Ultrasensitive Detection of Malachite Green Isothiocyanate Using Nanoporous Gold as SERS Substrate
Abstract
1. Introduction
2. Materials and Methods
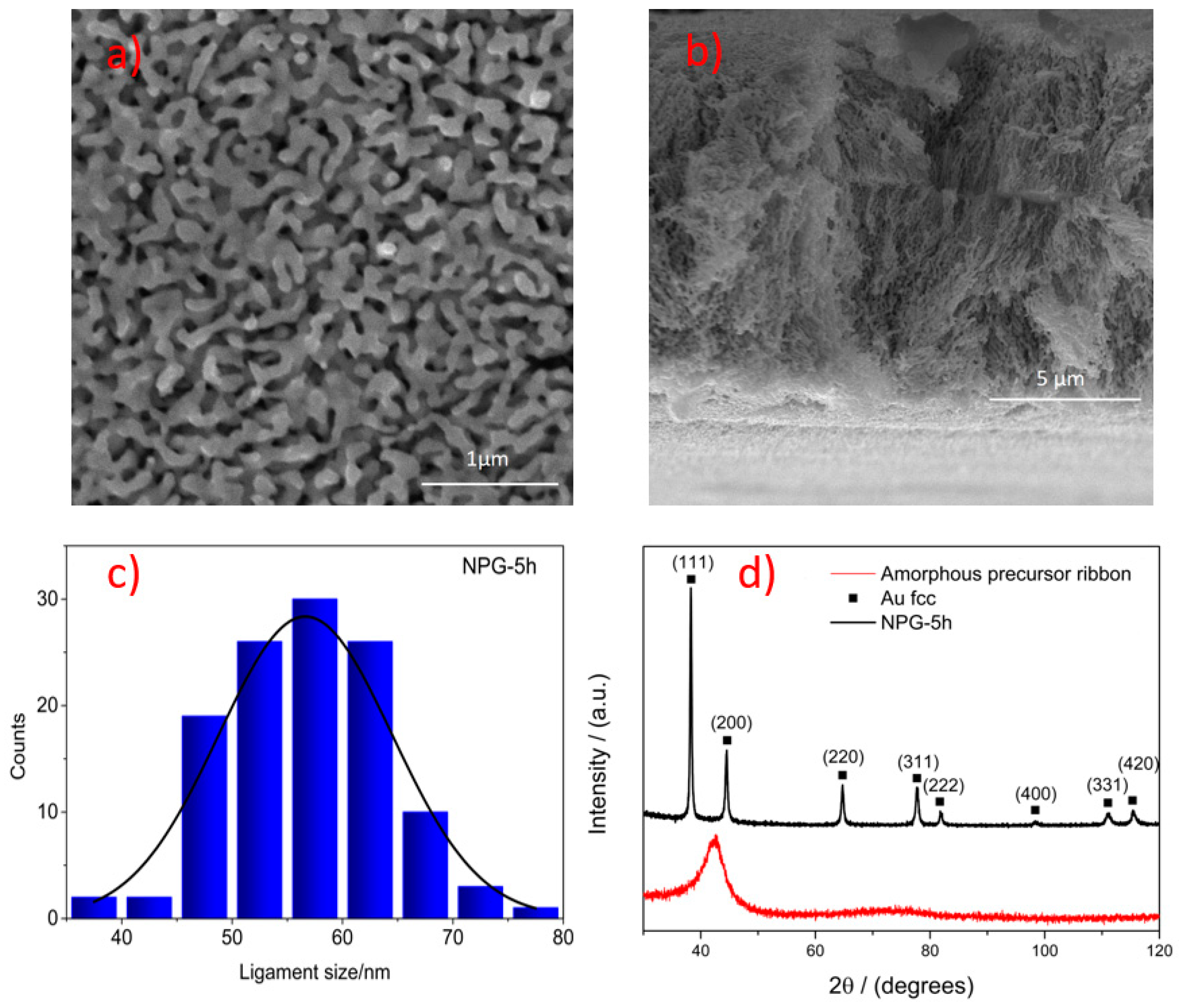
2.1. Synthesis of the Nanoporous Gold
2.2. Preparation of Probe Molecule Solutions
2.3. Data Analysis
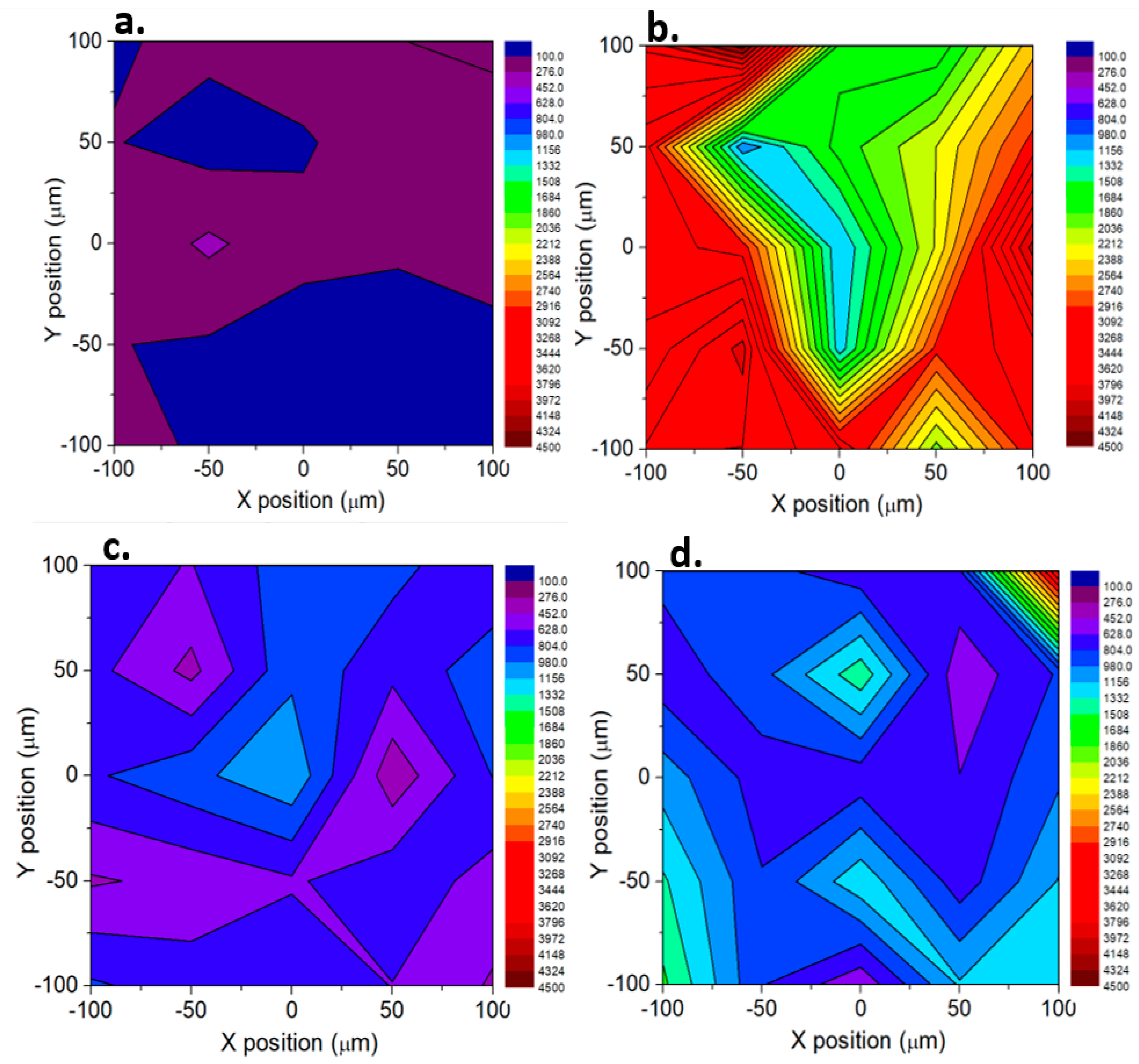
3. Results and Discussion
SERS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Meenakshi, S. Fabrication of Sulfur-Doped Biochar Derived from Tapioca Peel Waste with Superior Adsorption Performance for the Removal of Malachite Green and Rhodamine B Dyes. Surf. Interfaces 2021, 23, 100920. [Google Scholar] [CrossRef]
- Plakas, S.M.; Doerge, D.R.; Turnipseed, S.B. Disposition and Metabolism of Malachite Green and Other Therapeutic Dyes in Fish. In Xenobiotics in Fish; Springer: New York, NY, USA, 1999; pp. 149–166. [Google Scholar]
- Culp, S.J.; Beland, F.A. Malachite Green: A Toxicological Review. J. Am. Coll. Toxicol. 1996, 15, 219–238. [Google Scholar] [CrossRef]
- Thetford, D. Triphenylmethane and Related Dyes. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000; pp. 1–19. [Google Scholar]
- Yang, M.-C.; Fang, J.-M.; Kuo, T.-F.; Wang, D.-M.; Huang, Y.-L.; Liu, L.-Y.; Chen, P.-H.; Chang, T.-H. Production of Antibodies for Selective Detection of Malachite Green and the Related Triphenylmethane Dyes in Fish and Fishpond Water. J. Agric. Food Chem. 2007, 55, 8851–8856. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.L.; Ashwin, H.; Johnston, B.H.; Dallas, A.; Kazakov, S.A.; Tarbin, J.A.; Sharman, M.; Kay, J.; Keely, B.J. An RNA-Aptamer-Based Assay for the Detection and Analysis of Malachite Green and Leucomalachite Green Residues in Fish Tissue. Anal. Chem. 2010, 82, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, J.; Wan, L.; Lin, J.; Wang, X. Microwave-Assisted Covalent Modification of Graphene Nanosheets with Hydroxypropyl-β-Cyclodextrin and Its Electrochemical Detection of Phenolic Organic Pollutants. J. Mater. Chem. 2011, 21, 10463–10471. [Google Scholar] [CrossRef]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence Spectroscopy for Wastewater Monitoring: A Review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef]
- De Souza, D.; Machado, S.A.S. Electrochemical Detection of the Herbicide Paraquat in Natural Water and Citric Fruit Juices Using Microelectrodes. Anal. Chim. Acta 2005, 546, 85–91. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Environmental Applications of Plasmon Assisted Raman Scattering. Energy Environ. Sci. 2010, 3, 1011–1017. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.-Q. Advances in the Characterization and Monitoring of Natural Organic Matter Using Spectroscopic Approaches. Water Res. 2021, 190, 116759. [Google Scholar] [CrossRef]
- Yuan, Y.; Panwar, N.; Yap, S.H.K.; Wu, Q.; Zeng, S.; Xu, J.; Tjin, S.C.; Song, J.; Qu, J.; Yong, K.-T. SERS-Based Ultrasensitive Sensing Platform: An Insight into Design and Practical Applications. Coord. Chem. Rev. 2017, 337, 1–33. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.V.P.; Shruthi, S.; Vibha, B.; Reddy, B.A.A.; Kundu, T.K.; Narayana, C. Hot Spots in Ag Core–Au Shell Nanoparticles Potent for Surface-Enhanced Raman Scattering Studies of Biomolecules. J. Phys. Chem. C 2007, 111, 4388–4392. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Benefits, Trade-Offs and Future Developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Shih, W.-C.; Zhao, F.; Arnob, M. Label-Free Biomolecular Sensing by SERS on Nanoporous Gold Nanoparticle Arrays. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology (IEEE-NANO), Cork, Ireland, 23–26 July 2018; pp. 1–4. [Google Scholar]
- Meng, X.; Qiu, L.; Xi, G.; Wang, X.; Guo, L. Smart Design of High-performance Surface-enhanced Raman Scattering Substrates. SmartMat 2021, 2, 466–487. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Charron, G.; Cortés, E.; Kneipp, J.; de la Chapelle, M.L.; Langer, J.; Procházka, M.; Tran, V.; Schlücker, S. Towards Reliable and Quantitative Surface-enhanced Raman Scattering (SERS): From Key Parameters to Good Analytical Practice. Angew. Chem. Int. Ed. 2020, 59, 5454–5462. [Google Scholar] [CrossRef]
- Yuan, K.; Jurado-Sánchez, B.; Escarpa, A. Nanomaterials Meet Surface-Enhanced Raman Scattering towards Enhanced Clinical Diagnosis: A Review. J. Nanobiotechnol. 2022, 20, 537. [Google Scholar] [CrossRef]
- Qian, L.H.; Yan, X.Q.; Fujita, T.; Inoue, A.; Chen, M.W. Surface Enhanced Raman Scattering of Nanoporous Gold: Smaller Pore Sizes Stronger Enhancements. Appl. Phys. Lett. 2007, 90, 153120. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Celegato, F.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Gebert, A.; Oswald, S.; Wolff, U.; Mihaylov, L.; Spassov, T. The Mechanism of Generating Nanoporous Au by De-Alloying Amorphous Alloys. Acta Mater. 2016, 119, 177–183. [Google Scholar] [CrossRef]
- Rizzi, P.; Scaglione, F.; Battezzati, L. Nanoporous Gold by Dealloying of an Amorphous Precursor. J. Alloys Compd. 2014, 586, S117–S120. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Scaglione, F.; Rizzi, P.; Celegato, F.; Battezzati, L. Synthesis of Nanoporous Gold by Free Corrosion of an Amorphous Precursor. J. Alloys Compd. 2014, 615, S142–S147. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Celegato, F.; Denis, P.; Fecht, H.J.; Rizzi, P.; Battezzati, L. Shape Controlled Gold Nanostructures on De-Alloyed Nanoporous Gold with Excellent SERS Performance. Chem. Phys. Lett. 2018, 709, 46–51. [Google Scholar] [CrossRef]
- Scaglione, F.; Xue, Y.; Celegato, F.; Rizzi, P.; Battezzati, L. Amorphous Molybdenum Sulphide@ Nanoporous Gold as Catalyst for Hydrogen Evolution Reaction in Acidic Environment. J. Mater. Sci. 2018, 53, 12388–12398. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Scaglione, F.; Gebert, A.; Oswald, S.; Rizzi, P.; Battezzati, L. Partially and Fully De-Alloyed Glassy Ribbons Based on Au: Application in Methanol Electro-Oxidation Studies. J. Alloys Compd. 2016, 667, 302–309. [Google Scholar] [CrossRef]
- Scaglione, F.; Battezzati, L.; Rizzi, P. Breaking Down SERS Detection Limit: Engineering of a Nanoporous Platform for High Sensing and Technology. Nanomaterials 2022, 12, 1737. [Google Scholar] [CrossRef]
- Awada, C.; Dab, C.; Grimaldi, M.G.; Alshoaibi, A.; Ruffino, F. High Optical Enhancement in Au/Ag Alloys and Porous Au Using Surface-Enhanced Raman Spectroscopy Technique. Sci. Rep. 2021, 11, 4714. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; He, X.; Tang, C.; Du, K.; He, Z. Gradient Nanoporous Gold: A Novel Surface-Enhanced Raman Scattering Substrate. RSC Adv. 2017, 7, 15747–15753. [Google Scholar] [CrossRef]
- Li, W.; Ma, C.; Zhang, L.; Chen, B.; Chen, L.; Zeng, H. Tuning Localized Surface Plasmon Resonance of Nanoporous Gold with a Silica Shell for Surface Enhanced Raman Scattering. Nanomaterials 2019, 9, 251. [Google Scholar] [CrossRef]
- Scaglione, F.; Alladio, E.; Damin, A.; Turci, F.; Baggiani, C.; Giovannoli, C.; Bordiga, S.; Battezzati, L.; Rizzi, P. Functionalized Nanoporous Gold as a New Biosensor Platform for Ultra-Low Quantitative Detection of Human Serum Albumin. Sens. Actuators B Chem. 2019, 288, 460–468. [Google Scholar] [CrossRef]
- Tan, M.J.; Hong, Z.-Y.; Chang, M.-H.; Liu, C.-C.; Cheng, H.-F.; Loh, X.J.; Chen, C.-H.; Liao, C.-D.; Kong, K.V. Metal Carbonyl-Gold Nanoparticle Conjugates for Highly Sensitive SERS Detection of Organophosphorus Pesticides. Biosens. Bioelectron. 2017, 96, 167–172. [Google Scholar] [CrossRef]
- Ekmen, E.; Bilici, M.; Turan, E.; Tamer, U.; Zengin, A. Surface Molecularly-Imprinted Magnetic Nanoparticles Coupled with SERS Sensing Platform for Selective Detection of Malachite Green. Sens. Actuators B Chem. 2020, 325, 128787. [Google Scholar] [CrossRef]
- Chung, E.; Jeon, J.; Yu, J.; Lee, C.; Choo, J. Surface-Enhanced Raman Scattering Aptasensor for Ultrasensitive Trace Analysis of Bisphenol A. Biosens. Bioelectron. 2015, 64, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.L.; Zhen, S.J.; Huang, C.Z. One-Pot Green Synthesis of Graphene Oxide/Gold Nanocomposites as SERS Substrates for Malachite Green Detection. Analyst 2013, 138, 3075–3081. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.-Z.; Yin, P.-G.; You, T.-T.; Wang, H.; Guo, L. Three Dimensional Design of Large-Scale TiO2 Nanorods Scaffold Decorated by Silver Nanoparticles as SERS Sensor for Ultrasensitive Malachite Green Detection. ACS Appl. Mater. Interfaces 2012, 4, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Sivanesan, A.; Witkowska, E.; Gołąb, J.; Winiarska, M.; Nowis, D.; Dzięcielewski, I.; Weyher, J.L.; Waluk, J. Detection of DNA Mutations Using Novel SERS (Surface-Enhanced Raman Spectroscopy) Diagnostic Platform. J. Chem. Chem. Eng. 2013, 7, 972–978. [Google Scholar]
- Qin, M.; Wang, C.; Zhu, J.; Yong, L.; Wang, H.; Yang, L. Synthesis of Differently Sized Gold Nanoparticles for SERS Applications in the Detection of Malachite Green. Spectroscopy 2021, 36, 41–46, 54. [Google Scholar]
- Pérez-Gregorio, M.R.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Comparison of Sanitizing Technologies on the Quality Appearance and Antioxidant Levels in Onion Slices. Food Control 2011, 22, 2052–2058. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Yang, S.; Dai, X.; Stogin, B.B.; Wong, T.S. Ultrasensitive Surface-Enhanced Raman Scattering Detection in Common Fluids. Proc. Natl. Acad. Sci. USA 2016, 113, 268–273. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, J.; Li, M.; Zhang, X.; Luan, L.; Li, P.; Xu, W. Quantitative SERS Sensor Based on Self-Assembled Au@ Ag Heterogeneous Nanocuboids Monolayer with High Enhancement Factor for Practical Quantitative Detection. Anal. Bioanal. Chem. 2021, 413, 4207–4215. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, Y.; Miao, J.; Fan, Y.; Lai, K. A Highly Sensitive Surface-Enhanced Raman Scattering Sensor with MIL-100 (Fe)/Au Composites for Detection of Malachite Green in Fish Pond Water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 292, 122432. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chien, C.-H. Facile Fabrication of Micro/Nano Hierarchical SERS Sensor via Anisotropic Etching and Electrochemical Treatment for Malachite Green Detection. Appl. Sci. 2019, 9, 5237. [Google Scholar] [CrossRef]
- Xu, K.X.; Guo, M.H.; Huang, Y.P.; Li, X.D.; Sun, J.J. Rapid and Sensitive Detection of Malachite Green in Aquaculture Water by Electrochemical Preconcentration and Surface-Enhanced Raman Scattering. Talanta 2018, 180, 383–388. [Google Scholar] [CrossRef]
- Qiu, S.; Zhao, F.; Zenasni, O.; Li, J.; Shih, W.C. Nanoporous Gold Disks Functionalized with Stabilized G-Quadruplex Moieties for Sensing Small Molecules. ACS Appl. Mater. Interfaces 2016, 8, 29968–29976. [Google Scholar] [CrossRef]
- Cheng, Y.; Ding, Y.; Chen, J.; Xu, W.; Wang, W.; Xu, S. Au Nanoparticles Decorated Covalent Organic Framework Composite for SERS Analyses of Malachite Green and Thiram Residues in Foods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 281, 121644. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zou, S.; Zhang, H. Electrodeposition of Ag Nanodendrites SERS Substrates for Detection of Malachite Green. Microchem. J. 2019, 150, 104127. [Google Scholar] [CrossRef]
- Li, Z.H.; Bai, J.H.; Zhang, X.; Lv, J.M.; Fan, C.S.; Zhao, Y.M.; Wu, Z.L.; Xu, H.J. Facile Synthesis of Au Nanoparticle-Coated Fe3O4 Magnetic Composite Nanospheres and Their Application in SERS Detection of Malachite Green. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118532. [Google Scholar] [CrossRef]
- Kamińska, A.; Dzicielewski, I.; Weyher, J.L.; Waluk, J.; Gawinkowski, S.; Sashuk, V.; Fiałkowski, M.; Sawicka, M.; Suski, T.; Porowski, S.; et al. Highly Reproducible, Stable and Multiply Regenerated Surface-Enhanced Raman Scattering Substrate for Biomedical Applications. J. Mater. Chem. 2011, 21, 8662–8669. [Google Scholar] [CrossRef]
- Lang, X.Y.; Guan, P.F.; Zhang, L.; Fujita, T.; Chen, M.W. Characteristic Length and Temperature Dependence of Surface Enhanced Raman Scattering of Nanoporous Gold. J. Phys. Chem. C 2009, 113, 10956–10961. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Y.; Fujita, T.; Zhang, Y.; Chen, M.; Wang, T. Large Enhancement of Quantum Dot Fluorescence by Highly Scalable Nanoporous Gold. Adv. Mater. 2014, 26, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Hossain, M.A.; Park, J.Y.; Kim, S.-H.; Lee, D.; Suzuki, T.; Lee, J.; Park, E.Y. Metal Enhanced Fluorescence on Nanoporous Gold Leaf-Based Assay Platform for Virus Detection. Biosens. Bioelectron. 2014, 58, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pradel, J.S.; Tong, W.G. Determination of Malachite Green, Crystal Violet, Brilliant Green and Methylene Blue by Micro-Cloud-Point Extraction and Nonlinear Laser Wave-Mixing Detection Interfaced to Micellar Capillary Electrophoresis. Anal. Methods 2017, 9, 6411–6419. [Google Scholar] [CrossRef]

| at. % | Si | Cu | Pd | Ag | Au |
|---|---|---|---|---|---|
| Mean | 1.0 | 5.3 | 0.3 | 0.1 | 93.3 |
| SD | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 |
| SERS Substrate | Methods | Limit of Detection (LOD) | Enhancment Factor (EF) | References |
|---|---|---|---|---|
| Au@Ag NCs | SMG | 8.7 × 10−10 M | ------- | [44] |
| MIL-100(Fe)/Au | ST & EA | 10−13 M | 7.67 × 107 | [45] |
| Au NS @ SiPA | WE | 1.0 × 10−11 M | 4.05 × 108 | [46] |
| Ag NPs | EP | 2.4 × 10−16 M | ------- | [47] |
| NPG disk | NSL | 10−11 M | 5.49 × 108 | [48] |
| COF-AuNPs | EA | 6.2 × 10−10 M | 5.3 × 105 | [49] |
| Ag NDs | ED | 4 × 10−13 M. | 5.4 × 109 | [50] |
| Fe3O4@Au MCS | SMG & IR | 10−7 M | 1.1 × 105 | [51] |
| Au-GaN | PE | ------- | 2.8 × 106 | [52] |
| GO-AuNPs | RE | 2.5 × 10−6 M | 3.8 × 103 | [37] |
| (Ag/TiO2) | FTO | 1 × 10−12 M | 4.36 × 105 | [38] |
| NPG-5h | CD | 10−16 M | 7.9 × 109 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, D.; Tayyaba, N.; De Vita, G.; Scaglione, F.; Rizzi, P. Ultrasensitive Detection of Malachite Green Isothiocyanate Using Nanoporous Gold as SERS Substrate. Materials 2023, 16, 4620. https://doi.org/10.3390/ma16134620
Raj D, Tayyaba N, De Vita G, Scaglione F, Rizzi P. Ultrasensitive Detection of Malachite Green Isothiocyanate Using Nanoporous Gold as SERS Substrate. Materials. 2023; 16(13):4620. https://doi.org/10.3390/ma16134620
Chicago/Turabian StyleRaj, Deepti, Noor Tayyaba, Ginevra De Vita, Federico Scaglione, and Paola Rizzi. 2023. "Ultrasensitive Detection of Malachite Green Isothiocyanate Using Nanoporous Gold as SERS Substrate" Materials 16, no. 13: 4620. https://doi.org/10.3390/ma16134620
APA StyleRaj, D., Tayyaba, N., De Vita, G., Scaglione, F., & Rizzi, P. (2023). Ultrasensitive Detection of Malachite Green Isothiocyanate Using Nanoporous Gold as SERS Substrate. Materials, 16(13), 4620. https://doi.org/10.3390/ma16134620








