Solvent Extraction as a Method of Recovery and Separation of Platinum Group Metals
Abstract
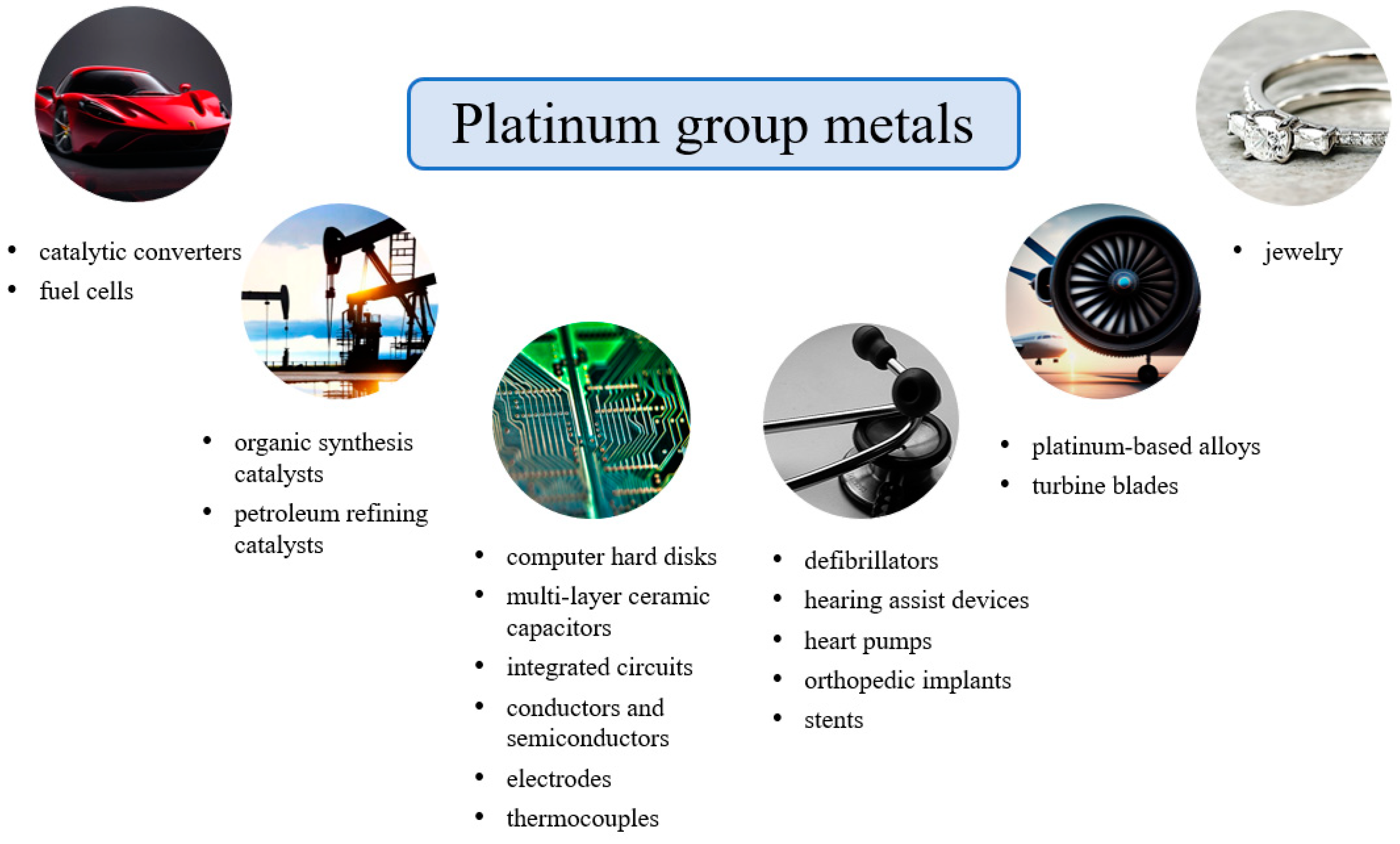
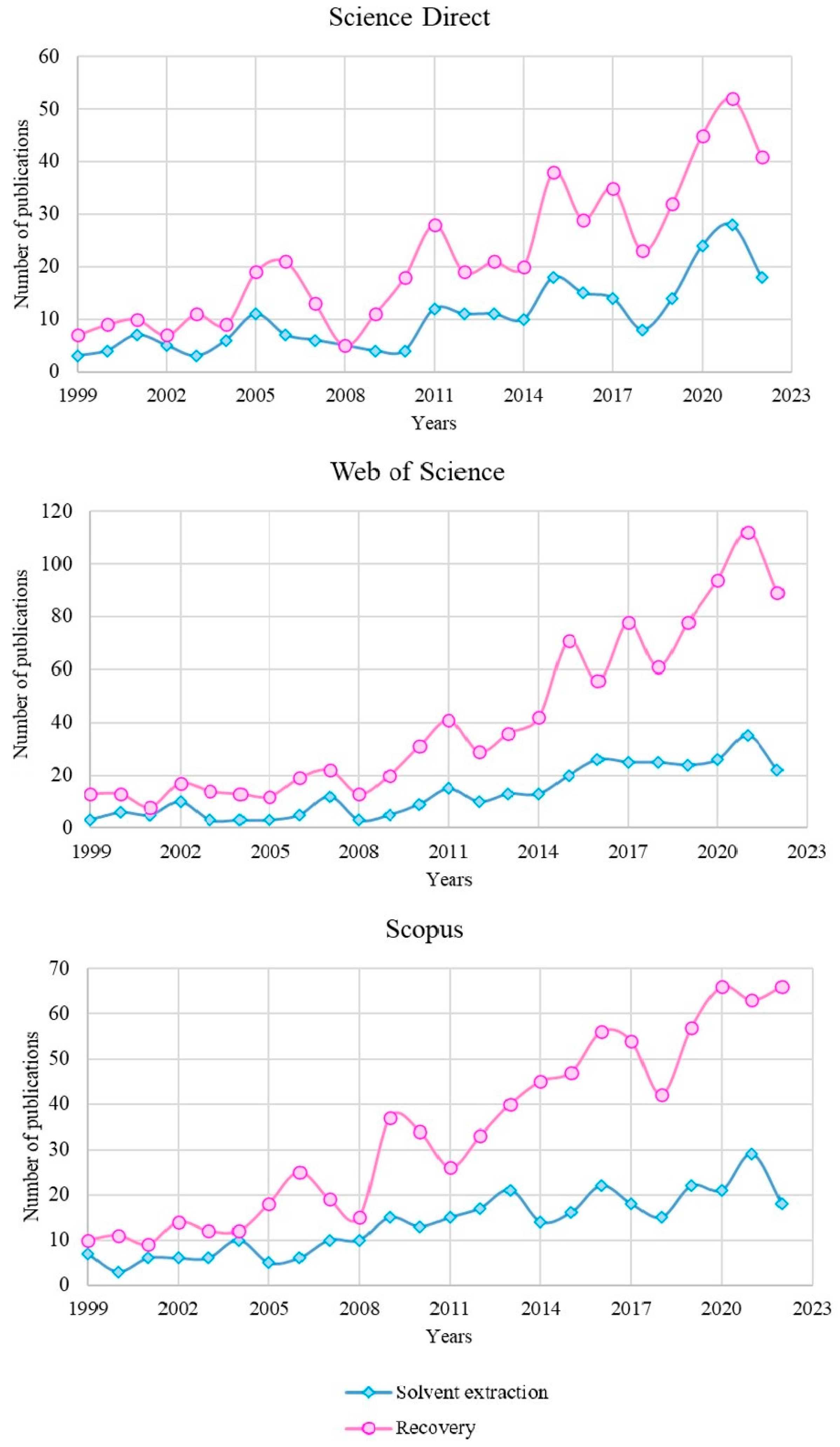
1. Introduction
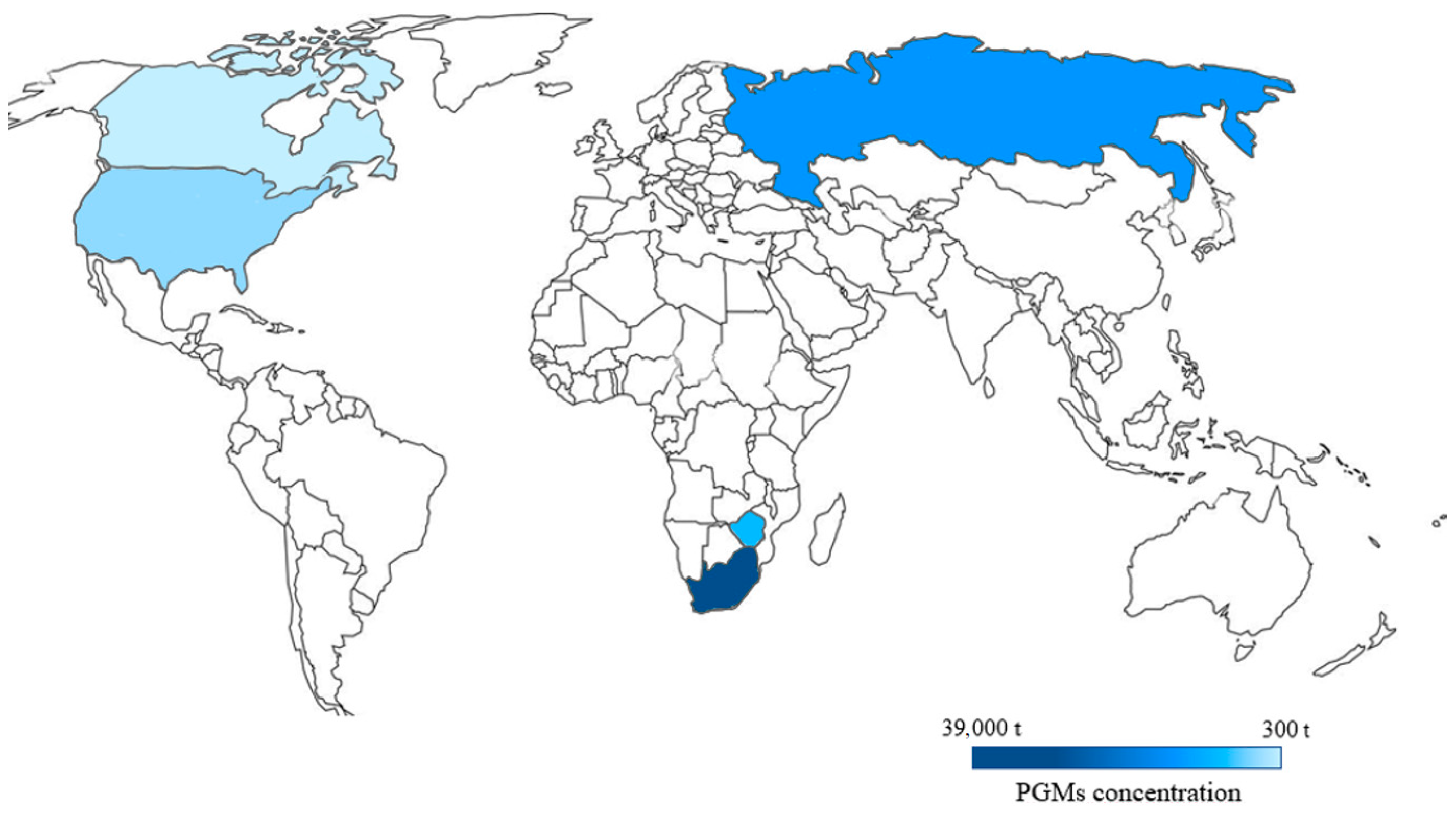
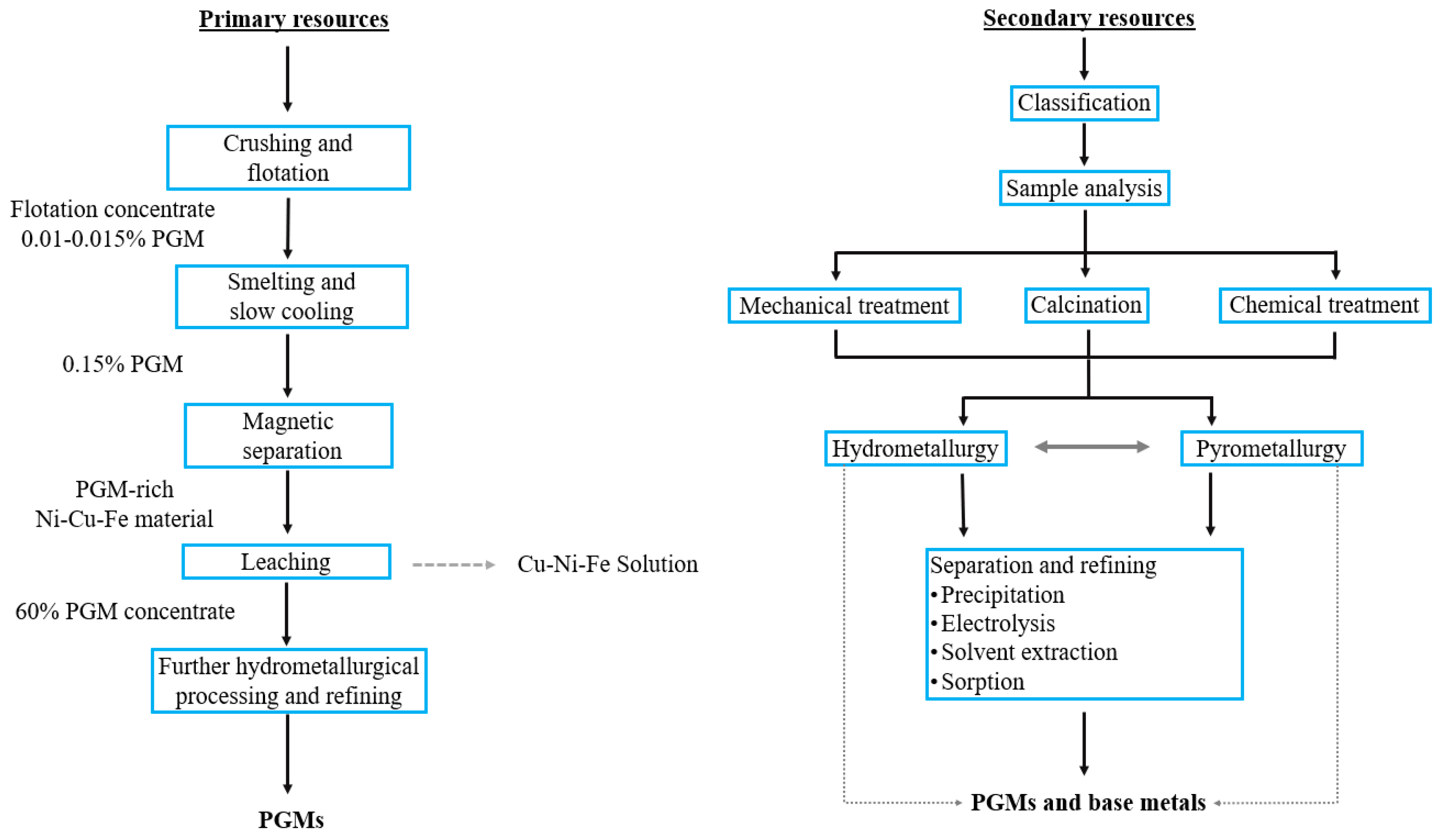
2. The Main Sources of PGMs
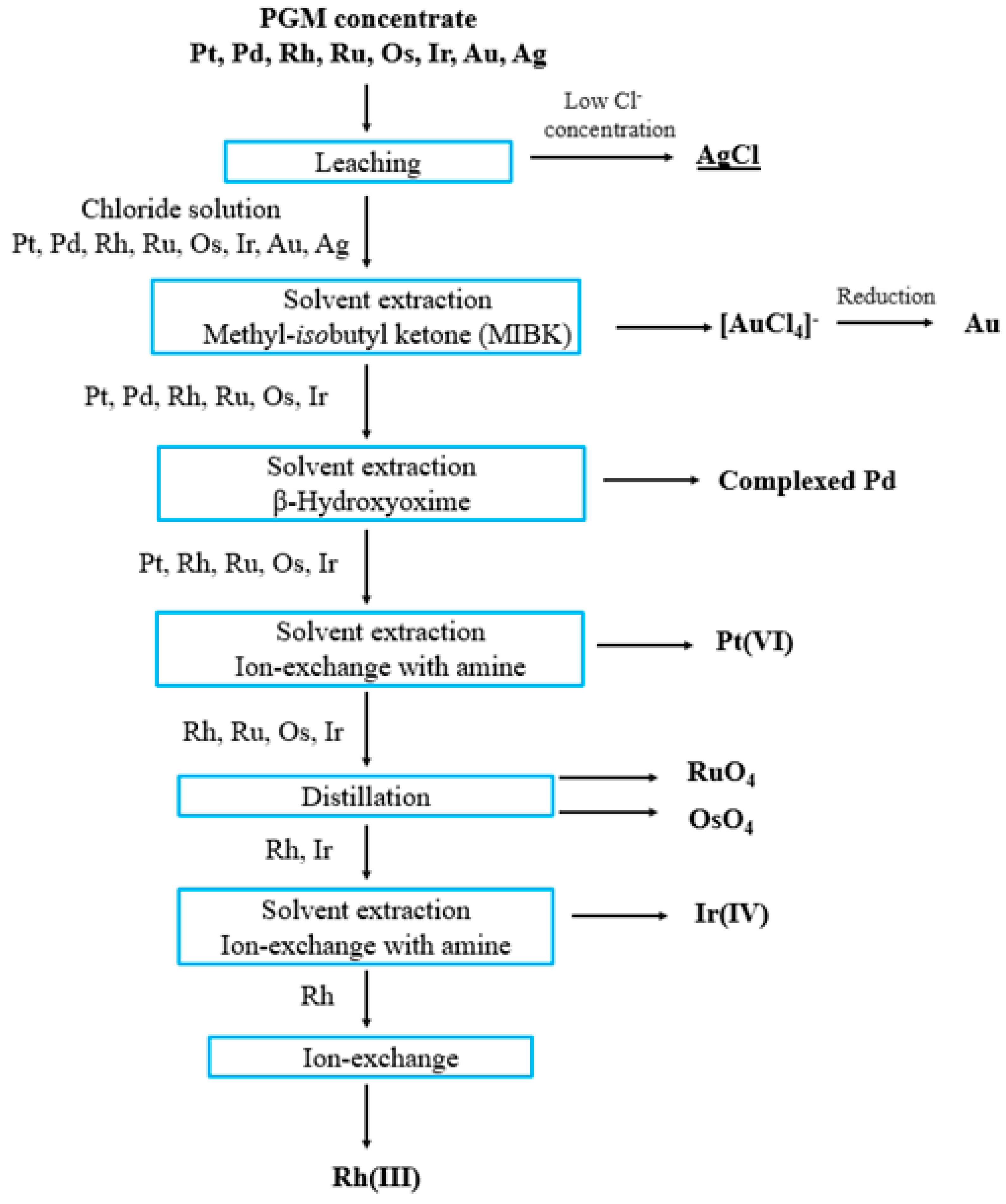
3. Industrial Methods for the Platinum Group Metals’ Separation
4. Solvent Extraction
4.1. Platinum Group Metals in Chloride Solutions
4.2. Solvent Extraction Mechanisms
- -
- solvation—where water molecules surrounding a metal are replaced by molecules of the extractant;
- -
- anion exchange—involving the formation of electrically neutral ion pairs between the anions of chlorocomplex MClxn− and the positively charged, basic molecules of the organic compound;
- -
- compound formation—a Pd-specific mechanism [27].
4.3. Popular Agents for the Extraction of Platinum Group Metals
- -
- organophosphorus extractants;
- -
- amine-based extractants;
- -
- sulfur-based extractants;
- -
- oxime extractants.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bielański, A. Podstawy Chemii Nieorganicznej Tom III; PWN: Warsaw, Poland, 1994; pp. 930–939. [Google Scholar]
- Nose, K.; Okabe, T.H. Treatise on Process Metallurgy Volume 3: Industrial Processes; Seetharaman, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1071–1097. [Google Scholar]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A Review of Methods of Separation of the Platinum-Group Metals through Their Chloro-Complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Woodward, B.K. Platinum Group Metals (PGMs) for Permanent Implantable Electronic Devices. In Precious Metals for Biomedical Applications; Baltzer, N., Copponnex, T., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 130–147. [Google Scholar]
- Panda, R.; Jha, M.K.; Pathak, D.D. Commercial Processes for the Extraction of Platinum Group Metals (PGMs). In Proceedings of the Minerals, Metals and Materials Series; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; Volume Part F5, pp. 119–130. [Google Scholar]
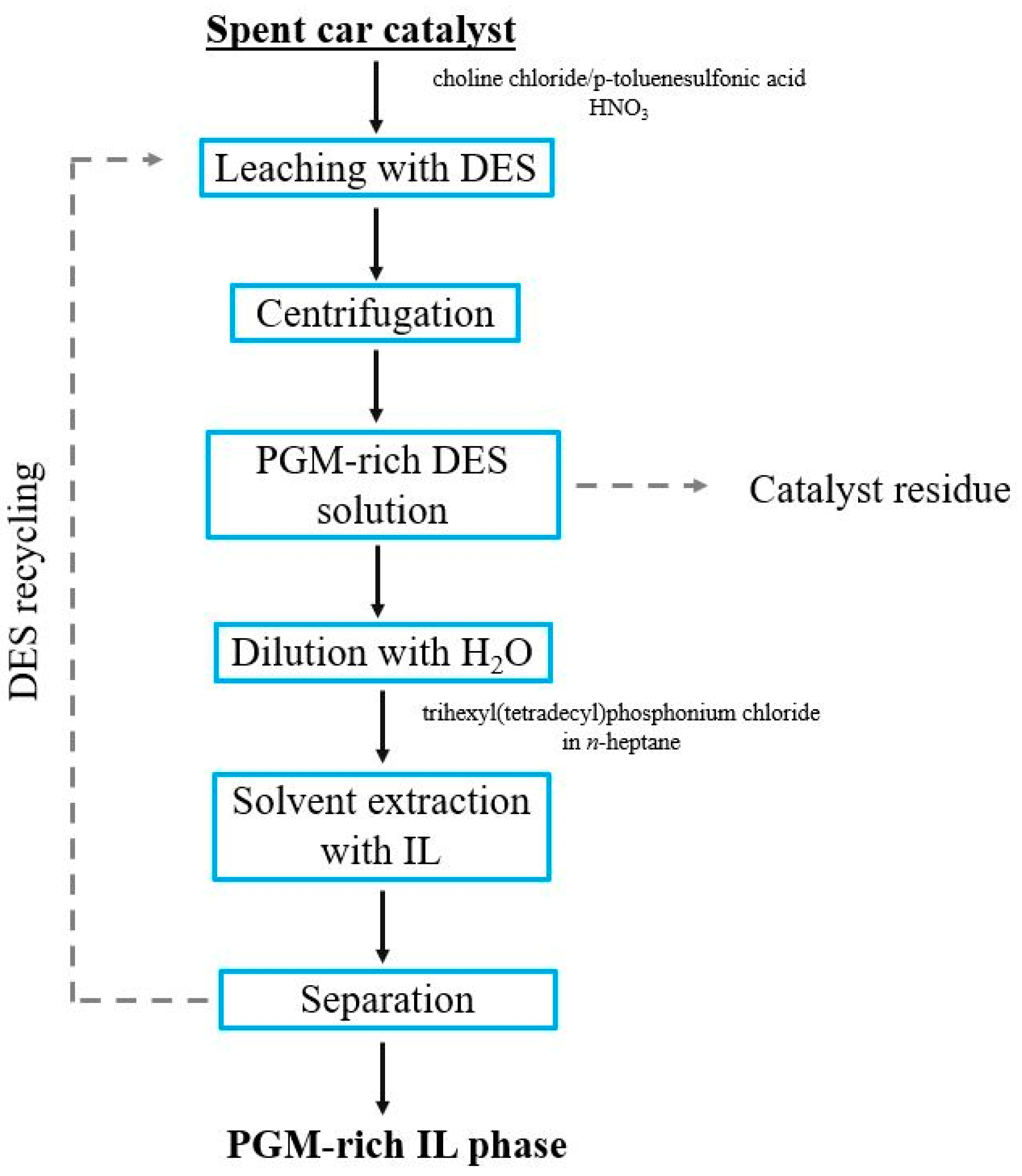
- Nguyen, V.T.; Riaño, S.; Aktan, E.; Deferm, C.; Fransaer, J.; Binnemans, K. Solvometallurgical Recovery of Platinum Group Metals from Spent Automotive Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 337–350. [Google Scholar] [CrossRef]
- Sole, K.C. Solvent Extraction in the Hydrometallurgical Processing and Purification of Metals: Process Design and Selected Applications. In Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials; CRC Press: Boca Raton, FL, USA, 2008; pp. 141–200. [Google Scholar]
- Sinisalo, P.; Lundström, M. Refining Approaches in the Platinum Group Metal Processing Value Chain—A Review. Metals 2018, 8, 203. [Google Scholar] [CrossRef]
- Available online: https://Matthey.Com/Products-and-Markets/Pgms-a (accessed on 10 April 2023).
- Cornish, L.A.; Süss, R.; Douglas, A.; Chown, L.H.; Glaner, L. The Platinum Development Initiative: Platinum-Based Alloys for High Temperature and Special Applications: Part I. Platin. Met. Rev. 2009, 53, 2–10. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of Platinum Group Metals from Spent Automotive Catalysts: A Review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Saguru, C.; Ndlovu, S.; Moropeng, D. A Review of Recent Studies into Hydrometallurgical Methods for Recovering PGMs from Used Catalytic Converters. Hydrometallurgy 2018, 182, 44–56. [Google Scholar] [CrossRef]
- Gervilla, F.; Kojonen, K. The Platinum-Group Minerals in the Upper Section of the Keivitsansarvi Ni-Cu-PGE Deposit, Northern Finland. Can. Mineral. 2002, 40, 377–394. [Google Scholar] [CrossRef]
- Available online: https://Matthey.Com/Documents/161599/509428/PGM-Market-Report-May-2022.Pdf/542bcada-F4ac-A673-5f95-Ad1bbfca5106?T=1655877358676 (accessed on 10 June 2023).
- Available online: https://www.Statista.Com/Statistics/273624/Platinum-Metal-Reserves-by-Country/ (accessed on 10 December 2022).
- Smith, W.; Maier, W.; Barnes, S.; Moorhead, G.; Reid, D.; Karykowski, B. Element Mapping the Merensky Reef of the Bushveld Complex. Geosci. Front. 2021, 12, 101101. [Google Scholar] [CrossRef]
- Mohamed, N.; Rifai, K.; Selmani, S.; Constantin, M.; Doucet, F.R.; Özcan, L.; Sabsabi, M.; Vidal, F. Chemical and Mineralogical Mapping of Platinum-Group Element Ore Samples Using Laser-Induced Breakdown Spectroscopy and Micro-X-Ray Fluorescence. Geostand. Geoanal. Res. 2021, 45, 539–550. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; Zhang, S. Separation and Purification of Platinum Group Metals from Aqueous Solution: Recent Developments and Industrial Applications. Resour. Conserv. Recycl. 2021, 167, 105417. [Google Scholar] [CrossRef]
- Sanuki, S.; Matsumoto, Y.; Majima, H. Preparation of Ammonium Chloroplatinate by a Precipitation Stripping of Pt(IV)-Loaded Alamine 336 or TBP. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1999, 30, 197–203. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Cieszyńska, A.; Wiśniewski, M. Extraction of Palladium (II) Ions from Chloride Solutions with Phosphonium Ionic Liquid CyphosIL101. Polish J. Chem. Technol. 2007, 9, 99–101. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of Pt(IV), Pd(II), Rh(III) and Ir(IV) from Concentrated Hydrochloric Acid Solutions by Solvent Extraction. Hydrometallurgy 2016, 164, 71–77. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of Platinum(IV) and Palladium(II) from Concentrated Hydrochloric Acid Solutions by Mixtures of Amines with Neutral Extractants. J. Ind. Eng. Chem. 2015, 32, 238–245. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Kubota, F.; Goto, M. Solvent Extraction of Pt(IV), Pd(II), and Rh(III) with the Ionic Liquid Trioctyl(Dodecyl) Phosphonium Chloride. J. Chem. Technol. Biotechnol. 2018, 93, 1714–1721. [Google Scholar] [CrossRef]
- Rydberg, J.; Cox, M.; Musikas, C.; Choppin, G.R. Solvent Extraction: Principles and Practice, Second Edition, Revised and Expanded Edited; Marcel Dekker: New York, NY, USA, 2004; Volume 1, pp. 457–507. [Google Scholar]
- Goc, K.; Kluczka, J.; Benke, G.; Malarz, J.; Pianowska, K.; Leszczyńska-Sejda, K. Application of Ion Exchange for Recovery of Noble Metals. Minerals 2021, 11, 1188. [Google Scholar] [CrossRef]
- Paiva, A.P. Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points. Metals 2017, 7, 505. [Google Scholar] [CrossRef]
- Lee, J.C.; Kurniawan, K.; Kim, S.; Nguyen, V.T.; Pandey, B.D. Ionic Liquids-Assisted Solvent Extraction of Precious Metals from Chloride Solutions. Sep. Purif. Rev. 2023, 52, 242–261. [Google Scholar] [CrossRef]
- Jha, M.K.; Gupta, D.; Lee, J.C.; Kumar, V.; Jeong, J. Solvent Extraction of Platinum Using Amine Based Extractants in Different Solutions: A Review. Hydrometallurgy 2014, 142, 60–69. [Google Scholar] [CrossRef]
- Mhaske, A.A.; Dhadke, P.M. Extraction Separation Studies of Rh, Pt and Pd Using Cyanex 921 in Toluene—A Possible Application to Recovery from Spent Catalysts. Hydrometallurgy 2001, 61, 143–150. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Liu, H.; Yu, F.; Wang, H. Extractant Structures and Their Performance for Palladium Extraction and Separation from Chloride Media: A Review. Miner. Eng. 2021, 163, 106798. [Google Scholar] [CrossRef]
- Rane, M.V. PGM Ore Processing: LIX Reagents for Palladium Extraction & Platinum Stripping from Alamine 336 Using NaOH-NaCl. Miner. Eng. 2019, 138, 119–124. [Google Scholar] [CrossRef]
- Marinho, R.S.; Afonso, J.C.; da Cunha, J.W.S.D. Recovery of Platinum from Spent Catalysts by Liquid-Liquid Extraction in Chloride Medium. J. Hazard. Mater. 2010, 179, 488–494. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Separation of Pt(IV), Pd(II), Ru(III) and Rh(III) from Model Chloride Solutions by Liquid-Liquid Extraction with Phosphonium Ionic Liquids. Sep. Purif. Technol. 2019, 212. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Paukszta, D.; Regel-Rosocka, M. Hydrometallurgical Recovery of Platinum Group Metals from Spent Automotive Converters. Physicochem. Probl. Miner. Process. 2021, 57, 83–94. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.C.; Chagnes, A.; Kim, M.S.; Jeong, J.; Cote, G. Highly Selective Separation of Individual Platinum Group Metals (Pd, Pt, Rh) from Acidic Chloride Media Using Phosphonium-Based Ionic Liquid in Aromatic Diluent. RSC Adv. 2016, 6, 62717–627728. [Google Scholar] [CrossRef]
- Svecova, L.; Papaiconomou, N.; Billard, I. Quantitative Extraction of Rh(III) Using Ionic Liquids and Its Simple Separation from Pd(II). Dalt. Trans. 2016, 45, 15162–15169. [Google Scholar] [CrossRef]
- Liu, R.; Geng, Y.; Tian, Z.; Wang, N.; Wang, M.; Zhang, G.; Yang, Y. Extraction of Platinum(IV) by Hydrophobic Deep Eutectic Solvents Based on Trioctylphosphine Oxide. Hydrometallurgy 2021, 199, 105521. [Google Scholar] [CrossRef]
- Devi, M.; Moral, R.; Thakuria, S.; Mitra, A.; Paul, S. Hydrophobic Deep Eutectic Solvents as Greener Substitutes for Conventional Extraction Media: Examples and Techniques. ACS Omega 2022, 8, 9702–9728. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Maksimova, V.; Shishov, A.; Shkinev, V. Extraction of Platinum Group Metals and Gold from Industrial Environments into Deep Eutectic Solvents. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Tang, N.; Liu, L.; Yin, C.; Zhu, G.; Huang, Q.; Dong, J.; Yang, X.; Wang, S. Environmentally Benign Hydrophobic Deep Eutectic Solvents for Palladium(II) Extraction from Hydrochloric Acid Solution. J. Taiwan Inst. Chem. Eng. 2021, 121, 92–100. [Google Scholar] [CrossRef]
- Lanaridi, O.; Platzer, S.; Nischkauer, W.; Limbeck, A.; Schnürch, M.; Bica-Schröder, K. A Combined Deep Eutectic Solvent–Ionic Liquid Process for the Extraction and Separation of Platinum Group Metals (Pt, Pd, Rh). Molecules 2021, 26, 7204. [Google Scholar] [CrossRef]
- Jaree, A.; Khunphakdee, N. Separation of Concentrated Platinum(IV) and Rhodium(III) in Acidic Chloride Solution via Liquid-Liquid Extraction Using Tri-Octylamine. J. Ind. Eng. Chem. 2011, 17, 243–247. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Kim, S.K.; Lee, J.C. Separation of Platinum and Palladium from Chloride Solution by Solvent Extraction Using Alamine 300. Hydrometallurgy 2010, 104, 1–7. [Google Scholar] [CrossRef]
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Reddy, B.R. Solvent Extraction Separation and Recovery of Palladium and Platinum from Chloride Leach Liquors of Spent Automobile Catalyst. Sep. Purif. Technol. 2010, 73, 213–218. [Google Scholar] [CrossRef]
- Paiva, A.P.; Piedras, F.V.; Rodrigues, P.G.; Nogueira, C.A. Hydrometallurgical Recovery of Platinum-Group Metals from Spent Auto-Catalysts—Focus on Leaching and Solvent Extraction. Sep. Purif. Technol. 2022, 286, 120474. [Google Scholar] [CrossRef]
- Pietrelli, L.; Fontana, D. Automotive Spent Catalysts Treatment and Platinum Recovery. Int. J. Environ. Waste Manag. 2013, 2, 2022–2232. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S.; Senanayake, G. Separation of Pt(IV), Rh(III) and Fe(III) in Acid Chloride Leach Solutions of Glass Scraps by Solvent Extraction with Various Extractants. Hydrometallurgy 2018, 175, 232–239. [Google Scholar] [CrossRef]
- Raju, B.; Kumar, J.R.; Lee, J.Y.; Kwonc, H.S.; Kantam, M.L.; Reddy, B.R. Separation of Platinum and Rhodium from Chloride Solutions Containing Aluminum, Magnesium and Iron Using Solvent Extraction and Precipitation Methods. J. Hazard. Mater. 2012, 227–228, 142–147. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M.; Wisniewski, M. Removal of Palladium(II) from Aqueous Chloride Solutions with Cyphos Phosphonium Ionic Liquids as Metal Ion Carriers for Liquid-Liquid Extraction and Transport across Polymer Inclusion Membranes. Physicochem. Probl. Miner. Process. 2015, 51, 150221. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wiśniewski, M. Extractive Recovery of Palladium(II) from Hydrochloric Acid Solutions with Cyphos®IL 104. Hydrometallurgy 2012, 113–114, 79–85. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Kubota, F.; Yoshida, W.; Goto, M. Application of a Novel Phosphonium-Based Ionic Liquid to the Separation of Platinum Group Metals from Automobile Catalyst Leach Liquor. Ind. Eng. Chem. Res. 2019, 58, 3845–3852. [Google Scholar] [CrossRef]
| Ru3+ | [RuCl6]3− | Rh3+ | [RhCl6]3− | Pd2+ | [PdCl4]2− |
| [RuCl5(H2O)]2− | [RhCl5(H2O)]2− | ||||
| [RuCl4(H2O)2]− | Pd4+ | [PdCl6]2− | |||
| [RuCl3(H2O)3] | Ir3+ | [IrCl6]3− | |||
| [IrCl5(H2O)]2− | Pt2+ | [PtCl4]2− | |||
| Ru4+ | [RuCl6]2− | ||||
| [Ru2OCl10]4− | Ir4+ | [IrCl4(H2O)2] | Pt4+ | [PtCl6]2− | |
| [Ru2OCl8(H2O)2]2− | [IrCl6]2− | ||||
| Os4+ | [OsCl6]2− |
| Solvation | MClyn− + nH+ + b[HA] → [HnMCly (HA)b] |
| Ion exchange | MClyn− + n[R3NHCl] → [(R3NH)n(MClyn−)] +nCl−MClyn− + nH+ + n[C][A] → [CnMCly] + nHA |
| Compound formation | PdCl42− +2[L] → [PdCl2L2] + 2Cl− |
| Extractant | Starting Solution | Extraction Efficiency | Stripping Solution | Stripping Efficiency | References |
|---|---|---|---|---|---|
| Trioctylamine C24H51N Trade names: Alamine 336; Alamine 300; Alamine 308 | Synthetic chloride solution containing Pt and Rh | 97% Pt 21% Rh | 8 M HNO3 (50 °C) | ~100% Pt <1%Rh | [42] |
| Synthetic chloride solution containing Pt and Pd | >99% Pt >99% Pd | (1) 0.1 M NaSCN (2) 0.1 M TU in 0.5 M HCl | (1) 99.9% Pt (2) 99.5% Pd | [43] | |
| Pt and Rh salts obtained by leaching of waste materials and dissolved in ethylene glycol | ~100% Pt | - | - | [6] | |
| Tributyl phosphate C12H27O4P | Synthetic chloride solution containing Pt, Pd, Mn, Fe, Cr | 99.9% Pd | 0.5 M TU in 0.1 M HCl | 99.8% | [44] |
| Triisobutylphosphine sulfide C12H27PS Trade name: Cyanex 471X | Chloride solution obtained after spent catalyst leaching | ~100% Pd | 0.1 M TU in 5% HCl | 46% | [45] |
| Trioctyl phosphine oxide C24H51OP Trade name: Cyanex 921 | Solution obtained after leaching of mixed spent catalyst samples in aqua regia | >95% Pt | (1) 5.2 mM citric acid (2) 5.5 mM phenanthroline (3) 1.6 mM (NaPO3)6 | (1) 98.1% (2) 63.7% (3) 72.6% | [46] |
| Mixture of trialkyl phosphine oxides with n-octyl and n-hexyl chains C42H90O2P2 Trade name: Cyanex 923 | Chloride solution obtained after leaching of glass industry scraps containing a small amount of Fe(III) | 85% Pt | (1) 0.1–0.5 M NaSCN (2) 0.01 M HCl | (1) ~100% Pt (2) ~100% Pt | [47] |
| 5,8-diethyl-7-hydroxyldodecane-6-oxime C16H33NO2 Trade name: LIX63 | Chloride solution containing Pd, Pt, Ir and Rh | 99.9% Pd | (1) 0.5 M TU (2) 0.5 M NaSCN | (1) 99.9% (2) 90.1% | [21] |
| 2-hydroxy-5-nonylacetophenoneoxime C17H27NO2 Trade name: LIX 84I | Synthetic chloride solution containing Pd | >95% Pd | - | - | [31] |
| Mixture of 5-nonylsalicylaldoxime and 2-hydroxy-5-nonylacetophenone oxime C16H25NO2; C17H27NO2 Trade name: LIX 984 | Synthetic chloride solution containing Pd | >97% Pd | - | - | [31] |
| Mixture of tricaprylylmethylammonium chloride and trioctylmethylammonium chloride C25H54ClN Trade name: Aliquat 336 | Synthetic chloride solution containing Pt, Rh, Al, Mg, Fe | 99.97% Pt | 0.1–0.5 M TU in 0.1–0.5 M HCl | 97–100% | [48] |
| Synthetic chloride solution containing Pt, Pd, Mn, Fe, Cr | 99.83% Pt | 0.5 M TU in 0.5 M HCl | 99.9% | [44] | |
| Solution obtained after spent catalyst leaching in aqua regia | >99% Pt | Na2S2O3 | >99.9% | [32] | |
| Pt and Rh salts obtained by leaching of waste materials and dissolved in ethylene glycol | ~100% Pt | 1.0 M TU | ~100% | [6] | |
| Trihexyl(tetradecyl)phosphonium chloride C32H68ClP Trade name: Cyphos IL 101 | Synthetic chloride solution containing Pt, Pd, Ru and Rh | >95% Pt/Pd ~60% Ru <15% Rh | (1) 0.1 M TU in 0.5 M HCl (2) 1.0 M HNO3 | (1) >90% Pd (2) >65% Pt | [33] |
| Chloride solution obtained after spent catalyst leaching | ~100% | 1.0 M HNO3(used to wash out other base metals, mainly Fe, Pb, Zn and Mg) | Pt 18.6% | [34] | |
| Synthetic chloride solution containing Pt, Pd, Rh | 99.9% Pt 98.1% Pd <2% Rh | (1) 0.02 M TU in 5% HCl (2) 0.1 M NaSCN (3) 0.01 M NH4OH | (1) ~100% Pd 10.5% Pt (2) 92.8% Pt <2% Pd (3) 94.6% Pt 81.9% Pd | [35] | |
| Synthetic chloride solution containing Rh and Pd | 99.9–89.7% Pd | - | - | [36] | |
| Pt and Rh salts obtained by leaching of waste materials and dissolved in ethylene glycol | ~100% Pt | 1.0 M TU | ~100% | [6] | |
| Trihexyl(tetradecyl)phosphonium bromide C32H68BrP Trade name: Cyphos IL 102 | Synthetic chloride solution containing Pd | >80~100% Pd | 0.5 M NH4OH | 84–90% | [49] |
| Trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate C48H102O2P2 Trade name: Cyphos IL 104 | Synthetic chloride solution containing Pd | 52–96% Pd | 0.5 M NH4OH | ~90% | [50] |
| Trioctyl(dodecyl) phosphonium chloride (P88812Cl) C36H74ClP | Synthetic chloride solution containing Pt, Pd and Rh | Pt, Pd: 99.9% Rh: 10.0–90.0% | (1) 5.0 M HNO3 (2) 1.0 M TU (3) 5.0 M HCl | (1) 74.9% Pt (2) 91.2% Pd (3) 73.7% Rh | [23] |
| Synthetic chloride solution containing Pd and Rh and contaminants including Al., Mg, Ce, Ba | ~100% Pd 80% Rh | (1) 1.0 M TU (2) 5. 0 M HCl | (1) >90% Pd (2) >75% Rh | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pianowska, K.; Kluczka, J.; Benke, G.; Goc, K.; Malarz, J.; Ochmański, M.; Leszczyńska-Sejda, K. Solvent Extraction as a Method of Recovery and Separation of Platinum Group Metals. Materials 2023, 16, 4681. https://doi.org/10.3390/ma16134681
Pianowska K, Kluczka J, Benke G, Goc K, Malarz J, Ochmański M, Leszczyńska-Sejda K. Solvent Extraction as a Method of Recovery and Separation of Platinum Group Metals. Materials. 2023; 16(13):4681. https://doi.org/10.3390/ma16134681
Chicago/Turabian StylePianowska, Karolina, Joanna Kluczka, Grzegorz Benke, Karolina Goc, Joanna Malarz, Michał Ochmański, and Katarzyna Leszczyńska-Sejda. 2023. "Solvent Extraction as a Method of Recovery and Separation of Platinum Group Metals" Materials 16, no. 13: 4681. https://doi.org/10.3390/ma16134681
APA StylePianowska, K., Kluczka, J., Benke, G., Goc, K., Malarz, J., Ochmański, M., & Leszczyńska-Sejda, K. (2023). Solvent Extraction as a Method of Recovery and Separation of Platinum Group Metals. Materials, 16(13), 4681. https://doi.org/10.3390/ma16134681