The Effect of Process Conditions on Sulfuric Acid Leaching of Manganese Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Leaching Experiments
2.3. Characterization of Materials and Products
3. Results
3.1. The Characteristics of Mn Sludge
3.2. Leachability of Mn Sludge
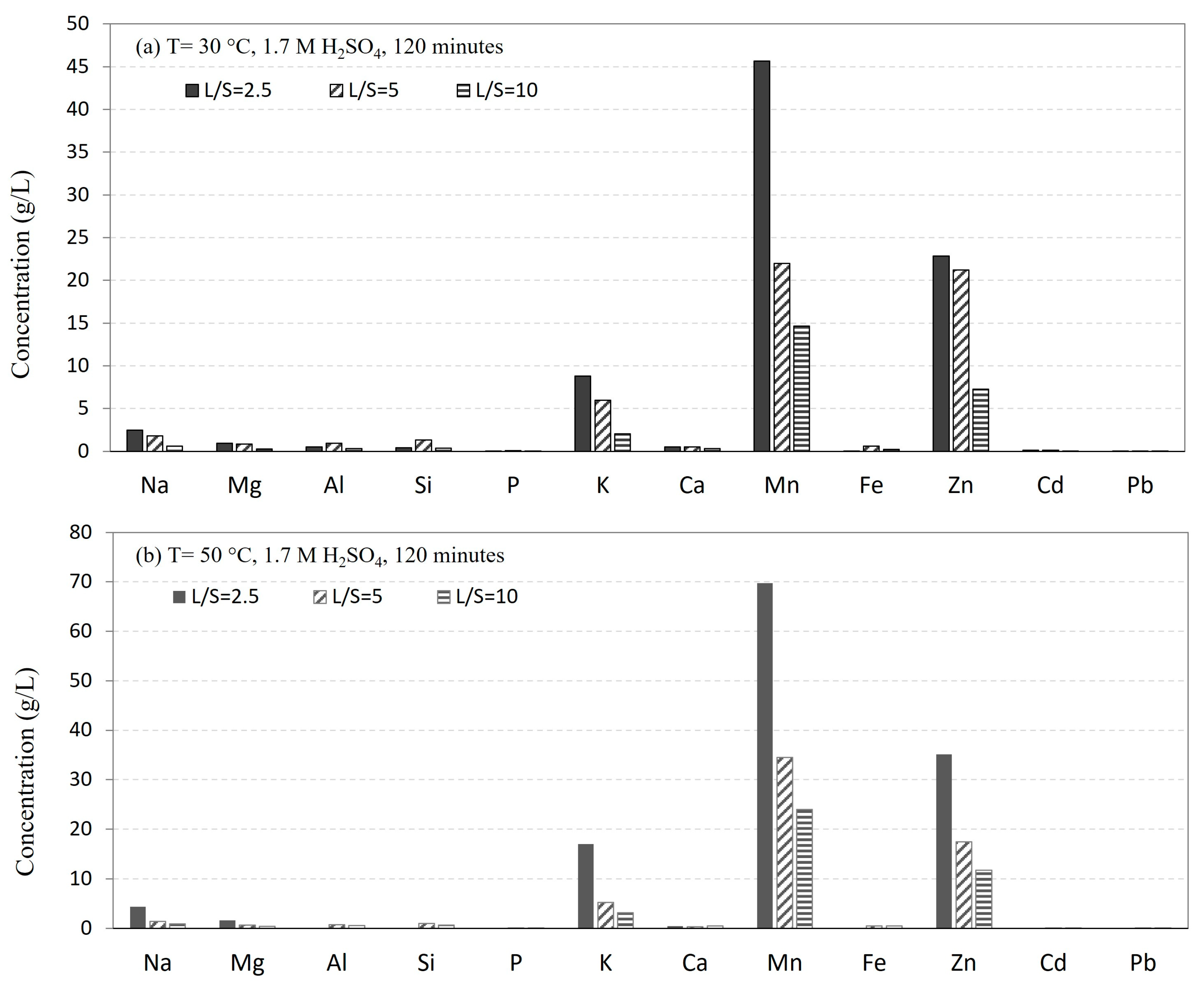
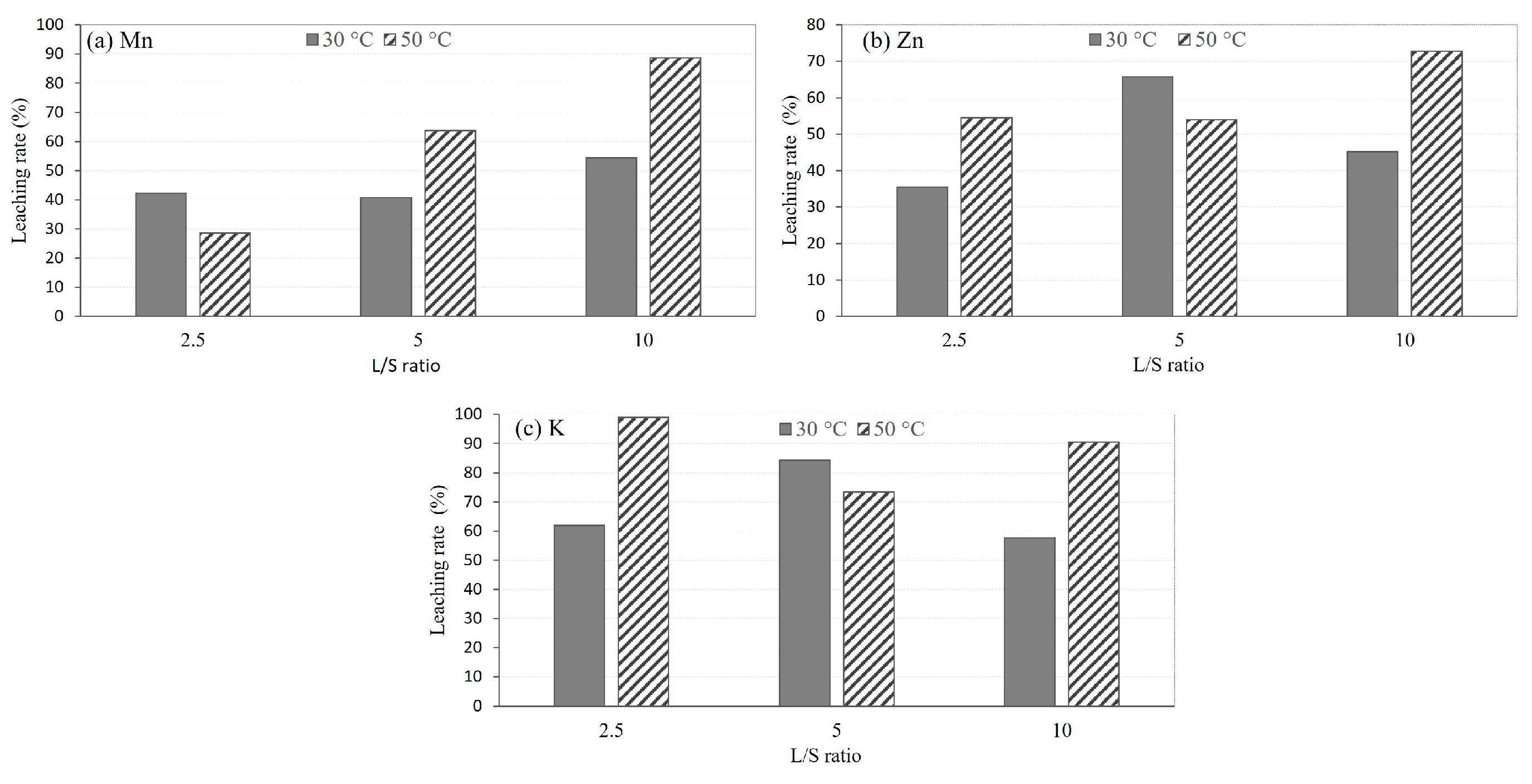
3.2.1. Effect of Temperature
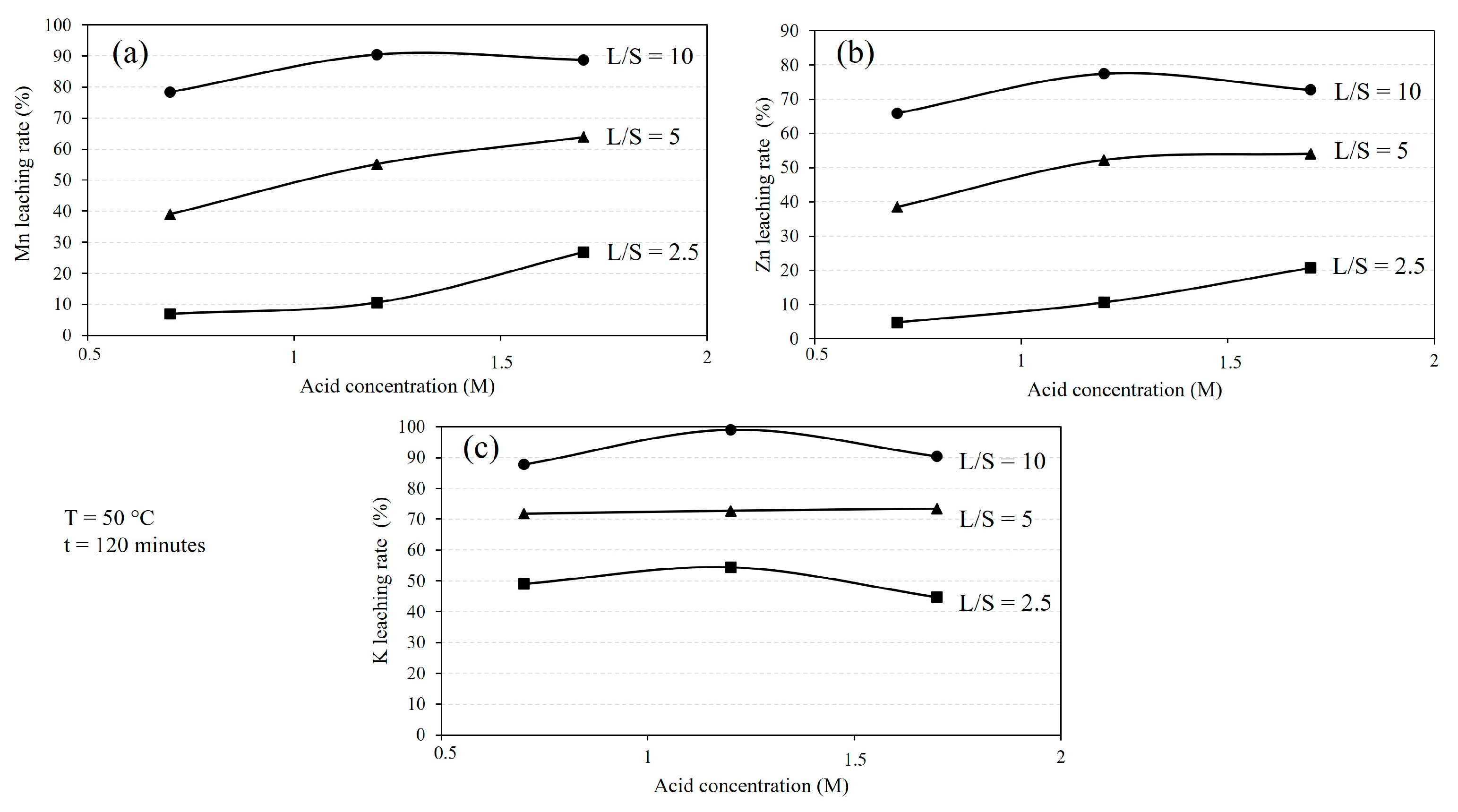
3.2.2. Effect of Solution Volume and Concentration
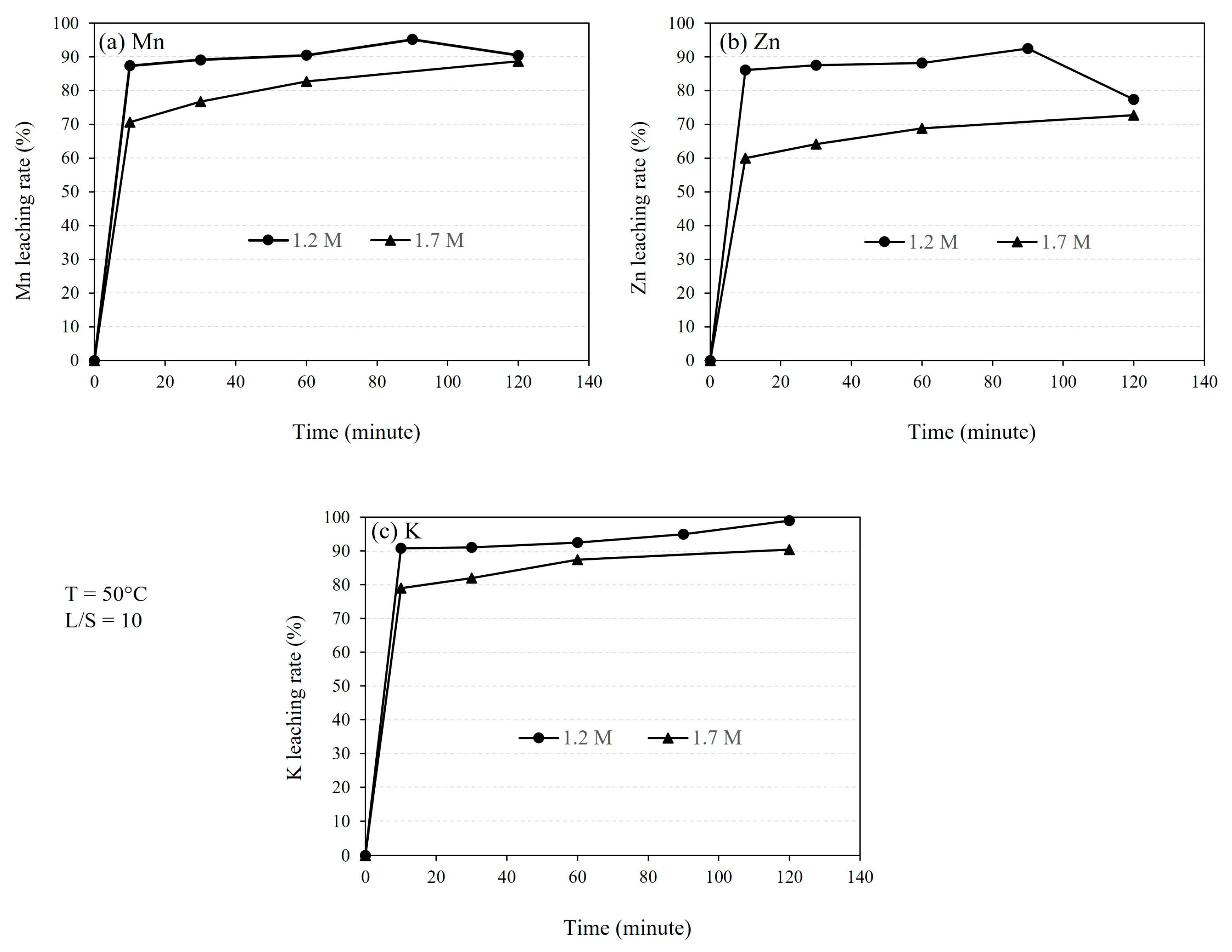
3.2.3. Effect of Leaching Duration
3.3. The Phase Analysis of Residue
3.4. The Microstructural Analysis of Residue
4. Discussion
4.1. Mn Sludge Composition
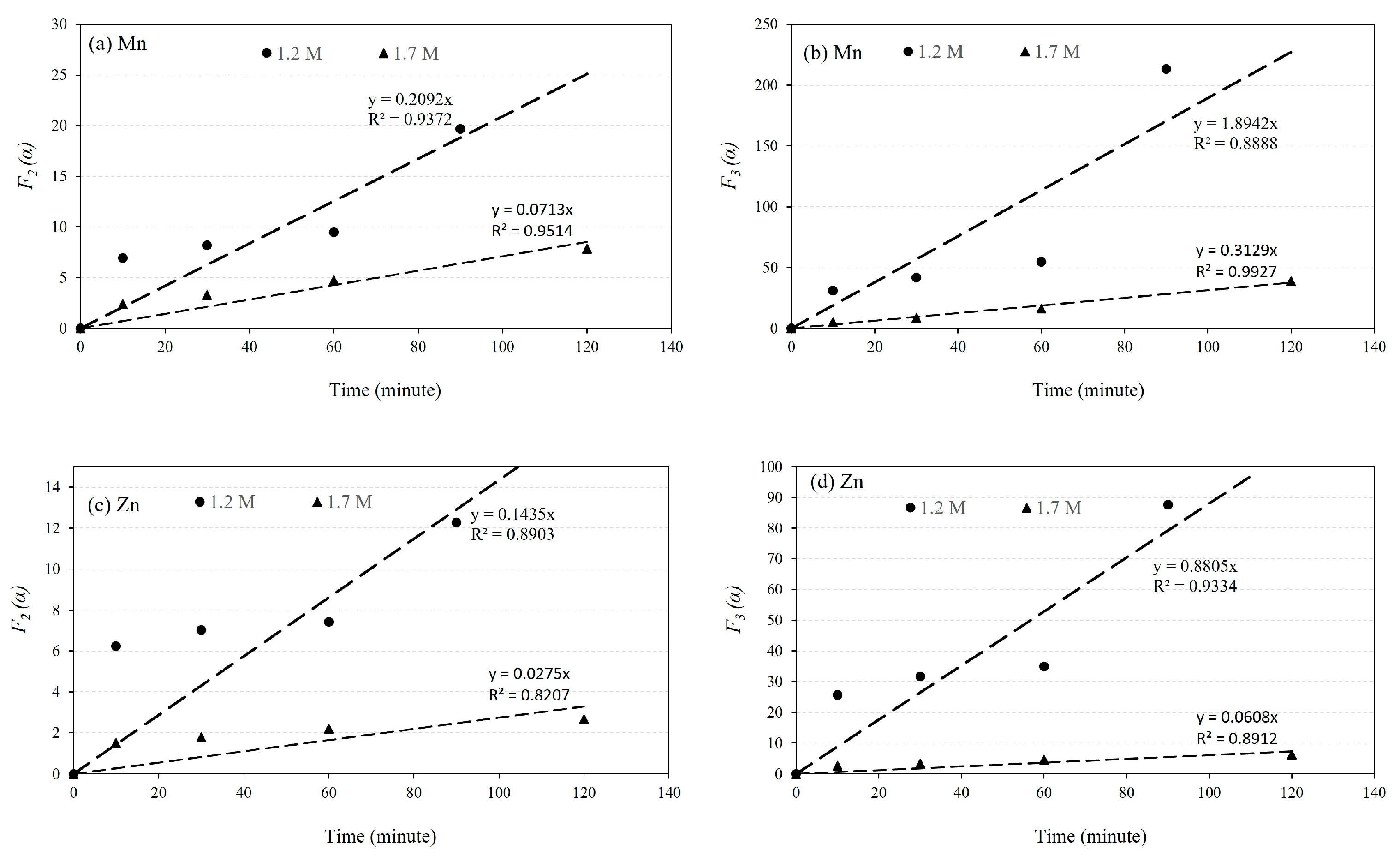
4.2. The Kinetics of Leaching
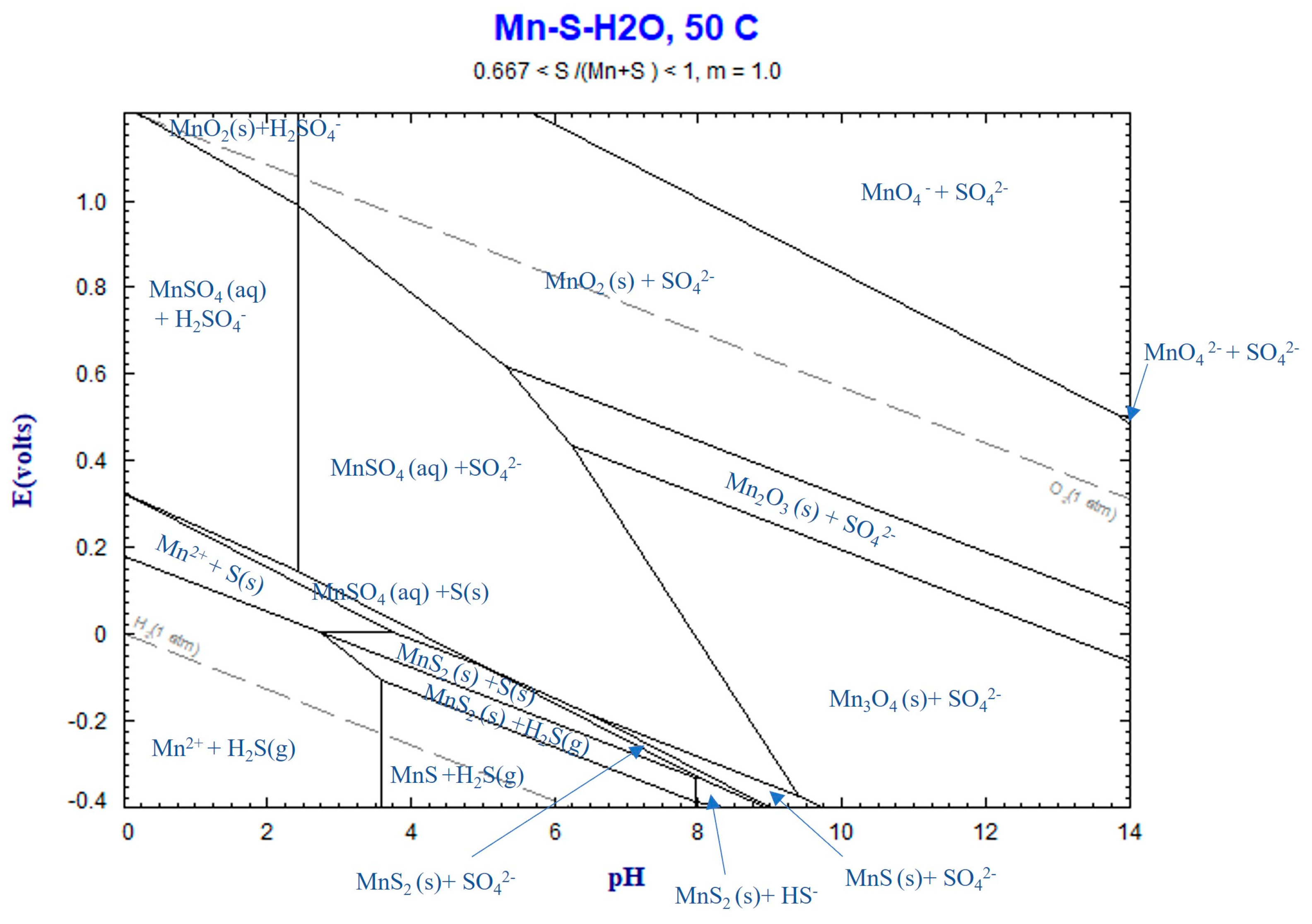
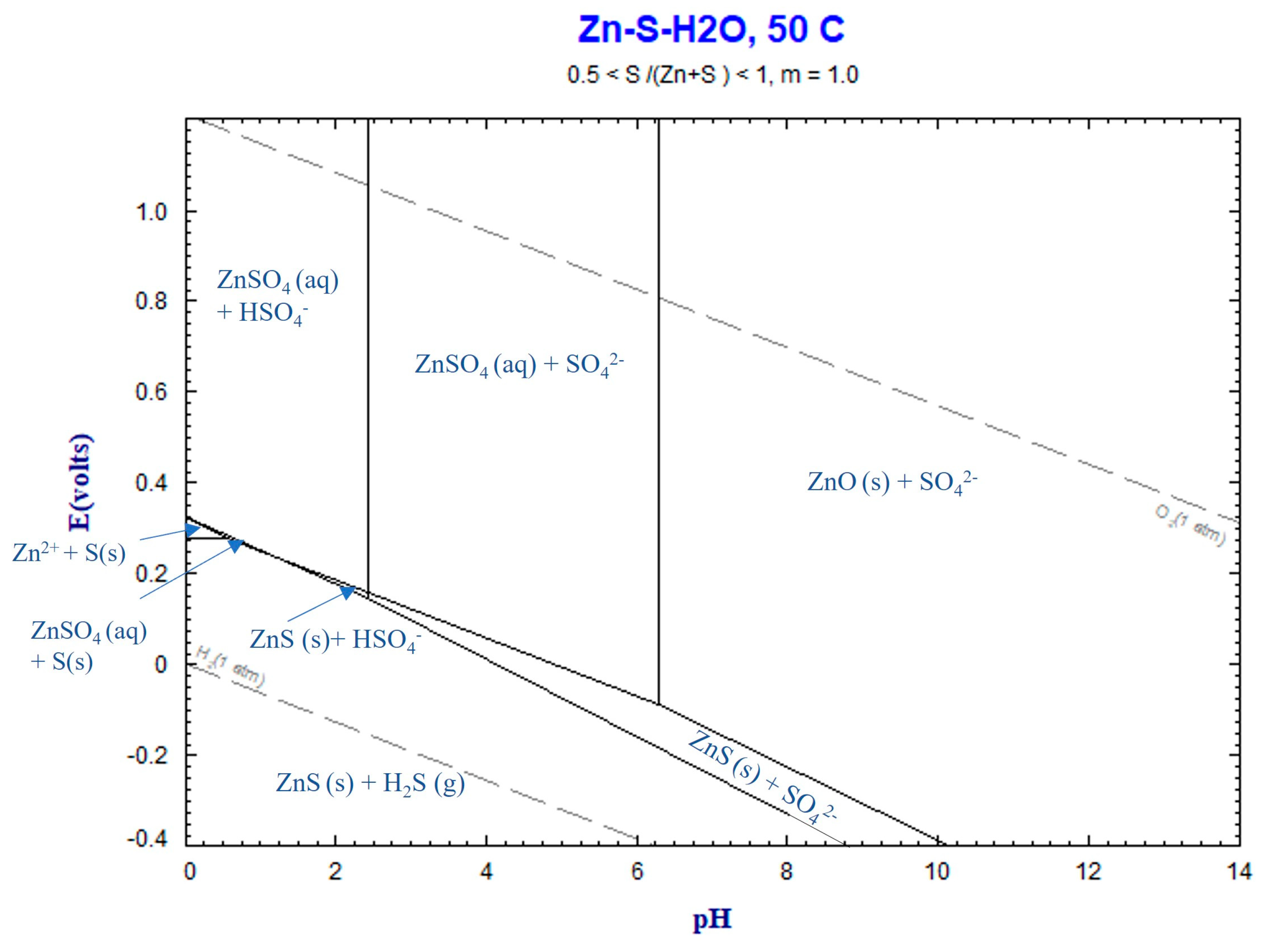
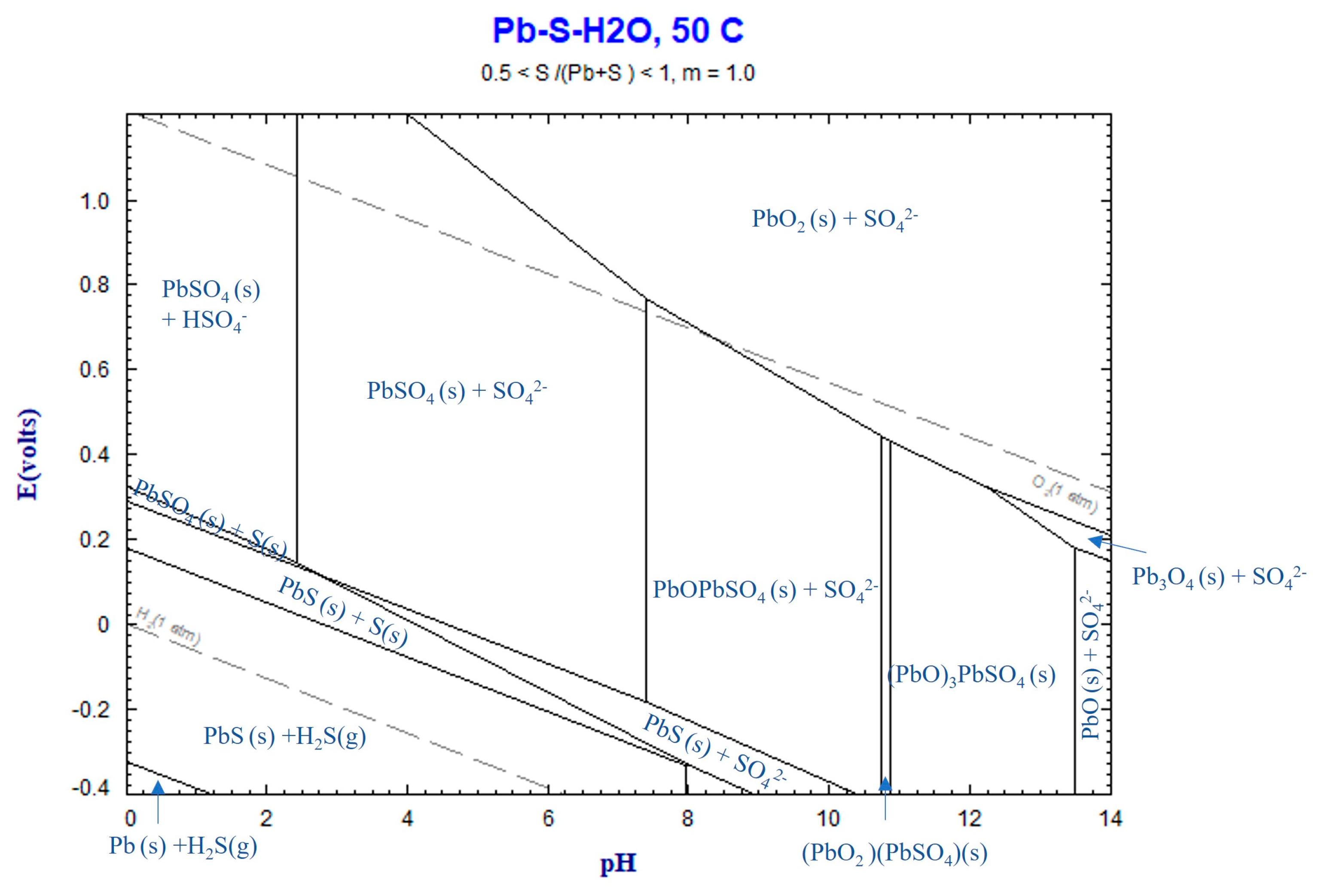
4.3. Thermochemistry of Reactions
4.3.1. Mn Leaching
4.3.2. Zn Leaching
4.3.3. Lead Sulfate Formation
5. Conclusions
- The leaching rate of Mn sludge is fast, resulting in high leaching rates of 90% for both Mn and Zn.
- The leaching rates of Mn, Zn, and K increase with increasing temperature, liquid to solid ratio, and duration. Mn exhibits a higher maximum leaching rate compared to Zn.
- Comparing acid solutions, the leaching rates of Mn and Zn are slightly higher using a 1.2 M H2SO4 solution compared to a 1.7 M H2SO4 solution, while the leaching rates are much lower with a 0.7 M acid solution.
- Kinetic studies of leaching with 1.2 M and 1.7 M H2SO4 acid solutions reveal that the reaction rate constant for Mn dissolution exceeds 1.2 × 10−3 s−1, while for Zn dissolution, it is higher than 5 × 10−4 s−1.
- During the leaching process, aqueous sulfate solutions of MnSO4 and ZnSO4 are formed from the dissolution of solid MnCO3 and ZnO (or ZnCO3) present in the sludge. This process is accompanied by the generation of CO2 gas and bubble formation.
- The Pb content in the sludge is fully converted to solid PbSO4 during leaching, and samples with high Mn and Zn leaching rates yield a residue of lead sulfate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Sintef: Trondheim, Norway; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Safarian, J. Duplex Process to Produce Ferromanganese and Direct Reduced Iron by Natural Gas. ACS Sustain. Chem. Eng. 2021, 9, 5010–5026. [Google Scholar] [CrossRef]
- Safarian, J. Kinetics and Mechanisms of Reduction of MnOcontaining Silicate Slags by Selected forms of Carbonaceous Materials. Ph.D. Thesis, NTNU, Trondheim, Norway, 2007. [Google Scholar]
- Gaal, S.; Tangstad, M.; Ravary, B. Recycling of waste materials from the production of FeMn and SiMn. Twelfth Int. Ferroalloys Congr. Hels. Finl. 2010, 81–88. [Google Scholar]
- Ravary, B.; Hunsbedt, L. Progress in recycling sludge from off-gas cleaning of manganese alloy furnaces. In Proceedings of the thirteenth International Ferroalloys Congress Efficient technologies in ferroalloy industry, Almaty, Kazakhstan, 9–13 June 2013; pp. 1023–1027. [Google Scholar]
- Shen, R.; Zhang, G.; Dell’Amico, M.; Brown, P.; Ostrovski, O. Characterisation of Manganese Furnace Dust and Zinc Balance in Production of Manganese Alloys. ISIJ Int. 2005, 45, 1248–1254. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Fernández, M.; Torres, V.M. Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review. J. Sustain. Met. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Mohanty, J.K.; Sahoo, P.K.; Nathsarma, K.C.; Panda, D.; Paramguru, R.K. Characterization and leaching of ferromanganese slag. Mining, Met. Explor. 1998, 15, 30–33. [Google Scholar] [CrossRef]
- Baumgartner, S.J. Groot. The recovery of manganese products from ferromanganese slag using a hydrometallurgical route. J. South. Afr. Inst. Min. Metall. 2014, 114, 331–340. [Google Scholar]
- Groot, D.; Kazadi, D.; Pollmann, H.; de Villiers, J.; Redtmann, T.; Steenkamp, J. The recovery of manganese and generation of a valuable residue from ferromanganese slags by a hydrometallurgical route. In Proceedings of the Thirteenth International Ferroalloys Congress Efficient Technologies in Ferroalloy Industry, Almaty, Kazakhstan, 9–13 June 2013; pp. 1051–1059. [Google Scholar]
- Kazadi, D.; Groot, D.; Pöllmann, H.; de Villiers, J.; Redtmann, T. Utilization of Ferromanganese slags for manganese extraction and as a cement additive. In Proceedings of the Advances in Cement and Concrete Technology in Africa Conference, Emperor’s Palace, Johannesburg, South Africa, 28–30 January 2013; pp. 983–995. [Google Scholar]
- Yan, S.; Qiu, Y.-R. Preparation of electronic grade manganese sulfate from leaching solution of ferromanganese slag. Trans. Nonferrous Met. Soc. China 2014, 24, 3716–3721. [Google Scholar] [CrossRef]
- Kazadi, D.; Groot, D.; Steenkamp, J.; Pöllmann, H. Control of silica polymerisation during ferromanganese slag sulphuric acid digestion and water leaching. Hydrometallurgy 2016, 166, 214–221. [Google Scholar] [CrossRef]
- Sancho, J.; Fernández Pérez, B.; Ayala, J.N.; Recio, J.C.; Rodríguez, C.; Bernardo, J.L. Method of obtaining electrolytic manganese from ferroalloy production waste. In Proceedings of the 1st Spanish National Conference on Advances in Materials Recycling and Eco—Energy, Madrid, Spain, 12–13 November 2009; pp. 126–128. [Google Scholar]
- Ghafarizadeh, B.; Rashchi, F. Separation of Manganese and Iron from Reductive Leaching Liquor of Electric Arc Furnace Dust of Ferromanganese Production Units by Solvent Extraction. Miner. Eng. 2011, 24, 174–176. [Google Scholar] [CrossRef]
- Sadeghi, S.M.; Ferreira, C.M.; Soares, H.M. Evaluation of two-step processes for the selective recovery of Mn from a rich Mn residue. Miner. Eng. 2019, 130, 148–155. [Google Scholar] [CrossRef]

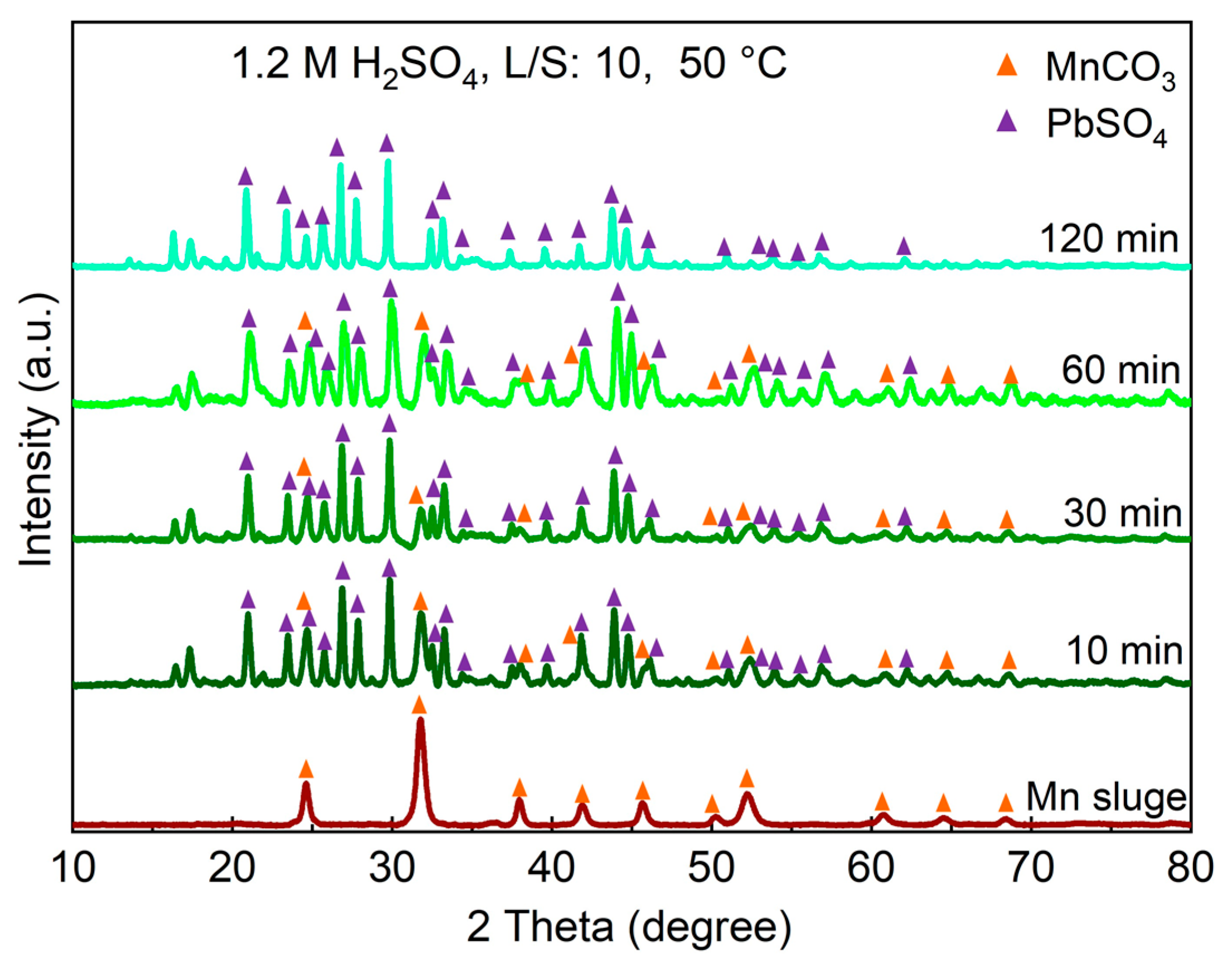

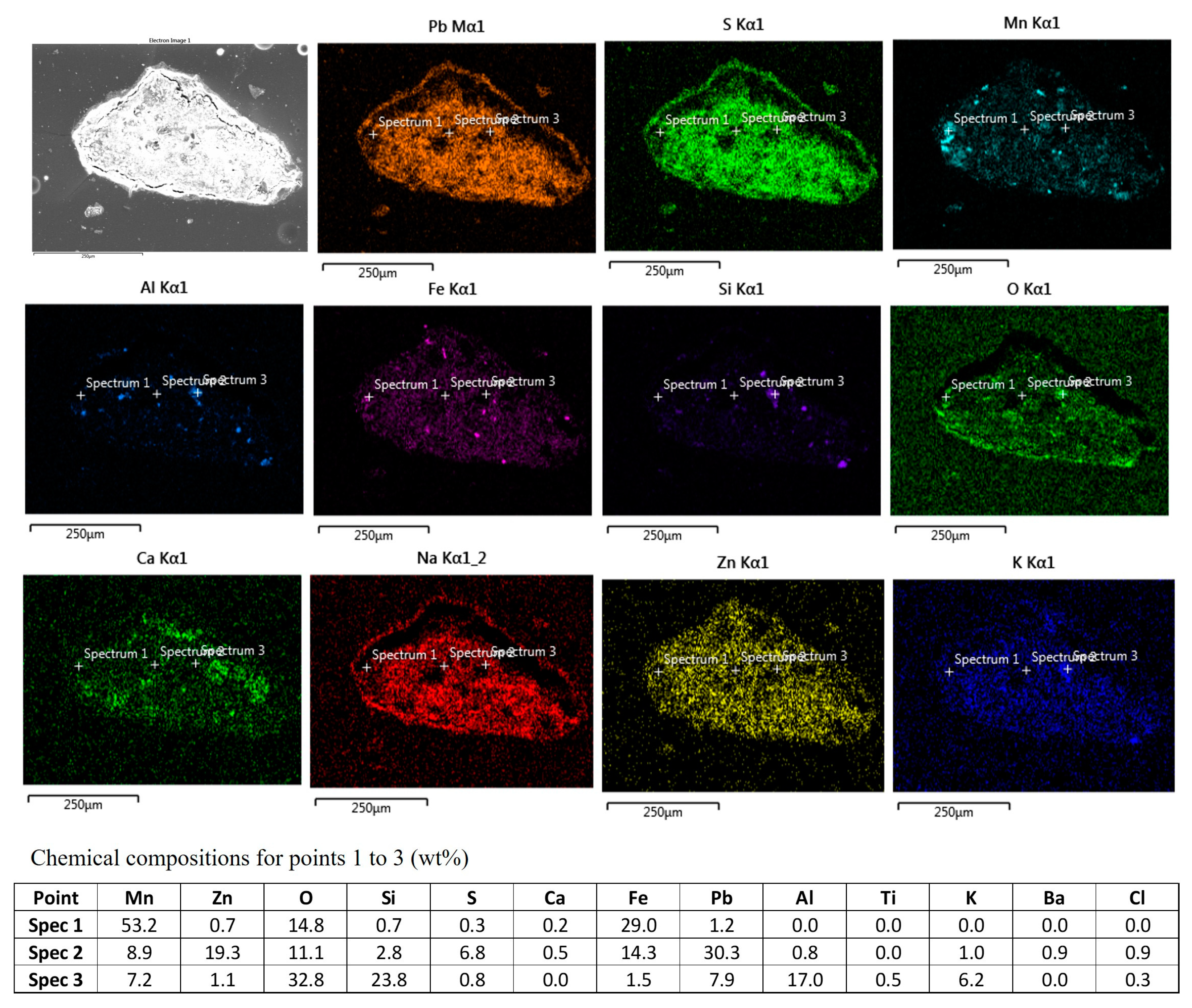
| MnCO3 | Fe2O3 | MgO | SiO2 | Al2O3 | CaO | TiO2 | BaO | ZnO | PbO | Na2O | K2O | P2O5 | CdO | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63.65 | 1.92 | 0.61 | 1.75 | 1.14 | 0.96 | 0.03 | 0.09 | 20.08 | 1.66 | 2.88 | 4.28 | 0.09 | 0.09 | 0.79 |
| Solution Concentration | Rate Constant k2 (s−1) | Rate Constant k3 (s−1) | ||
|---|---|---|---|---|
| Mn | Zn | Mn | Zn | |
| 1.2 M | 3.5 × 10−3 | 2.4 × 10−3 | 3.16 × 10−2 | 1.47 × 10−2 |
| 1.7 M | 1.2 × 10−3 | 5 × 10−4 | 5.2 × 10−3 | 1.0 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarian, J.; Eini, A.S.; Pedersen, M.A.E.; Haghdani, S. The Effect of Process Conditions on Sulfuric Acid Leaching of Manganese Sludge. Materials 2023, 16, 4591. https://doi.org/10.3390/ma16134591
Safarian J, Eini AS, Pedersen MAE, Haghdani S. The Effect of Process Conditions on Sulfuric Acid Leaching of Manganese Sludge. Materials. 2023; 16(13):4591. https://doi.org/10.3390/ma16134591
Chicago/Turabian StyleSafarian, Jafar, Ariel Skaug Eini, Markus Antonius Elinsønn Pedersen, and Shokouh Haghdani. 2023. "The Effect of Process Conditions on Sulfuric Acid Leaching of Manganese Sludge" Materials 16, no. 13: 4591. https://doi.org/10.3390/ma16134591
APA StyleSafarian, J., Eini, A. S., Pedersen, M. A. E., & Haghdani, S. (2023). The Effect of Process Conditions on Sulfuric Acid Leaching of Manganese Sludge. Materials, 16(13), 4591. https://doi.org/10.3390/ma16134591






