Design and Development of Low- and Medium-Viscosity Alginate Beads Loaded with Pluronic® F-127 Nanomicelles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
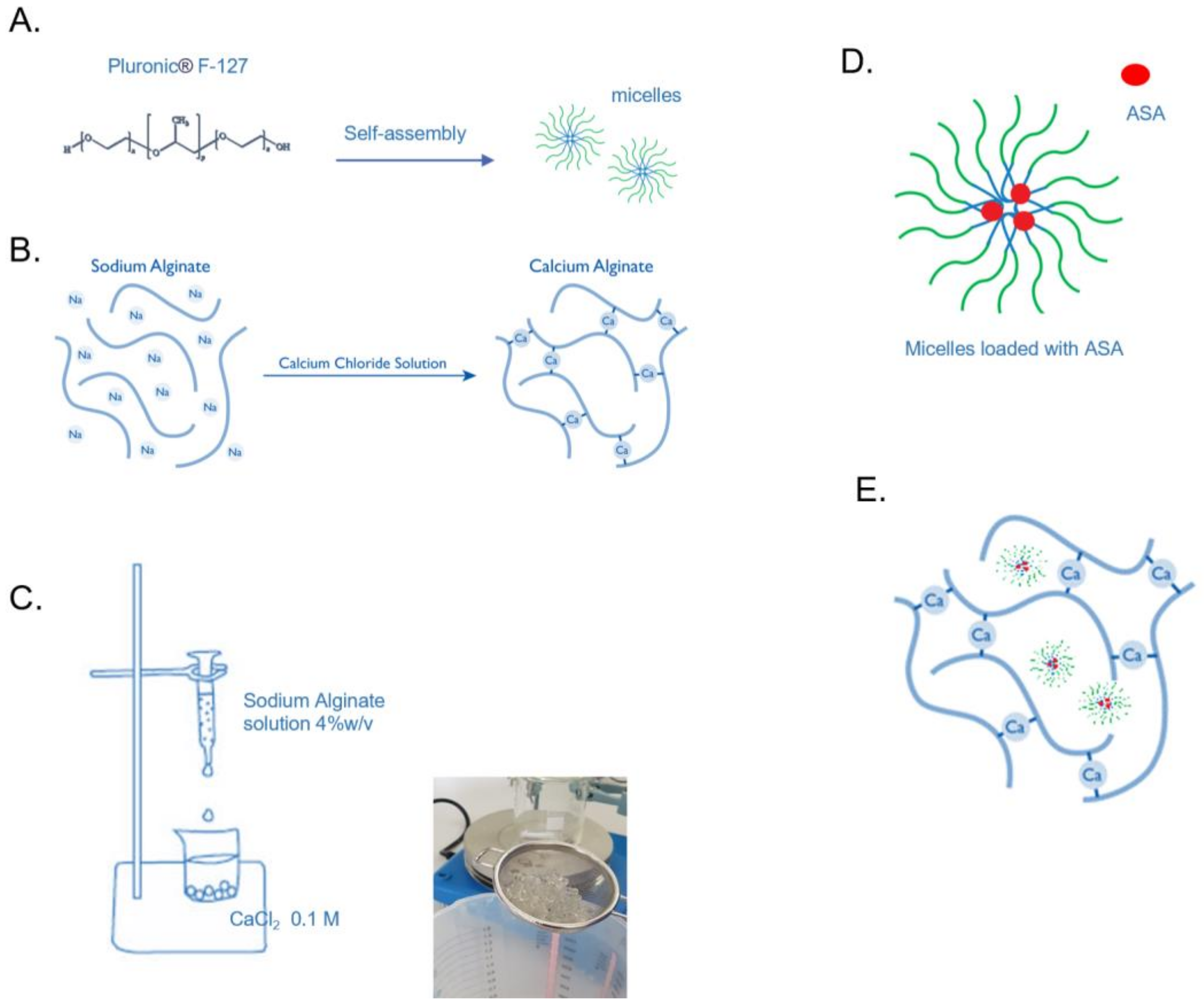
2.2.1. Preparation and Physicochemical Characterization of Micelles
2.2.2. Preparation of sodium-alginate beads
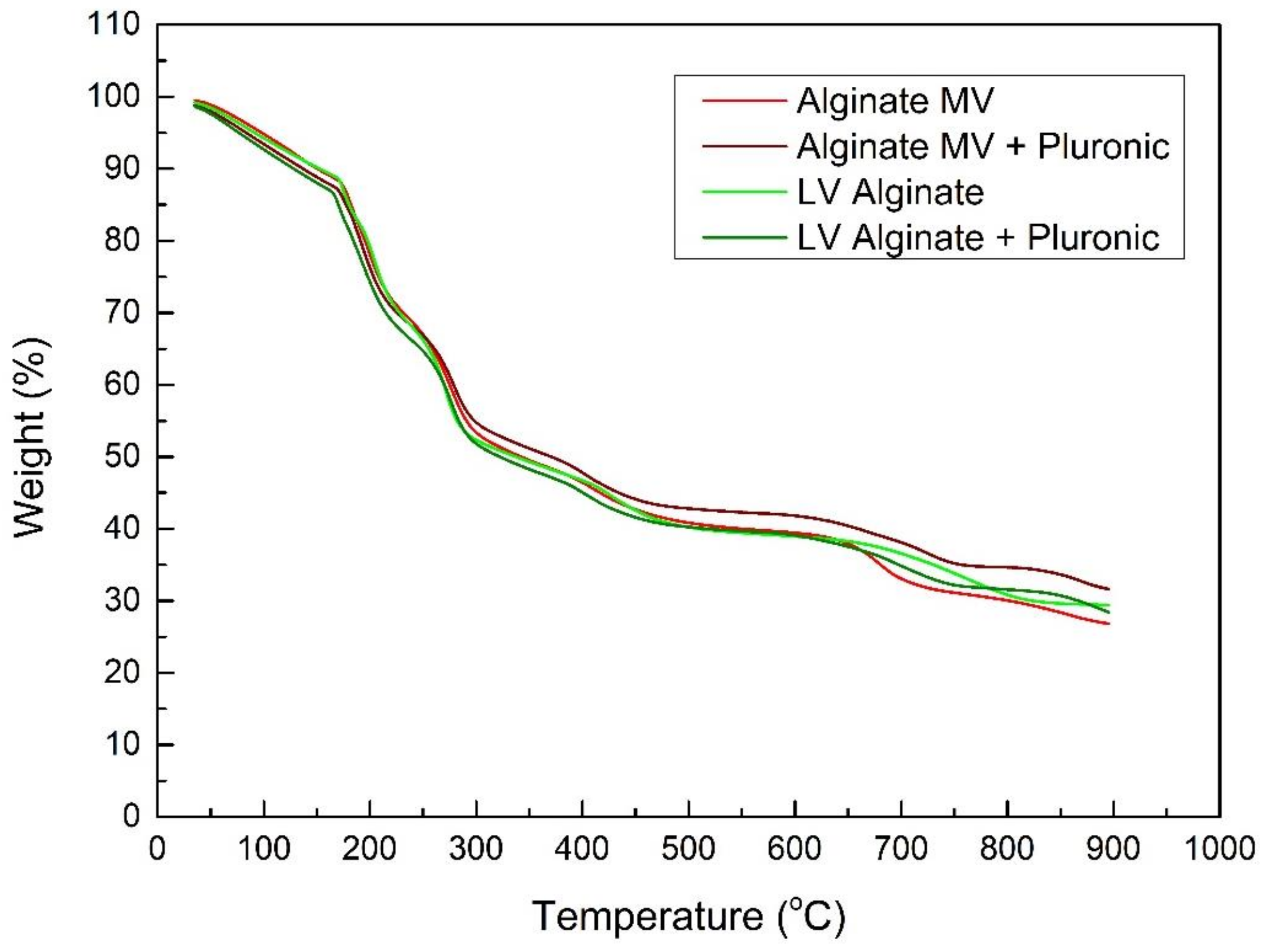
2.2.3. Thermogravimetric Analysis
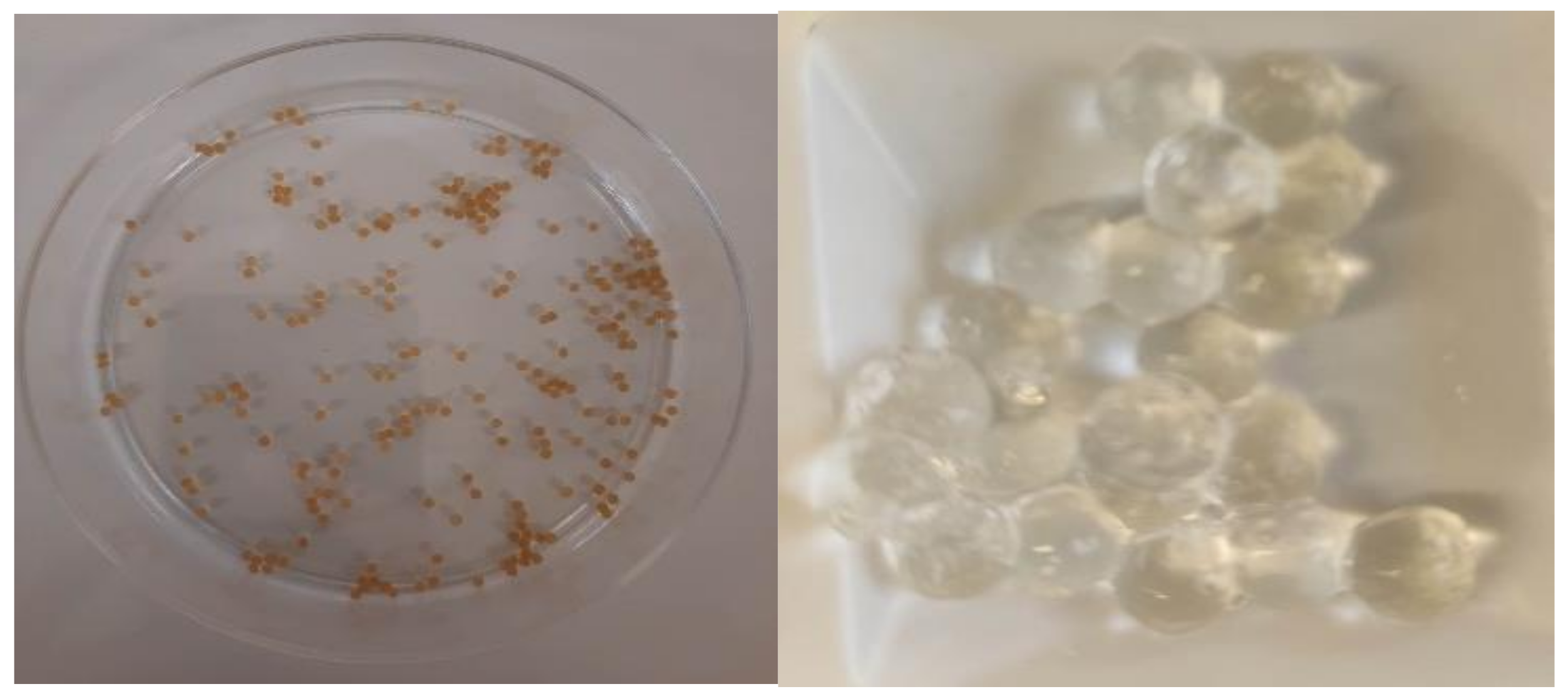
2.2.4. Morphological Characterization
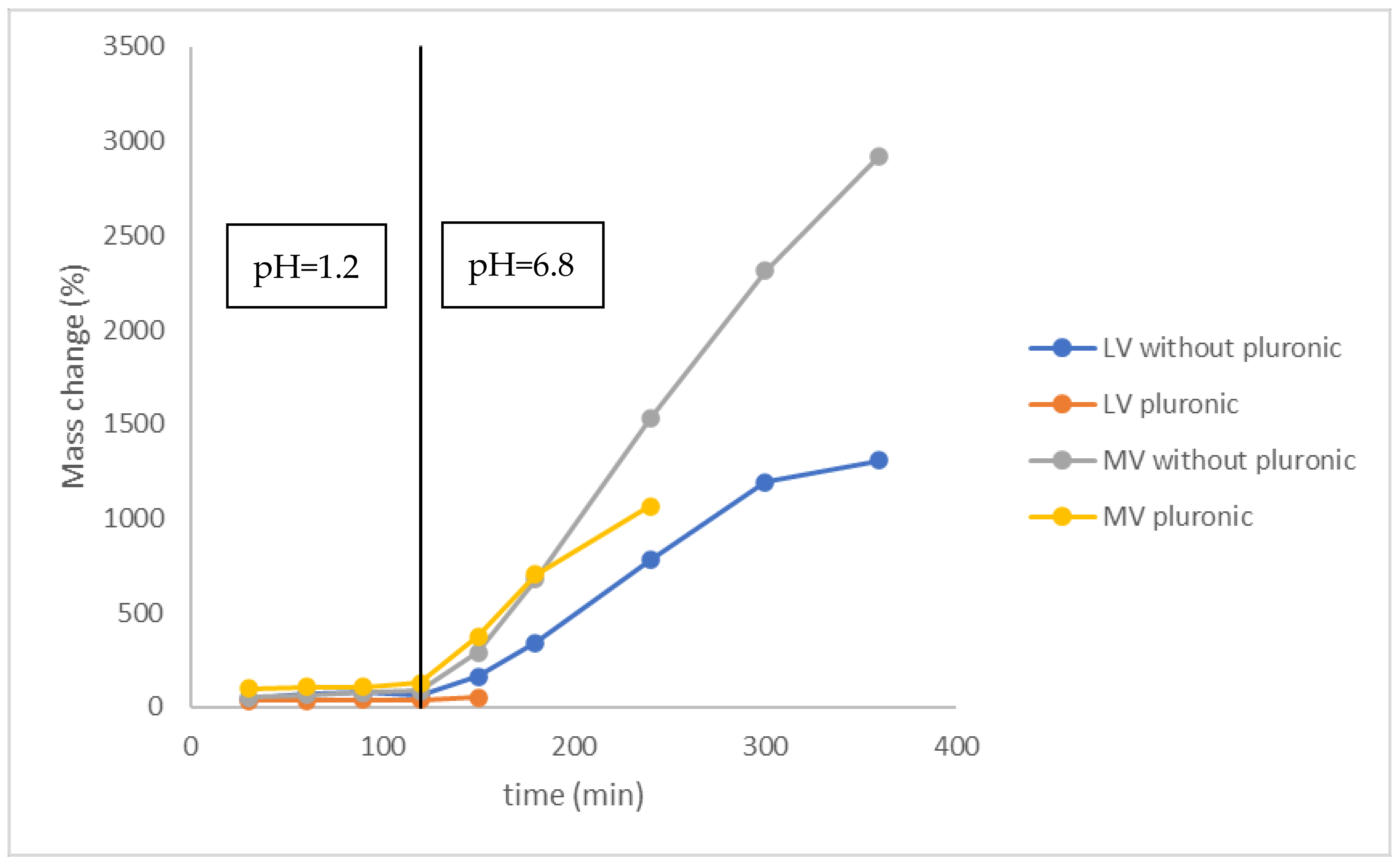
2.2.5. Swelling Studies
2.2.6. Dissolution Studies
2.2.7. Statistical Analysis
3. Results
3.1. Physicochemical and Thermotropic Characterization of the Prepared Systems
3.2. Swelling Studies
3.3. Morphological Characterization
3.4. Dissolution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khlibsuwan, R.; Khunkitti, W.; Pongjanyakul, T. Alginate-Poloxamer Beads for Clotrimazole Delivery: Molecular Interactions, Mechanical Properties, and Anticandidal Activity. Int. J. Biol. Macromol. 2020, 148, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Sentoukas, T.; Pispas, S.; Demetzos, C.; Papalois, A.; Bouropoulos, N. PH-Responsive Polymeric Nanoassemblies Encapsulated into Alginate Beads: Morphological Characterization and Swelling Studies. J. Polym. Res. 2018, 25, 117. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural Polymer-Based Stimuli-Responsive Hydrogels. Curr. Med. Chem. 2019, 27, 2631–2657. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile Synthesis of PH-Responsive Sodium Alginate/Carboxymethyl Chitosan Hydrogel Beads Promoted by Hydrogen Bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef]
- Takka, S.; Gürel, A. Evaluation of Chitosan/Alginate Beads Using Experimental Design: Formulation and in Vitro Characterization. AAPS PharmSciTech 2010, 11, 460–466. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, J.; Liu, Y.; Liu, L.; Yu, J.; Fan, Y. Controlled Delivery of Aspirin from Nanocellulose-Sodium Alginate Interpenetrating Network Hydrogels. Ind. Crops Prod. 2023, 192, 116081. [Google Scholar] [CrossRef]
- Naharros-Molinero, A.; Caballo-González, M.Á.; de la Mata, F.J.; García-Gallego, S. Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics 2022, 14, 2628. [Google Scholar] [CrossRef]
- Aslam, M.; Barkat, K.; Shamshad Malik, N.; Alqahtani, M.S.; Anjum, I.; Khalid, I.; Ruqia Tulain, U.; Gohar, N.; Zafar, H.; Cláudia Paiva-Santos, A.; et al. PH Sensitive Pluronic Acid/Agarose-Hydrogels as Controlled Drug Delivery Carriers: Design, Characterization and Toxicity Evaluation. Pharmaceutics 2022, 14, 1218. [Google Scholar] [CrossRef]
- Islam, N.; Irfan, M.; Khan, S.U.D.; Syed, H.K.; Iqbal, M.S.; Khan, I.U.; Mahdy, A.; Raafat, M.; Hossain, M.A.; Inam, S.; et al. Poloxamer-188 and d-α-Tocopheryl Polyethylene Glycol Succinate (Tpgs-1000) Mixed Micelles Integrated Orodispersible Sublingual Films to Improve Oral Bioavailability of Ebastine in Vitro and in Vivo Characterization. Pharmaceutics 2021, 13, 54. [Google Scholar] [CrossRef]
- Broome, T.A.; Brown, M.P.; Gronwall, R.R.; Casey, M.F.; Meritt, K.A. Pharmacokinetics and Plasma Concentrations of Acetylsalicylic Acid after Intravenous, Rectal, and Intragastric Administration to Horses. Can. J. Vet. Res. 2003, 67, 297. [Google Scholar]
- Smrdel, P.; Bogataj, M.; Podlogar, F.; Planinšek, O.; Zajc, N.; Mazaj, M.; Kaučič, V.; Mrhar, A. Characterization of Calcium Alginate Beads Containing Structurally Similar Drugs. Drug Dev. Ind. Pharm. 2006, 32, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.M.K.; Poon, C.S.P.; Law, B.K.B.; Pang, L.M.; Chu, W.C.W.; Ma, T.K.F. Fine Needle Aspiration Cytology of Granulomatous Mastitis. J. Clin. Pathol. 2003, 56, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Dissolution Testing of Immediate Release Solid Oral Dosage Forms|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms (accessed on 23 June 2023).
- Vlachou, M.; Siamidi, A.; Anagnostopoulou, D.; Christodoulou, E.; Bikiaris, N.N. Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-Lactic Acid) and Its PLA-Co-PEAd and PLA-Co-PBAd Copolymers. Polymers 2022, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, N.; Kanik, A.E.; Baykara, T. Comparison of in Vitro Dissolution Profiles by ANOVA-Based, Model-Dependent and -Independent Methods. Int. J. Pharm. 2000, 209, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Shaarani, S.; Hamid, S.S.; Kaus, N.H.M. The Influence of Pluronic F68 and F127 Nanocarrier on Physicochemical Properties, In Vitro Release, and Antiproliferative Activity of Thymoquinone Drug. Pharmacogn. Res. 2017, 9, 12. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Cha, C. Dual-Functional Alginate Crosslinker: Independent Control of Crosslinking Density and Cell Adhesive Properties of Hydrogels via Separate Conjugation Pathways. Carbohydr. Polym. 2021, 252, 117128. [Google Scholar] [CrossRef]
- Herrero, E.P.; Martín Del Valle, E.M.; Galán, M.A. Development of a New Technology for the Production of Microcapsules Based in Atomization Processes. Chem. Eng. J. 2006, 117, 137–142. [Google Scholar] [CrossRef]
- Salisu, A.; Sanagi, M.M.; Abu Naim, A.; Abd Karim, K.J.; Wan Ibrahim, W.A.; Abdulganiyu, U. Alginate Graft Polyacrylonitrile Beads for the Removal of Lead from Aqueous Solutions. Polym. Bull. 2016, 73, 519–537. [Google Scholar] [CrossRef]
- Li, S.S.; Song, Y.L.; Yang, H.R.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Modifying Alginate Beads Using Polycarboxyl Component for Enhanced Metal Ions Removal. Int. J. Biol. Macromol. 2020, 158, 493–501. [Google Scholar] [CrossRef]
- Dos Santos Araújo, P.; Belini, G.B.; Mambrini, G.P.; Yamaji, F.M.; Waldman, W.R. Thermal Degradation of Calcium and Sodium Alginate: A Greener Synthesis towards Calcium Oxide Micro/Nanoparticles. Int. J. Biol. Macromol. 2019, 140, 749–760. [Google Scholar] [CrossRef]
- Klokk, T.I.; Melvik, J.E. Controlling the Size of Alginate Gel Beads by Use of a High Electrostatic Potential. J. Microencapsul. 2002, 19, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.J.; Huang, Y.Y.; Lo, S.H.; Hsu, T.F.; Huang, W.Y.; Huang, S.L.; Lin, Y.S. Effects of PH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Shi, J.; Alves, N.M.; Mano, J.F. Drug Release of PH/Temperature-Responsive Calcium Alginate/Poly(N-Isopropylacrylamide) Semi-IPN Beads. Macromol. Biosci. 2006, 6, 358–363. [Google Scholar] [CrossRef]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Kovačević, M.M.; Janković, M.G.; Stanić, Z.D. PH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2011, 37, 106–126. [Google Scholar] [CrossRef]
- Hong, Y.; Song, H.W.; Kang, J.Y.; Yoon, D.S.; Jin, L.; Hong, Y.-C.; Pyo, J.-W.; Song, H.; Lee, S.W.; Kim, B.-M.; et al. Monitoring of Swelling and Degrading Behavior of Alginate Beads Using Optical Tweezers Application of Cell Membrane in Bio/Nano-Medical Technology View Project Development of High Throughput Drug Screening System for Alzheimer’s Disease View Project. BioChip J. 2009, 3, 213. [Google Scholar]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrcková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef]
- Dressman, J.B.; Nair, A.; Abrahamsson, B.; Barends, D.M.; Groot, D.W.; Kopp, S.; Langguth, P.; Polli, J.E.; Shah, V.P.; Zimmer, M. Biowaiver Monograph for Immediate-Release Solid Oral Dosage Forms: Acetylsalicylic Acid. J. Pharm. Sci. 2012, 101, 2653–2667. [Google Scholar] [CrossRef]
- Voo, W.P.; Ooi, C.W.; Islam, A.; Tey, B.T.; Chan, E.S. Calcium Alginate Hydrogel Beads with High Stiffness and Extended Dissolution Behaviour. Eur. Polym. J. 2016, 75, 343–353. [Google Scholar] [CrossRef]


| Formulations | Dh (nm) 1 | PDI 2 | Ζ-Potential (mV) |
|---|---|---|---|
| Pluronic® F-127 | 120 ± 12.5 | 0.37 ± 0.06 | −4.8 ± 1.9 |
| Pluronic® F-127: ASA | 102.6 ± 11.6 | 0.30 ± 0.02 | −2.9 ± 0.9 |
| Formulations | t20% | t50% | t90% | DE% | MDT | n |
|---|---|---|---|---|---|---|
| Low-viscosity alginate beads | 20 | 84 | 164 | 39.16 | 85.11 | 0.57 |
| Medium-viscosity alginate beads | 28 | 134 | 171 | 27.10 | 110.89 | 0.41 |
| Formulations | Formulation of Beads | Morphology | Thermotropic Behavior | Swelling Behavior | Release Kinetics |
|---|---|---|---|---|---|
| Low-viscosity alginate beads | Low viscosity | porous | ≈60% water loss | Rapid and extensive | Fast |
| Medium-viscosity alginate beads | Medium viscosity | dense | ≈60% water loss | Control and gradual | Slow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogeropoulou, F.; Papailiou, D.; Protopapa, C.; Siamidi, A.; Tziveleka, L.-A.; Pippa, N.; Vlachou, M. Design and Development of Low- and Medium-Viscosity Alginate Beads Loaded with Pluronic® F-127 Nanomicelles. Materials 2023, 16, 4715. https://doi.org/10.3390/ma16134715
Kalogeropoulou F, Papailiou D, Protopapa C, Siamidi A, Tziveleka L-A, Pippa N, Vlachou M. Design and Development of Low- and Medium-Viscosity Alginate Beads Loaded with Pluronic® F-127 Nanomicelles. Materials. 2023; 16(13):4715. https://doi.org/10.3390/ma16134715
Chicago/Turabian StyleKalogeropoulou, Flora, Dimitra Papailiou, Chrystalla Protopapa, Angeliki Siamidi, Leto-Aikaterini Tziveleka, Natassa Pippa, and Marilena Vlachou. 2023. "Design and Development of Low- and Medium-Viscosity Alginate Beads Loaded with Pluronic® F-127 Nanomicelles" Materials 16, no. 13: 4715. https://doi.org/10.3390/ma16134715
APA StyleKalogeropoulou, F., Papailiou, D., Protopapa, C., Siamidi, A., Tziveleka, L.-A., Pippa, N., & Vlachou, M. (2023). Design and Development of Low- and Medium-Viscosity Alginate Beads Loaded with Pluronic® F-127 Nanomicelles. Materials, 16(13), 4715. https://doi.org/10.3390/ma16134715










