Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
- -
- The coil’s applied current frequency was 13.56 MHz.
- -
- The RF power used was 18 W, and the plasma chamber’s working gas pressure (Air) was 33 Pa.
2.2. Surface Characterization
3. Results and Discussion
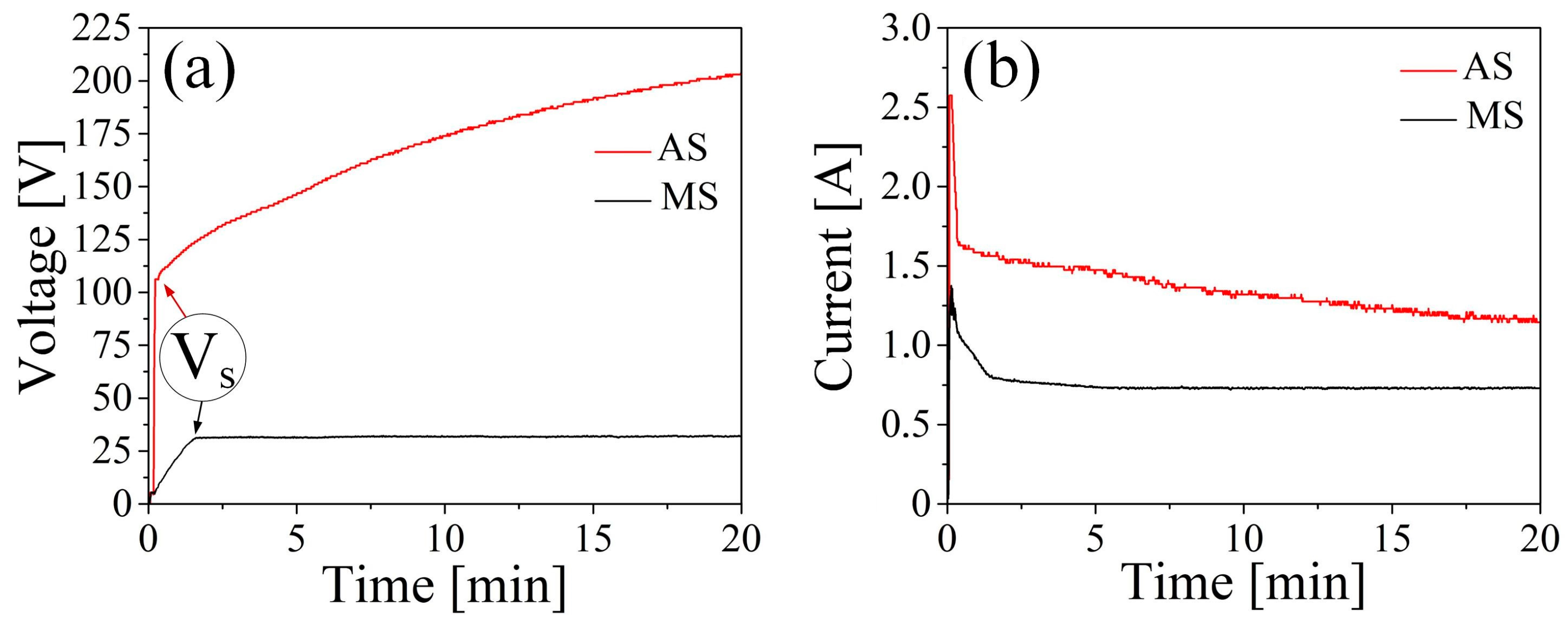
3.1. Plasma Electrolytic Oxidation Processing
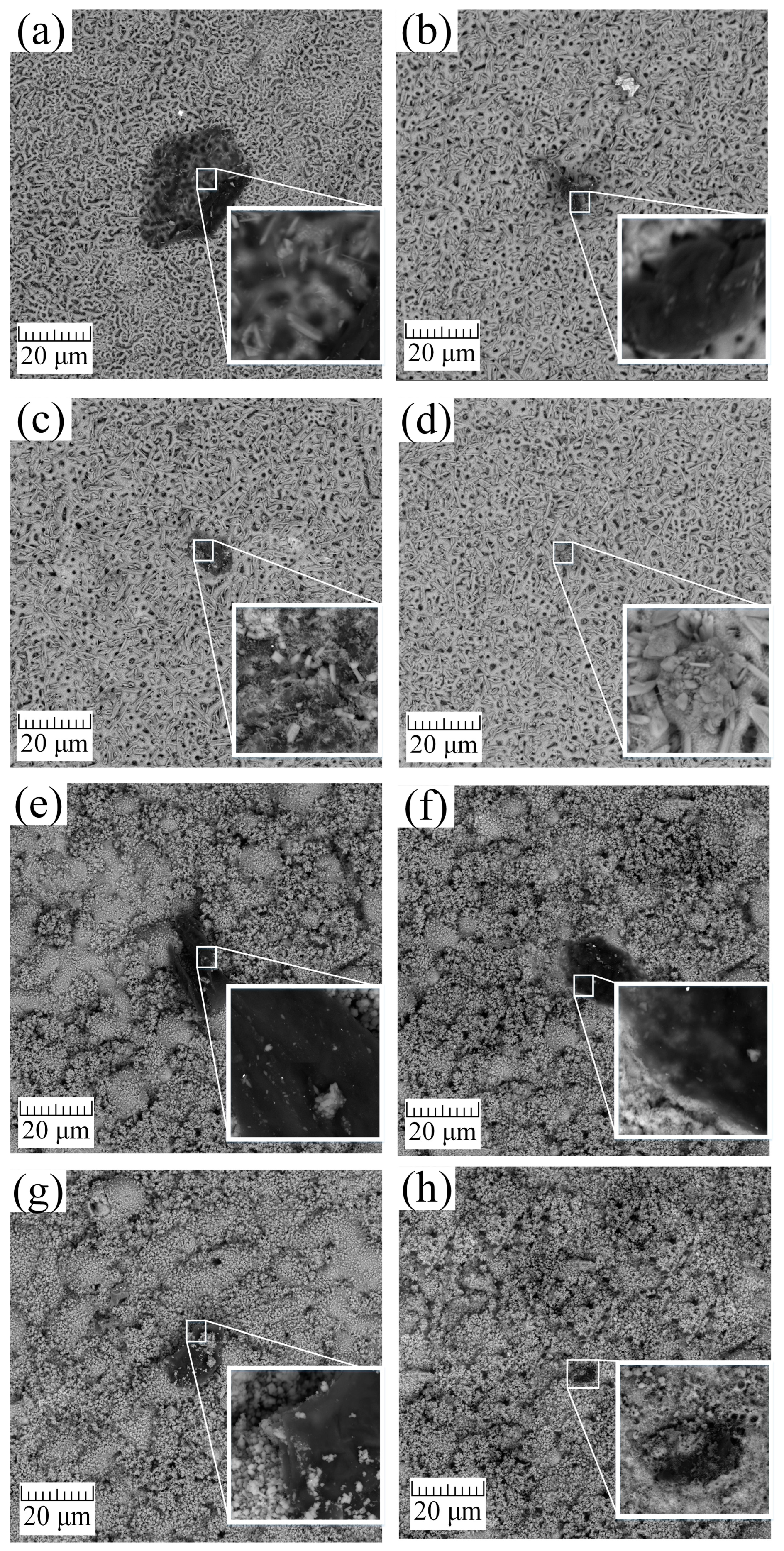
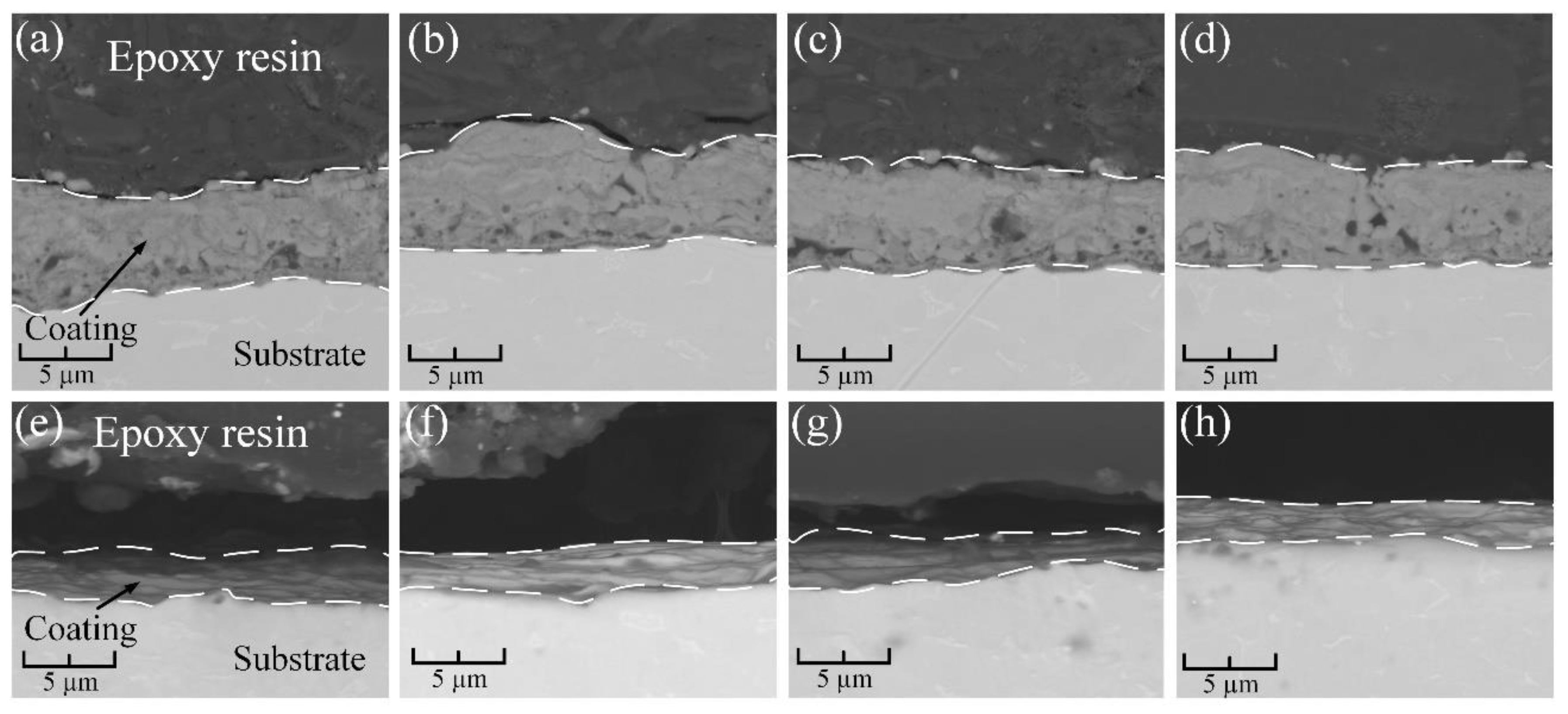
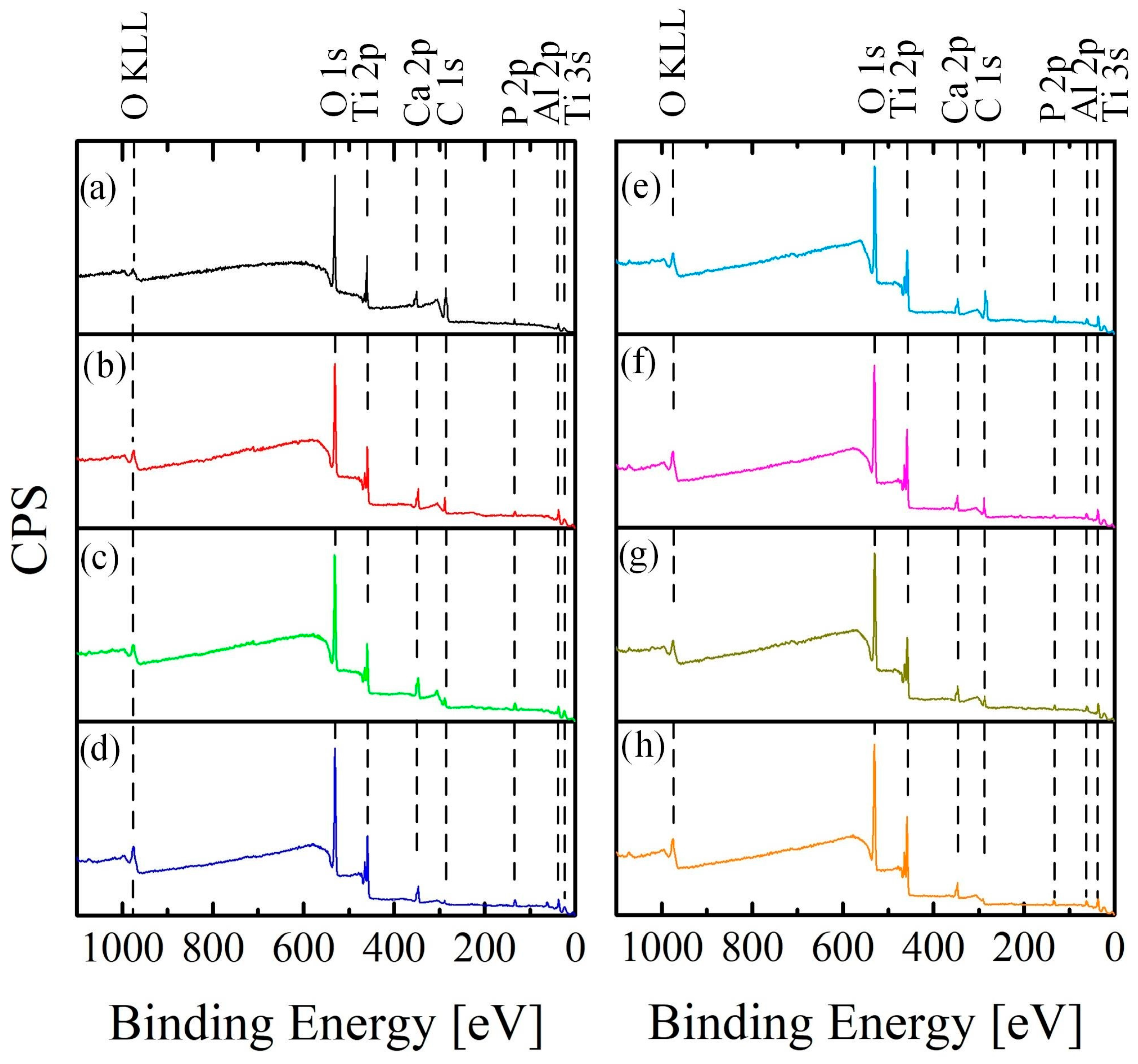
3.2. Microstructural Characterization and Chemical Analysis
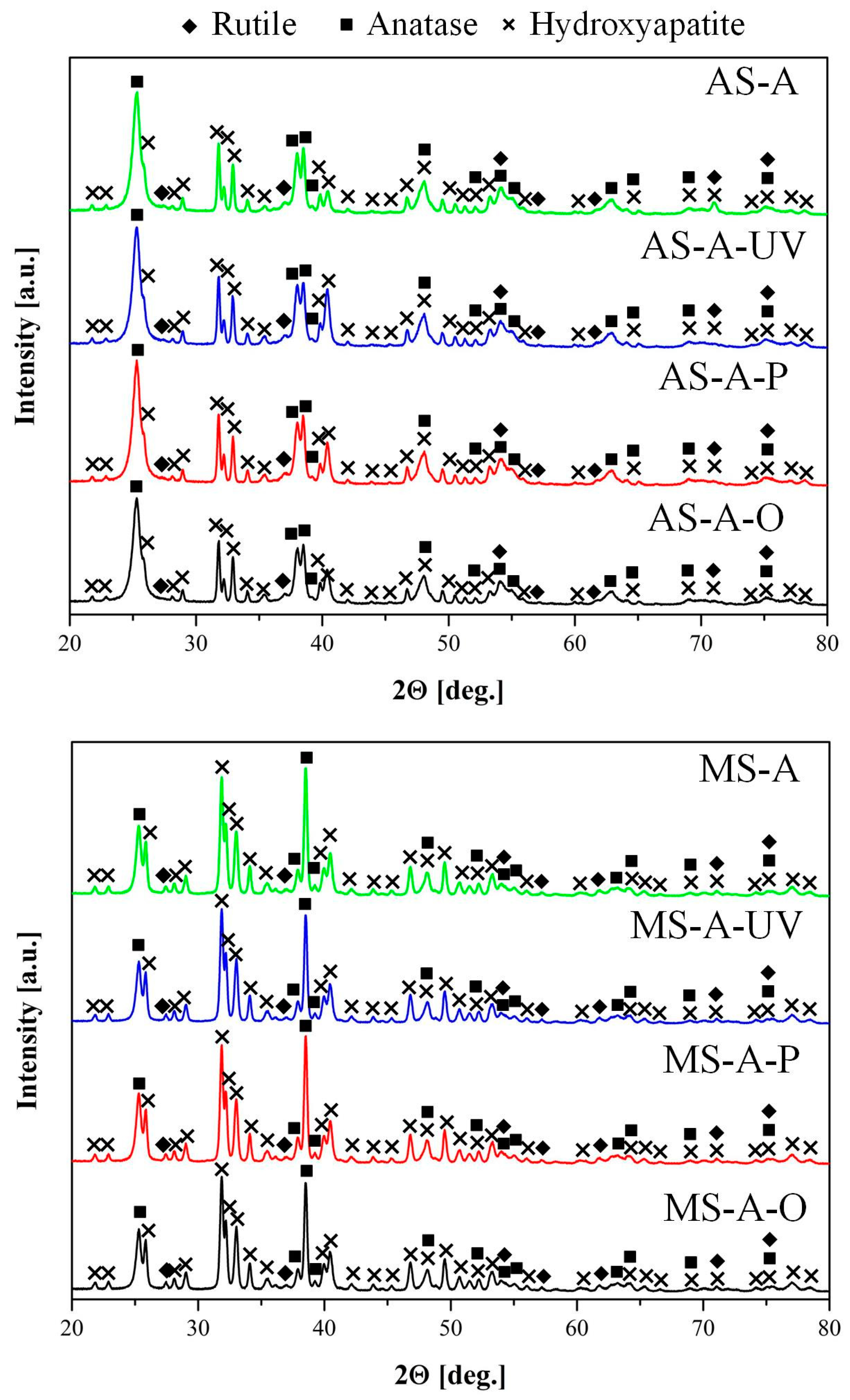
3.3. Phase Characterization
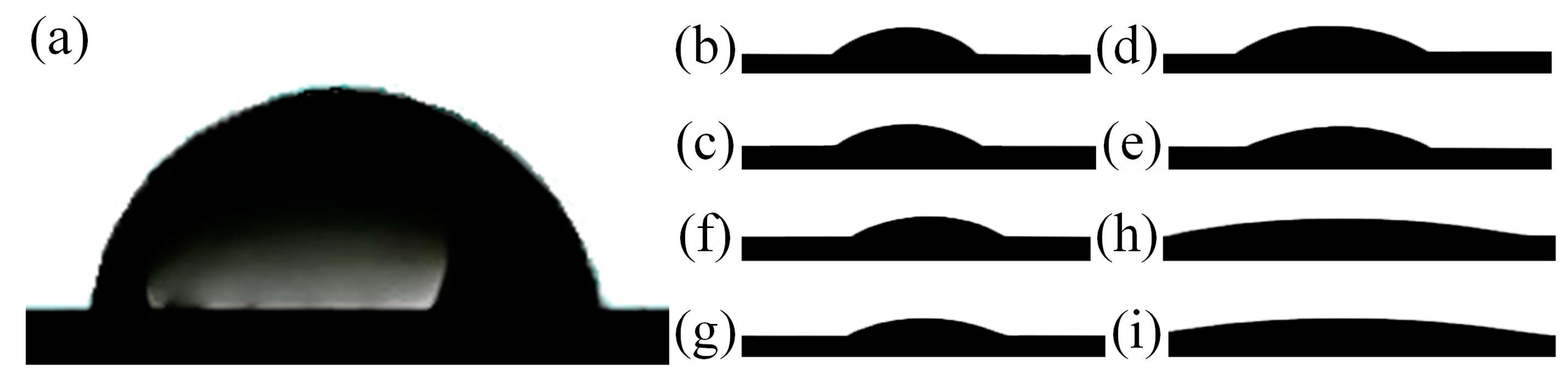
3.4. Wettability
4. Conclusions
- The coating obtained using molten salt exhibited a more uniform distribution of hydroxyapatite crystals across the surface than similar coatings produced using an aqueous electrolyte. Additionally, the concentration of hydroxyapatite crystals, as determined by EDS, and the phase composition, as determined by XRD, were found to be 1.5 and 2.2 times higher, respectively, in the coating obtained in molten salt compared to the aqueous electrolyte coating.
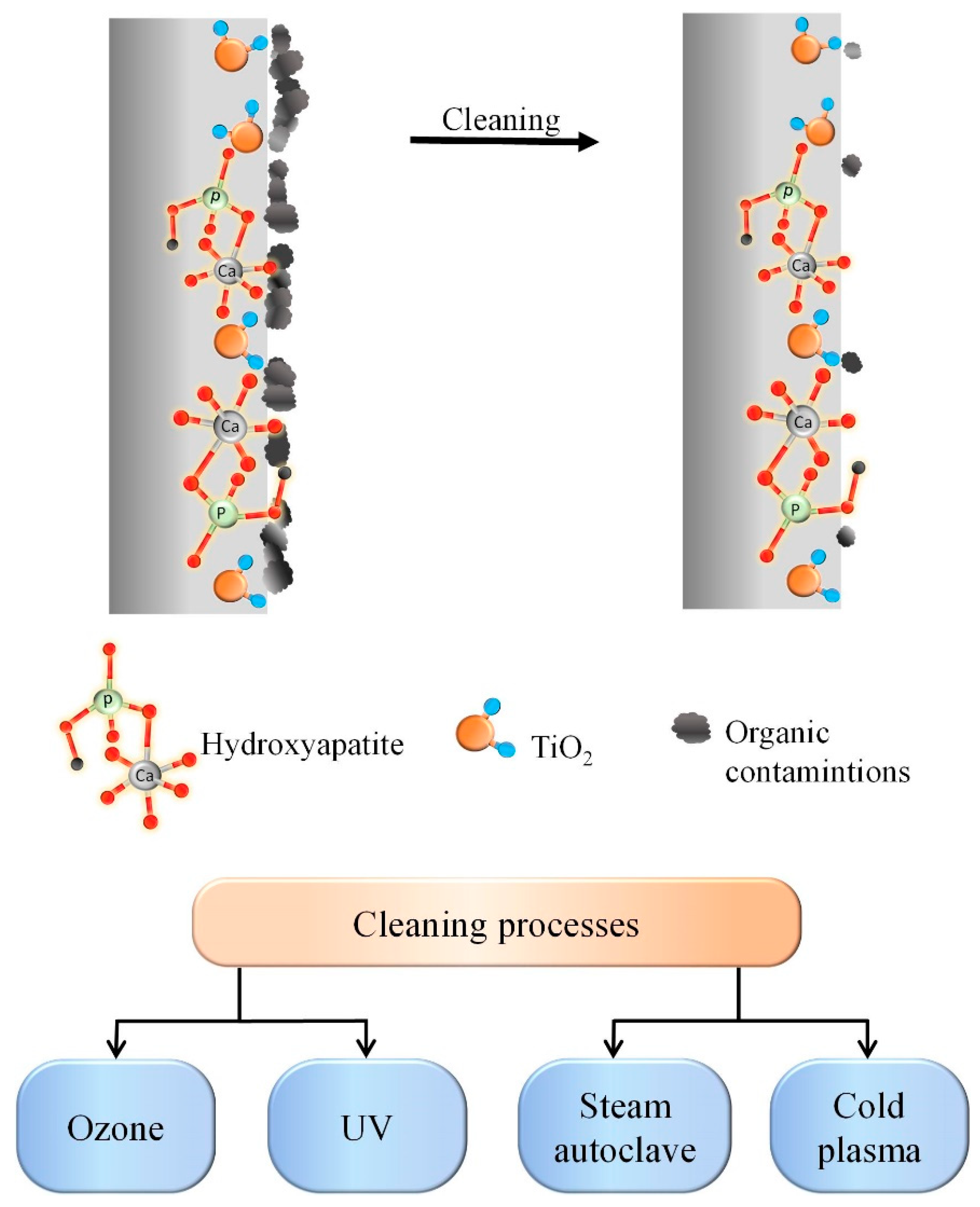
- During the cleaning process of samples from organic contaminants using autoclaving (A), UV, cold plasma (P), and ozone (O), a comprehensive analysis was performed using scanning electron microscopy (SEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS). Scanning electron microscopy (SEM), X-ray diffraction (XRF), energy dispersive X-ray spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS) were used for complex analysis of the samples cleaned from organic contaminants using autoclaving (HTO), UV, cold plasma (P), and ozone (O). The results revealed that A, UV, and P treatments exhibited a moderate cleaning capability but also led to the degradation of the bioactive coating. On the other hand, ozone treatment proved to be highly effective in removing organic contaminants without adversely affecting the structural and morphological properties of the coating.
- The sessile drop method was used to study the hydrophilic properties of the coatings after their cleaning by the A, UV, P, and O methods. The coating obtained in molten salt and treated for organic contaminants by the ozone cleaning method was found to have the highest hydrophilicity (CA = 7.5 ± 0.7°).
- It has been established that the coatings formed on Ti-6Al-4V alloys by the PEO method in molten salts have an increased uniformity and content of HAP and can be promising for medical implantology, and ozone treatment is an effective and sparing procedure for removing organic contaminants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Nutt, M.J.; Jablokov, V.R.; Freese, H.L.; Richelsoph, M.E. The Application of Ti-15Mo Beta Titanium Alloy in High-Strength Structural Orthopaedic Applications. In Titanium, Niobium, Zirconium, and Tantalum for Medical and Surgical Applications; Abstract book; ASTM Symposium: West Conshohocken, PA, USA, 2004; pp. 9–10. [Google Scholar]
- Devaraj, A.; Joshi, V.V.; Srivastava, A.; Manandhar, S.; Moxson, V.; Duz, V.A.; Lavender, C. A low-cost hierarchical nanostructured beta-titanium alloy with high strength. Nat. Commun. 2016, 7, 11176. [Google Scholar] [CrossRef] [PubMed]
- Maehara, K.; Doi, K.; Matsushita, T.; Sasaki, Y. Application of Vanadium-Free Titanium Alloys to Artificial Hip Joints. Mater. Trans. 2002, 43, 2936–2942. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D. Chemical and biological functionalization of titanium for dental implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Guillemot, F. Recent advances in the design of titanium alloys for orthopedic applications. Expert Rev. Med. Devices 2005, 2, 741–748. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Kitagawa, I.L.; Miyazaki, C.M.; Pitol-Palin, L.; Okamoto, R.; de Vasconcellos, L.M.R.; Constantino, C.J.L.; Lisboa-Filho, P.N. Titanium-Based Alloy Surface Modification with TiO2 and Poly(sodium 4-styrenesulfonate) Multilayers for Dental Implants. ACS Appl. Bio Mater. 2021, 4, 3055–3066. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B. Calcium Phosphate Nanocomposites for Biomedical and Dental Applications: Recent Developments. In Handbook of Composites from Renewable Materials; Wiley: New Jersey, NJ, USA, 2017; pp. 423–450. [Google Scholar] [CrossRef]
- Harel, N.; Moses, O.; Palti, A.; Ormianer, Z. Long-Term Results of Implants Immediately Placed into Extraction Sockets Grafted with β-Tricalcium Phosphate: A Retrospective Study. J. Oral Maxillofac. Surg. 2013, 71, e63–e68. [Google Scholar] [CrossRef]
- Gan, L.; Pilliar, R. Calcium phosphate sol–gel-derived thin films on porous-surfaced implants for enhanced osteoconductivity. Part I: Synthesis and characterization. Biomaterials 2004, 25, 5303–5312. [Google Scholar] [CrossRef]
- Tisdel, C.L.; Goldberg, V.M.; Parr, J.A.; Bensusan, J.S.; Staikoff, L.S.; Stevenson, S. The influence of a hydroxyapatite and tricalcium-phosphate coating on bone growth into titanium fiber-metal implants. J. Bone Jt. Surg. 1994, 76, 159–171. [Google Scholar] [CrossRef]
- Knabe, C.; Berger, G.; Gildenhaar, R.; Klar, F.; Zreiqat, H. The modulation of osteogenesis in vitro by calcium titanium phosphate coatings. Biomaterials 2004, 25, 4911–4919. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite–PCL composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef]
- Wang, C.; Duan, Y.; Markovic, B.; Barbara, J.; Howlett, C.; Zhang, X.; Zreiqat, H. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials 2004, 25, 2507–2514. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. BioMed Res. Int. 2016, 2016, 1–3. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Mechanical Properties of Bioceramic Coatings on Medical Implants. In Bioceramic Coatings for Medical Implants; Wiley: New Jersey, NJ, USA, 2013; pp. 311–321. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Vladescu, A.; Surmeneva, M.A.; Cotrut, C.M.; Surmenev, R.A.; Antoniac, I.V. Bioceramic Coatings for Metallic Implants. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 703–733. [Google Scholar] [CrossRef]
- Zambrano, D.; Barrios, A.; Tobón, L.; Serna, C.; Gómez, P.; Osorio, J.; Toro, A. Thermal properties and phase stability of Yttria-Stabilized Zirconia (YSZ) coating deposited by Air Plasma Spray onto a Ni-base superalloy. Ceram. Int. 2018, 44, 3625–3635. [Google Scholar] [CrossRef]
- Zamani, P.; Valefi, Z. Microstructure, phase composition and mechanical properties of plasma sprayed Al2O3, Cr2O3 and Cr2O3-Al2O3 composite coatings. Surf. Coatings Technol. 2017, 316, 138–145. [Google Scholar] [CrossRef]
- Ohmori, A.; Park, K.-C.; Inuzuka, M.; Arata, Y.; Inoue, K.; Iwamoto, N. Electrical conductivity of plasma-sprayed titanium oxide (rutile) coatings. Thin Solid Film. 1991, 201, 1–8. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing Treatments for Magnesium Alloys and Their Effect on Corrosion Resistance in Various Environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Suay, J.; Giménez, E.; Rodríguez, T.; Habbib, K.; Saura, J. Characterization of anodized and sealed aluminium by EIS. Corros. Sci. 2003, 45, 611–624. [Google Scholar] [CrossRef]
- Vetter, J.; Stüber, M.; Ulrich, S. Growth effects in carbon coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2003, 168, 169–178. [Google Scholar] [CrossRef]
- Santecchia, E.; Hamouda, A.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; Spigarelli, S. Wear resistance investigation of titanium nitride-based coatings. Ceram. Int. 2015, 41, 10349–10379. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Rameshbabu, N.; Venkateswarlu, K.; Sreekanth, D.; Subrahmanyam, C. Surface morphology, corrosion resistance and in vitro bioactivity of P containing ZrO2 films formed on Zr by plasma electrolytic oxidation. J. Alloys Compd. 2013, 553, 324–332. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Wang, Q.; Bu, S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Nahum, E.Z.; Lugovskoy, S.; Lugovskoy, A.; Kazanski, B.; Sobolev, A. The study of hydroxyapatite growth kinetics on CP–Ti and Ti65Zr treated by Plasma electrolytic oxidation process. J. Mater. Res. Technol. 2023, 24, 2169–2186. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohedano, M.; Pardo, A.; Merino, M.C.; Rivero, E. Stability of plasma electrolytic oxidation coating on titanium in artificial saliva. J. Mater. Sci. Mater. Med. 2012, 24, 37–51. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite Coating on Ti-6Al-7Nb Alloy by Plasma Electrolytic Oxidation in Salt-Based Electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef]
- Casanova, L.; La Padula, M.; Pedeferri, M.; Diamanti, M.V.; Ormellese, M. An insight into the evolution of corrosion resistant coatings on titanium during bipolar plasma electrolytic oxidation in sulfuric acid. Electrochim. Acta 2021, 379, 138190. [Google Scholar] [CrossRef]
- Sobolev, A.; Bograchev, D.; Borodianskiy, K.; Zinigrad, M. Kinetics and mechanism of corrosion of oxide coating fabricated on aluminum alloy by the plasma electrolytic oxidation in molten salt. Corros. Sci. 2022, 208, 110604. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Kohli, R.R.; Mittal, K.L. Developments in Surface Contamination and Cleaning: Methods for Assessment and Verification of Cleanliness of Surfaces and Characterization of Surface Contaminants; Elsevier: Amsterdam, The Netherlands, 2019; Volume 12, pp. 1–276. [Google Scholar]
- Calderon, J.G.; Harsch, A.; Gross, G.W.; Timmons, R.B. Stability of plasma-polymerized allylamine films with sterilization by autoclaving. J. Biomed. Mater. Res. 1998, 42, 597–603. [Google Scholar] [CrossRef]
- Sorrentino, L.; Carrino, L. 2024 aluminium alloy wettability and superficial cleaning improvement by air cold plasma treatment. J. Mater. Process. Technol. 2009, 209, 1400–1409. [Google Scholar] [CrossRef]
- Takakuwa, Y.; Nogawa, M.; Niwano, M.; Katakura, H.; Matsuyoshi, S.; Ishida, H.; Kato, H.; Miyamoto, N. Low-Temperature Cleaning of HF-Passivated Si(111) Surface with VUV Light. Jpn. J. Appl. Phys. 1989, 28 Pt 2, L1274. [Google Scholar] [CrossRef]
- Masotti, F.; Vallone, L.; Ranzini, S.; Silvetti, T.; Morandi, S.; Brasca, M. Effectiveness of air disinfection by ozonation or hydrogen peroxide aerosolization in dairy environments. Food Control. 2019, 97, 32–38. [Google Scholar] [CrossRef]
- Doundoulakis, J.H. Surface analysis of titanium after sterilization: Role in implant-tissue interface and bioadhesion. J. Prosthet. Dent. 1987, 58, 471–478. [Google Scholar] [CrossRef]
- Couvert, A.; Grandguillot, G.; Féliers, C. Investigation of byproduct formation during the irradiation of drinking water with a medium pressure lamp. Environ. Technol. 2007, 28, 841–851. [Google Scholar] [CrossRef]
- Vig, J.R. UV/ozone cleaning of surfaces. J. Vac. Sci. Technol. A 1985, 3, 1027–1034. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L. Investigation of the formation mechanisms of plasma electrolytic oxidation coatings on Mg alloy AM50 using particles. Electrochim. Acta 2016, 196, 680–691. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Xue, W.; Qu, Y.; Yang, C.; Wang, B.; Wu, X. Discharge behaviors during plasma electrolytic oxidation on aluminum alloy. Mater. Chem. Phys. 2014, 148, 284–292. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, G.; Lv, Y.; Wu, Y.; Dong, Z. Growth mechanism of titania on titanium substrate during the early stage of plasma electrolytic oxidation. Surf. Coat. Technol. 2020, 400, 126202. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Wang, X.; Kuang, Q.; Ji, D.; Yuan, R.; Jing, X. Effect of phosphate additive on the morphology and anti-corrosion performance of plasma electrolytic oxidation coatings on magnesium―Lithium alloy. Corros. Sci. 2019, 157, 295–304. [Google Scholar] [CrossRef]
- Allegrini, S.; Rumpel, E.; Kauschke, E.; Fanghänel, J.; König, B. Hydroxyapatite grafting promotes new bone formation and osseointegration of smooth titanium implants. Ann. Anat. -Anat. Anz. 2006, 188, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, Y.; Wang, F.; Shimizu, T.; Igarashi, K.; Zhao, L. Formation characterization of hydroxyapatite on titanium by microarc oxidation and hydrothermal treatment. J. Biosci. Bioeng. 2005, 100, 100–104. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low Temperature Synthesis and Photocatalytic Activity of Rutile TiO2 Nanorod Superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Farina, F.E.; Azmi, W.S.B.; Harafuji, K. Ultraviolet-ozone anode surface treatment and its effect on organic solar cells. Thin Solid Film. 2017, 623, 72–83. [Google Scholar] [CrossRef]
- Shahi, S.; Özcan, M.; Dizaj, S.M.; Sharifi, S.; Husain, N.A.-H.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Hu, J.; Wang, B.; Li, Y.; Wang, L.; Li, G.; Zhang, J.; Zhou, Z. Effect of hydroxyapatite content and particle size on the mechanical behaviors and osteogenesis in vitro of polyetheretherketone–hydroxyapatite composite. Polym. Compos. 2021, 42, 6512–6522. [Google Scholar] [CrossRef]
- Pang, Y.; Bao, X. Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 1697–1704. [Google Scholar] [CrossRef]
- Albrektsson, T.; Albrektsson, B. Osseointegration of bone implants: A review of an alternative mode of fixation. Acta Orthop. 1987, 58, 567–577. [Google Scholar] [CrossRef]
- Wolff, J.; Narra, N.; Antalainen, A.-K.; Valášek, J.; Kaiser, J.; Sándor, G.K.; Marcián, P. Finite element analysis of bone loss around failing implants. Mater. Des. 2014, 61, 177–184. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Neo, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Osteoinduction of porous bioactive titanium metal. Biomaterials 2004, 25, 443–450. [Google Scholar] [CrossRef]
- Kilpadi, K.L.; Chang, P.-L.; Bellis, S.L. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J. Biomed. Mater. Res. 2001, 57, 258–267. [Google Scholar] [CrossRef]



| Chemical Element | Fe | V | Al | Ti |
|---|---|---|---|---|
| Weight % | <0.3 | 3.5–4.5 | 5.5–6.5 | Balance |
| Samples | Treatment Description |
|---|---|
| AS-A | PEO in aqueous solution + A |
| MS-A | PEO in molten salt + A |
| AS-A-UV | PEO in aqueous solution + A + UV 5 min |
| MS-A-UV | PEO in molten salt + A + UV 5 min |
| AS-A-P | PEO in aqueous solution + A + cold plasma 5 min |
| MS-A-P | PEO in molten salt + A + cold plasma 5 min |
| AS-A-O | PEO in aqueous solution + A + ozone 5 min |
| MS-A-O | PEO in molten salt + A + ozone 5 min |
| Sample | Ti | Ca | P | O | C |
|---|---|---|---|---|---|
| AS-A | 22.9 ± 0.1 | 6.3 ± 0.1 | 3.8 ± 0.1 | balance | 5.3 ± 0.1 |
| AS-A-UV | 22.5 ± 0.1 | 4.3 ± 0.1 | 2.6 ± 0.1 | balance | 2.1 ± 0.1 |
| AS-A-P | 22.6 ± 0.1 | 5.7 ± 0.1 | 3.6 ± 0.1 | balance | 1.1 ± 0.1 |
| AS-A-O | 22.6 ± 0.1 | 6.2± 0.1 | 3.6 ± 0.1 | balance | - |
| MS-A | 22.4 ± 0.1 | 9.8 ± 0.1 | 5.9 ± 0.1 | balance | 4.7 ± 0.1 |
| MS-A-UV | 22.3 ± 0.1 | 5.9 ± 0.1 | 3.5 ± 0.1 | balance | 1.6 ± 0.1 |
| MS-A-P | 22.5 ± 0.1 | 8.2 ± 0.1 | 4.9 ± 0.1 | balance | 0.8 ± 0.1 |
| MS-A-O | 22.8 ± 0.1 | 9.6 ± 0.1 | 5.7 ± 0.1 | balance | - |
| Sample | Ti | Ca | P | O | C |
|---|---|---|---|---|---|
| AS-A | 22.91 ± 0.01 | 6.32 ± 0.01 | 3.84 ± 0.01 | balance | 5.21 ± 0.08 |
| AS-A-UV | 22.43 ± 0.01 | 4.27 ± 0.01 | 2.63 ± 0.01 | balance | 2.13 ± 0.02 |
| AS-A-P | 22.61 ± 0.01 | 5.72 ± 0.01 | 3.61 ± 0.01 | balance | 1.01 ± 0.01 |
| AS-A-O | 22.67 ± 0.01 | 6.14 ± 0.01 | 3.65 ± 0.01 | balance | 0.11 ± 0.03 |
| MS-A | 22.44 ± 0.01 | 9.86 ± 0.01 | 5.91 ± 0.01 | balance | 4.63 ± 0.06 |
| MS-A-UV | 22.31 ± 0.01 | 5.93 ± 0.01 | 3.54 ± 0.01 | balance | 1.63 ± 0.07 |
| MS-A-P | 22.52 ± 0.01 | 8.22 ± 0.01 | 4.96 ± 0.01 | balance | 0.86 ± 0.02 |
| MS-A-O | 22.84 ± 0.01 | 9.62 ± 0.01 | 5.74 ± 0.01 | balance | 0.12 ± 0.02 |
| Sample | Anatase, wt.% | Rutile, wt.% | Hydroxyapatite, wt.% | Rwp, % |
|---|---|---|---|---|
| AS-A | 60.6 ± 0.6 | 5.9 ± 0.7 | 33.5 ± 0.5 | 8.75 |
| AS-A-UV | 57.2 ± 1.2 | 11.7 ± 1.9 | 31.1 ± 0.8 | 8.08 |
| AS-A-P | 67.4 ± 0.7 | 0.8 ± 0.7 | 31.7 ± 0.5 | 8.31 |
| AS-A-O | 54.4 ± 1.9 | 12.5 ± 1.2 | 33.1 ± 1.1 | 10.00 |
| MS-A | 21.2 ± 1.2 | 3.0 ± 0.6 | 75.8 ± 1.2 | 11.41 |
| MS-A-UV | 22.1 ± 1.6 | 1.8 ± 0.5 | 76.1 ± 1.4 | 9.93 |
| MS-A-P | 21.1 ± 1.6 | 3.4 ± 0.9 | 75.5 ± 1.6 | 13.81 |
| MS-A-O | 23.1 ± 1.5 | 2.4 ± 0.7 | 74.5 ± 1.3 | 11.45 |
| Sample | Ti-6Al-4V | AS-A | AS-A-UV | AS-A-P | AS-A-O |
|---|---|---|---|---|---|
| CA [°] | 78.2 ± 1.1 | 35.2 ± 1.5 | 28.3 ± 0.8 | 26.1 ± 1.4 | 16.6 ± 0.7 |
| Sample | MS-A | MS-A-UV | MS-A-P | MS-A-O | |
| CA [°] | 25.3 ± 1.1 | 19.5 ± 0.9 | 10.5 ± 0.4 | 7.5 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, A.; Kossenko, A.; Zinigrad, M.; Danchuk, V.; Sobolev, A. Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials 2023, 16, 4624. https://doi.org/10.3390/ma16134624
Schwartz A, Kossenko A, Zinigrad M, Danchuk V, Sobolev A. Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials. 2023; 16(13):4624. https://doi.org/10.3390/ma16134624
Chicago/Turabian StyleSchwartz, Avital, Alexey Kossenko, Michael Zinigrad, Viktor Danchuk, and Alexander Sobolev. 2023. "Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations" Materials 16, no. 13: 4624. https://doi.org/10.3390/ma16134624
APA StyleSchwartz, A., Kossenko, A., Zinigrad, M., Danchuk, V., & Sobolev, A. (2023). Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials, 16(13), 4624. https://doi.org/10.3390/ma16134624









