Effect of Antirotational Two-Piece Titanium Base on the Vertical Misfit, Fatigue Behavior, Stress Concentration, and Fracture Load of Implant-Supported Zirconia Crowns
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Vertical Misfit
2.3. Capacity to Maintain Placement Torque
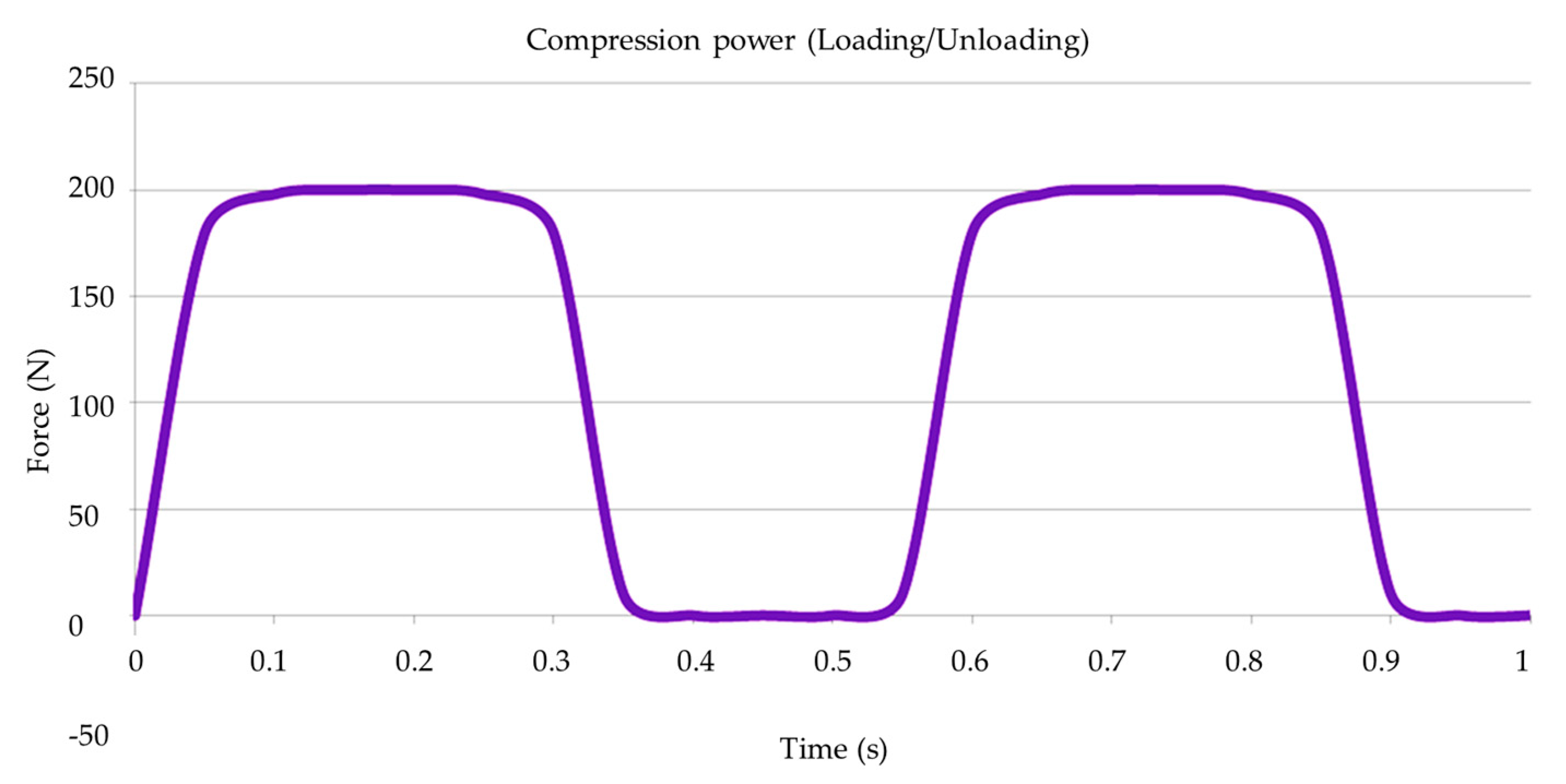
2.4. Mechanical Fatigue
2.5. Post-Fatigue Fracture Load
2.6. Finite Element Analysis
2.7. Statistical Analysis
3. Results
3.1. Measurement of Vertical Misfit
3.2. Torque Maintenance Capacity
3.3. Compression Test
3.4. FEA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soegiantho, P.; Suryawinata, P.G.; Tran, W.; Kujan, O.; Koyi, B.; Khzam, N.; Algarves Miranda, L. Survival of Single Immediate Implants and Reasons for Loss: A Systematic Review. Prosthesis 2023, 5, 378–424. [Google Scholar] [CrossRef]
- Bushra, S.; Khierla, L.; Guindy, J.E.; Fahmy, N.; Nahass, H.E. Esthetic Patient Satisfaction, Marginal Bone Loss and Peri-Implant Tissue Success in Esthetic Zone: Systematic Review. Braz. Dent. Sci. 2022, 25, e2514. [Google Scholar] [CrossRef]
- Yoshiyasu, R.H.; Cartelli, C.A.; Vianna, C.P.; Caldas, W.; Bernardes, S.R.; Trojan, L.C.; Freitas, R.M. de Peri-Implant Bone Level Assessment of Early Loaded Dental Implants Submitted to Different Prosthetic Protocols: A Case Report. Braz. Dent. Sci. 2023, 26, e3569. [Google Scholar] [CrossRef]
- Giordano, M.; Ausiello, P.; Martorelli, M. Accuracy evaluation of surgical guides in implant dentistry by non-contact reverse engineering techniques. Dent. Mater. 2012, 28, e178–e185. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Werner, A.; Blom, E.J. Failed Dental Implant: When Titanium Fractures. Diagnostics 2023, 13, 2123. [Google Scholar] [CrossRef]
- Ortensi, L.; Vitali, T.; Mirra, R.; Ortensi, M.; Borromeo, C. Ageing-Oriented Prosthetic Treatment Plan: A Case Report. Prosthesis 2023, 5, 496–508. [Google Scholar] [CrossRef]
- Deeb, J.G.; Reddy, N.G.; Hopfensperger, L.J.; Harris, A.L.; Bencharit, S. Same-Day Digital Dentistry Restorative Workflow for Single Immediate Provisionalization of Narrow-Diameter Implants: An Exploratory Prospective Study. Prosthesis 2023, 5, 197–207. [Google Scholar] [CrossRef]
- Valsan, I.M.; Pauna, M.R.; Petre, A.E.; Oancea, L. Biologic and Esthetic Outcome of CAD/CAM Custom Ceramic Implant Abutment: A Clinical Report. Maedica 2021, 16, 145–148. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Boșca, A.B.; Babtan, A.M.; Feurden, C.N.; Ionel, A.; Vermeșan, H.; Popa, C.O.; Ilea, A. Optimization of the Manufacturing Process by Molding Cobalt-Chrome Alloys in Assembled Dental Frameworks. Prosthesis 2021, 3, 245–260. [Google Scholar] [CrossRef]
- Adolfi, D.; Tribst, J.; Borges, A.; Bottino, M. Torque Maintenance Capacity, Vertical Misfit, Load to Failure, and Stress Concentration of Zirconia Restorations Cemented or Notched to Titanium Bases. Int. J. Oral Maxillofac. Implants 2020, 35, 357–365. [Google Scholar] [CrossRef]
- Tallarico, M.; Meloni, S.M.; Park, C.-J.; Zadrożny, Ł.; Scrascia, R.; Cicciù, M. Implant Fracture: A Narrative Literature Review. Prosthesis 2021, 3, 267–279. [Google Scholar] [CrossRef]
- Tiossi, R.; Lin, L.; Rodrigues, R.C.S.; Heo, Y.C.; Conrad, H.J.; de da Mattos, M.G.C.; Ribeiro, R.F.; Fok, A.S.L. Digital Image Correlation Analysis of the Load Transfer by Implant-Supported Restorations. J. Biomech. 2011, 44, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Satwalekar, P.; Chander, K.S.; Reddy, B.A.; Sandeep, N.; Sandeep, N.; Satwalekar, T. A Simple and Cost Effective Method Used for Removal of a Fractured Implant Abutment Screw: A Case Report. J. Int. Oral Health 2013, 5, 120–123. [Google Scholar]
- Pelivan, I.; Šeparović, I.; Vuletić, M.; Dulčić, N.; Gabrić, D. Radiological and Periodontal Evaluation of Stock and Custom CAD/CAM Implant Abutments—A One-Year Follow-up Study. Prosthesis 2023, 5, 437–452. [Google Scholar] [CrossRef]
- Çin, V.; İzgi, A.D.; Kale, E.; Yilmaz, B. Marginal and Internal Fit of Monolithic Zirconia Crowns Fabricated by Using Two Different CAD-CAM Workflows: An in Vitro Study. Prosthesis 2023, 5, 35–47. [Google Scholar] [CrossRef]
- Lo Giudice, R.; Galletti, C.; Tribst, J.P.M.; Melenchón, L.P.; Matarese, M.; Miniello, A.; Cucinotta, F.; Salmeri, F. In Vivo Analysis of Intraoral Scanner Precision Using Open-Source 3D Software. Prosthesis 2022, 4, 554–563. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, H.; Zhang, Y.; Zhang, F.; Gao, L.; Omran, M.; Chen, G. Microstructure and Phase Transformation Behavior of Al2O3–ZrO2 under Microwave Sintering. Ceram. Int. 2023, 49, 4855–4862. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, Y.; Huang, W.; Peng, J.; Chen, J.; Gao, L.; Omran, M.; Li, N.; Chen, G. Sintering Properties of Tetragonal Zirconia Nanopowder Preparation of the NaCl + KCl Binary System by the Sol–Gel–Flux Method. ACS Sustain. Chem. Eng. 2023, 11, 1067–1077. [Google Scholar] [CrossRef]
- Martins, R.G.; Castro, T.S.d.; Dib, L.L.; Gehrke, S.A.; Mesquita, A.M.M. Influence of Restorative Material on the Distribution of Loads to the Bone in Hybrid Abutment Crowns—In Vitro Study. Medicina 2023, 59, 1188. [Google Scholar] [CrossRef]
- Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. CAD/CAM Abutments versus Stock Abutments: An Update Review. Prosthesis 2022, 4, 468–479. [Google Scholar] [CrossRef]
- de Matos, J.D.M.; da Lopes, G.R.S.; Queiroz, D.A.; Pereira, A.L.J.; Sinhoreti, M.A.C.; de Ramos, N.C.; Lino, V.; de Oliveira, F.R.; Borges, A.L.S.; Bottino, M.A. Influence of the Peek Abutments on Mechanical Behavior of the Internal Connections Single Implant. Materials 2022, 15, 8133. [Google Scholar] [CrossRef] [PubMed]
- Favasuli, L.; Mascarenhas, P.S.; Mauricio, P. Laboratory Fracture Resilience of Hybrid Abutments Used in Oral Rehabilitation: A Systematic Review. J. Funct. Biomater. 2022, 13, 120. [Google Scholar] [CrossRef]
- Ožiūnas, R.; Sakalauskienė, J.; Staišiūnas, L.; Žekonis, G.; Žilinskas, J.; Janužis, G. Physical and Mechanical Changes on Titanium Base of Three Different Types of Hybrid Abutment after Cyclic Loading. J. Adv. Prosthodont. 2023, 15, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Demachkia, A.M.; Sichi, L.G.B.; Rodrigues, J.V.M.; Junior, L.N.; de Araújo, R.M.; de Ramos, N.C.; Bottino, M.A.; Tribst, J.P.M. Implant-Supported Restoration with Straight and Angled Hybrid Abutments: Digital Image Correlation and 3D-Finite Element Analysis. European J. Gen. Dent. 2022, 11, 23–31. [Google Scholar] [CrossRef]
- Menini, M.; Pesce, P.; Corvino, E.; Iannello, G.; Baldi, D.; Canullo, L. Clinical Outcomes of Dental Implants with Two Different Internal Connection Configurations—A RCT. Prosthesis 2022, 4, 564–574. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Trinkner, A.; Burkhardt, F.; Patzelt, S.B.M.; Vach, K.; Kušter, M.; Abram, A.; Kocjan, A.; Nold, J. Long-Term Stability of Hydrothermally Aged and/or Dynamically Loaded One-Piece Diameter Reduced Zirconia Oral Implants. J. Funct. Biomater. 2023, 14, 123. [Google Scholar] [CrossRef]
- Fiorillo, L.; Meto, A.; Cicciù, M. Bioengineering Applied to Oral Implantology, a New Protocol: “Digital Guided Surgery”. Prosthesis 2023, 5, 234–250. [Google Scholar] [CrossRef]
- Dib-Zaitum, I.; Guadilla-González, Y.; Flores-Fraile, J.; Dib-Zakkour, J.; Benito-Garzón, L.; Montero, J. Effect Morphology and Surface Treatment of the Abutments of Dental Implants on the Dimension and Health of Peri-Implant Biological Space. Materials 2022, 15, 4422. [Google Scholar] [CrossRef]
- Nakano, L.J.N.; Gomes, L.C.L.; de Queiroz, T.S.; Paes-Junior, T.J. de A. Effect of Abutment Type and Tightening Sequence on Torque Maintenance Capacity after Mechanical Cycling in Splinted Implant-Supported Restorations. Oral 2021, 1, 300–306. [Google Scholar] [CrossRef]
- Fiorillo, L.; Milone, D.; D’Andrea, D.; Santonocito, D.; Risitano, G.; Cervino, G.; Cicciù, M. Finite Element Analysis of Zirconia Dental Implant. Prosthesis 2022, 4, 490–499. [Google Scholar] [CrossRef]
- de Ramos, N.C.; Ramos, G.F.; Penteado, M.M.; de Melo, R.M.; Borges, A.L.S.; Bottino, M.A.; Tribst, J.P.M. Comparative Stress Evaluation between Bilayer, Monolithic and Cutback All-Ceramic Crown Designs: 3D Finite Element Study. Prosthesis 2021, 3, 173–180. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Terranova, A.; Risitano, G.; Raffaele, M.; Cucinotta, F.; Santonocito, D.; Fiorillo, L. Prosthetic and Mechanical Parameters of the Facial Bone under the Load of Different Dental Implant Shapes: A Parametric Study. Prosthesis 2019, 1, 41–53. [Google Scholar] [CrossRef]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental Luting Cements: An Updated Comprehensive Review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef]
- Sichi, L.G.B.; Pierre, F.Z.; Arcila, L.V.C.; de Andrade, G.S.; Tribst, J.P.M.; Ausiello, P.; di Lauro, A.E.; Borges, A.L.S. Effect of Biologically Oriented Preparation Technique on the Stress Concentration of Endodontically Treated Upper Central Incisor Restored with Zirconia Crown: 3D-FEA. Molecules 2021, 26, 6113. [Google Scholar] [CrossRef]
- Vélez, J.; Peláez, J.; López-Suárez, C.; Agustín-Panadero, R.; Tobar, C.; Suárez, M.J. Influence of Implant Connection, Abutment Design and Screw Insertion Torque on Implant-Abutment Misfit. J. Clin. Med. 2020, 9, 2365. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.S.; Sordi, M.B.; Magini, R.S.; Calazans Duarte, A.R.; Souza, J.C.M. Abutment Misfit in Implant-Supported Prostheses Manufactured by Casting Technique: An Integrative Review. Eur. J. Dent. 2017, 11, 553–558. [Google Scholar] [CrossRef]
- Hein, D.; Joly, J.C.; Napimoga, M.H.; Peruzzo, D.C.; Martinez, E.F. Influence of Abutment Angulation on Loss of Prosthetic Abutment Torque under Mechanical Cycling. J. Prosthet. Dent. 2021, 125, 349.e1–349.e6. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.-G.; Kwon, H.-B.; Lim, Y.-J.; Koak, J.-Y.; Kim, M.-J. The Influence of a Positioning Hex on Abutment Rotation in Tapered Internal Implants: A 3D Finite Element Model Study. Int. J. Oral Maxillofac. Implants 2020, 35, 281–288. [Google Scholar] [CrossRef]
- Barbosa, G.A.S.; Bernardes, S.R.; das Neves, F.D.; Fernandes Neto, A.J.; de da Mattos, M.G.C.; Ribeiro, R.F. Relation between Implant/Abutment Vertical Misfit and Torque Loss of Abutment Screws. Braz. Dent. J. 2008, 19, 358–363. [Google Scholar] [CrossRef]
- Alonso-Pérez, R.; Bartolomé, J.F.; Ferreiroa, A.; Salido, M.P.; Pradíes, G. Original vs. Non-Original Abutments for Screw-Retained Single Implant Crowns: An in Vitro Evaluation of Internal Fit, Mechanical Behaviour and Screw Loosening. Clin. Oral Implants Res. 2018, 29, 1230–1238. [Google Scholar] [CrossRef]
- de Matos, J.D.M.; Gomes, L.S.; de Carvalho Ramos, N.; Queiroz, D.A.; Tribst, J.P.M.; Campos, T.M.B.; Borges, A.L.S.; da Rocha Scalzer Lopes, G.; Bottino, M.A.; Paes Junior, T.J.A. Influence of CAD/CAM Abutment Heights on the Biomechanical Behavior of Zirconia Single Crowns. Metals 2022, 12, 2025. [Google Scholar] [CrossRef]
- Suralik, K.; Sun, J.; Chen, C.-Y.; Lee, S. Effect of Fabrication Method on Fracture Strength of Provisional Implant-Supported Fixed Dental Prostheses. Prosthesis 2020, 2, 325–332. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; López-Jarana, P.; Ríos-Santos, J.V.; Herrero-Climent, M. Review of the Mechanical Behavior of Different Implant-Abutment Connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef] [PubMed]
- Fontijn-Tekamp, F.A.; Slagter, A.P.; Van Der Bilt, A.; Van ’T Hof, M.A.; Witter, D.J.; Kalk, W.; Jansen, J.A. Biting and Chewing in Overdentures, Full Dentures, and Natural Dentitions. J. Dent. Res. 2000, 79, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-T.; Koak, J.-Y.; Lim, Y.-J.; Kim, S.-K.; Kwon, H.-B.; Kim, M.-J. Stress Shielding and Fatigue Limits of Poly-Ether-Ether-Ketone Dental Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1044–1052. [Google Scholar] [CrossRef]
- Koc, D.; Dogan, A.; Bek, B. Bite Force and Influential Factors on Bite Force Measurements: A Literature Review. Eur. J. Dent. 2010, 4, 223–232. [Google Scholar] [CrossRef]
- Fabris, D.; Moura, J.P.A.; Fredel, M.C.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Biomechanical Analyses of One-Piece Dental Implants Composed of Titanium, Zirconia, PEEK, CFR-PEEK, or GFR-PEEK: Stresses, Strains, and Bone Remodeling Prediction by the Finite Element Method. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 79–88. [Google Scholar] [CrossRef]
- García-Braz, S.H.; Prados-Privado, M.; Zanatta, L.C.S.; Calvo-Guirado, J.L.; Prados-Frutos, J.C.; Gehrke, S.A. A Finite Element Analysis to Compare Stress Distribution on Extra-Short Implants with Two Different Internal Connections. J. Clin. Med. 2019, 8, 1103. [Google Scholar] [CrossRef]
- Chang, H.-C.; Chang, C.-H.; Li, H.-Y.; Wang, C.-H. Biomechanical Analysis of the Press-Fit Effect in a Conical Morse Taper Implant System by Using an in Vitro Experimental Test and Finite Element Analysis. J. Prosthet. Dent. 2022, 127, 601–608. [Google Scholar] [CrossRef]
- Grande, M.F.; Lopes, G.D.; Teixeira, M.L.; Pelegrine, A.A.; De Matos, J.D.; Nishioka, R.S. Mechanical Behavior of Implant-Supported Full-Arch Prostheses in Different Locations in the Maxilla: 3D-FEA and Strain Gauge Analysis. Braz. Dent. Sci. 2023, 26, 346–359. [Google Scholar] [CrossRef]
- Ausiello, P.; Franciosa, P.; Martorelli, M.; Watts, D.C. Effects of Thread Features in Osseo-Integrated Titanium Implants Using a Statistics-Based Finite Element Method. Dent. Mater. 2012, 28, 919–927. [Google Scholar] [CrossRef]
- Epifania, E.; di Lauro, A.E.; Ausiello, P.; Mancone, A.; Garcia-Godoy, F.; Mendes Tribst, J.P. Effect of Crown Stiffness and Prosthetic Screw Absence on the Stress Distribution in Implant-Supported Restoration: A 3D Finite Element Analysis. PLoS ONE 2023, 18, e0285421. [Google Scholar] [CrossRef]
- Ausiello, P.; Tribst, J.P.M.; Ventre, M.; Salvati, E.; di Lauro, A.E.; Martorelli, M.; Lanzotti, A.; Watts, D.C. The Role of Cortical Zone Level and Prosthetic Platform Angle in Dental Implant Mechanical Response: A 3D Finite Element Analysis. Dent. Mater. 2021, 37, 1688–1697. [Google Scholar] [CrossRef]
- Ausiello, P.; Di Lauro, A.E.; Tribst, J.P.M.; Watts, D.C. Stress Distribution in Resin-Based CAD-CAM Implant-Supported Crowns. Dent. Mater. 2023, 39, 114–122. [Google Scholar] [CrossRef]
- Kang, M.; Smanio Neto, H.; Pelegrine, A.A.; Turssi, C.P.; Clemente-Napimoga, J.T.; Napimoga, M.H. Survival Rate of Dental Implants Installed by Postgraduate Students Attending an Implantology Program in Brazil: A 52-Month Retrospective Analysis. Front. Dent. Med. 2023, 4, 1170253. [Google Scholar] [CrossRef]
- Desai, S.R.; Koulgikar, K.D.; Alqhtani, N.R.; Alqahtani, A.R.; Alqahtani, A.S.; Alenazi, A.; Heboyan, A.; Fernandes, G.V.O.; Mustafa, M. Three-Dimensional FEA Analysis of the Stress Distribution on Titanium and Graphene Frameworks Supported by 3 or 6-Implant Models. Biomimetics 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Van Oers, R.F.M.; Feilzer, A.J. Abutment-to-Fixture Load Transfer and Peri-Implant Bone Stress. Am. J. Dent. 2015, 28, 247–250. [Google Scholar] [PubMed]
- Datte, C.E.; Rodrigues, V.A.; Datte, F.B.; da Scalzer Lopes, G.R.; Borges, A.L.S.; Nishioka, R.S. The Effect of Different Bone Level and Prosthetic Connection on the Biomechanical Response of Unitary Implants: Strain Gauge and Finite Element Analyses. Int. J. Adv. Eng. Res. Sci. 2021, 8, 218–224. [Google Scholar] [CrossRef]
- Al-Asad, H.M.; El Afandy, M.H.; Mohamed, H.T.; Mohamed, M.H. Hybrid Prosthesis versus Overdenture: Effect of BioHPP Prosthetic Design Rehabilitating Edentulous Mandible. Int. J. Dent. 2023, 2023, 4108679. [Google Scholar] [CrossRef]
- Tokgöz, E.; Carro, M.A. Biomechanics of Facial Plastic Surgery Applications. In Cosmetic and Reconstructive Facial Plastic Surgery; Springer Nature: Cham, Switzerland, 2023; pp. 257–279. ISBN 9783031311673. [Google Scholar]
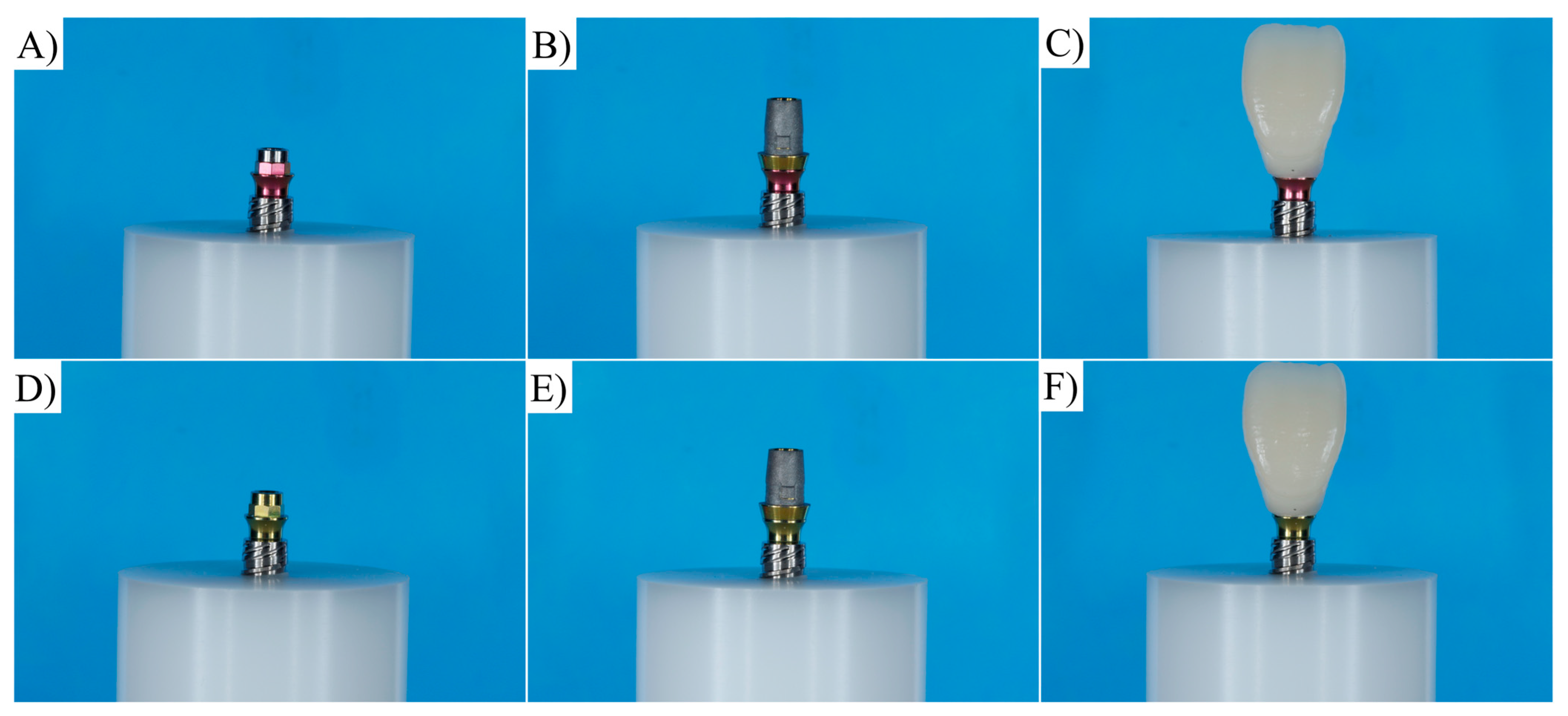
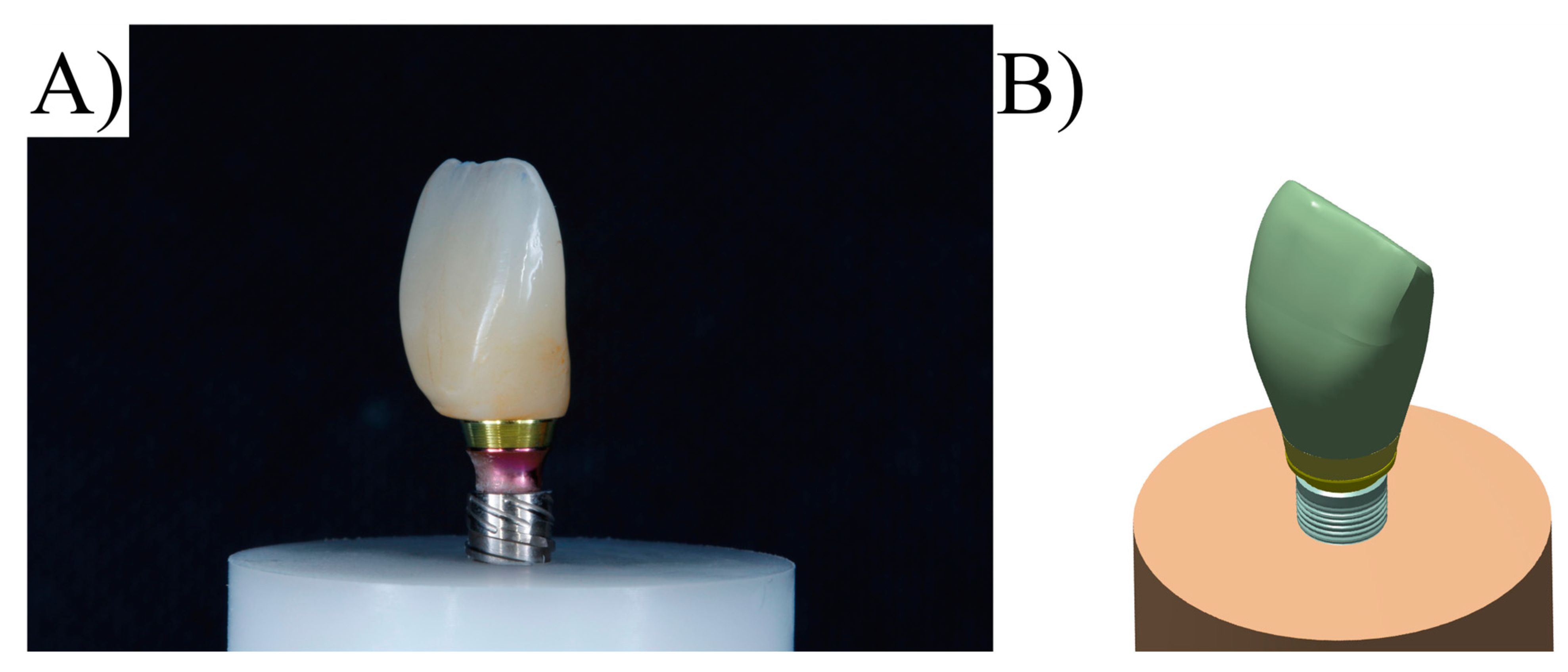
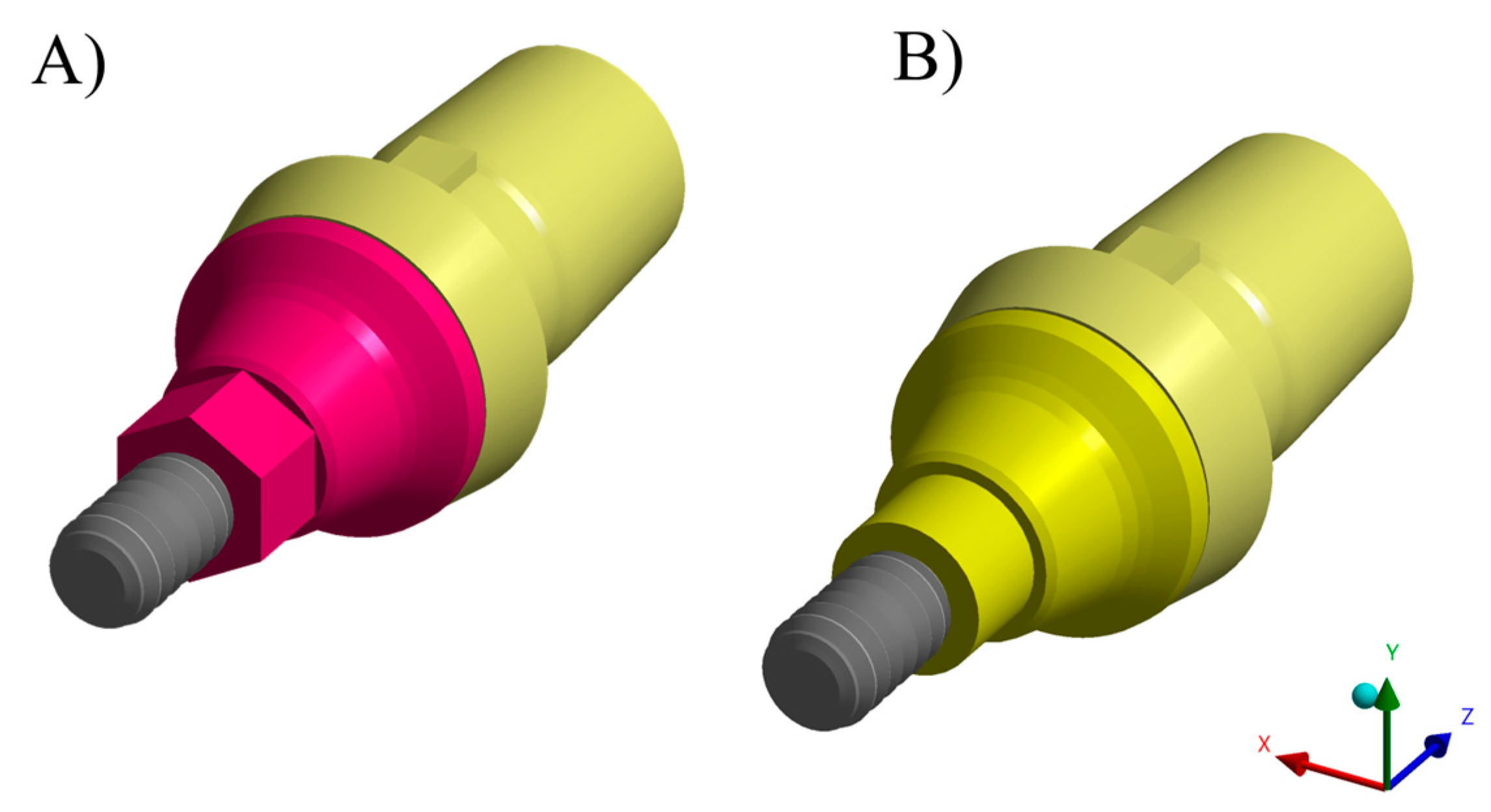
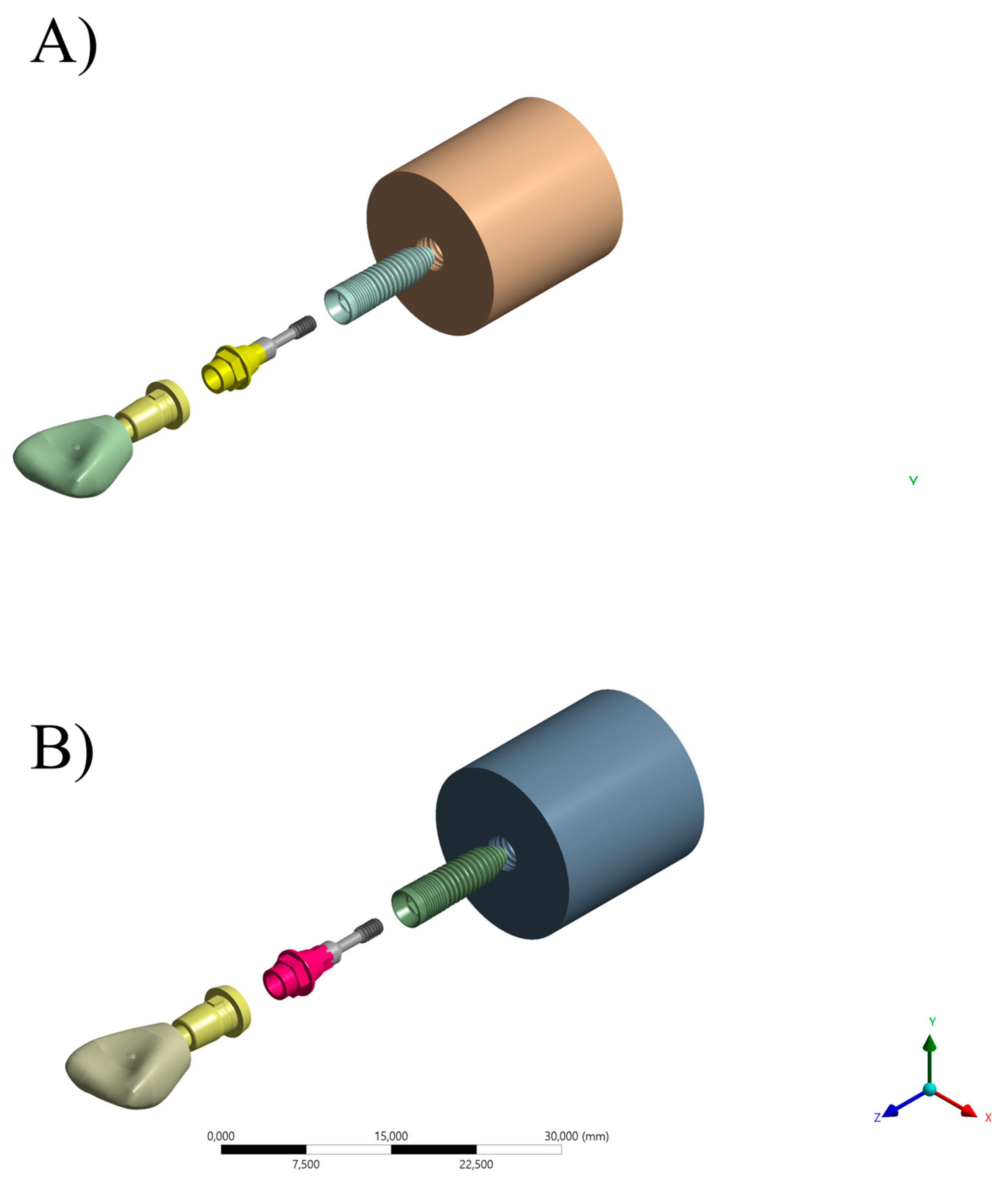

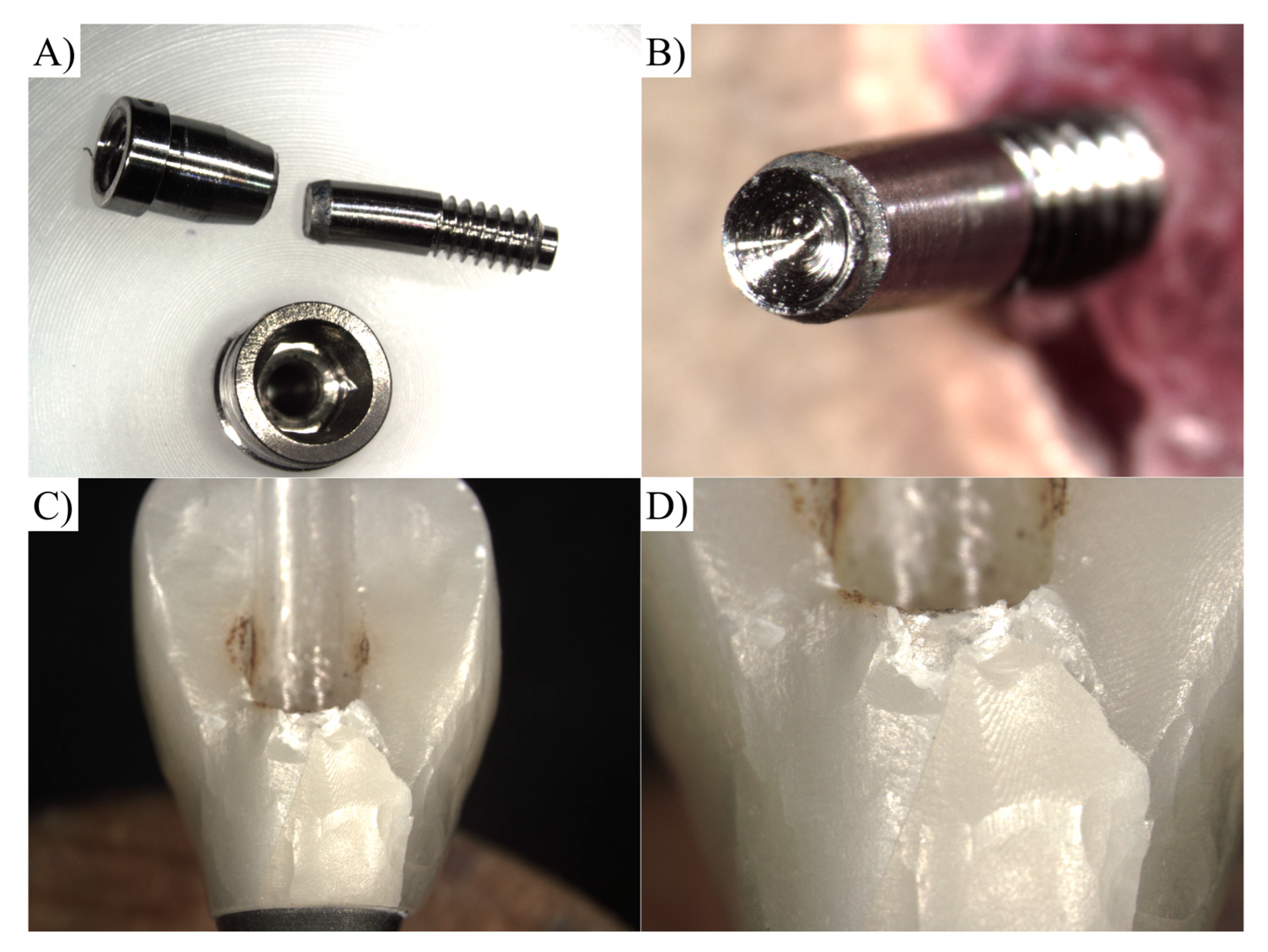

| Material | Commercial Name | Manufacturer |
|---|---|---|
| Titanium | Implant postextracción conexión interna hexagonal 4 × 12 mm platform 4 mm (batch 202009029) Cod IPX 4012 | New Galimplant, Sarria, Lugo, Spain |
| Titanium | Abutment multi-posicíon recto anti-rotacional solidario altura 2 mm CI hexagonal (batch 202010078) Cod KMUSA S04020 | |
| Titanium | Abutment multi-posicíon recto anti-rotacional altura 2 mm CI hexagonal (batch 202005016) Cod KMUSA 04020 | |
| Titanium | Interfase compatible con Sistema Cerec para multi-posicíon estético anti-rotacional (batch 202005001) Cod EPCERCMUA 40 | |
| Self-curing acrylic resin | Unifast | GC America; Alsip, IL, USA |
| Inclusion resin | POM (Delrin) (batch 166976) | Dupont; Wilmington, DE, USA |
| Zirconia disk for mesostructure | 3D pro ML (batch W200604ATB2M-01-P) | Aidite Technology Co., Ltd., Shenzhen, China |
| Cementing agent | Panavia F 2.0 Light (batch 000133) | Kuraray Noritake Dental Inc., Okayama, Japan |
| Primer for metal | Alloy Primer (batch AA0096) | Kuraray Noritake Dental Inc., Okayama, Japan |
| Primer for zirconia | Ceramic Primer Plus (batch 3P0053) | Kuraray Noritake Dental Inc., Okayama, Japan |
| Material | Elastic Modulus (GPa) | Poisson Coefficient | Reference |
|---|---|---|---|
| Titanium | 105 | 0.33 | [32] |
| Zirconia | 205 | 0.3 | [30,31] |
| Fixation resin | 2.8 | 0.3 | [10] |
| Resin cement | 11.8 | 0.3 | [33] |
| Region | Aging | Marginal Misfit |
|---|---|---|
| Buccal | No | 95.61 ± 45.21 |
| Yes | 99.91 ± 50.38 | |
| Distal | No | 94.84 ± 45.56 |
| Yes | 97.24 ± 49.02 | |
| Palatal | No | 96.07 ± 47.29 |
| Yes | 98.24 ± 50.43 | |
| Mesial | No | 94.47 ± 45.24 |
| Yes | 97.52 ± 49.69 |
| Region | Aging | Marginal Misfit |
|---|---|---|
| Buccal | No | 99.72 ± 32.3 |
| Yes | 104.30 ± 55.06 | |
| Distal | No | 97.95 ± 30.02 |
| Yes | 96.12 ± 37.48 | |
| Palatal | No | 99.33 ± 34.08 |
| Yes | 96.57 ± 35.68 | |
| Mesial | No | 98.92 ± 33.52 |
| Yes | 98.02 ± 37.85 |
| Group*Aging | Torque (N·cm) | Grouping | ||
|---|---|---|---|---|
| ARs*No | 20.00 | A | ||
| RTs*No | 20.00 | A | ||
| RTs*Yes | 13.45 ± 2.18 | B | ||
| ARs*Yes | 12.05 ± 2.08 | C | ||
| Model | Structure | Stress Peak (MPa) |
|---|---|---|
| AR | Abutment | 818.56 |
| Implant | 754.35 | |
| RT | Abutment | 994.36 |
| Implant | 755.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adolfi, D.; Grangeiro, M.T.V.; Ausiello, P.; Bottino, M.A.; Tribst, J.P.M. Effect of Antirotational Two-Piece Titanium Base on the Vertical Misfit, Fatigue Behavior, Stress Concentration, and Fracture Load of Implant-Supported Zirconia Crowns. Materials 2023, 16, 4848. https://doi.org/10.3390/ma16134848
Adolfi D, Grangeiro MTV, Ausiello P, Bottino MA, Tribst JPM. Effect of Antirotational Two-Piece Titanium Base on the Vertical Misfit, Fatigue Behavior, Stress Concentration, and Fracture Load of Implant-Supported Zirconia Crowns. Materials. 2023; 16(13):4848. https://doi.org/10.3390/ma16134848
Chicago/Turabian StyleAdolfi, Dario, Manassés Tercio Vieira Grangeiro, Pietro Ausiello, Marco Antonio Bottino, and João Paulo Mendes Tribst. 2023. "Effect of Antirotational Two-Piece Titanium Base on the Vertical Misfit, Fatigue Behavior, Stress Concentration, and Fracture Load of Implant-Supported Zirconia Crowns" Materials 16, no. 13: 4848. https://doi.org/10.3390/ma16134848
APA StyleAdolfi, D., Grangeiro, M. T. V., Ausiello, P., Bottino, M. A., & Tribst, J. P. M. (2023). Effect of Antirotational Two-Piece Titanium Base on the Vertical Misfit, Fatigue Behavior, Stress Concentration, and Fracture Load of Implant-Supported Zirconia Crowns. Materials, 16(13), 4848. https://doi.org/10.3390/ma16134848










