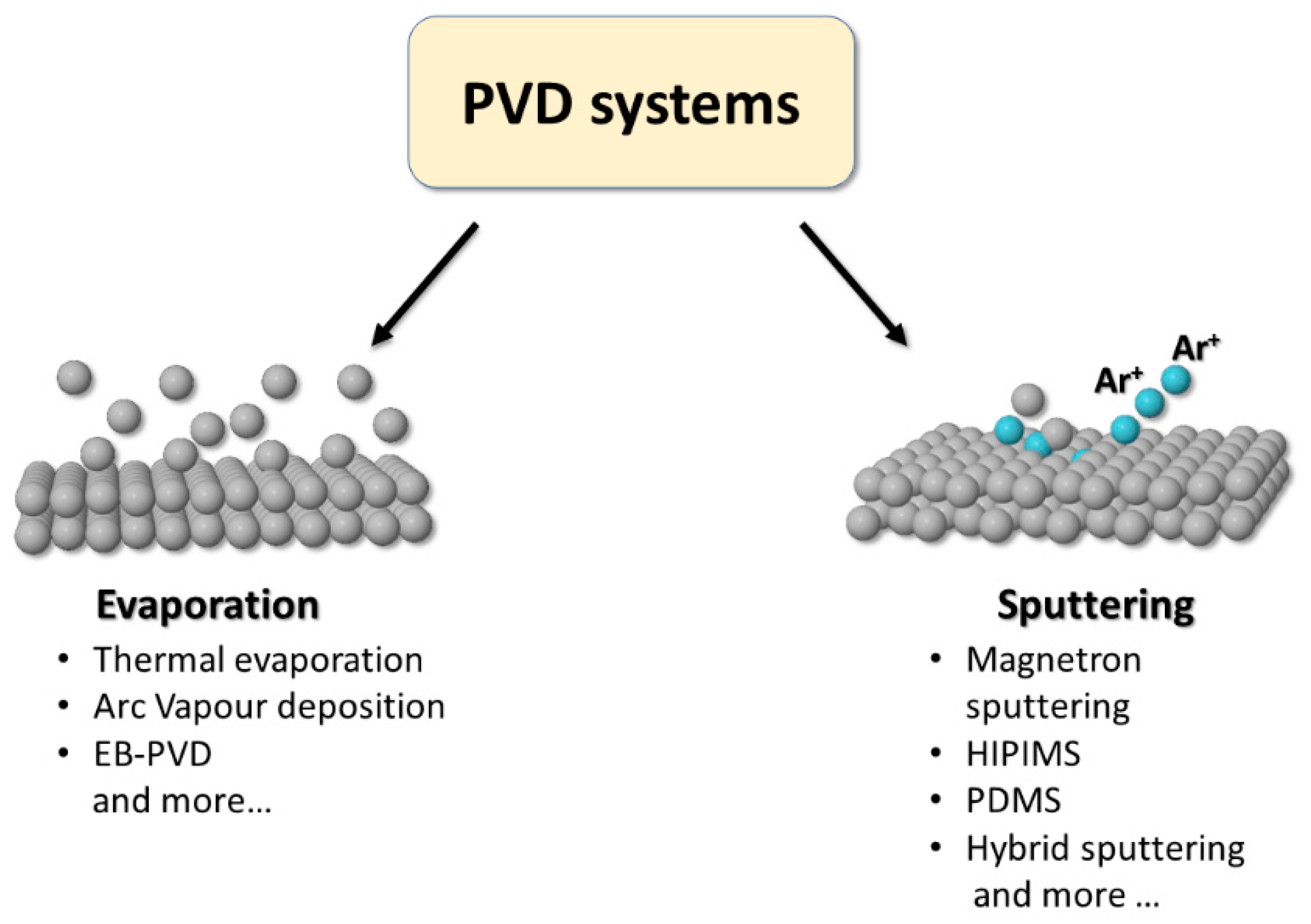
2. Fundamentals of Physical Vapor Deposition (PVD)
2.1. Evaporation Systems
2.2. Sputtering Systems
2.3. PVD + Galvanic Hybrid Systems
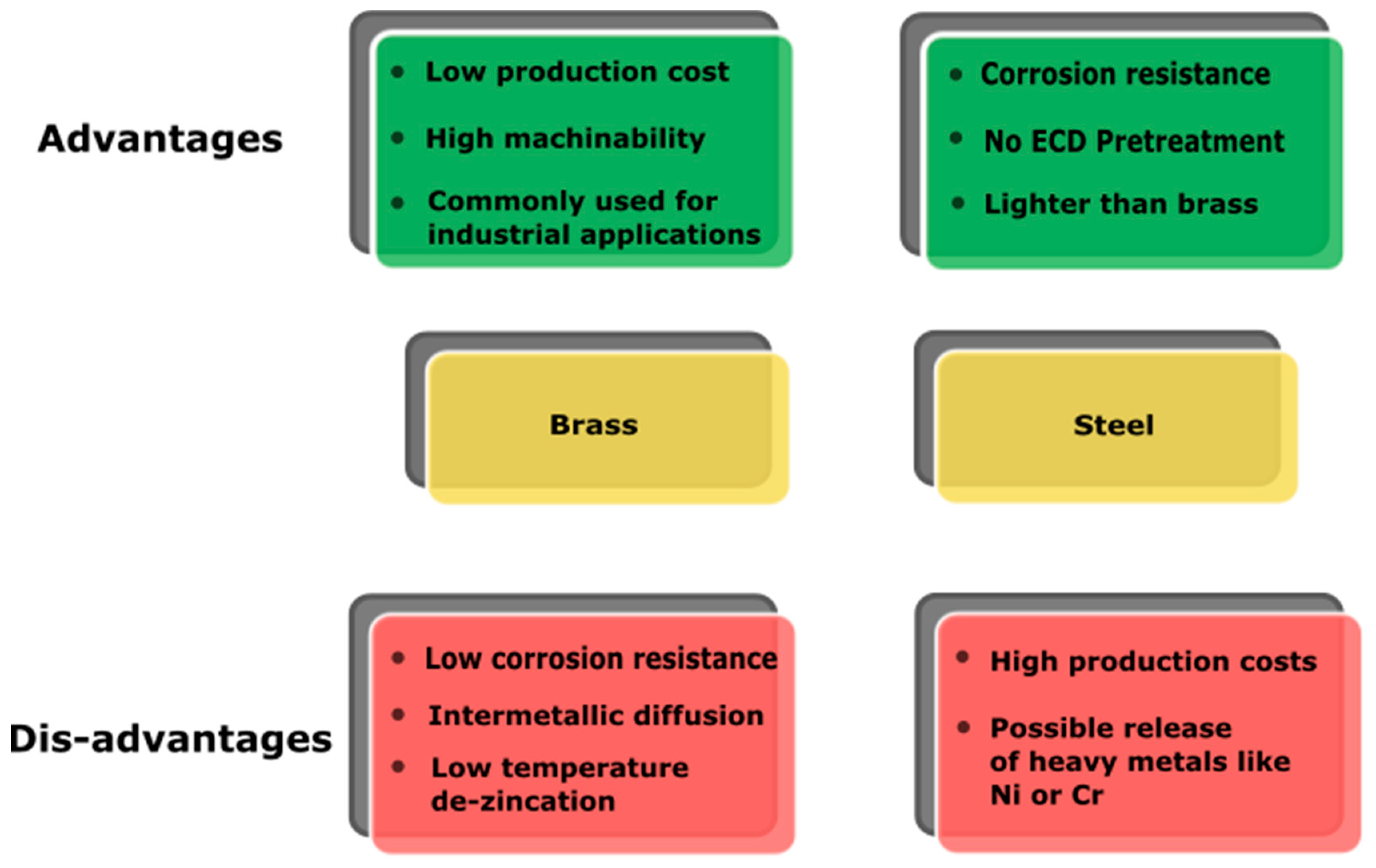
3. Substrates
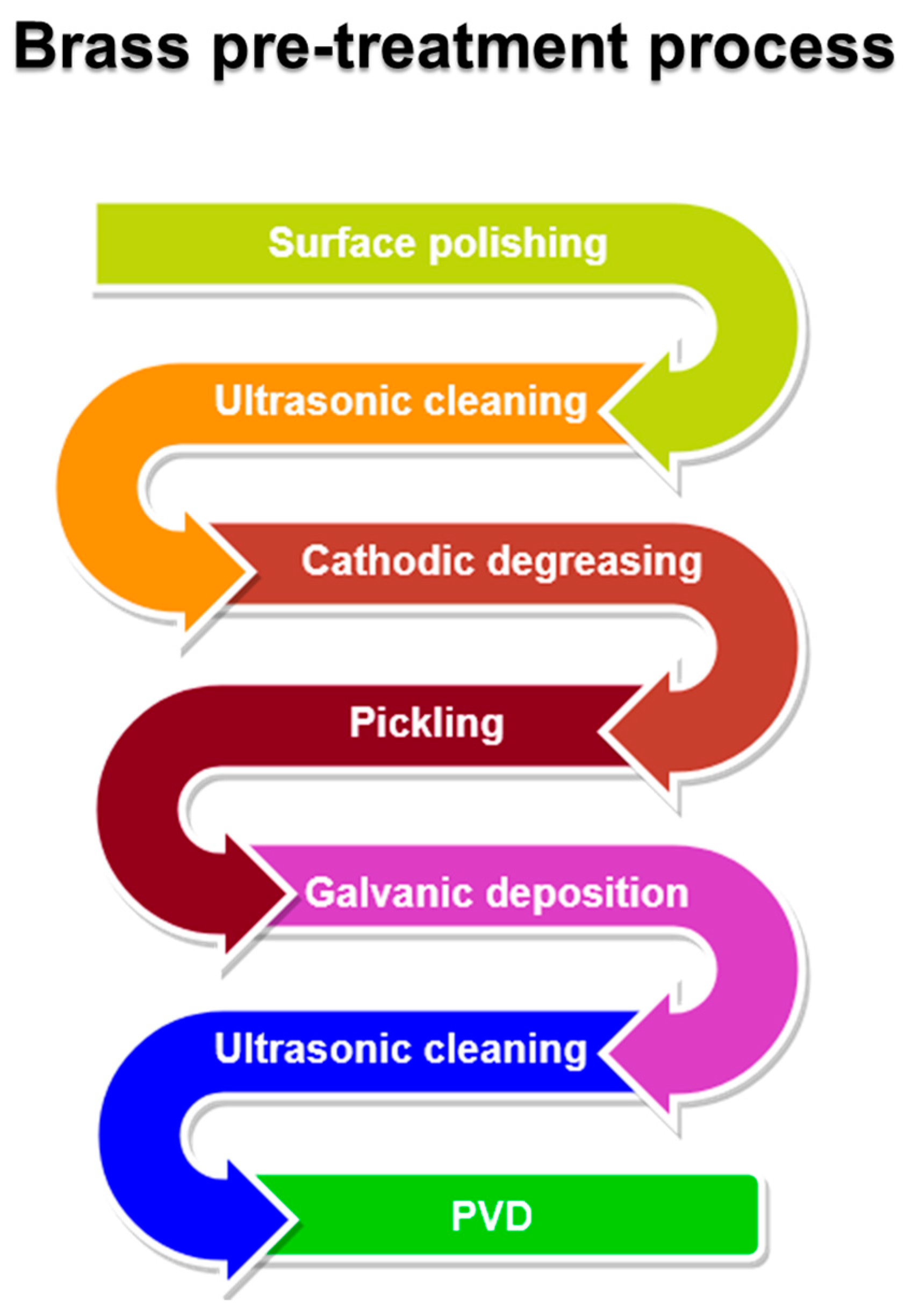
3.1. Brass
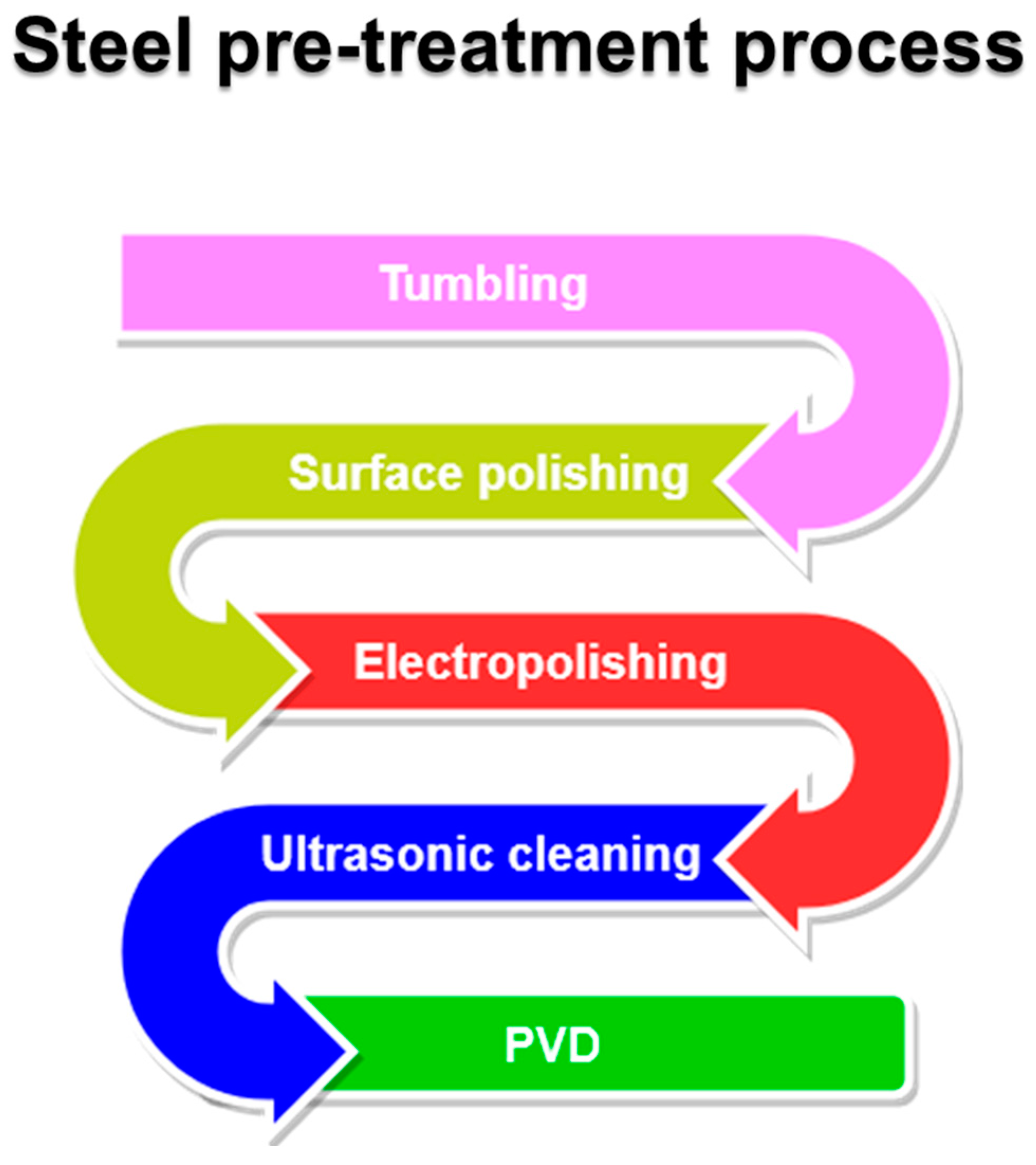
3.2. Stainless Steel
4. Surface Finishing
4.1. Titanium-Based Coatings
4.2. Chromium-Based Coatings
4.3. Zirconium-Based Coatings
4.4. DLC Coatings
5. Characterization of PVD Coatings
5.1. Thickness Determination
5.2. Mechanical Properties and Defects Analysis
5.3. Color Evaluation
5.4. Corrosion Tests
5.5. Heavy Metals’ Release
6. Recent Trends and Perspectives in the Decorative PVD Industry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawaid, A.; Koksal, S.; Sharif, S. Cutting Performance and Wear Characteristics of PVD Coated and Uncoated Carbide Tools in Face Milling Inconel 718 Aerospace Alloy. J. Mater. Process. Technol. 2001, 116, 2–9. [Google Scholar] [CrossRef]
- Baptista, A.; Pinto, G.; Silva, F.J.G.; Ferreira, A.A.; Pinto, A.G.; Sousa, V.F.C. Wear Characterization of Chromium PVD Coatings on Polymeric Substrate for Automotive Optical Components. Coatings 2021, 11, 555. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Sputter Deposition for Semiconductor Manufacturing. IBM J. Res. Dev. 1999, 43, 163–179. [Google Scholar] [CrossRef]
- Bandinelli, R.; Fani, V.; Bindi, B. Electroplating and Pvd Finishing Technologies in the Fashion Industry: Perspectives and Scenarios. Sustainability 2021, 13, 4453. [Google Scholar] [CrossRef]
- Faraday, M.X. The Bakerian Lecture—Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Nahrwold, R. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Ann. Phys. 1887, 31, 467. [Google Scholar]
- Kundt, A. Ann. Phys. 1888, 34, 473.
- Holland, L. Vacuum Deposition of Thin Films; Chapman & Hall: London, UK, 1958; ISBN 9780412053801. [Google Scholar]
- Mattox, D.M.; McDonald, J.E. Interface Formation during Thin Film Deposition. J. Appl. Phys. 1963, 34, 2493–2494. [Google Scholar] [CrossRef]
- Bunshah, R.F.; Juntz, R.S. Influence of Condensation Temperature on Microstructure and Tensile Properties of Titanium Sheet Produced by High-Rate Physical Vapor Deposition Process. Metall. Trans. 1973, 4, 21–26. [Google Scholar] [CrossRef]
- Meyers, R.G.; Morgan, R.P. Transactions Vacuum Metallurgy Conference; American Vacuum Society: New York, NY, USA, 1966; p. 271. [Google Scholar]
- Smith, H.R.; Kennedy, K.; Boericke, F.S. Metallurgical characteristics of titanium-alloy foil prepared by electron-beam evaporation. J. Vac. Sci. Technol. 1970, 7, 48–51. [Google Scholar] [CrossRef]
- Bunshah, R.F. Physical vapor deposition of metals, alloys and compounds. In New Trends in Materials Processing; American Society for Metals: Metals Park, OH, USA, 1976; p. 200. [Google Scholar]
- Paton, B.A.; Movchan, B.A.; Demchishin, A.V. Structure and properties of electron-beam evaporated massive vacuum deposits. In Proceedings of the 4th International Conference on Vacuum Metallurgy, Tokyo, Japan, 4–8 June 1973; Iron and Steel Institute of Japan: Tokyo, Japan, 1973; p. 251. [Google Scholar]
- North Atlantic Treaty Organization, Advisory Group for Aerospace Research and Development. Consultant and Exchange Programme. In Materials Coating Techniques; North Atlantic Teaty Organization, Advisory Group for Aerospace Research and Development: Brussels, Belgium, 1980; ISBN 9283513576. [Google Scholar]
- Bernus, F.V.; Freller, H.; Günther, K.G. Vapour-Deposited Films and Industrial Applications. Thin Solid Films 1978, 50, 39–48. [Google Scholar] [CrossRef]
- Nakamura, K.; Inagawa, K.; Tsuruoka, K.; Komiya, S. Applications of Wear-Resistant Thick Films Formed by Physical Vapor Deposition Processes. Thin Solid Films 1977, 40, 155–167. [Google Scholar] [CrossRef]
- Van Stappen, M.; Stals, L.M.; Kerkhofs, M.; Quaeyhaegens, C. State of the Art for the Industrial Use of Ceramic PVD Coatings. Surf. Coat. Technol. 1995, 74, 629–633. [Google Scholar] [CrossRef]
- Moll, E.; Bergmann, E. Hard Coatings by Plasma-Assisted PVD Technologies: Industrial Practice. Surf. Coat. Technol. 1989, 37, 483–509. [Google Scholar] [CrossRef]
- Constantin, R.; Miremad, B. Performance of Hard Coatings, Made by Balanced and Unbalanced Magnetron Sputtering, for Decorative Applications. Surf. Coat. Technol. 1999, 120–121, 728–733. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G.; Fernandes, L. On the Physical Vapour Deposition (PVD): Evolution of Magnetron Sputtering Processes for Industrial Applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Oehr, C.; Hegemann, D.; Liehr, M.; Wohlfart, P. Cost Structure and Resource Efficiency of Plasma Processes. Plasma Process. Polym. 2022, 19, 2200022. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Multilayer Coatings for Tribology: A Mini Review. Nanomaterials 2022, 12, 1388. [Google Scholar] [CrossRef]
- Fortune Business Insights Physical Vapor Deposition (PVD) Market Size & Growth 2023–2028. Available online: https://www.fortunebusinessinsights.com/physical-vapour-deposition-pvd-market-102364 (accessed on 18 May 2023).
- Kumar, V.; Dwivedi, S.K. Toxicity Potential of Electroplating Wastewater and Its Bioremediation Approaches: A Review. Environ. Technol. Rev. 2021, 10, 238–254. [Google Scholar] [CrossRef]
- Kamar, M.T.; Elattar, H.; Mahmoud, A.S.; Peters, R.W.; Mostafa, M.K. A Critical Review of State-of-the-Art Technologies for Electroplating Wastewater Treatment. Int. J. Environ. Anal. Chem. 2022, 102, 1–34. [Google Scholar] [CrossRef]
- Comparini, A.; Del Pace, I.; Giurlani, W.; Emanuele, R.; Verrucchi, M.; Bonechi, M.; Innocenti, M. Electroplating on Al6082 Aluminium: A New Green and Sustainable Approach. Coatings 2022, 13, 13. [Google Scholar] [CrossRef]
- Mariani, E.; Giurlani, W.; Bonechi, M.; Dell’Aquila, V.; Innocenti, M. A Systematic Study of Pulse and Pulse Reverse Plating on Acid Copper Bath for Decorative and Functional Applications. Sci. Rep. 2022, 12, 18175. [Google Scholar] [CrossRef]
- Giurlani, W.; Fidi, A.; Anselmi, E.; Pizzetti, F.; Bonechi, M.; Carretti, E.; Lo Nostro, P.; Innocenti, M. Specific Ion Effects on Copper Electroplating. Colloids Surf. B Biointerfaces 2023, 225, 113287. [Google Scholar] [CrossRef]
- Giurlani, W.; Dell’Aquila, V.; Vizza, M.; Calisi, N.; Lavacchi, A.; Irrera, A.; Lo Faro, M.J.; Leonardi, A.A.; Morganti, D.; Innocenti, M. Electrodeposition of Nanoparticles and Continuous Film of CdSe on N-Si (100). Nanomaterials 2019, 9, 1504. [Google Scholar] [CrossRef]
- Passaponti, M.; Lari, L.; Bonechi, M.; Bruni, F.; Giurlani, W.; Sciortino, G.; Rosi, L.; Fabbri, L.; Vizza, M.; Lazarov, V.K.; et al. Optimisation Study of Co Deposition on Chars from MAP of Waste Tyres as Green Electrodes in ORR for Alkaline Fuel Cells. Energies 2020, 13, 5646. [Google Scholar] [CrossRef]
- Dalirian, F.; Afzali, N.; Keshavarzi, R. TiCuN Coating on Brass Faucets: From Beautifully Colored Appearance to Antibacterial Properties. Chem. Pap. 2022, 77, 3471–3479. [Google Scholar] [CrossRef]
- Arenas, M.A.; Conde, A.; García, I.; de Damborenea, J.J. PVD Hard Coatings on Ceramic Tiles for Aesthetic Applications: Surface Characterisation and Corrosion Properties. Ceram. Int. 2022, 48, 21835–21843. [Google Scholar] [CrossRef]
- Osés, J.; Fuentes, G.G.; Palacio, J.F.; Esparza, J.; García, J.A.; Rodríguez, R. Antibacterial Functionalization of PVD Coatings on Ceramics. Coatings 2018, 8, 197. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron Sputtering: A Review of Recent Developments and Applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Martinuzzi, S.M.; Donati, L.; Giurlani, W.; Pizzetti, F.; Galvanetto, E.; Calisi, N.; Innocenti, M.; Caporali, S. A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings. Coatings 2022, 12, 257. [Google Scholar] [CrossRef]
- Digital Sciences & Research Solutions, Inc. PVD Pubblications over Year. Available online: https://app.dimensions.ai/analytics/publication/overview/timeline?search_mode=content&search_text=PVD%20over%20year&search_type=kws&search_field=full_search&year_from=1974&year_to=2022 (accessed on 18 April 2023).
- Sun, Z.; He, G.; Meng, Q.; Li, Y.; Tian, X. Corrosion Mechanism Investigation of TiN/Ti Coating and TC4 Alloy for Aircraft Compressor Application. Chin. J. Aeronaut. 2020, 33, 1824–1835. [Google Scholar] [CrossRef]
- Meier, S.M.; Gupta, D.K. The Evolution of Thermal Barrier Coatings in Gas Turbine Engine Applications. J. Eng. Gas Turbine Power 1994, 116, 250–257. [Google Scholar] [CrossRef]
- Ferreira, A.A.; Silva, F.J.G.; Pinto, A.G.; Sousa, V.F.C. Characterization of Thin Chromium Coatings Produced by PVD Sputtering for Optical Applications. Coatings 2021, 11, 215. [Google Scholar] [CrossRef]
- Singh, J.; Wolfe, D.E. Review Nano and Macro-Structured Component Fabrication by Electron Beam-Physical Vapor Deposition (EB-PVD). J. Mater. Sci. 2005, 40, 1–26. [Google Scholar] [CrossRef]
- Krella, A. Resistance of PVD Coatings to Erosive and Wear Processes: A Review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- Duminica, F.D.; Belchi, R.; Libralesso, L.; Mercier, D. Investigation of Cr(N)/DLC Multilayer Coatings Elaborated by PVD for High Wear Resistance and Low Friction Applications. Surf. Coat. Technol. 2018, 337, 396–403. [Google Scholar] [CrossRef]
- Che, J.; Yi, P.; Peng, L.; Lai, X. Impact of Pressure on Carbon Films by PECVD toward High Deposition Rates and High Stability as Metallic Bipolar Plate for PEMFCs. Int. J. Hydrogen Energy 2020, 45, 16277–16286. [Google Scholar] [CrossRef]
- Xiao, Q.; He, H.; Shao, S.; Shao, J.; Fan, Z. Influences of Deposition Rate and Oxygen Partial Pressure on Residual Stress and Microstructure of YSZ Thin Films. Thin Solid Films 2009, 517, 4295–4298. [Google Scholar] [CrossRef]
- Li, N.; Allain, J.P.; Ruzic, D.N. Enhancement of Aluminum Oxide Physical Vapor Deposition with a Secondary Plasma. Surf. Coat. Technol. 2002, 149, 161–170. [Google Scholar] [CrossRef]
- Aouadi, K.; Tlili, B.; Nouveau, C.; Besnard, A.; Chafra, M.; Souli, R. Influence of Substrate Bias Voltage on Corrosion and Wear Behavior of Physical Vapor Deposition CrN Coatings. J. Mater. Eng. Perform. 2019, 28, 2881–2891. [Google Scholar] [CrossRef]
- Schulz, U.; Miinzer, J.; Kaclen, U. Influence of Deposition Conditions on Density and Microstructure of EB–PVD TBCs. In 26th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: B: Ceramic Engineering and Science Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 353–360. [Google Scholar] [CrossRef]
- Mahne, N.; Čekada, M.; Panjan, M. Total and Differential Sputtering Yields Explored by SRIM Simulations. Coatings 2022, 12, 1541. [Google Scholar] [CrossRef]
- Strijckmans, K.; Schelfhout, R.; Depla, D. Tutorial: Hysteresis during the Reactive Magnetron Sputtering Process. J. Appl. Phys. 2018, 124, 241101. [Google Scholar] [CrossRef]
- Särhammar, E.; Strijckmans, K.; Nyberg, T.; Van Steenberge, S.; Berg, S.; Depla, D. A Study of the Process Pressure Influence in Reactive Sputtering Aiming at Hysteresis Elimination. Surf. Coat. Technol. 2013, 232, 357–361. [Google Scholar] [CrossRef]
- Zubizarreta, C.; Hernández, O.; Fernández-Martínez, I.; Ciarsolo, I.; Díez-Sierra, J.; Carreras, L.; Barriga, J. The Effect of Bias Configuration on the Properties of AlCrN Based Thick Coatings Deposited by Cathodic Arc Evaporation. Appl. Surf. Sci. 2023, 610, 155543. [Google Scholar] [CrossRef]
- Shuangquan, R.; Jun, H.; Hongjun, W.; Canxin, T.; Liping, G.; Dejun, F. Effects of Bias Voltage on the Structure and Mechanical Properties of Thick CrN Coatings Deposited by Mid-Frequency Magnetron Sputtering. Plasma Sci. Technol. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Stevenson, P.; Matthews, A. PVD Equipment Design: Concepts for Increased Production Throughput. Surf. Coat. Technol. 1995, 74–75, 770–780. [Google Scholar] [CrossRef]
- Gilewicz, A.; Jedrzejewski, R.; Myslinski, P.; Warcholinski, B. Structure, Morphology, and Mechanical Properties of AlCrN Coatings Deposited by Cathodic Arc Evaporation. J. Mater. Eng. Perform. 2019, 28, 1522–1531. [Google Scholar] [CrossRef]
- Ürgen, M.; Eryilmaz, O.L.; Çakir, A.F.; Kayali, E.S.; Nilüfer, B.; Işik, Y. Characterization of Molybdenum Nitride Coatings Produced by Arc-PVD Technique. Surf. Coat. Technol. 1997, 94–95, 501–506. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, Y.; Jin, S.; Wang, J.; Liu, R.; Liu, H.; Shi, W.; Kolawole, S.K.; Ren, L.; Yu, B.; et al. Antibacterial TiCu/TiCuN Multilayer Films with Good Corrosion Resistance Deposited by Axial Magnetic Field-Enhanced Arc Ion Plating. ACS Appl. Mater. Interfaces 2019, 11, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Bakan, E.; Mack, D.E.; Mauer, G.; Vaßen, R.; Lamon, J.; Padture, N.P. 1—High-Temperature Materials for Power Generation in Gas Turbines; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780081027264. [Google Scholar]
- Rossnagel, S.M. Directional and Preferential Sputtering-Based Physical Vapor Deposition. Thin Solid Films 1995, 263, 1–12. [Google Scholar] [CrossRef]
- Liu, M.-J.; Zhang, M.; Zhang, X.-F.; Li, G.-R.; Zhang, Q.; Li, C.-X.; Li, C.-J.; Yang, G.-J. Transport and Deposition Behaviors of Vapor Coating Materials in Plasma Spray-Physical Vapor Deposition. Appl. Surf. Sci. 2019, 486, 80–92. [Google Scholar] [CrossRef]
- Wang, P.; He, W.; Mauer, G.; Mücke, R.; Vaßen, R. Monte Carlo Simulation of Column Growth in Plasma Spray Physical Vapor Deposition Process. Surf. Coat. Technol. 2018, 335, 188–197. [Google Scholar] [CrossRef]
- Karabacak, T.; Zhao, Y.-P.; Wang, G.-C.; Lu, T.-M. Growth-Front Roughening in Amorphous Silicon Films by Sputtering. Phys. Rev. B 2001, 64, 085323. [Google Scholar] [CrossRef]
- Greczynski, G.; Jensen, J.; Hultman, L. Mitigating the Geometrical Limitations of Conventional Sputtering by Controlling the Ion-to-Neutral Ratio during High Power Pulsed Magnetron Sputtering. Thin Solid Films 2011, 519, 6354–6361. [Google Scholar] [CrossRef]
- Biswas, B.; Purandare, Y.; Sugumaran, A.; Khan, I.; Hovsepian, P.E. Effect of Chamber Pressure on Defect Generation and Their Influence on Corrosion and Tribological Properties of HIPIMS Deposited CrN/NbN Coatings. Surf. Coat. Technol. 2018, 336, 84–91. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and Technology of Magnetron Sputtering Discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Keraudy, J.; Viloan, R.P.B.; Raadu, M.A.; Brenning, N.; Lundin, D.; Helmersson, U. Bipolar HiPIMS for Tailoring Ion Energies in Thin Film Deposition. Surf. Coat. Technol. 2019, 359, 433–437. [Google Scholar] [CrossRef]
- Tiron, V.; Ursu, E.-L.; Cristea, D.; Munteanu, D.; Bulai, G.; Ceban, A.; Velicu, I.-L. Overcoming the Insulating Materials Limitation in HiPIMS: Ion-Assisted Deposition of DLC Coatings Using Bipolar HiPIMS. Appl. Surf. Sci. 2019, 494, 871–879. [Google Scholar] [CrossRef]
- Brenning, N.; Hajihoseini, H.; Rudolph, M.; Raadu, M.A.; Gudmundsson, J.T.; Minea, T.M.; Lundin, D. HiPIMS Optimization by Using Mixed High-Power and Low-Power Pulsing. Plasma Sources Sci. Technol. 2021, 30, 015015. [Google Scholar] [CrossRef]
- Ghailane, A.; Makha, M.; Larhlimi, H.; Alami, J. Design of Hard Coatings Deposited by HiPIMS and DcMS. Mater. Lett. 2020, 280, 128540. [Google Scholar] [CrossRef]
- Greczynski, G.; Jensen, J.; Böhlmark, J.; Hultman, L. Microstructure Control of CrNx Films during High Power Impulse Magnetron Sputtering. Surf. Coat. Technol. 2010, 205, 118–130. [Google Scholar] [CrossRef]
- Dong, X.; Su, Y.; Wu, Z.; Xu, X.; Xiang, Z.; Shi, Y.; Chen, W.; Dai, J.; Huang, Z.; Wang, T.; et al. Reactive Pulsed DC Magnetron Sputtering Deposition of Vanadium Oxide Thin Films: Role of Pulse Frequency on the Film Growth and Properties. Appl. Surf. Sci. 2021, 562, 150138. [Google Scholar] [CrossRef]
- Geng, D.; Li, H.; Chen, Z.; Xu, Y.X.; Wang, Q. Microstructure, Oxidation Behavior and Tribological Properties of AlCrN/Cu Coatings Deposited by a Hybrid PVD Technique. J. Mater. Sci. Technol. 2022, 100, 150–160. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kwon Hwangbo, C. Influence of Ar Ion-Beam Assistance and Annealing Temperatures on Properties of TiO2 Thin Films Deposited by Reactive DC Magnetron Sputtering. Thin Solid Films 2005, 475, 155–159. [Google Scholar] [CrossRef]
- Dai, W.; Zheng, H.; Wu, G.; Wang, A. Effect of Bias Voltage on Growth Property of Cr-DLC Film Prepared by Linear Ion Beam Deposition Technique. Vacuum 2010, 85, 231–235. [Google Scholar] [CrossRef]
- Körber, F.-J. The Role of Ion Implantation in Industrial Sputtering Processes for Tribological Applications. Surf. Coat. Technol. 1995, 74–75, 168–172. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.J.; Sproul, W.D.; Mishra, B.; Wu, Z.; Wang, J. The Structure and Properties of Chromium Nitride Coatings Deposited Using Dc, Pulsed Dc and Modulated Pulse Power Magnetron Sputtering. Surf. Coat. Technol. 2010, 204, 2230–2239. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive High Power Impulse Magnetron Sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Haye, E.; Colaux, J.L.; Moskovkin, P.; Pireaux, J.-J.; Lucas, S. Wide Range Investigation of Duty Cycle and Frequency Effects on Bipolar Magnetron Sputtering of Chromium Nitride. Surf. Coat. Technol. 2018, 350, 84–94. [Google Scholar] [CrossRef]
- Ou, Y.X.; Ouyang, X.P.; Liao, B.; Zhang, X.; Zhang, S. Hard yet Tough CrN/Si3N4 Multilayer Coatings Deposited by the Combined Deep Oscillation Magnetron Sputtering and Pulsed Dc Magnetron Sputtering. Appl. Surf. Sci. 2020, 502, 144168. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Guo, X.; Wang, C.; Sun, L. Preparation of Micro-Textures on Cemented Carbide Substrate Surface by Plasma-Assisted Laser Machining to Enhance the PVD Tool Coatings Adhesion. J. Mater. Process Technol. 2021, 288, 116870. [Google Scholar] [CrossRef]
- Esparza-Contro, C.; Berthomé, G.; Renou, G.; Robaut, F.; Coindeau, S.; Vachey, C.; Cambin, J.; Mantel, M.; Latu-Romain, L. Microstructures of Titanium Oxide Thin Films Grown Continuously on Stainless Steel Wires by PVD in an Inverted Cylindrical Magnetron: Towards an Industrial Process. Surf. Coat. Technol. 2020, 389, 125643. [Google Scholar] [CrossRef]
- Bertorelle, E. Manuale Di Galvanotecnica; Hoepli: Milan, Italy, 2016; ISBN 9788820374983. [Google Scholar]
- Antonov, M.; Hussainova, I.; Sergejev, F.; Kulu, P.; Gregor, A. Assessment of Gradient and Nanogradient PVD Coatings Behaviour under Erosive, Abrasive and Impact Wear Conditions. Wear 2009, 267, 898–906. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Lukaszkowicz, K. Comparison of Structure and Properties of the PVD, Hybrid (Galvanic + PVD), and Galvanic Coatings Deposited onto the Brass Substrate. Mater. Sci. Forum 2008, 591–593, 860–864. [Google Scholar] [CrossRef]
- Ponte, F.; Sharma, P.; Figueiredo, N.M.; Ferreira, J.; Carvalho, S. Decorative Chromium Coatings on Polycarbonate Substrate for the Automotive Industry. Materials 2023, 16, 2315. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Johansson, J. Towards Sustainable Production of Brass Components Characterisation, Machinability and Solid-State-Cutting Machining Mechanism; Lund University: Lund, Sweden, 2022; ISBN 9789180392716. [Google Scholar]
- Pantazopoulos, G. A Review of Defects and Failures in Brass Rods and Related Components. J. Fail. Anal. Prev. 2003, 3, 14–22. [Google Scholar] [CrossRef]
- Johansson, J.; Alm, P.; M’Saoubi, R.; Malmberg, P.; Ståhl, J.E.; Bushlya, V. On the Function of Lead (Pb) in Machining Brass Alloys. Int. J. Adv. Manuf. Technol. 2022, 120, 7263–7275. [Google Scholar] [CrossRef]
- Pantazopoulos, G. Leaded Brass Rods C 38500 for Automatic Machining Operations: A Technical Report. J. Mater. Eng. Perform. 2002, 11, 402–407. [Google Scholar] [CrossRef]
- Stavroulakis, P.; Toulfatzis, A.I.; Pantazopoulos, G.A.; Paipetis, A.S. Machinable Leaded and Eco-Friendly Brass Alloys for High Performance Manufacturing Processes: A Critical Review. Metals 2022, 12, 246. [Google Scholar] [CrossRef]
- Pantazopoulos, G.; Vazdirvanidis, A. Characterization of the Microstructural Aspects of Machinable α–β Phase Brass. Microsc. Anal. 2008, 22, 13–16. [Google Scholar]
- Reiners, G.; Beck, U.; Jehn, H.A. Decorative Optical Coatings. Thin Solid Films 1994, 253, 33–40. [Google Scholar] [CrossRef]
- Langer, B. Methods to Improve the PVD Coatability of Brass by Using Diffusion Barriers. Doctoral Dissertation, Sheffield Hallam University, Sheffield, UK, 1995. [Google Scholar]
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and Development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Lukaszkowicz, K.; Zarychta, A.; Cunha, L. Corrosion Resistance of Multilayer Coatings Deposited by PVD Techniques onto the Brass Substrate. J. Mater. Process. Technol. 2005, 164–165, 816–821. [Google Scholar] [CrossRef]
- Dobrzańska-Danikiewicz, A.D.; Lukaszkowicz, K. Technology Validation of Coatings Deposition onto the Brass Substrate. Arch. Mater. Sci. Eng. 2010, 46, 5–38. [Google Scholar]
- EN 10027-1:2016; Designation Systems for Steels—Part 1: Steel Names. European Committee for Standardization: Brussels, Belgium, 2016.
- ASM International Handbook Committee. ASM Handbook, Volume 1, Properties and Selection: Irons, Steels, and High Performance Alloys; ASM International: West Conshohocken, PA, USA, 1993; Volume 1, ISBN 0-87170-377-7. [Google Scholar]
- Davis, J.R.; ASM International; Handbook Committee. ASM Specialty Handbook® Stainless Steels; ASM International: Conshohocken, PA, USA, 1994; ISBN 0871705036. [Google Scholar]
- Aggen, G.; Akstens, F.W.; Michael, C.; Adjelian, A.; Rubeli, A.; Avery Consultant, L.H.S.; Babu, P.; Bayer, A.M.; Vasco, T.; Bello, F.; et al. ASM Handbook, Volume 1, Properties and Selection: Irons, Steels, and High Performance Alloys Section: Publication Information and Contributors Publication Information and Contributors Authors and Reviewers; ASM International: West Conshohocken, PA, USA, 2005; Volume 1. [Google Scholar]
- ASM International. ASM HANDBOOK Volume 9: Metallography and Microstructures; Vander Voort, G.F., Ed.; ASM International: West Conshohocken, PA, USA, 2004; Volume 9, ISBN 978-0-87170-706-2. [Google Scholar]
- Outokumpu Stainless, A.B. Handbook of Stainless Steel; Avesta Resarch Centre: Avesta, Sweden, 2013. [Google Scholar]
- Sutcliffe, J.M.; Fessler, R.R.; Boyd, W.K.; Parkins, R.N. Stress Corrosion Cracking of Carbon Steel in Carbonate Solutions. Corrosion 1972, 28, 313–320. [Google Scholar] [CrossRef]
- Awan, A.; Pasha, R.A.; Butt, M.S.; Malik, R.A.; Alarifi, I.M.; Alzaid, M.; Latif, M.; Naseer, A.; Saleem, M.; Alrobei, H. Corrosion and Wear Behavior of TiN PVD Coated 304 Stainless-Steel. J. Mech. Sci. Technol. 2020, 34, 3227–3232. [Google Scholar] [CrossRef]
- Hoche, H.; Pusch, C.; Oechsner, M. Corrosion and Wear Protection of Mild Steel Substrates by Innovative PVD Coatings. Surf. Coat. Technol. 2020, 391, 125659. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Ananth, A.; Khan, J.; Srinivas, G. Ar+H2 Plasma Etching for Improved Adhesion of PVD Coatings on Steel Substrates. Vacuum 2012, 86, 1165–1173. [Google Scholar] [CrossRef]
- Mohamadian Samim, P.; Fattah-Alhosseini, A.; Elmkhah, H.; Imantalab, O. Structure and Corrosion Behavior of ZrN/CrN Nano-Multilayer Coating Deposited on AISI 304 Stainless Steel by CAE-PVD Technique. J. Asian Ceram. Soc. 2020, 8, 460–469. [Google Scholar] [CrossRef]
- Taweesup, K.; Lertsakwanit, R.; Jiravanichkul, J.; Yongvanich, N.; Lothongkum, G.; Visuttipitukul, P. Effect of Type and Coating Time of Interlayer on Properties and Morphology of Cr-Zr-N Film Prepared by DC Magnetron Sputtering PVD on H13 Steel. Key Eng. Mater. 2015, 658, 111–119. [Google Scholar] [CrossRef]
- Petrogalli, C.; Montesano, L.; Gelfi, M.; La Vecchia, G.M.; Solazzi, L. Tribological and Corrosion Behavior of CrN Coatings: Roles of Substrate and Deposition Defects. Surf. Coat. Technol. 2014, 258, 878–885. [Google Scholar] [CrossRef]
- Kopecki, E.S. Cleaning Stainless Steel; ASTM International: West Conshohocken, PA, USA, 1973; Volume 538. [Google Scholar]
- Nikolova, M.P.; Nikolova, V.; Ivanova, V.L.; Valkov, S.; Petrov, P.; Apostolova, M.D. Mechanical Properties and in Vitro Biocompatibility Evaluation of TiN/TiO2 Coated Ti6Al4V Alloy. Mater. Today Proc. 2020, 33, 1781–1786. [Google Scholar] [CrossRef]
- Nose, M.; Zhou, M.; Honbo, E.; Yokota, M.; Saji, S. Colorimetric Properties of ZrN and TiN Coatings Prepared by DC Reactive Sputtering. Surf. Coat. Technol. 2001, 142–144, 211–217. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Terek, P.; Miletić, A.; Čekada, M.; Panjan, M. Comparative Study of Tribological Behavior of TiN Hard Coatings Deposited by Various PVD Deposition Techniques. Coatings 2022, 12, 294. [Google Scholar] [CrossRef]
- Silva, F.C.; Schön, G. Influence of Substrate Stiffness and of PVD Parameters on the Influence of and Substrate Stiffness and of PVD Parameters on the Microstructure Tension Fracture Characteristics of TiN Thin Films and a Tension Fracture Characteri. Procedia Struct. Integr. 2018, 13, 658–663. [Google Scholar] [CrossRef]
- Tsai, D.C.; Chang, Z.C.; Kuo, B.H.; Liu, Y.C.; Chen, E.C.; Shieu, F.S. Structural, Electro-Optical, and Mechanical Properties of Reactively Sputtered (TiZrHf)N Coatings. Ceram. Int. 2016, 42, 14257–14265. [Google Scholar] [CrossRef]
- Vaz, F.; Machado, P.; Rebouta, L.; Cerqueira, P.; Goudeau, P.; Rivière, J.P.; Alves, E.; Pischow, K.; de Rijk, J. Mechanical Characterization of Reactively Magnetron-Sputtered TiN Films. Surf. Coat. Technol. 2003, 174–175, 375–382. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez, E.; González-Hernández, A.; Brachetti-Sibaja, S.B.; Dorantes-Rosales, H.J.; López-Oyama, A.B. Effect of Deposition Parameters on Structural, Mechanical and Electrochemical Properties in Ti/TiN Thin Films on AISI 316L Substrates Produced by r. f. Magnetron Sputtering. J. Alloys Compd. 2018, 746, 688–698. [Google Scholar] [CrossRef]
- Combadiere, L.; Machet, J. Reactive Magnetron Sputtering Deposition of TiN Films. I. Influence of the Substrate Temperature on Structure, Composition and Morphology of the Films. Surf. Coat. Technol. 1997, 88, 17–27. [Google Scholar] [CrossRef]
- Chunyan, Y.; Linhai, T.; Yinghui, W.; Shebin, W.; Tianbao, L.; Bingshe, X. The Effect of Substrate Bias Voltages on Impact Resistance of CrAlN Coatings Deposited by Modified Ion Beam Enhanced Magnetron Sputtering. Appl. Surf. Sci. 2009, 255, 4033–4038. [Google Scholar] [CrossRef]
- Zalnezhad, E.; Sarhan, A.A.D.; Hamdi, M. Optimizing the PVD TiN Thin Film Coating’s Parameters on Aerospace AL7075-T6 Alloy for Higher Coating Hardness and Adhesion with Better Tribological Properties of the Coating Surface. Int. J. Adv. Manuf. Technol. 2013, 64, 281–290. [Google Scholar] [CrossRef]
- Richter, N.A.; Yang, B.; Barnard, J.P.; Niu, T.; Sheng, X.; Shaw, D.; Watanabe, M.; Rane, G.; Krause, U.; Dürrenfeld, P.; et al. Significant Texture and Wear Resistance Improvement of TiN Coatings Using Pulsed DC Magnetron Sputtering. Appl. Surf. Sci. 2023, 635, 157709. [Google Scholar] [CrossRef]
- Richey, B.; Burch, M. Applications for Decorative and Protective Coatings. In Polymer Dispersions and Their Industrial Applications; Wiley: Hoboken, NJ, USA, 2002; pp. 123–161. [Google Scholar]
- Gupta, P.; Fang, F.; Rubanov, S.; Loho, T.; Koo, A.; Swift, N.; Fiedler, H.; Leveneur, J.; Murmu, P.P.; Markwitz, A.; et al. Decorative Black Coatings on Titanium Surfaces Based on Hard Bi-Layered Carbon Coatings Synthesized by Carbon Implantation. Surf. Coat. Technol. 2019, 358, 386–393. [Google Scholar] [CrossRef]
- Sanchéz, J.E.; Sanchéz, O.M.; Ipaz, L.; Aperador, W.; Caicedo, J.C.; Amaya, C.; Landaverde, M.A.H.; Beltran, F.E.; Muñoz-Saldaña, J.; Zambrano, G. Mechanical, Tribological, and Electrochemical Behavior of Cr1−xAlxN Coatings Deposited by r.f. Reactive Magnetron Co-Sputtering Method. Appl. Surf. Sci. 2010, 256, 2380–2387. [Google Scholar] [CrossRef]
- Quazi, M.M.; Ishak, M.; Arslan, A.; Nasir Bashir, M.; Ali, I. Scratch Adhesion and Wear Failure Characteristics of PVD Multilayer CrTi/CrTiN Thin Film Ceramic Coating Deposited on AA7075-T6 Aerospace Alloy. J. Adhes. Sci. Technol. 2018, 32, 625–641. [Google Scholar] [CrossRef]
- He, J.; Lan, X.; Liu, Z.; Jiao, D.; Zhong, X.; Cheng, Y.; Tang, C.; Qiu, W. Modification of Cr/CrN Composite Structure by Fe Addition and Its Effect on Decorative Performance and Corrosion Resistance. Ceram. Int. 2021, 47, 23888–23894. [Google Scholar] [CrossRef]
- Lorenzo-Martin, C.; Ajayi, O.; Erdemir, A.; Fenske, G.R.; Wei, R. Effect of Microstructure and Thickness on the Friction and Wear Behavior of CrN Coatings. Wear 2013, 302, 963–971. [Google Scholar] [CrossRef]
- Hessel, E.V.S.; Staal, Y.C.M.; Piersma, A.H.; den Braver-Sewradj, S.P.; Ezendam, J. Occupational Exposure to Hexavalent Chromium. Part I. Hazard Assessment of Non-Cancer Health Effects. Regul. Toxicol. Pharmacol. 2021, 126, 105048. [Google Scholar] [CrossRef]
- Ul-Hamid, A. Microstructure, Properties and Applications of Zr-Carbide, Zr-Nitride and Zr-Carbonitride Coatings: A Review. Mater. Adv. 2020, 1, 1012–1037. [Google Scholar] [CrossRef]
- Johnson, P.C.; Randhawa, H. Zirconium Nitride Films Prepared by Cathodic Arc Plasma Deposition Process. Surf. Coat. Technol. 1987, 33, 53–62. [Google Scholar] [CrossRef]
- Budke, E.; Krempel-Hesse, J.; Maidhof, H.; Schüssler, H. Decorative Hard Coatings with Improved Corrosion Resistance. Surf. Coat. Technol. 1999, 112, 108–113. [Google Scholar] [CrossRef]
- Ul-Hamid, A. The Effect of Deposition Conditions on the Properties of Zr-Carbide, Zr-Nitride and Zr-Carbonitride Coatings-a Review. Mater. Adv. 2020, 1, 988–1011. [Google Scholar] [CrossRef]
- Klumdoung, P.; Buranawong, A.; Chaiyakun, S.; Limsuwan, P. Variation of Color in Zirconium Nitride Thin Films Prepared at High Ar Flow Rates with Reactive Dc Magnetron Sputtering. Procedia Eng. 2012, 32, 916–921. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Lapitskaya, V.; Khabarava, A.; Chizhik, S.; Warcholinski, B.; Gilewicz, A. The Influence of Nitrogen on the Morphology of ZrN Coatings Deposited by Magnetron Sputtering. Appl. Surf. Sci. 2020, 522, 146508. [Google Scholar] [CrossRef]
- Navinšek, B.; Panjan, P.; Milošev, I. PVD Coatings as an Environmentally Clean Alternative to Electroplating and Electroless Processes. Surf. Coat. Technol. 1999, 116–119, 476–487. [Google Scholar] [CrossRef]
- Chou, W.J.; Yu, G.P.; Huang, J.H. Corrosion Resistance of ZrN Films on AISI 304 Stainless Steel Substrate. Surf. Coat. Technol. 2003, 167, 59–67. [Google Scholar] [CrossRef]
- Rizzo, A.; Signore, M.A.; Valerini, D. Protective Multilayer Coatings for Tribological Applications Nowadays Protective Coatings Are Identifi Ed as a Fundamental Key to Guarantee Strong Mechanical. EAI—Energ. Ambiente Innov. 2012, 3, 102–108. [Google Scholar]
- Kuprin, A.S.; Gilewicz, A.; Kuznetsova, T.A.; Lapitskaya, V.A.; Tolmachova, G.N.; Warcholinski, B.; Aizikovich, S.M.; Sadyrin, E.V. Structure and Properties of Zron Coatings Synthesized by Cathodic Arc Evaporation. Materials 2021, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.A.; Lapitskaya, V.A.; Chizhik, S.A.; Warcholinski, B.; Gilewicz, A.; Kuprin, A.S. Influence of the Third Element Additives on the Surface Morphology of the Wear-Resistant ZrN Coatings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 443, 3–8. [Google Scholar] [CrossRef]
- Warcholinski, B.; Kuznetsova, T.A.; Gilewicz, A.; Zubar, T.I.; Lapitskaya, V.A.; Chizhik, S.A.; Komarov, A.I.; Komarova, V.I.; Kuprin, A.S.; Ovcharenko, V.D.; et al. Structural and Mechanical Properties of Zr-Si-N Coatings Deposited by Arc Evaporation at Different Substrate Bias Voltages. J. Mater. Eng. Perform. 2018, 27, 3940–3950. [Google Scholar] [CrossRef]
- Behrangi, S.; Sedláček, I.; Štěrba, J.; Suková, G.; Czigány, Z.; Buršíková, V.; Souček, P.; Sochora, V.; Balázsi, K.; Vašina, P. An Assessment of the Bactericidal and Virucidal Properties of ZrN-Cu Nanostructured Coatings Deposited by an Industrial PVD System. Coatings 2022, 12, 1330. [Google Scholar] [CrossRef]
- Etiemble, A.; Der Loughian, C.; Apreutesei, M.; Langlois, C.; Cardinal, S.; Pelletier, J.M.; Pierson, J.F.; Steyer, P. Innovative Zr-Cu-Ag Thin Film Metallic Glass Deposed by Magnetron PVD Sputtering for Antibacterial Applications. J. Alloys Compd. 2017, 707, 155–161. [Google Scholar] [CrossRef]
- Constantin, R.; Steinmann, P.-A.; Manasterski, C. Decorative PVD Coatings. In Nanomaterials and Surface Engineering; Takadoum, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 109–162. [Google Scholar]
- Robertson, J. Properties of Diamond-like Carbon. Surf. Coat. Technol. 1992, 50, 185–203. [Google Scholar] [CrossRef]
- Varade, A.; Niranjan Reddy, K.; Sasen, D.; Krishna, A.; Chellamalai, M.; Shashi Kumar, P.V. Detailed Raman Study of DLC Coating on Si (100) Made by RF-PECVD. Procedia Eng. 2014, 97, 1452–1456. [Google Scholar] [CrossRef]
- Miki, Y.; Nishimoto, A.; Sone, T.; Araki, Y. Residual Stress Measurement in DLC Films Deposited by PBIID Method Using Raman Microprobe Spectroscopy. Surf. Coat. Technol. 2015, 283, 274–280. [Google Scholar] [CrossRef]
- Januś, M. DLC Layers Created Using CVD Techniques and Their Application. Chem. Vap. Depos. Nanotechnol. 2019. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Muller, I.L. Electrodeposition of Diamond-like Carbon (DLC) Films on Ti. Appl. Surf. Sci. 2009, 255, 4082–4086. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- Vetter, J. 60years of DLC Coatings: Historical Highlights and Technical Review of Cathodic Arc Processes to Synthesize Various DLC Types, and Their Evolution for Industrial Applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Gómez, I.; Claver, A.; Santiago, J.A.; Fernandez, I.; Palacio, J.F.; Diaz, C.; Mändl, S.; Garcia, J.A. Improved Adhesion of the Dlc Coating Using Hipims with Positive Pulses and Plasma Immersion Pretreatment. Coatings 2021, 11, 1070. [Google Scholar] [CrossRef]
- Christopher, M.; Lowson, R.; Peck, H. Creating Agile Supply Chains in the Fashion Industry. Int. J. Retail. Distrib. Manag. 2004, 32, 367–376. [Google Scholar] [CrossRef]
- Brun, A.; Moretto, A. Organisation and Supply Chain for Quality Control in Luxury Companies. J. Fash. Mark. Manag. 2014, 18, 206–230. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Innocenti, M.; Lavacchi, A. Measuring the Thickness of Metal Coatings: A Review of the Methods. Coatings 2020, 10, 1211. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Ren, X.; Wang, B.; Cai, Y.; Song, Q.; Wan, Y. Coating-Thickness-Dependent Physical Properties and Cutting Temperature for Cutting Inconel 718 with TiAlN Coated Tools. J. Adv. Res. 2022, 38, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Schmid, M. Thickness Measurement Methods for Physical Vapor Deposited Aluminum Coatings in Packaging Applications: A Review. Coatings 2017, 7, 9. [Google Scholar] [CrossRef]
- Holleck, H.; Schier, V. Multilayer PVD Coatings for Wear Protection. Surf. Coat. Technol. 1995, 76–77, 328–336. [Google Scholar] [CrossRef]
- Stueber, M.; Holleck, H.; Leiste, H.; Seemann, K.; Ulrich, S.; Ziebert, C. Concepts for the Design of Advanced Nanoscale PVD Multilayer Protective Thin Films. J. Alloys Compd. 2009, 483, 321–333. [Google Scholar] [CrossRef]
- ISO 21874:2019; PVD Multi-Layer Hard Coatings—Composition, Structure and Properties. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 26423:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics). International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 3497:2000; Measurement of Coating Thickness. International Organization for Standardization: Geneva, Switzerland, 2000.
- Quinones-Salinas, M.A.; Mercado-Solis, R.D. Comparative Study of Three Methods for Measuring Thickness of PVD Hard Coatings. Int. J. Surf. Sci. Eng. 2015, 9, 493–509. [Google Scholar] [CrossRef]
- Sokolov, A.; Hasikova, J.; Pecerskis, A.; Gostilo, V. Industrial X-Ray Fluorescence Analyzer for Real-Time Thickness Measurements of Aluminium Coatings on Rolled Steel. Coatings 2019, 9, 425. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Innocenti, M.; Lavacchi, A. Coating Thickness Determination Using X-Ray Fluorescence Spectroscopy: Monte Carlo Simulations as an Alternative to the Use of Standards. Coatings 2019, 9, 79. [Google Scholar] [CrossRef]
- Giurlani, W.; Innocenti, M.; Lavacchi, A. X-ray Microanalysis of Precious Metal Thin Films: Thickness and Composition Determination. Coatings 2018, 8, 84. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Lavacchi, A.; Innocenti, M. Thickness Determination of Metal Multilayers by ED-XRF Multivariate Analysis Using Monte Carlo Simulated Standards. Anal. Chim. Acta 2020, 1130, 72–79. [Google Scholar] [CrossRef]
- Isern, L.; Waddie, A.J.; Chalk, C.; Moore, A.J.; Nicholls, J.R. Non-Destructive Thickness Measurement of Thermal Barrier Coatings Using Terahertz Radiation. Emergent Mater. 2021, 4, 1547–1557. [Google Scholar] [CrossRef]
- Cruz, J.P.N.; Garzón, C.M.; Recco, A.A.C. An Analytic Equation for Assessing the Thickness of Titanium Nitride Coatings by Energy Dispersive X-Ray Spectroscopy in the Scanning Electron Microscope. Microsc. Microanal. 2023, 29, 938–952. [Google Scholar] [CrossRef]
- Daure, J.L.; Carrington, M.J.; Shipway, P.H.; McCartney, D.G.; Stewart, D.A. A Comparison of the Galling Wear Behaviour of PVD Cr and Electroplated Hard Cr Thin Films. Surf. Coat. Technol. 2018, 350, 40–47. [Google Scholar] [CrossRef]
- Bergmann, E.; Vogel, J. Criteria for the Choice of a PVD Treatment for the Solution of Wear Problems; Pergamon Books Ltd.: London, UK, 1987. [Google Scholar]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement Andmarket Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Fukui, H. Evolutional History of Coating Technologies for Cemented Carbide Inserts—Chemical Vapor Deposition and Physical Vapor Deposition. SEI Tech. Rev. 2016, 82, 39–45. [Google Scholar]
- Jehn, H.A.; Thiergarten, F.; Ebersbach, E.; Fabian, D. Characterization of PVD (Ti, Cr)Nx Hard Coatings. Surf. Coat. Technol. 1991, 50, 45–52. [Google Scholar] [CrossRef]
- ASTM G99-17; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. Society for Testing and Materials International: West Conshohocken, PA, USA, 2017.
- DIN 50324:1992-07 TRIBOLOGY; Testing of Friction and Wear Model Test for Sliding Friction of Solids (Ball on Disc System). German Institute for Standardisation (Deutsches Institut für Normung): Berlin, Germany, 2013.
- ISO 18535:2016; Diamond-like Carbon Films—Determination of Friction and Wear Characteristics of Diamond-like Carbon Films by Ball-on-Disc Method. International Organization for Standardization: Geneva, Switzerland, 2016.
- Chen, Z.; Zheng, Y.; Huang, Y.; Gao, Z.; Sheng, H.; Bartosik, M.; Mayrhofer, P.H.; Zhang, Z. Atomic-Scale Understanding of the Structural Evolution of TiN/AlN Superlattice during Nanoindentation—Part 1: Deformation. Acta Mater. 2022, 234, 118008. [Google Scholar] [CrossRef]
- ISO 14577-1:2015; Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method. International Organization for Standardization: Geneva, Switzerland, 2015.
- ASTM C1624-22; Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2022.
- Verein Deutscher Ingenieure. VDI 3198 Beschichten von Werkzeugen Der Kaltmassivumformung; CVD-Und PVD-Verfahren; Verein Deutscher Ingenieure: Dusseldorf, Germany, 1992. [Google Scholar]
- Lenz, B.; Hasselbruch, H.; Mehner, A. Automated Evaluation of Rockwell Adhesion Tests for PVD Coatings Using Convolutional Neural Networks. Surf. Coat. Technol. 2020, 385, 125365. [Google Scholar] [CrossRef]
- ISO 2819:2017; Metallic Coatings on Metallic Substrates—Electrodeposited and Chemically Deposited Coatings—Review of Methods Available for Testing Adhesion. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11644:2022; IULTCS/IUF 470 Leather—Test for Adhesion of Finish. International Organization for Standardization: Geneva, Switzerland, 2022.
- Domanowski, P.; Betiuk, M. Recatest—A Technique for Qualitative and Quantitative Assessment of Deferment and Degraded Pvd Coatings and Cvd Layers in the Deformed Area in the Scratch Test. Materials 2021, 14, 2625. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Pakuła, D.; Hajduczek, E. Structure and Properties of the Multi-Component TiAlSiN Coatings Obtained in the PVD Process in the Nitride Tool Ceramics. J. Mater. Process. Technol. 2004, 157–158, 331–340. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Lukaszkowicz, K.; Križ, A. Properties of the Multi-Layer Ti/CrN and Ti/TiAlN Coatings Deposited with the PVD Technique onto the Brass Substrate. J. Mater. Process. Technol. 2003, 143–144, 832–837. [Google Scholar] [CrossRef]
- Steinvall, A. Anthropology of Color; MacLaury, R.E., Paramei, G.V., Dedrick, D., Eds.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2007; ISBN 978-90-272-3243-4. [Google Scholar]
- Kodžoman, D. The Psychology of Clothing: Meaning of Colors, Body Image and Gender Expression in Fashion. Text. Leather Rev. 2019, 2, 90–103. [Google Scholar] [CrossRef]
- ISO/CIE 11664-1:2019; Colorimetry—Part 1: CIE Standard Colorimetric Observers. International Organization for Standardization: Geneva, Switzerland, 2019.
- Hiller, G. Libro 3—Gestione Del Colore. In Datacolor; Datacolor AG Europe: Rotkreuz, Switzerland; pp. 1–14.
- Capitán-Vallvey, L.F.; López-Ruiz, N.; Martínez-Olmos, A.; Erenas, M.M.; Palma, A.J. Recent Developments in Computer Vision-Based Analytical Chemistry: A Tutorial Review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef]
- Eppeldauer, G. Spectral Response Based Calibration Method of Tristimulus Colorimeters. J. Res. Natl. Inst. Stand. Technol. 1998, 103, 615–619. [Google Scholar] [CrossRef]
- Giurlani, W.; Gambinossi, F.; Salvietti, E.; Passaponti, M.; Innocenti, M. Color Measurements in Electroplating Industry: Implications for Product Quality Control. ECS Trans. 2017, 80, 757–766. [Google Scholar] [CrossRef]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of Uniform Color Spaces Developed after the Adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar]
- Giurlani, W.; Sergi, L.; Crestini, E.; Calisi, N.; Poli, F.; Soavi, F.; Innocenti, M. Electrochemical Stability of Steel, Ti, and Cu Current Collectors in Water-in-Salt Electrolyte for Green Batteries and Supercapacitors. J. Solid State Electrochem. 2022, 26, 85–95. [Google Scholar] [CrossRef]
- Fenker, M.; Jackson, N.; Spolding, M.; Nicole, P.; Schönhut, K.; Gregory, G.; Hovsepian, P.E.; Münz, W.D. Corrosion Performance of PVD-Coated and Anodised Materials for the Decorative Market. Surf. Coat. Technol. 2004, 188–189, 466–472. [Google Scholar] [CrossRef]
- Jehn, H.A.; Rother, B. Homogeneity of Multi-Component PVD Hard Coatings Deposited by Multi-Source Arrangements. Surf. Coat. Technol. 1999, 112, 103–107. [Google Scholar] [CrossRef]
- ISO 9227:2022; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 3160-2:2015; Watch-Cases and Accessories—Gold Alloy Coverings—Part 2: Determination of Fineness, Thickness, Corrosion Resistance and Adhesion. International Organization for Standardization: Geneva, Switzerland, 2015.
- Association Francaise de Normalisation. NFS 80 772: 2010 Horology—Gold-Plated Watch-Cases—Test Methods for Coatings; Association Francaise de Normalisation: Saint-Denis, France, 2010. [Google Scholar]
- ISO/TR 16208:2014; Corrosion of Metals and Alloys—Test Method for Corrosion of Materials by Electrochemical Impedance Measurements. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 16773:2016; Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 17475:2005; Corrosion of Metals and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 4538:1978; Metallic Coatings—Thioacetamide Corrosion Test (TAA Test). International Organization for Standardization: Geneva, Switzerland, 1978.
- ISO 4524:2000; Metallic Coatings—Test Methods for Electrodeposited Gold and Gold Alloy Coatings. International Organization for Standardization: Geneva, Switzerland, 2000.
- ISO 17228:2015; IULTCS/IUF 412. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 4611:2010; Plastics—Determination of the Effects of Exposure to Damp Heat, Water Spray and Salt Mist. International Organization for Standardization: Geneva, Switzerland, 2010.
- Hedberg, Y.S.; Lidén, C. Chromium(III) and Chromium(VI) Release from Leather during 8 Months of Simulated Use. Contact Dermat. 2016, 75, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.S. Chromium and Leather: A Review on the Chemistry of Relevance for Allergic Contact Dermatitis to Chromium. J. Leather Sci. Eng. 2020, 2, 20. [Google Scholar] [CrossRef]
- Bobrowski, A.; Królicka, A.; Śliwa, J.; Zarębski, J.; Januś, M.; Kyzioł, K. PVD Fabrication of Lead Film Electrodes and Their Catalytic Adsorptive Stripping Voltammetric Performance in the Presence of Oxidants. Electrochem. Commun. 2018, 94, 49–54. [Google Scholar] [CrossRef]
- Elrefaey, A.; Wojarski, L.; Janczak-Rusch, J.; Tillmann, W. Vacuum Brazing Titanium Using Thin Nickel Layer Deposited by PVD Technique. Mater. Sci. Eng. A 2013, 565, 180–186. [Google Scholar] [CrossRef]
- García, P.; Rivera, S.; Palacios, M.; Belzunce, J. Comparative Study of the Parameters Influencing the Machinability of Leaded Brasses. Eng. Fail. Anal. 2010, 17, 771–776. [Google Scholar] [CrossRef]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public. Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Whittington, C.M.; Lo, W.Y. ‘Nickel Allergy’ Arising from Decorative Nickel Plated and Alloyed Articles: Prevention at Source. Trans. Inst. Met. Finish. 2019, 97, 64–66. [Google Scholar] [CrossRef]
- EN 12472:2020; Method for the Simulation of Accelerated Wear and Corrosion for the Detection of Nickel Release from Coated Items. European Committee for Standardization: Brussels, Belgium, 2020.
- EN 1811:2011+A1:2015; Reference Test Method for Release of Nickel from All Post Assemblies Which Are Inserted into Pierced Parts of the Human Body and Articles Intended to Come into Direct and Prolonged Contact with the Skin. European Committee for Standardization: Brussels, Belgium, 2015.
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- ASTM F2999-19; Standard Consumer Safety Specification for Adult Jewelry. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2019.
- ASTM F2923-14; Standard Specification for Consumer Product Safety for Children’s Jewelry. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2014.
- Negev, M.; Berman, T.; Goulden, S.; Reicher, S.; Barnett-Itzhaki, Z.; Ardi, R.; Shammai, Y.; Diamond, M.L. Lead in Children’s Jewelry: The Impact of Regulation. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 10–16. [Google Scholar] [CrossRef]
- United States Consumer Product Safety Commission. Test Method: CPSC-CH-E1001-08.3; United States Consumer Product Safety Commission: Bethesda, MD, USA, 2012; pp. 1–8.
- ISO 26482:2010; Hardmetals—Determination of Lead and Cadmium Content. International Organization for Standardization: Geneva, Switzerland, 2010.
- United States Environmental Protection Agency EPA. Method 6010D (SW-846): Inductively Coupled Plasma—Atomic Emission Spectrometry; United States Environmental Protection Agency: Washington, DC, USA, 2014.
- Benseler Holding GmbH Co. KG Future-Proof Coatings with PVD. IST Int. Surf. Technol. 2020, 13, 42–43. [Google Scholar] [CrossRef]
- United Nations (UN). Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations (UN): New York, NY, USA, 2015. [Google Scholar]
- Legg, K.O.; Graham, M.; Chang, P.; Rastagar, F.; Gonzales, A.; Sartwell, B. The Replacement of Electroplating. Surf. Coat. Technol. 1996, 81, 99–105. [Google Scholar] [CrossRef]
- Vergason, G.; Fitch, M.; Smith, R.; Brazil, M.; Jochum, T. PVD Chromium Coatings Replacing Decorative Chromium Electroplated Coatings on Plastics. Soc. Vac. Coaters 2016, 58, 403–410. [Google Scholar] [CrossRef]
- Hu, Z.; Ren, L.; Zhao, K.; Wei, G.; Zhang, Z.; Han, T.; Zhong, F.; Yuan, M. A Novel Diffusion Barrier of Electrodeposited CoWP Layer between Copper and Silicon: Preparation and Performance. Surf. Interfaces 2022, 30, 101925. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef]
- An, B.-S.; Kwon, Y.; Oh, J.-S.; Lee, C.; Choi, S.; Kim, H.; Lee, M.; Pae, S.; Yang, C.-W. Characteristics of an Amorphous Carbon Layer as a Diffusion Barrier for an Advanced Copper Interconnect. ACS Appl. Mater. Interfaces 2020, 12, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Giurlani, W.; Biffoli, F.; Fei, L.; Pizzetti, F.; Bonechi, M.; Fontanesi, C.; Innocenti, M. Analytic Procedure for the Evaluation of Copper Intermetallic Diffusion in Electroplated Gold Coatings with Energy Dispersive X-ray Microanalysis. Anal. Chim. Acta 2023, 1269, 341428. [Google Scholar] [CrossRef]
- Jimenez, M.J.M.; Antunes, V.G.; Zagonel, L.F.; Figueroa, C.A.; Wisnivesky, D.; Alvarez, F. Effect of the Period of the Substrate Oscillation in the Dynamic Glancing Angle Deposition Technique: A Columnar Periodic Nanostructure Formation. Surf. Coat. Technol. 2020, 383, 125237. [Google Scholar] [CrossRef]
- Habibi, M.; Mirzaei, S.; Arman, A.; Jurečka, S.; Sadeghi, M.; Zelati, A.; Shakoury, R.; Tanhaee, E.; Ghobadi, N.; Ehteram, H.; et al. Microstructure, Fractal Geometry and Corrosion Properties of CrN Thin Films: The Effect of Shot Number and Angular Position. Mater. Today Commun. 2022, 32, 104072. [Google Scholar] [CrossRef]
- Mareus, R.; Mastail, C.; Anğay, F.; Brunetière, N.; Abadias, G. Study of Columnar Growth, Texture Development and Wettability of Reactively Sputter-Deposited TiN, ZrN and HfN Thin Films at Glancing Angle Incidence. Surf. Coat. Technol. 2020, 399, 126130. [Google Scholar] [CrossRef]
- Alliott, G.T.; Higginson, R.L.; Wilcox, G.D. Producing a Thin Coloured Film on Stainless Steels—A Review. Part 2: Non-Electrochemical and Laser Processes. Trans. IMF 2023, 101, 72–78. [Google Scholar] [CrossRef]
- Eberhardt, M.G.; Hodge, A.M.; Branicio, P.S. Atomistic Modeling of Physical Vapor Deposition on Complex Topology Substrates. Comput. Mater. Sci. 2022, 203, 111111. [Google Scholar] [CrossRef]
- Pinto, G.; Silva, F.; Porteiro, J.; Míguez, J.; Baptista, A. Numerical Simulation Applied to PVD Reactors: An Overview. Coatings 2018, 8, 410. [Google Scholar] [CrossRef]
- Kubečka, M.; Obrusník, A.; Zikán, P.; Jílek, M.; Vencels, J.; Bonaventura, Z. Predictive Simulation of Antenna Effect in PVD Processes Using Fluid Models. Surf. Coat. Technol. 2019, 379, 125045. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.; Shao, T.; Cheng, B.; Zhao, Q.; Shang, H. Simulation of Magnetic-Field-Induced Ion Motion in Vacuum Arc Deposition for Inner Surfaces of Tubular Workpiece. Coatings 2020, 10, 1053. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, Q.; Yuan, L.; Qin, Y.; Chen, M.; Shan, D. Molecular Dynamics Study of the Interactions of Incident N or Ti Atoms with the TiN(001) Surface. Appl. Surf. Sci. 2016, 360, 946–952. [Google Scholar] [CrossRef]
- Li, L.; Long, R.; Prezhdo, O.V. Why Chemical Vapor Deposition Grown MoS2 Samples Outperform Physical Vapor Deposition Samples: Time-Domain Ab Initio Analysis. Nano Lett. 2018, 18, 4008–4014. [Google Scholar] [CrossRef]
- Guo, F.; Holec, D.; Wang, J.; Li, S.; Du, Y. Impact of V, Hf and Si on Oxidation Processes in Ti–Al–N: Insights from Ab Initio Molecular Dynamics. Surf. Coat. Technol. 2020, 381, 125125. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, G.; Liu, Q.; Sui, X.; Liu, Y.; Chen, Y.; Luo, S.; Li, Z. Atomic Level Insights into the Ti2AlC Oxidation Mechanism by the Combination of Density Functional Theory and Ab Initio Molecular Dynamics Calculations. Corros. Sci. 2021, 191, 109756. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Amini, H.; Davoodi, J.; Poursaeidi, E. Molecular Dynamic Simulation of Crack Growth in Ti/TiN Multilayer Coatings. Mater. Today Commun. 2022, 30, 103059. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Gajewska-Midziałek, A.; Buczko, Z.; Łożyńska, M.; Życki, M.; Barszcz, W.; Ciciszwili, T.; Dąbrowski, A.; Kasierot, S.; Charasińska, J.; et al. Circular Economy Approach in Treatment of Galvanic Wastewater Employing Membrane Processes. Membranes 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyova, M.; Biffoli, F.; Giurlani, W.; Martinuzzi, S.M.; Linser, M.; Caneschi, A.; Innocenti, M. PVD for Decorative Applications: A Review. Materials 2023, 16, 4919. https://doi.org/10.3390/ma16144919
Vorobyova M, Biffoli F, Giurlani W, Martinuzzi SM, Linser M, Caneschi A, Innocenti M. PVD for Decorative Applications: A Review. Materials. 2023; 16(14):4919. https://doi.org/10.3390/ma16144919
Chicago/Turabian StyleVorobyova, Mariya, Fabio Biffoli, Walter Giurlani, Stefano Mauro Martinuzzi, Maximilian Linser, Andrea Caneschi, and Massimo Innocenti. 2023. "PVD for Decorative Applications: A Review" Materials 16, no. 14: 4919. https://doi.org/10.3390/ma16144919
APA StyleVorobyova, M., Biffoli, F., Giurlani, W., Martinuzzi, S. M., Linser, M., Caneschi, A., & Innocenti, M. (2023). PVD for Decorative Applications: A Review. Materials, 16(14), 4919. https://doi.org/10.3390/ma16144919










