Analysis of Nano-ZnO-Modified Asphalt Compatibility Based on Molecular Dynamics
Abstract
1. Introduction
2. Simulation Methodology
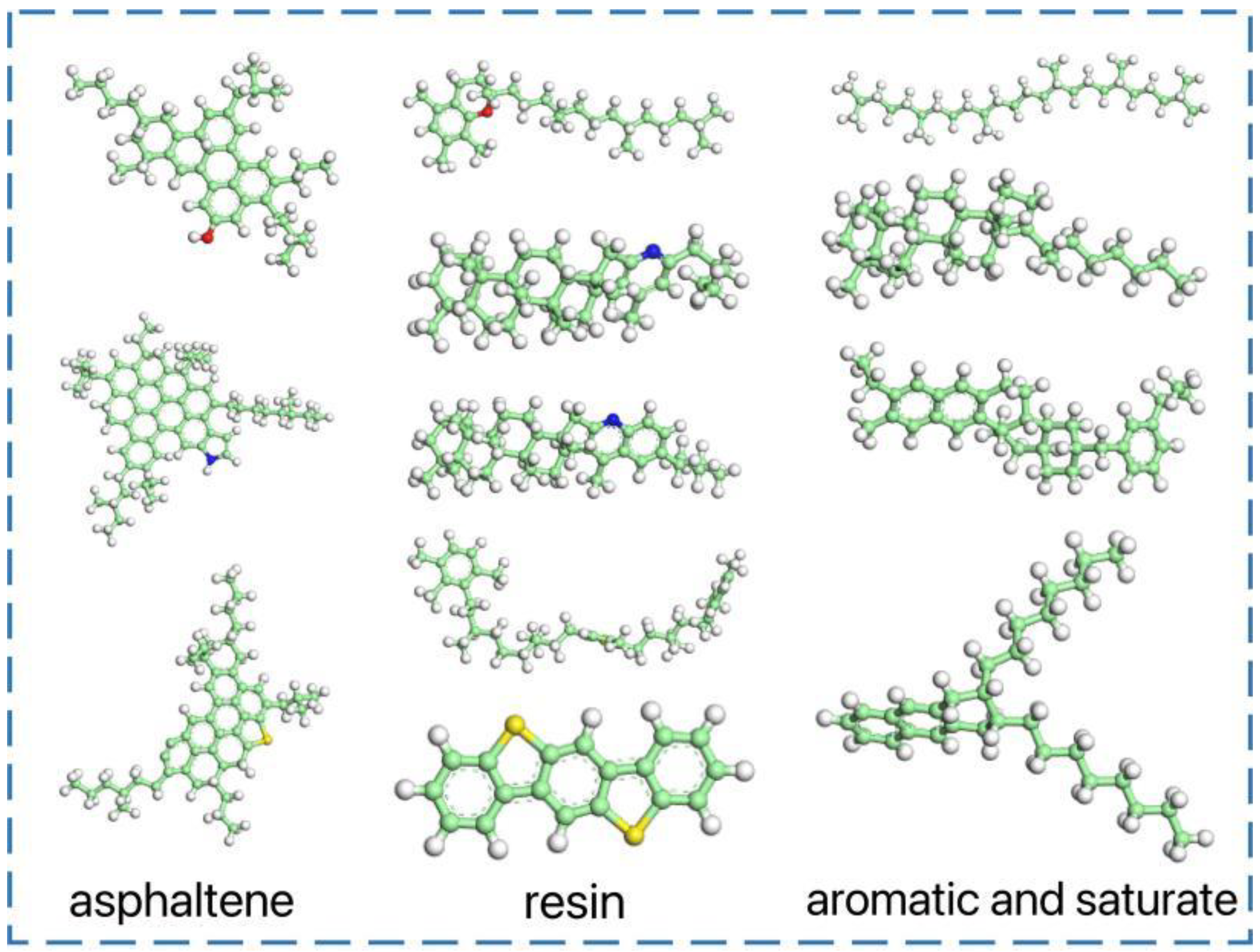
2.1. Establish a Molecular Model of Matrix Asphalt
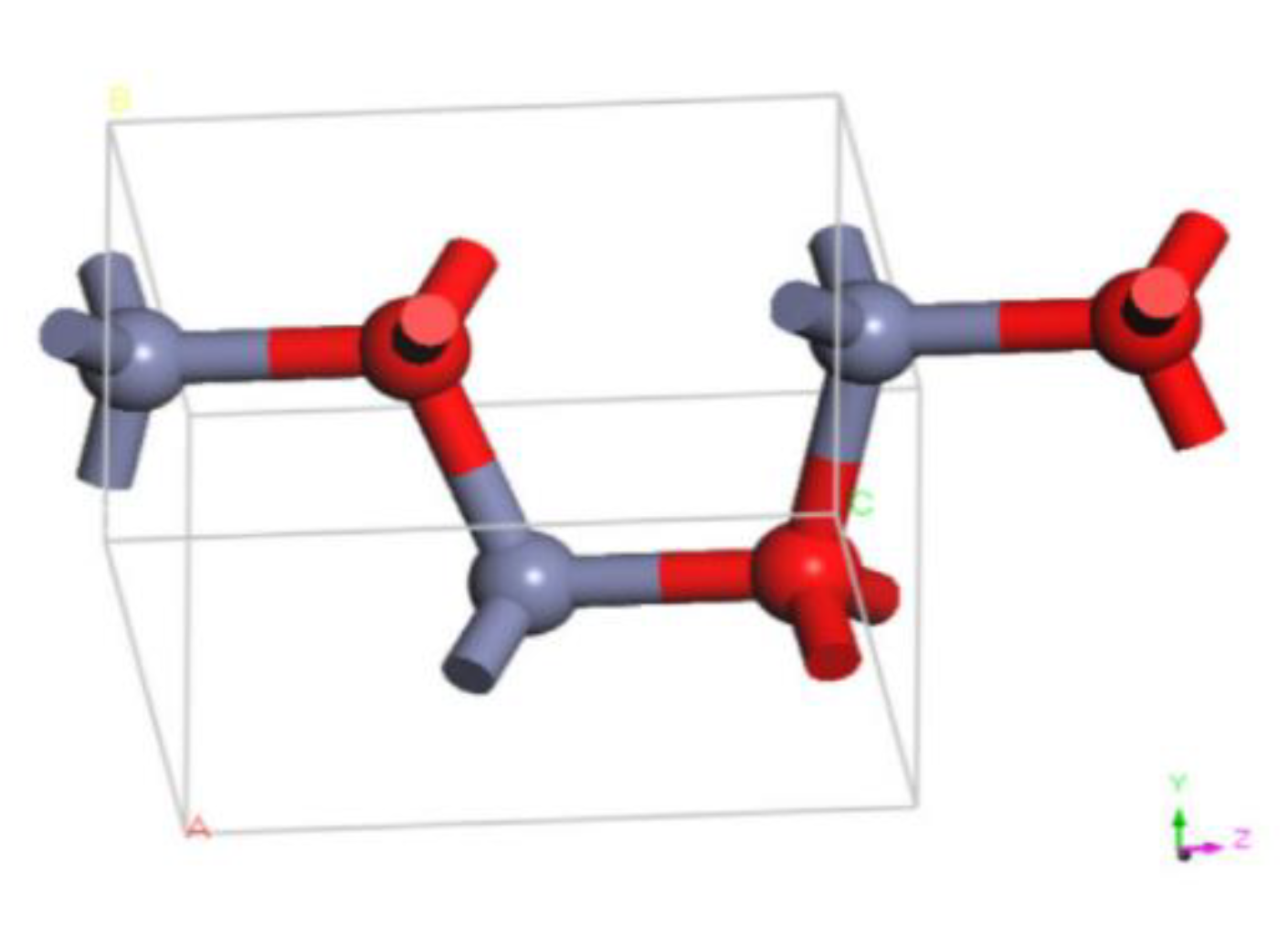
2.2. Molecular Modeling of Nano-ZnO Molecules
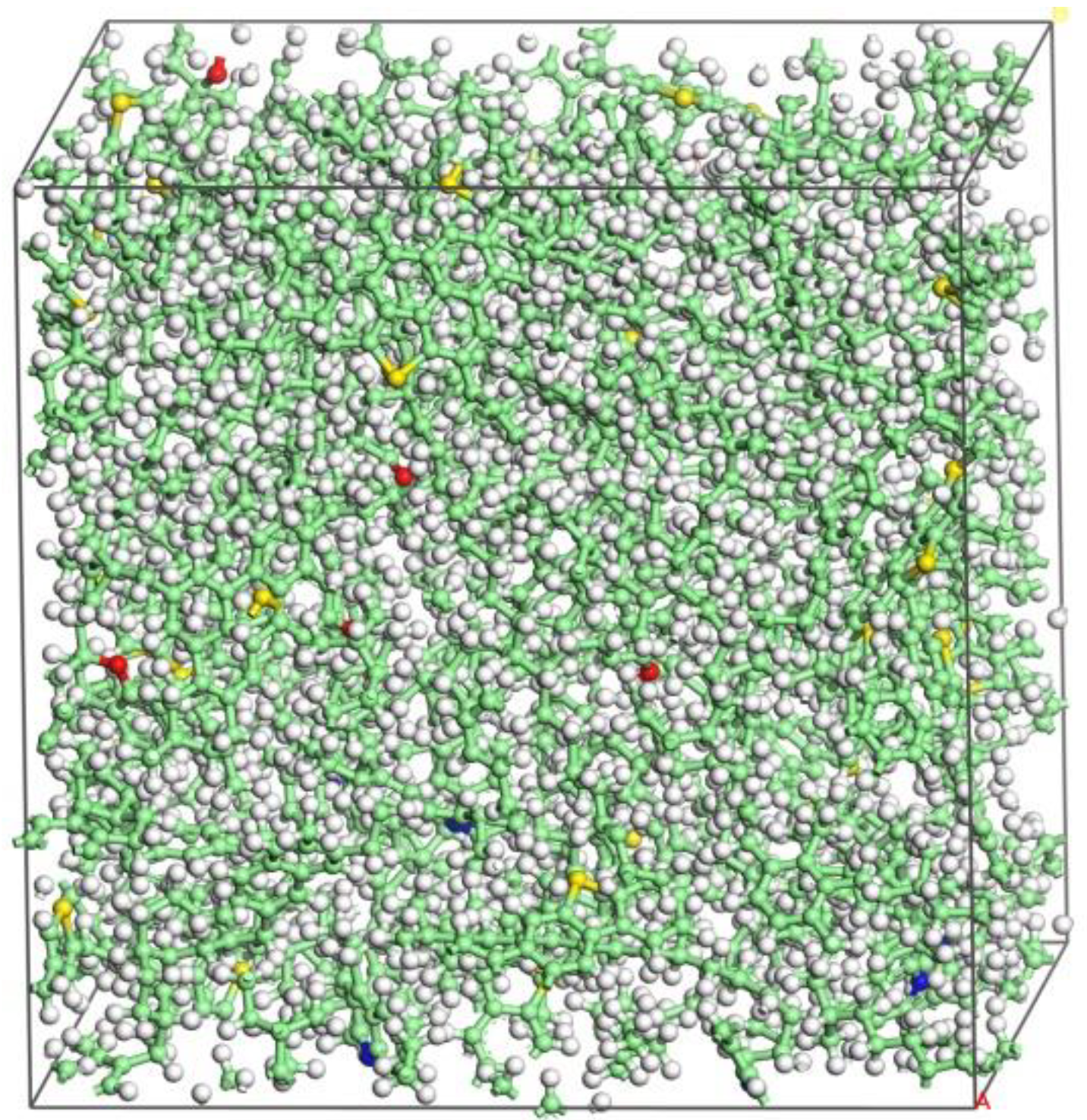
2.3. Molecular Modeling of Nano-ZnO-modified Asphalt
2.4. Molecular Dynamics Calculation of Solubility Parameters
2.5. Interaction Energy
2.6. Mean Square Displacement
2.7. Radial Distribution Function
2.8. Radius of Gyration
3. Results and Discussion
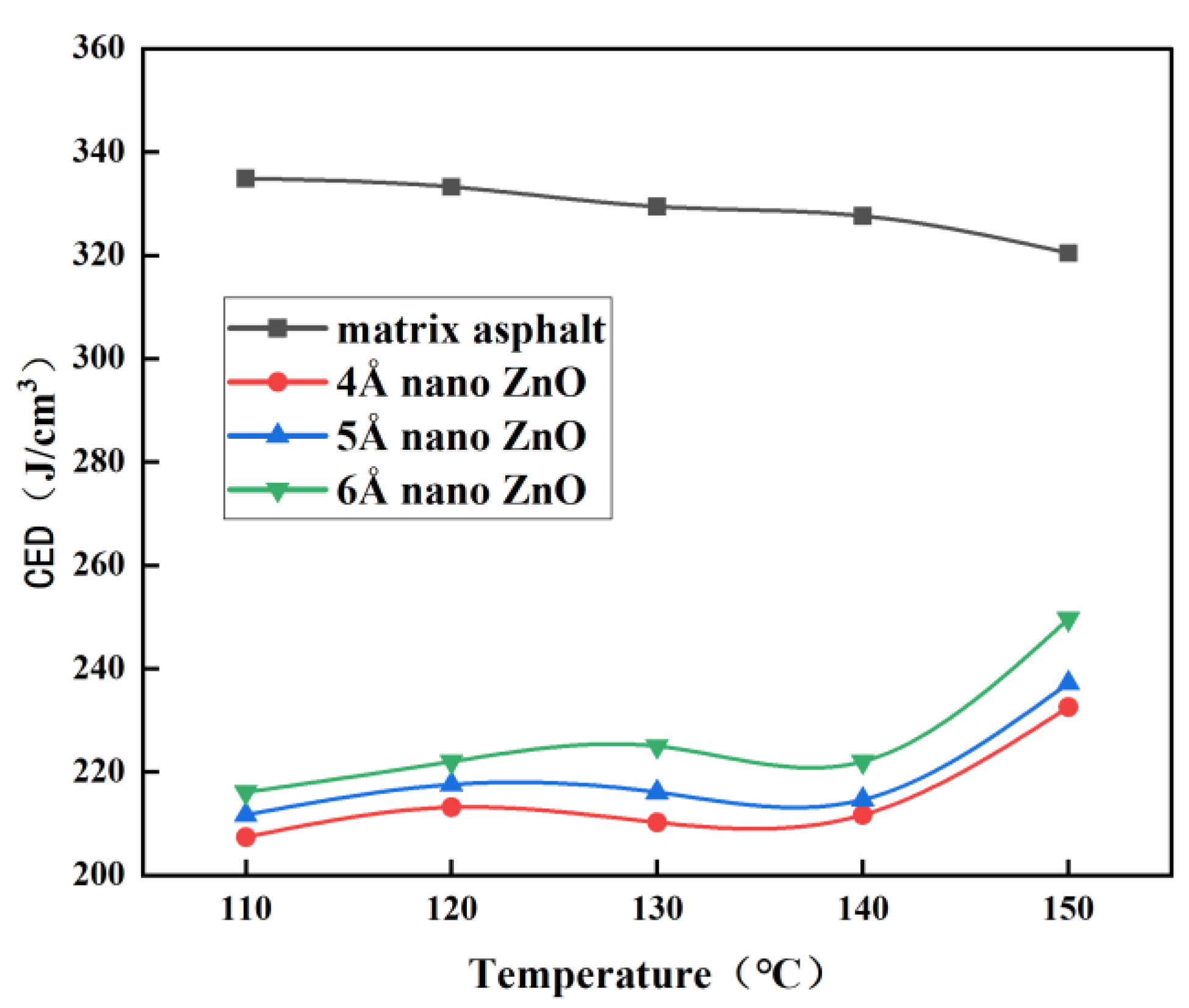
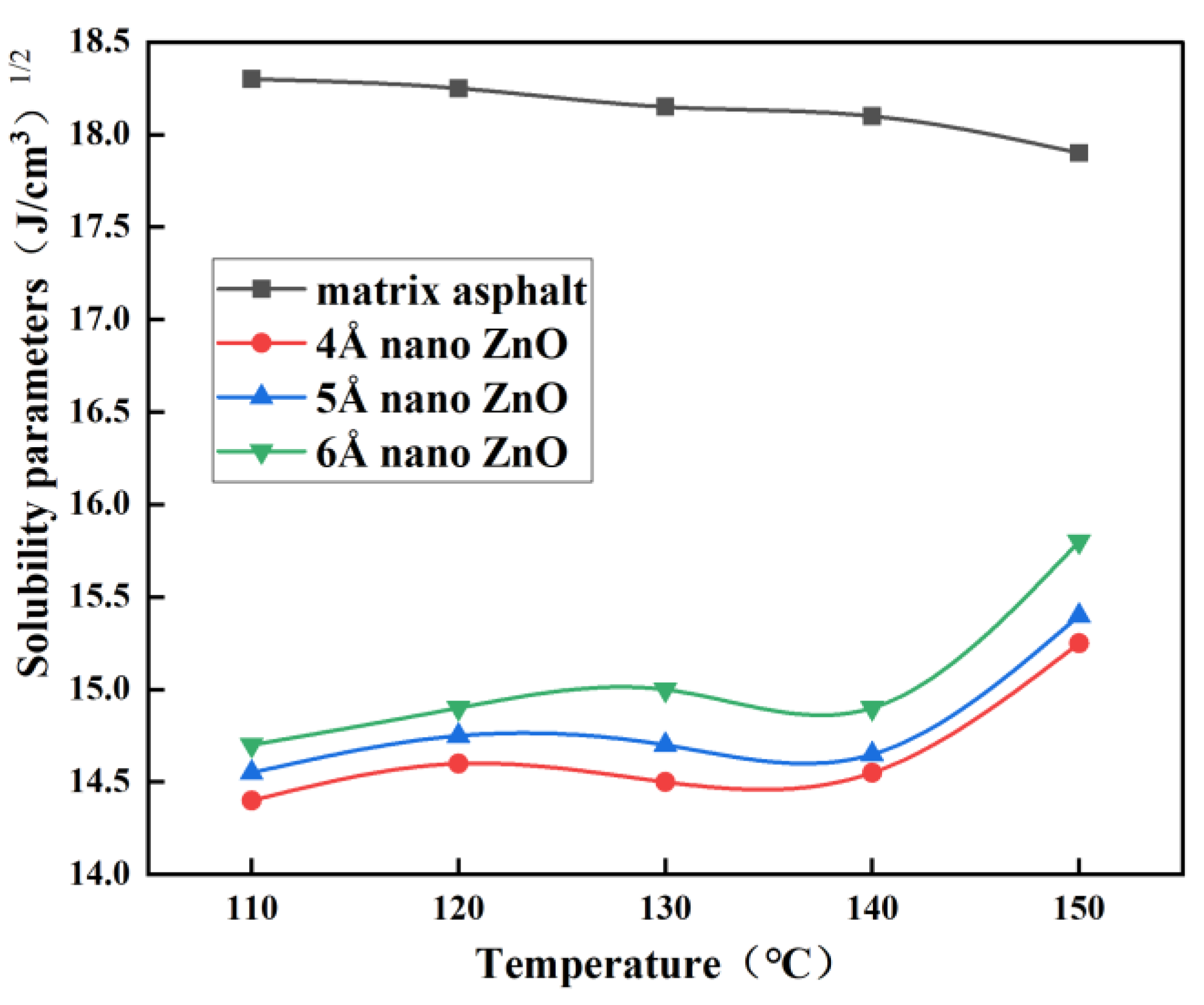
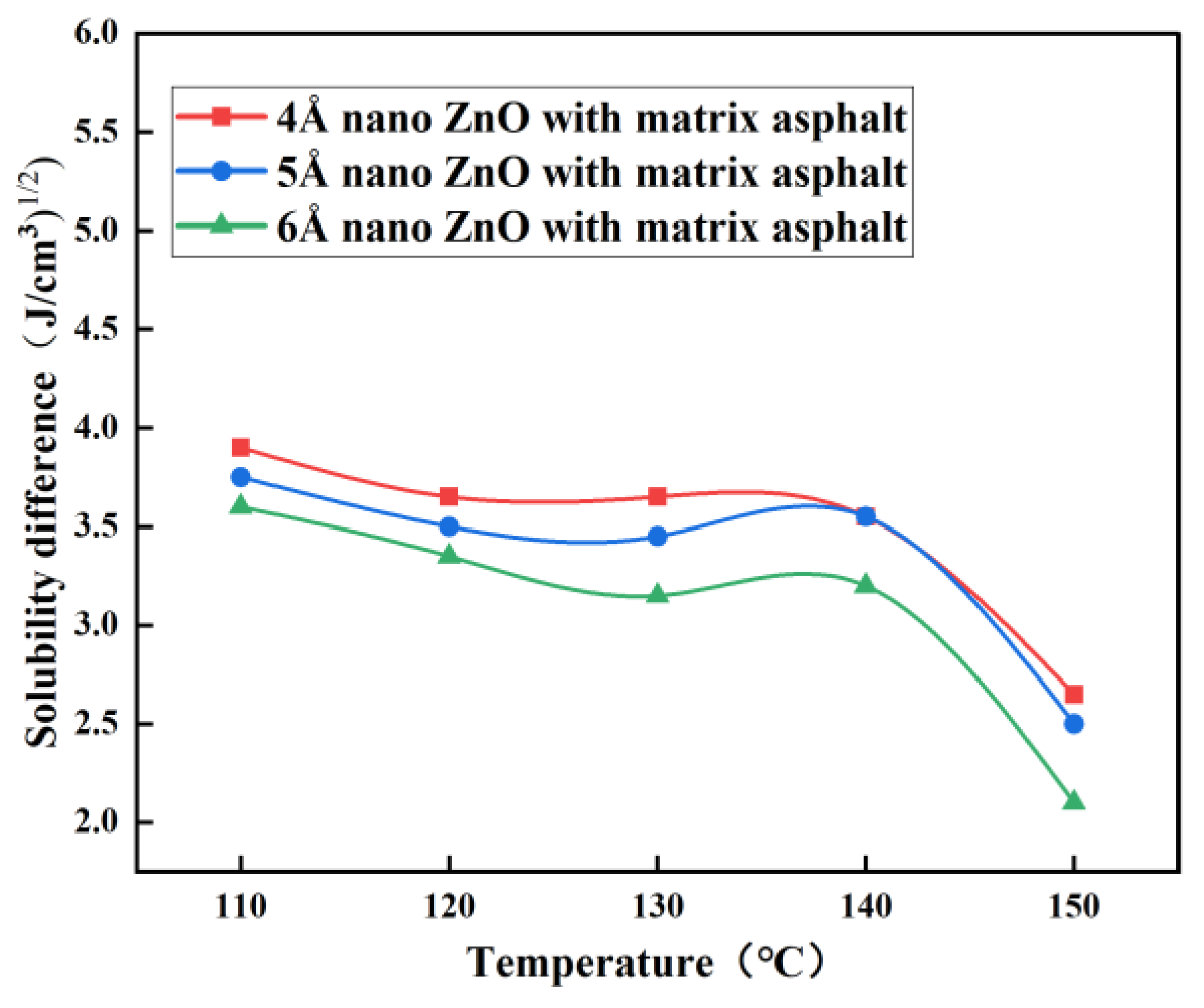
3.1. Solubility Parameters
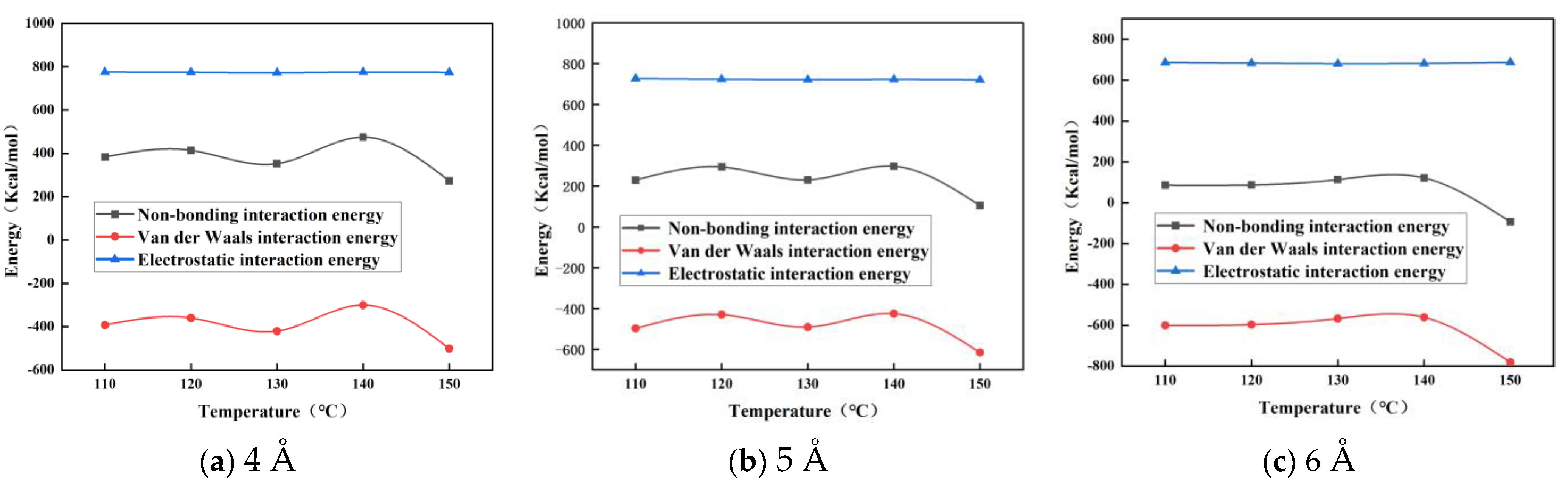
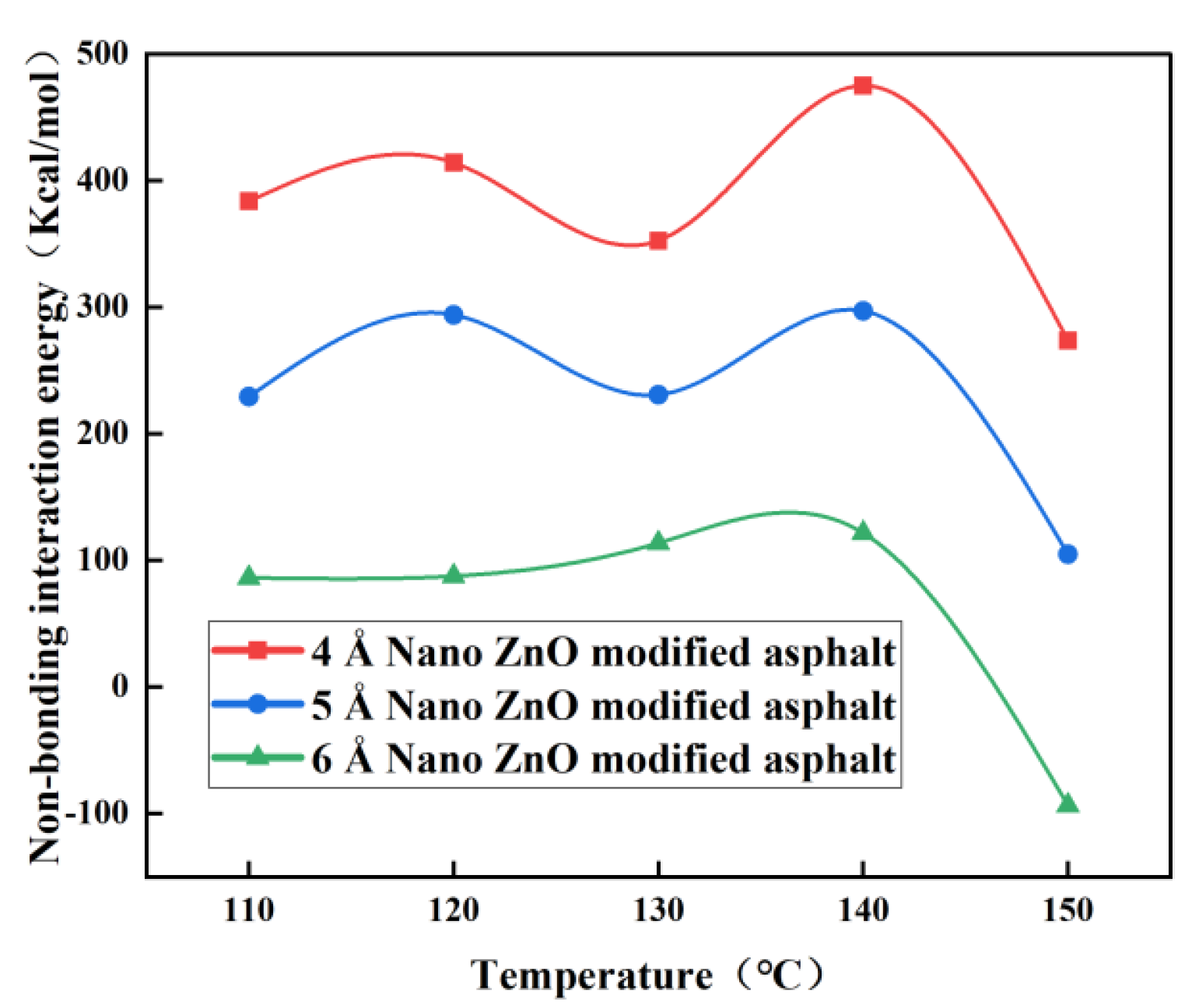
3.2. Effect of Nano-ZnO on the Interaction Energy of Modified Asphalt
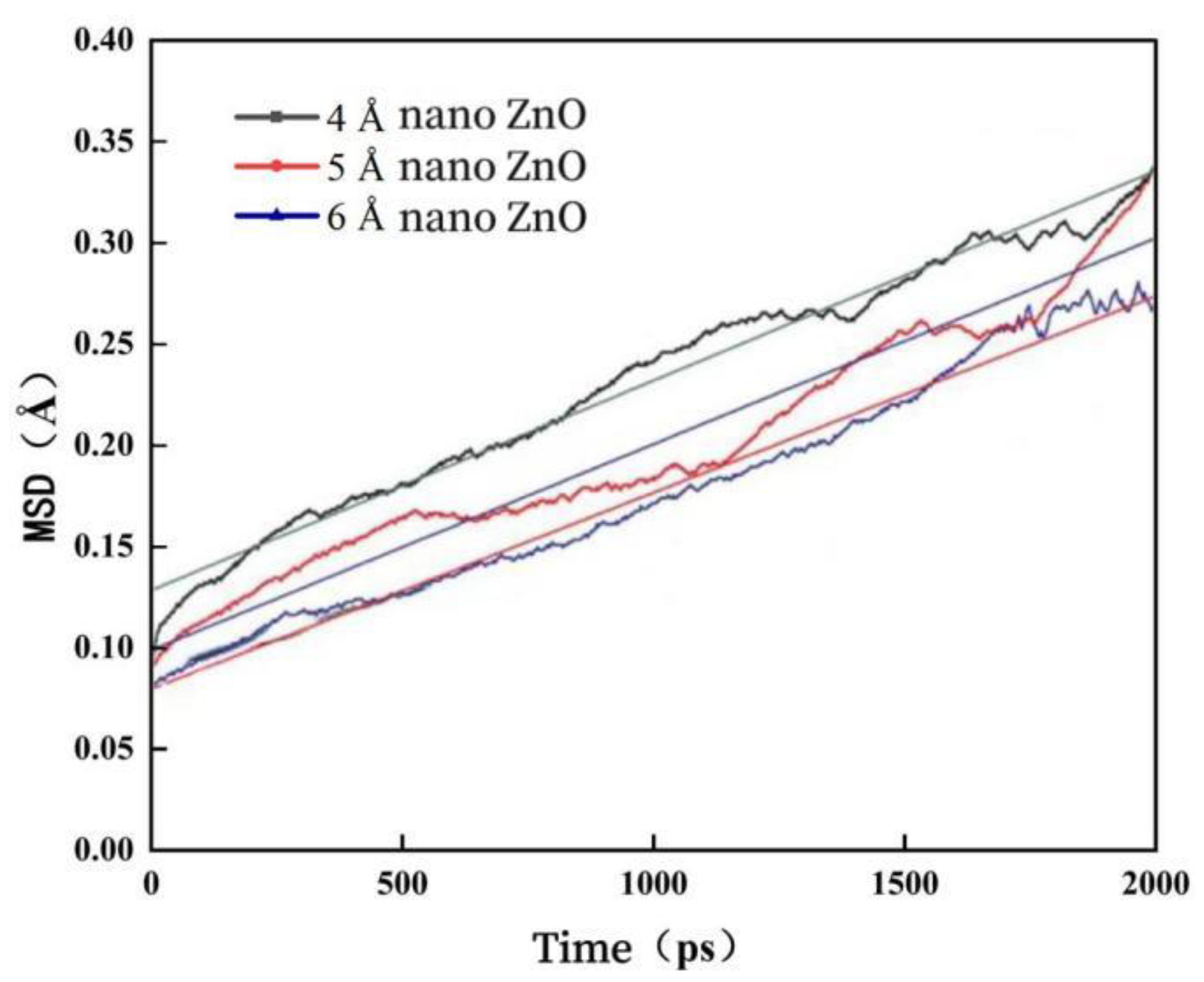
3.3. Effect of Nano-ZnO on the Mean Square Displacement of Modified Asphalt
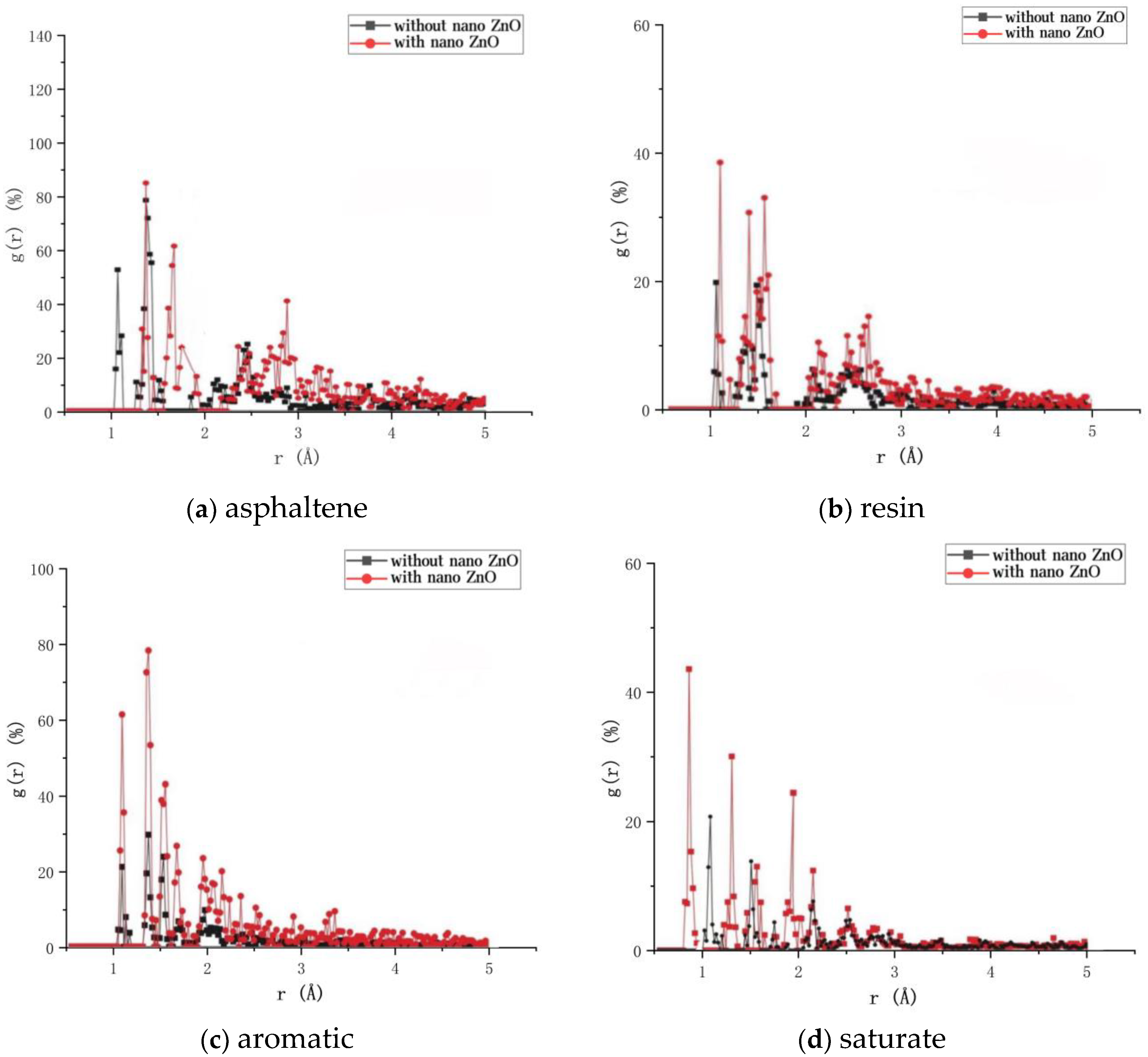
3.4. Effect of Nano-ZnO on the Radial Distribution Function of Four Components of Asphalt
3.5. Effect of Nano-ZnO on the Radius of Gyration of Asphalt Four Components
4. Conclusions
- The difference in solubility parameters indicates the best compatibility between nano-ZnO and matrix asphalt at 150 °C, especially at a nano-ZnO particle size of 6 Å.
- The particle size of nano-ZnO has little effect on the temperature at which the nano-ZnO-modified asphalt is most structurally stable, with the most stable and compatible system at around 150 °C.
- When the diameter of nano-ZnO becomes larger, its diffusion ability in asphalt is weakened. From the perspective of improving the diffusion ability of nano-ZnO in asphalt, smaller diameter nano-ZnO should be selected.
- The addition of nano-ZnO enhances the intensity of the first peak of the radial distribution function of the four components of the asphalt, the orderliness of the molecules, the ductility of the branched chains of the aromatic and saturated fractions of the asphalt, the addition of nano-ZnO improves the denseness of the molecular structure of the asphalt and makes the asphalt structure more stable.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, M.; Hu, T.; Rose, J.G. Geometric composition, structural behavior and material design for asphalt trackbed: A review. Constr. Build. Mater. 2020, 262, 120755. [Google Scholar] [CrossRef]
- Ding, X.; Ma, T.; Gu, L.; Zhang, Y. Investigation of surface micro-crack growth behavior of asphalt mortar based on the designed innovative mesoscopic test. Mater. Des. 2020, 185, 108238. [Google Scholar] [CrossRef]
- Maadani, O.; Shafiee, M.; Egorov, I. Climate change challenges for flexible pavement in Canada: An overview. J. Cold Reg. Eng. 2021, 35, 03121002. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, J.; Guo, D.; Sun, G.; Zhou, Y.; Zhang, W. Preparation and repeated repairability evaluation of sunflower oil-type microencapsulated filling materials. J. Nanosci. Nanotechnol. 2020, 20, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Liu, G.; Pei, J. Effects of material characteristics on asphalt and filler interaction ability. Int. J. Pavement Eng. 2019, 20, 928–937. [Google Scholar] [CrossRef]
- De Sousa Neto, V.F.; Lucena LC, F.L.; de Barros, A.G.; Lucena, A.E.D.F.L.; Filho, P.G.T.M. Rheological evaluation of asphalt binder modified with zinc oxide nanoparticles. Case Stud. Constr. Mater. 2022, 17, e01224. [Google Scholar]
- Duan, H.; Long, J.; Zhang, H.; Luo, H.; Cao, J. Effects of different carriers for zinc oxide (ZnO) particles on microstructure of ZnO/layered silicate composite and aging resistance of composite modified asphalt. Constr. Build. Mater. 2022, 349, 128773. [Google Scholar] [CrossRef]
- Qing, Z.; Qi-Cheng, L.; Peng, L.; Chuan-Sheng, C.; Jiang-Rong, K. Study on modification mechanism of nano-ZnO/polymerised styrene butadiene composite-modified asphalt using density functional theory. Road Mater. Pavement Des. 2018, 21, 1426–1438. [Google Scholar] [CrossRef]
- Shu, J.; Wang, X.; Yang, B.; Wang, X. Research on a New Loading Method for Nano TiO2 Photocatalytic Asphalt Pavement. Sustainability 2022, 14, 11977. [Google Scholar] [CrossRef]
- Enieb, M.; Cengizhan, A.; Karahancer, S.; Eltwati, A. Evaluation of Physical-Rheological Properties of Nano Titanium Dioxide Modified Asphalt Binder and Rutting Resistance of Modified Mixture. Int. J. Pavement Res. Technol. 2022, 16, 285–303. [Google Scholar] [CrossRef]
- Li, Z.; Guo, T.; Chen, Y.; Liu, Q.; Chen, Y. The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt. Nanotechnol. Rev. 2021, 10, 1253–1265. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Kuang, D.; Wu, S. Temperature regulation and rheological properties assessment of asphalt binders modified with paraffin/SiO2 micro-encapsulated phase change materials. Constr. Build. Mater. 2023, 368, 130377. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, S.; Liu, Q.; Yang, C.; Zou, Y.; Wan, P. Feasibility assessment of CeO2 nanoparticles as aging-resistant agent of asphalt. Constr. Build. Mater. 2022, 330, 127245. [Google Scholar] [CrossRef]
- Zhu, Q. Preparation and properties of nano-ZnO modified asphalt. Appl. Chem. 2019, 48, 1031–1034. [Google Scholar]
- Zhang, M. Study on Nano-Zinc Oxide Modified Asphalt and Its Anti-Aging Properties. Master’s Thesis, Chang’an University, Xi’an, China, 2015. [Google Scholar]
- Chen, Y.; Chen, A.; Li, C.; Li, Z. Analysis of performance for nano-ZnO. modified asphalt mixture. China J. Highw. Transp. 2017, 30, 25–32. [Google Scholar]
- Liu, H.Y.; Zhang, H.L.; Hao, P.W.; Zhu, C.Z. The effect of surface modifiers on ultraviolet aging properties of nano-zinc oxide modified bitumen. Pet. Sci. Technol. 2015, 33, 72–78. [Google Scholar] [CrossRef]
- Wang, P.; Zhai, F.; Dong, Z.J.; Wang, L.; Liao, J.; Li, G. Micromorphology of asphalt modified by polymer and carbon nanotubes through molecular dynamics simulation and experiments: Role of strengthened interfacial interactions. Energy Fuels 2018, 32, 1179–1187. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Yang, L. Study on the compatibility of mastic powder and asphalt in mastic powder modified asphalt based on molecular dynamics. J. Constr. Mater. 2018, 21, 689–694. [Google Scholar]
- Wang, L.; Zhang, L.; Yang, L. Molecular dynamics study on the compatibility of asphalt and mastic powder before and after aging. J. Constr. Mater. 2019, 22, 474–479. [Google Scholar]
- Su, M.M.; Si, C.D.; Zhang, Z.P.; Zhang, H. Molecular dynamics study on influence of Nano-ZnO/SBS on physical properties and molecular structure of asphalt binder. Fuel 2020, 263, 116777. [Google Scholar] [CrossRef]
- Su, M.M.; Si, C.D.; Zhang, H.L.; Zhang, H. Molecular dynamics simulation study of nano ZnO modified asphalt. J. Chongqing Jiaotong Univ. (Nat. Sci. Ed.) 2021, 40, 118–127. [Google Scholar]
- Guo, F.; Zhang, J.; Pei, J.; Zhou, B.; Falchetto, A.C.; Hu, Z. Investigating the interaction behavior between asphalt binder and rubber in rubber asphalt by molecular dynamics simulation. Constr. Build. Mater. 2020, 252, 118956. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, J.; Pei, J.; Ma, W.; Hu, Z.; Guan, Y. Evaluation of the compatibility between rubber and asphalt based on molecular dynamics simulation. Front. Struct. Civ. Eng. 2020, 14, 435–445. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Si, J.; Mei, J.; Ding, G.; Li, J. Research on compatibility mechanism of biobased cold-mixed epoxy asphalt binder. Constr. Build. Mater. 2020, 250, 118868. [Google Scholar] [CrossRef]
- Wei, J.; Dong, F.; Li, Y.; Zhang, Y. Relationship analysis between surface free energy and chemical composition of asphalt binder. Constr. Build. Mater. 2014, 71, 116–123. [Google Scholar] [CrossRef]
- Dahham, N.A.; Fares, A.H.; Najem, K.A. Modeling and simulation of mechanical and physical properties of Barium orthotitanate (Ba2TiO4) composite by Materials Studio (MS). Tikrit J. Pure Sci. 2018, 22, 61–65. [Google Scholar] [CrossRef]
- Ji, J.; Yao, H.; Yang, X.; Xu, Y.; Suo, Z.; You, Z. Performance analysis of direct coal liquefaction residue (DCLR) and Trinidad lake asphalt (TLA) for the purpose of modifying traditional asphalt. Arab. J. Sci. Eng. 2016, 41, 3983–3993. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Li, X.; Zhang, Y.; Yuan, S. A Molecular Dynamics Study on the Suitable Compatibility Conditions of CO2-cosolvent-light hydrocarbon system by Calculating the Solubility Parameters. Energy Fuels 2020, 34, 3483–3492. [Google Scholar] [CrossRef]
- Dudek, G.; Borys, P. A simple methodology to estimate the diffusion coefficient in pervaporation-based purification experiments. Polymers 2019, 11, 343. [Google Scholar] [CrossRef]
- Tong, Z.F.; Xie, Y.B.; Zhang, Y.H. Molecular dynamics simulation on the interaction between polymer inhibitors and β-dicalcium silicate surface. J. Mol. Liq. 2018, 259, 65–75. [Google Scholar] [CrossRef]

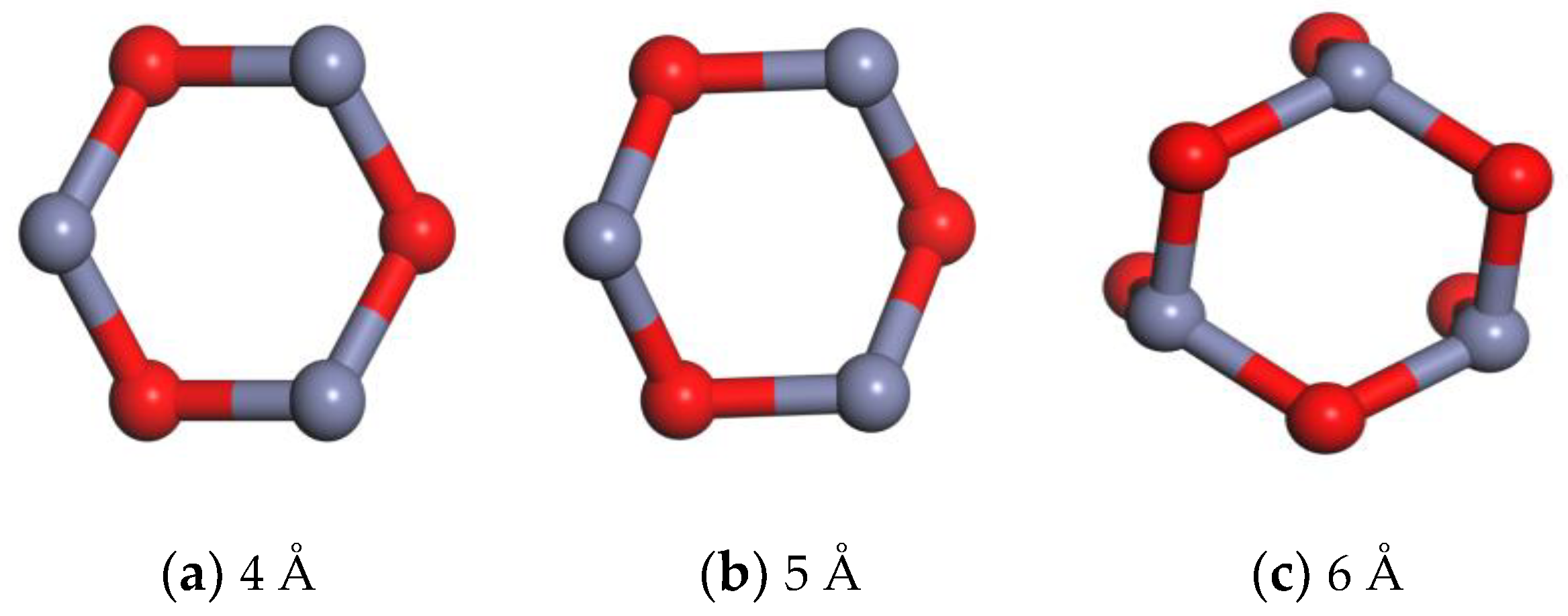




| Nano-ZnO Cluster Diameter/Å | Number of Nano-ZnO Clusters/pc | Nano-ZnO Content/% |
|---|---|---|
| 4 Å | 6 | 4.2981 |
| 5 Å | 6 | 4.2861 |
| 6 Å | 5 | 4.2933 |
| Temperature (°C) | 110 | 120 | 130 | 140 | 150 |
|---|---|---|---|---|---|
| 4 Å ZnO nanoparticle-modified asphalt | 383.800 | 414.372 | 352.614 | 475.145 | 273.801 |
| 5 Å ZnO nanoparticle-modified asphalt | 229.424 | 293.941 | 230.889 | 297.209 | 104.934 |
| 4 Å ZnO nanoparticle-modified asphalt | 86.292 | 87.644 | 113.705 | 121.750 | −93.479 |
| Temperature (°C) | 110 | 120 | 130 | 140 | 150 |
|---|---|---|---|---|---|
| 4 Å ZnO nanoparticle-modified asphalt | −392.209 | −360.007 | −420.083 | −300.408 | −500.259 |
| 5 Å ZnO nanoparticle-modified asphalt | −497.402 | −430.073 | −490.781 | −425.506 | −615.478 |
| 4 Å ZnO nanoparticle-modified asphalt | −600.606 | −596.358 | −567.335 | −560.932 | −781.241 |
| Temperature (°C) | 110 | 120 | 130 | 140 | 150 |
|---|---|---|---|---|---|
| 4 Å ZnO nanoparticle-modified asphalt | 776.009 | 774.379 | 772.697 | 775.553 | 774.060 |
| 5 Å ZnO nanoparticle-modified asphalt | 726.826 | 724.014 | 721.670 | 722.715 | 720.412 |
| 4 Å ZnO nanoparticle-modified asphalt | 686.898 | 684.002 | 681.040 | 682.682 | 687.726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Yu, P.; Zhai, M. Analysis of Nano-ZnO-Modified Asphalt Compatibility Based on Molecular Dynamics. Materials 2023, 16, 4710. https://doi.org/10.3390/ma16134710
Xie Y, Yu P, Zhai M. Analysis of Nano-ZnO-Modified Asphalt Compatibility Based on Molecular Dynamics. Materials. 2023; 16(13):4710. https://doi.org/10.3390/ma16134710
Chicago/Turabian StyleXie, Yunlan, Pandeng Yu, and Ming Zhai. 2023. "Analysis of Nano-ZnO-Modified Asphalt Compatibility Based on Molecular Dynamics" Materials 16, no. 13: 4710. https://doi.org/10.3390/ma16134710
APA StyleXie, Y., Yu, P., & Zhai, M. (2023). Analysis of Nano-ZnO-Modified Asphalt Compatibility Based on Molecular Dynamics. Materials, 16(13), 4710. https://doi.org/10.3390/ma16134710





