Eco-Friendly Approach for the Construction of Superhydrophobic Coating on Stainless Steel Metal Based on Biological Metal–Organic Framework and Its Corrosion Resistance Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Construction of Cu-As MOF
2.3. Superhydrophobic Coating Manufacture
2.4. Surface Characterization
2.5. Chemical Stability
2.6. Mechanical Abrasion
2.7. Corrosion Test
3. Results and Discussion
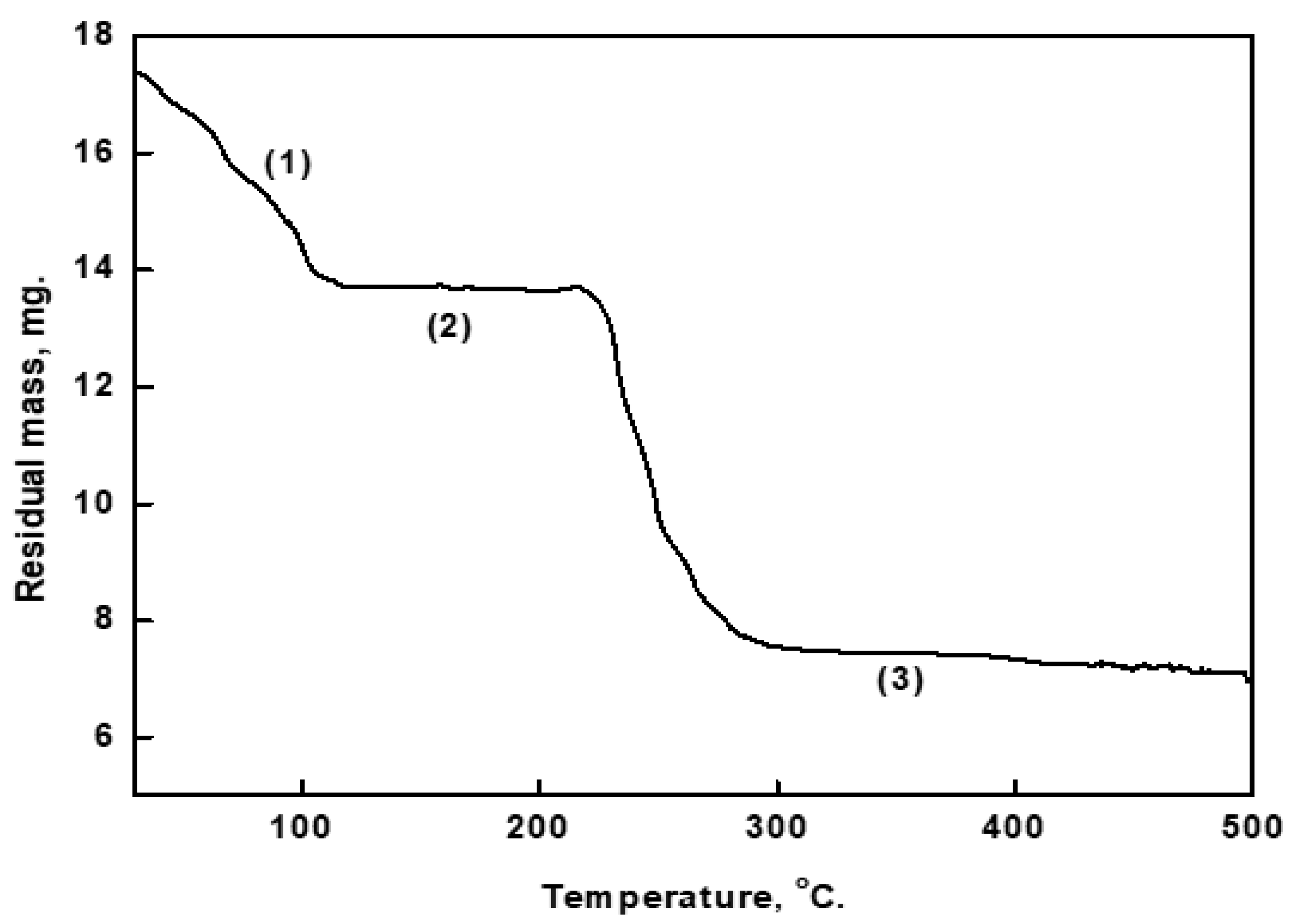
3.1. Thermogravimetric Results of the Prepared Cu-As MOF
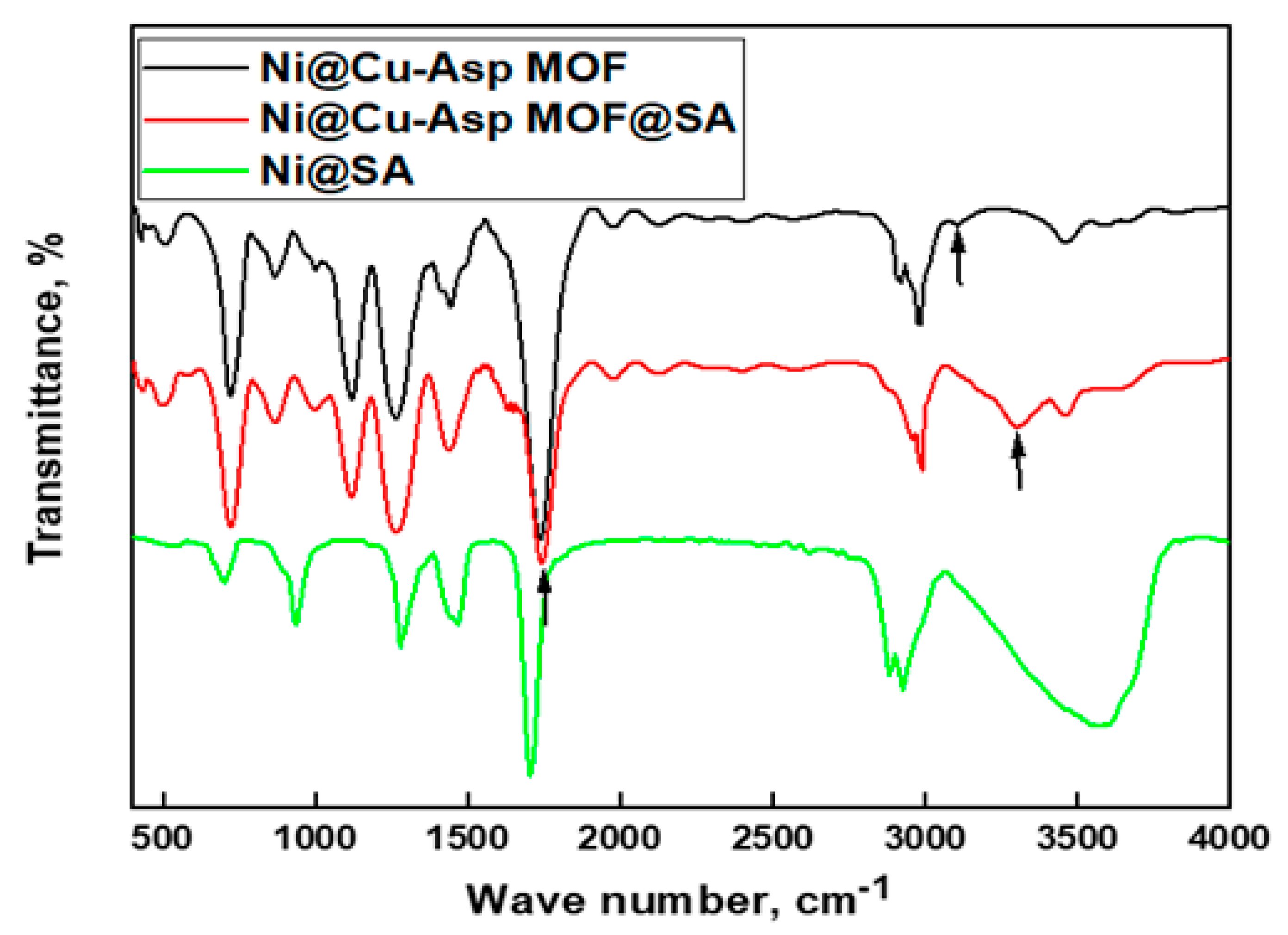
3.2. FTIR Results
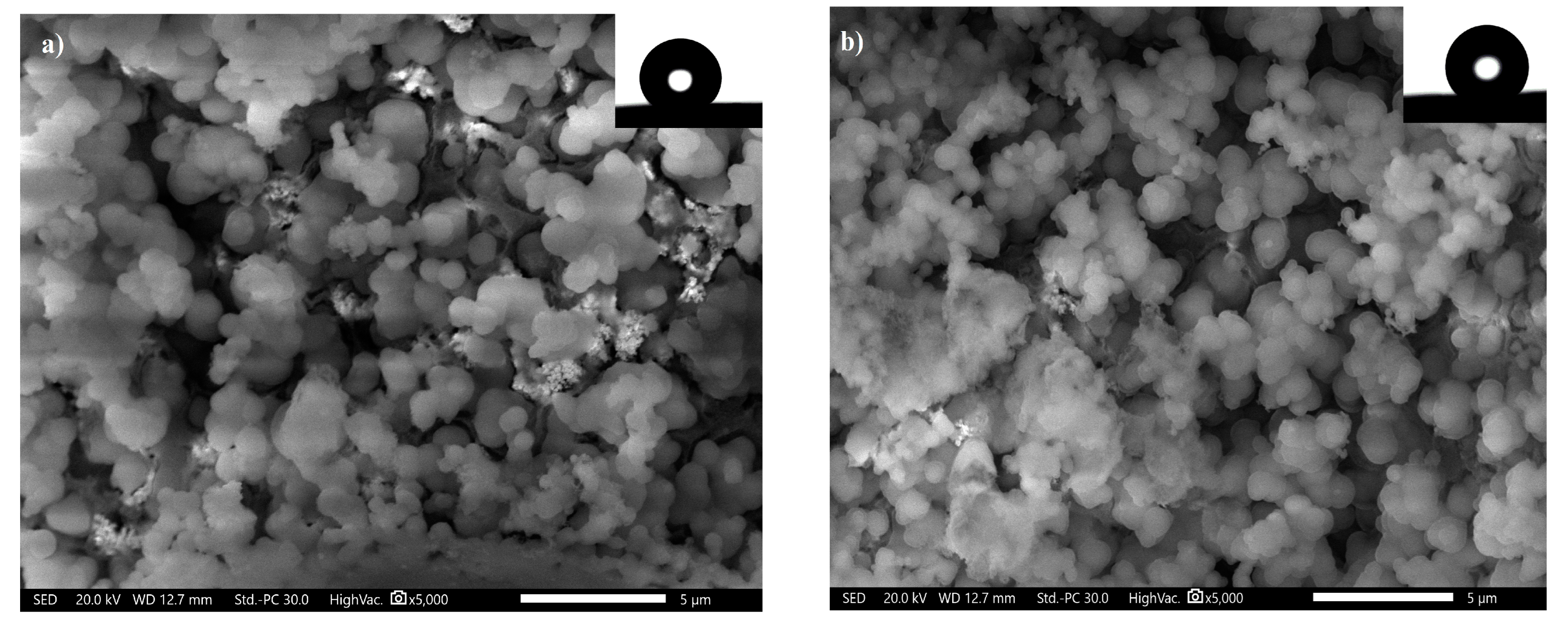
3.3. SEM and Wettability
3.4. Chemical Stability
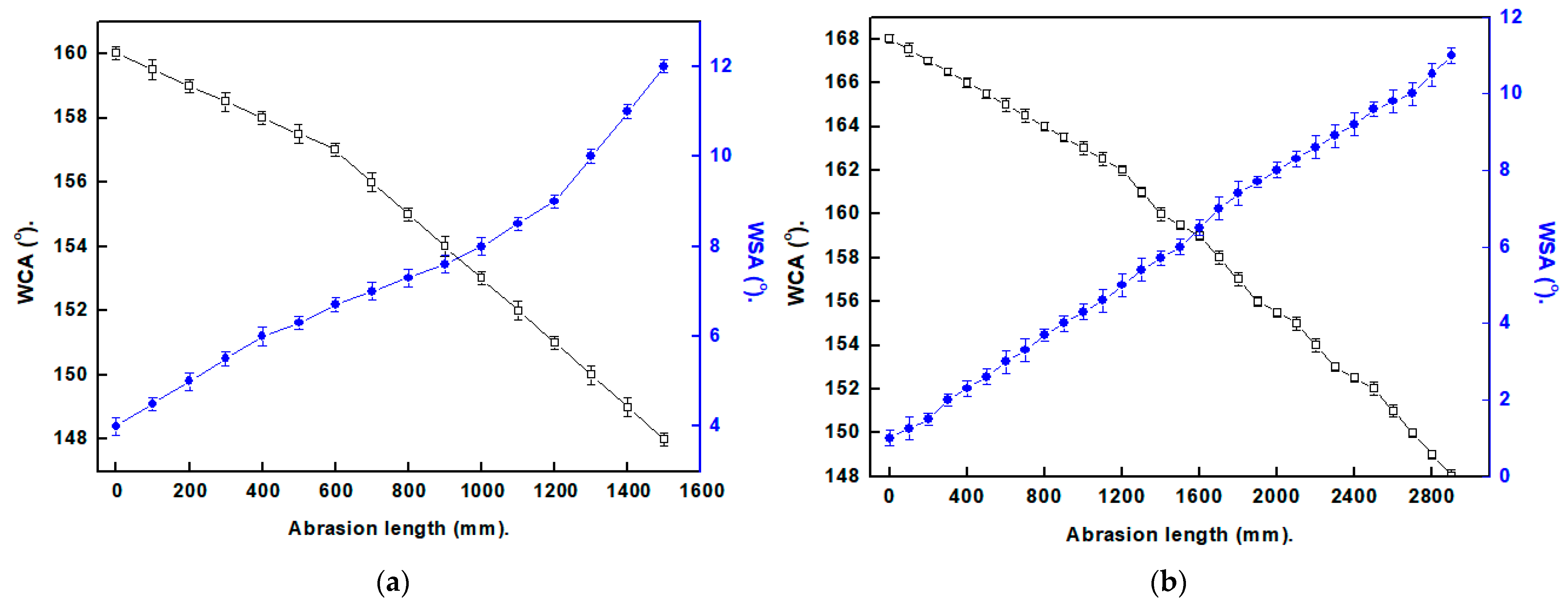
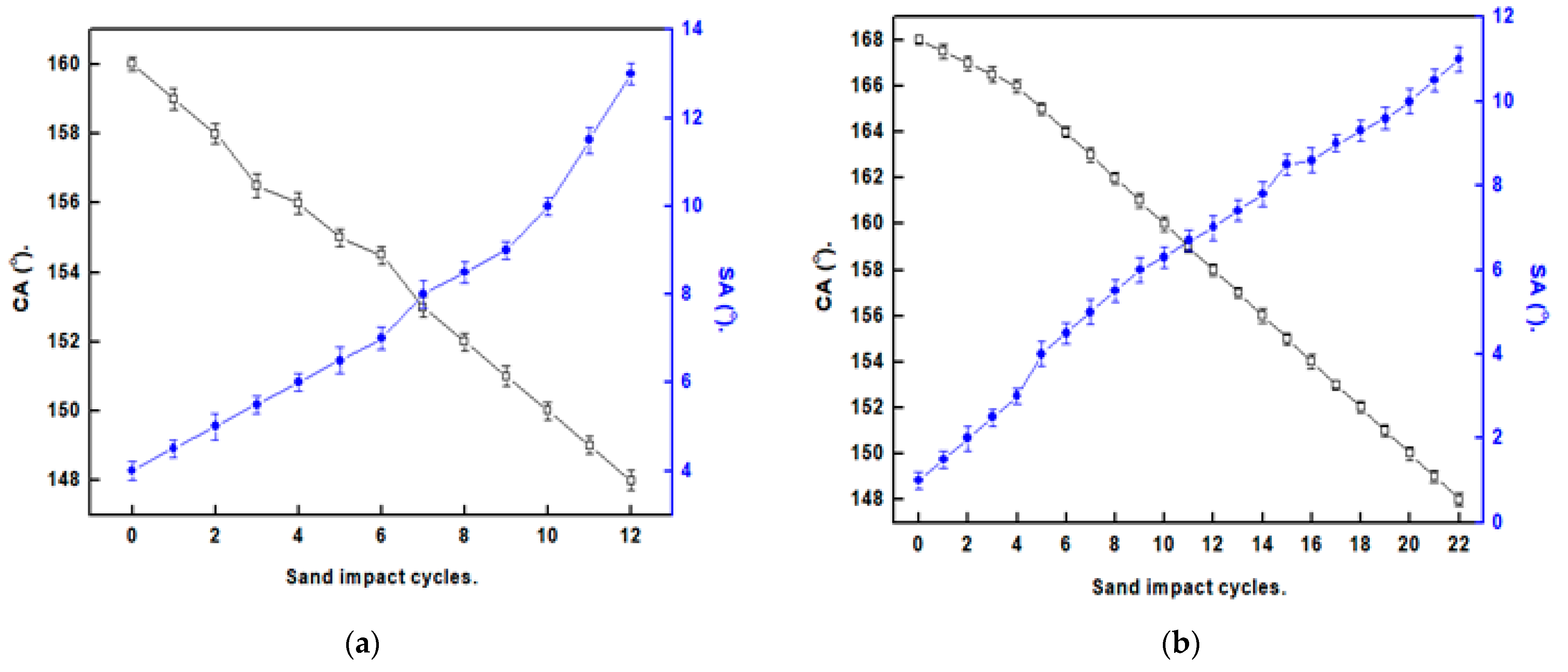
3.5. Mechanical Stability
3.6. Corrosion Measurements
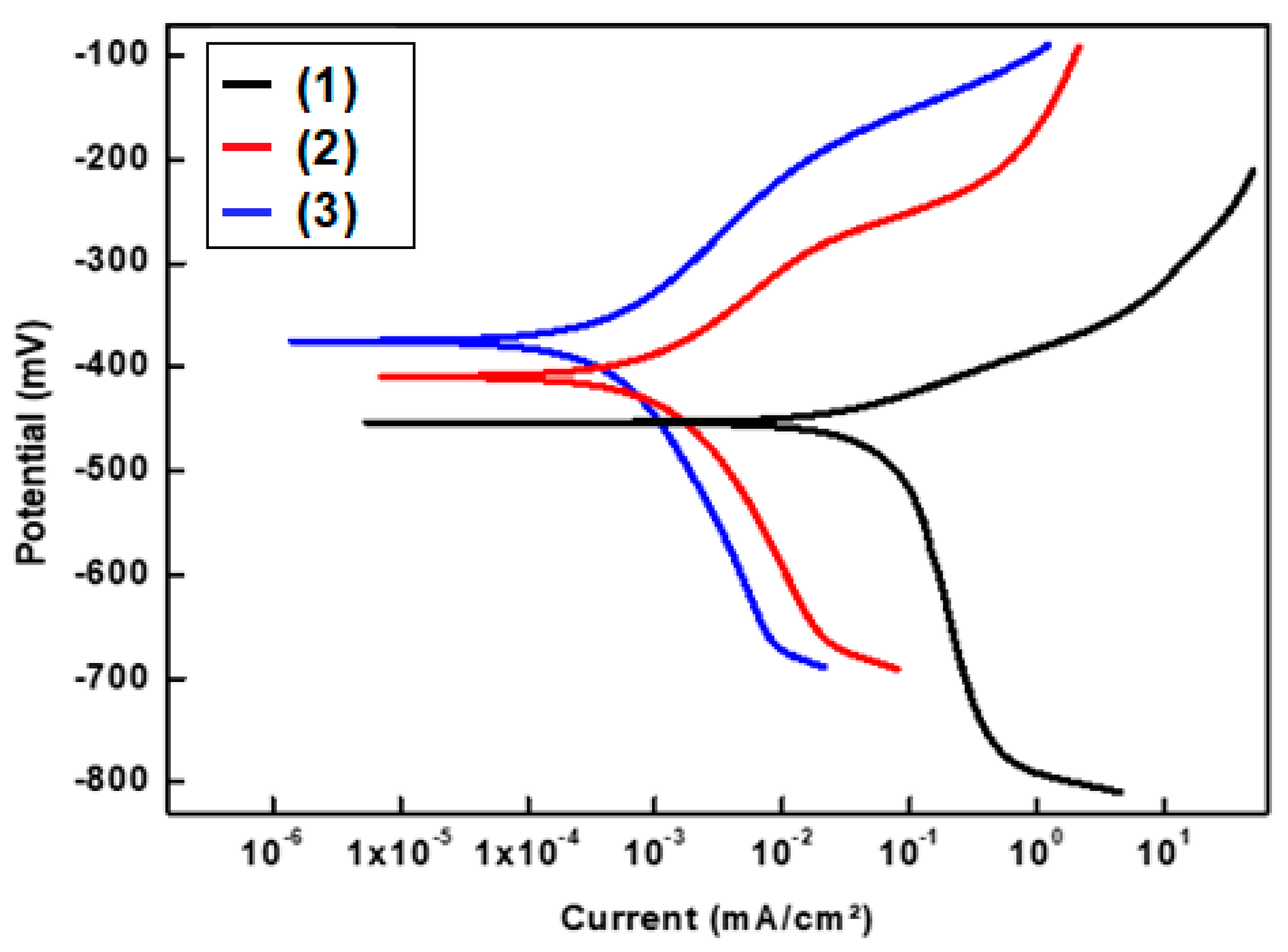
3.6.1. Potentiodynamic Polarization Results
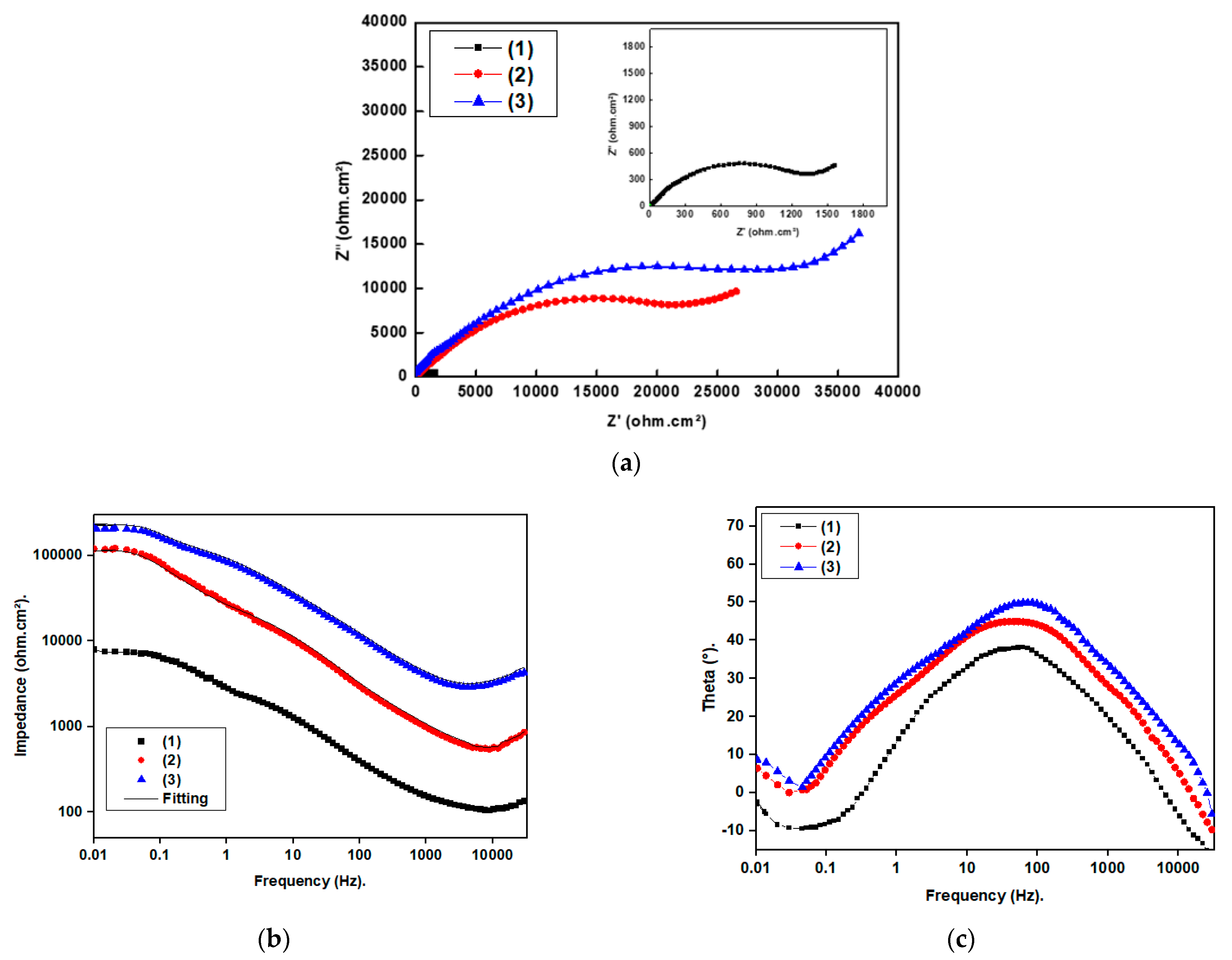
3.6.2. Electrochemical Impedance Spectroscopy Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, M.E.; Abd-El-Nabey, B.A. Fabrication of a Biological Metal–Organic Framework Based Superhydrophobic Textile Fabric for Efficient Oil/Water Separation. Sci. Rep. 2022, 12, 15483. [Google Scholar] [CrossRef]
- Liu, J.; Ye, L.; Sun, Y.; Hu, M.; Chen, F.; Wegner, S.; Mailänder, V.; Steffen, W.; Kappl, M.; Butt, H. Elastic Superhydrophobic and Photocatalytic Active Films Used as Blood Repellent Dressing. Adv. Mater. 2020, 32, 1908008. [Google Scholar] [CrossRef]
- Li, Z.; Marlena, J.; Pranantyo, D.; Nguyen, B.L.; Yap, C.H. A Porous Superhydrophobic Surface with Active Air Plastron Control for Drag Reduction and Fluid Impalement Resistance. J. Mater. Chem. A 2019, 7, 16387–16396. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Ezzat, A.; Gaber, A.M.A. Fabrication of Eco-Friendly Graphene-Based Superhydrophobic Coating on Steel Substrate and Its Corrosion Resistance, Chemical and Mechanical Stability. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D.; Fedchenko, Y.I.; Gyoshev, S.D.; Lazarov, Y.; Chaushev, T.A.; Grakov, T. On the Development of Ultradurable Extremely Water-Repellent and Oleophobic Soot-Based Fabrics with Direct Relevance to Sperm Cryopreservation. ACS Appl. Bio Mater. 2022, 5, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic Electrospun Nanofibers. J. Mater. Chem. A 2013, 1, 1929–1946. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Gao, P.; Ma, H.; Wei, Q. Superhydrophobic Hybrid Films Prepared from Silica Nanoparticles and Ionic Liquids via Layer-by-Layer Self-Assembly. Thin Solid Films 2014, 570, 27–32. [Google Scholar] [CrossRef]
- Qing, Y.; Long, C.; An, K.; Liu, C. Natural Rosin-Grafted Nanoparticles for Extremely-Robust and Eco-Friendly Antifouling Coating with Controllable Liquid Transport. Compos. Part B Eng. 2022, 236, 109797. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhao, S.; Sun, B.; Wang, Z.; Wang, J. Robust Superhydrophobic Mesh Coated by PANI/TiO2 Nanoclusters for Oil/Water Separation with High Flux, Self-Cleaning, Photodegradation and Anti-Corrosion. Sep. Purif. Technol. 2020, 235, 116166. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An Introduction to Superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 1–25. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, J.; Guo, Z.; Liu, W. Asymmetric Superwetting Stainless Steel Meshes for On-Demand and Highly Effective Oil-Water Emulsion Separation. Sep. Purif. Technol. 2021, 273, 118994. [Google Scholar] [CrossRef]
- Xu, P.; Li, X. Fabrication of TiO2/SiO2 superhydrophobic Coating for Efficient Oil/Water Separation. J. Environ. Chem. Eng. 2021, 9, 105538. [Google Scholar] [CrossRef]
- Xu, B.; Cai, Z. Fabrication of a Superhydrophobic ZnO Nanorod Array Film on Cotton Fabrics via a Wet Chemical Route and Hydrophobic Modification. Appl. Surf. Sci. 2008, 254, 5899–5904. [Google Scholar] [CrossRef]
- Cao, C.; Wang, F.; Lu, M. Superhydrophobic CuO Coating Fabricated on Cotton Fabric for Oil/Water Separation and Photocatalytic Degradation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125033. [Google Scholar] [CrossRef]
- Wan, F.; Yang, D.Q.; Sacher, E. Repelling Hot Water from Superhydrophobic Surfaces Based on Carbon Nanotubes. J. Mater. Chem. A 2015, 3, 16953–16960. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Huang, W.; Zhang, S.; Huang, J.; Yang, H.; Zhou, Y.; Xu, W.; Gu, S. Fabrication of Multifunctional Textiles with Durable Antibacterial Property and Efficient Oil-Water Separation via in Situ Growth of Zeolitic Imidazolate Framework-8 (ZIF-8) on Cotton Fabric. Appl. Surf. Sci. 2020, 503, 144079. [Google Scholar] [CrossRef]
- Yang, H.M.; Liu, X.; Song, X.L.; Yang, T.L.; Liang, Z.H.; Fan, C.M. In Situ Electrochemical Synthesis of MOF-5 and Its Application in Improving Photocatalytic Activity of BiOBr. Trans. Nonferrous Met. Soc. China 2015, 25, 3987–3994. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Maurya, S.; Rathod, V.K. Polysaccharide Based Metal Organic Frameworks (Polysaccharide–MOF): A Review. Coord. Chem. Rev. 2019, 396, 1–21. [Google Scholar] [CrossRef]
- Hang, P.; Zhao, B.; Zhou, J.; Ding, Y. Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment. Materials 2023, 16, 3952. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Revilla, R.I.; Vaglio, E.; Totis, G.; Sortino, M.; De Graeve, I.; Fedrizzi, L. Effect of Thermal Treatment on Corrosion Behavior of AISI 316L Stainless Steel Manufactured by Laser Powder Bed Fusion. Materials 2022, 15, 6768. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Pang, S.; Pang, D.; Tian, F.; Yu, Y. The Microstructure and Pitting Corrosion Behavior of K-TIG Welded Joints of the UNS S32101 Duplex Stainless Steel. Materials 2023, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Maicas-Esteve, H.; Taji, I.; Wilms, M.; Gonzalez-Garcia, Y.; Johnsen, R. Corrosion and Microstructural Investigation on Additively Manufactured 316L Stainless Steel: Experimental and Statistical Approach. Materials 2022, 15, 1605. [Google Scholar] [CrossRef] [PubMed]
- Ijaola, A.O.; Farayibi, P.K.; Asmatulu, E. Superhydrophobic Coatings for Steel Pipeline Protection in Oil and Gas Industries: A Comprehensive Review. J. Nat. Gas Sci. Eng. 2020, 83, 103544. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Mohseni-Dargah, M.; Firoozirad, K.; Aryai, V.; Razmjou, A.; Abbassi, R.; Garaniya, V.; Beheshti, A.; Asadnia, M. Recent Advances in Sensing and Assessment of Corrosion in Sewage Pipelines. Process Saf. Environ. Prot. 2021, 147, 192–213. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, H.; Zou, Y.; Ye, Y.; Zhao, H. Corrosion Protective Mechanism of Smart Graphene-Based Self-Healing Coating on Carbon Steel. Corros. Sci. 2020, 174, 108825. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.A.; Ashour, M.; Aly, A.M.; Mohamed, M.E. Fabrication of Robust Superhydrophobic Nickel Films on Steel Surface with High Corrosion Resistance, Mechanical and Chemical Stability. J. Eng. Mater. Technol. 2022, 144, 021007. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion Resistance and Long-Term Durability of Super-Hydrophobic Nickel Film Prepared by Electrodeposition Process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Li, L.; Zeng, Z.; Zhao, W.; Wang, G.; Guan, X. Simple and Green Fabrication of a Superhydrophobic Surface by One-Step Immersion for Continuous Oil/Water Separation. J. Phys. Chem. A 2016, 120, 5617–5623. [Google Scholar] [CrossRef]
- Jena, G.; Thinaharan, C.; George, R.P.; Philip, J. Robust Nickel-Reduced Graphene Oxide-Myristic Acid Superhydrophobic Coating on Carbon Steel Using Electrochemical Codeposition and Its Corrosion Resistance. Surf. Coat. Technol. 2020, 397, 125942. [Google Scholar] [CrossRef]
- Du, C.; He, X.; Tian, F.; Bai, X.; Yuan, C. Preparation of Superhydrophobic Steel Surfaces with Chemical Stability and Corrosion. Coatings 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, Z.; Kang, Y.; Sun, M.; Ma, L. Preparation of a Superhydrophobic TiN/PTFE Composite Film toward Self-Cleaning and Corrosion Protection Applications. J. Mater. Sci. 2021, 56, 1413–1425. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, L.; Ge, F.; Wang, G.; Zeng, Z. Sprayable Superhydrophobic Coating with High Mechanical/Chemical Robustness and Anti-Corrosion. Surf. Coat. Technol. 2022, 443, 128609. [Google Scholar] [CrossRef]
- Nanda, D.; Sahoo, A.; Kumar, A.; Bhushan, B. Facile Approach to Develop Durable and Reusable Superhydrophobic/Superoleophilic Coatings for Steel Mesh Surfaces. J. Colloid Interface Sci. 2019, 535, 50–57. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, Z.; Zhang, X.; Wu, S.; Wang, R.; Zhu, Y. A Novel Electrodeposition Route for Fabrication of the Superhydrophobic Surface with Unique Self-Cleaning, Mechanical Abrasion and Corrosion Resistance Properties. Chem. Eng. J. 2016, 303, 37–47. [Google Scholar] [CrossRef]
- Tan, C.; Li, Q.; Cai, P.; Yang, N.; Xi, Z. Fabrication of Color-Controllable Superhydrophobic Copper Compound Coating with Decoration Performance. Appl. Surf. Sci. 2015, 328, 623–631. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Chen, J.; Liu, L.; Lei, J.; Li, N.; Liu, G.; Pan, F. One-Step Spraying Method to Construct Superhydrophobic Magnesium Surface with Extraordinary Robustness and Multi-Functions. J. Magnes. Alloy. 2021, 9, 668–675. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of Corrosion Rate from Polarisation Curves Not Exhibiting a Tafel Region. Corros. Sci. 2005, 47, 3034–3052. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of Corrosion Rates Measured by the Tafel Extrapolation Method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Fetouh, H.A.; Abd-El-Nabey, B.; Goher, Y.M.; Karam, M.S. An Electrochemical Investigation in the Anticorrosive Properties of Silver Nanoparticles for the Acidic Corrosion of Aluminium. J. Electrochem. 2018, 24, 89–100. [Google Scholar]
- Ou, J.; Liu, M.; Li, W.; Wang, F.; Xue, M.; Li, C. Corrosion Behavior of Superhydrophobic Surfaces of Ti Alloys in NaCl Solutions. Appl. Surf. Sci. 2012, 258, 4724–4728. [Google Scholar] [CrossRef]
- Ghiamati Yazdi, E.; Ghahfarokhi, Z.S.; Bagherzadeh, M. Protection of Carbon Steel Corrosion in 3.5% NaCl Medium by Aryldiazonium Grafted Graphene Coatings. New J. Chem. 2017, 41, 12470–12480. [Google Scholar] [CrossRef]
- Qu, J.E.; Yu, C.; Nie, C.; Wang, H.; Cao, Z.; Li, Y.; Wang, X. A New Environmentally Friendly Approach to Prepare Superhydrophobic Colored Stainless Steel Surface for Decoration, Anti-Corrosion and Self-Cleaning. J. Mater. Sci. 2021, 56, 854–869. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Liang, H.; Zhao, B.; He, Y.; Cui, X. A Facile Method for Constructing a Superhydrophobic Zinc Coating on a Steel Surface with Anti-Corrosion and Drag-Reduction Properties. Appl. Surf. Sci. 2021, 562, 150192. [Google Scholar] [CrossRef]
- Varshney, P.; Mohapatra, S.S.; Kumar, A. Durable and Regenerable Superhydrophobic Coating on Steel Surface for Corrosion Protection. J. Bio-Tribo-Corrosion 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Fedchenko, Y.I. Colloids and Surfaces A: Physicochemical and Engineering Aspects Superhydrophobic Soot as a Mechanically Robust, Heat Insulating and Anti-Corrosion Protective Coating for Concrete Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131723. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Yan, J.; Li, Z.; Huang, S.; Weng, Y. Preparation of Superhydrophobic Coating with Anti-Corrosion and Anti-Fouling Properties on the Surface of Low Manganese Steel by Electrodeposition. Surf. Coat. Technol. 2023, 460, 129412. [Google Scholar] [CrossRef]
- Daneshnia, A.; Raeissi, K.; Salehikahrizsangi, P. Rapid One-Step Electrodeposition of Robust Superhydrophobic and Oleophobic Ni Coating with Anti-Corrosion and Self-Cleaning Properties. Surf. Coat. Technol. 2022, 450, 129007. [Google Scholar] [CrossRef]



| Factor | Ni@SA | Ni@Cu-As MOF@SA |
|---|---|---|
| NiCl2.6H2O | 40 gL−1 | 40 gL−1 |
| NiSO4 | 176 gL−1 | 176 gL−1 |
| H3BO3 | 60 gL−1 | 60 gL−1 |
| Bio-MOF | 0.0 gL−1 | 0.4 gL−1 |
| Time of electrodeposition | 6.0 min | 6.0 min |
| Deposition potential | 11.0 Volt | 11.0 Volt |
| Deposit | -Ecorr mV | βa mV/Decade | -βc mV/Decade | icorr µA/cm2 | %P |
|---|---|---|---|---|---|
| Bare stainless steel | 455.03 | 44.323 | 467.05 | 0.0710569 | --- |
| stainless steel + Ni@SA | 434.56 | 95.915 | 211.46 | 0.0041915 | 94.1 |
| stainless steel + Ni@Cu-As MOF@SA | 392.42 | 112.02 | 181.69 | 0.0007017 | 99.0 |
| Coat | Rs (Ohm.cm2) | n1 | CPEdl × 10−6 (sn Ω−1 cm2) | W × 10−4 | Rct (Ohm.cm2) | %P |
|---|---|---|---|---|---|---|
| Bare stainless steel | 2.1 | 0.77 | 311.1 | 444.4 | 1312 | --- |
| Stainless steel + Ni@SA | 3.9 | 0.74 | 56.2 | 22.3 | 28,420 | 95.3 |
| Stainless steel + Ni@Cu-As MOF@SA | 4.4 | 0.72 | 35.3 | 18.9 | 47,512 | 97.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almufarij, R.S.; Fetouh El Sayed, H.A.; Mohamed, M.E. Eco-Friendly Approach for the Construction of Superhydrophobic Coating on Stainless Steel Metal Based on Biological Metal–Organic Framework and Its Corrosion Resistance Performance. Materials 2023, 16, 4728. https://doi.org/10.3390/ma16134728
Almufarij RS, Fetouh El Sayed HA, Mohamed ME. Eco-Friendly Approach for the Construction of Superhydrophobic Coating on Stainless Steel Metal Based on Biological Metal–Organic Framework and Its Corrosion Resistance Performance. Materials. 2023; 16(13):4728. https://doi.org/10.3390/ma16134728
Chicago/Turabian StyleAlmufarij, Rasmiah Saad, Howida Abouel Fetouh El Sayed, and Mohamed Elshahat Mohamed. 2023. "Eco-Friendly Approach for the Construction of Superhydrophobic Coating on Stainless Steel Metal Based on Biological Metal–Organic Framework and Its Corrosion Resistance Performance" Materials 16, no. 13: 4728. https://doi.org/10.3390/ma16134728
APA StyleAlmufarij, R. S., Fetouh El Sayed, H. A., & Mohamed, M. E. (2023). Eco-Friendly Approach for the Construction of Superhydrophobic Coating on Stainless Steel Metal Based on Biological Metal–Organic Framework and Its Corrosion Resistance Performance. Materials, 16(13), 4728. https://doi.org/10.3390/ma16134728






