The Effect of the Iridium Alloying and Hydrogen Sorption on the Physicochemical and Electrochemical Properties of Palladium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Sample Analysis
2.2.3. Electrochemical Measurements
3. Results
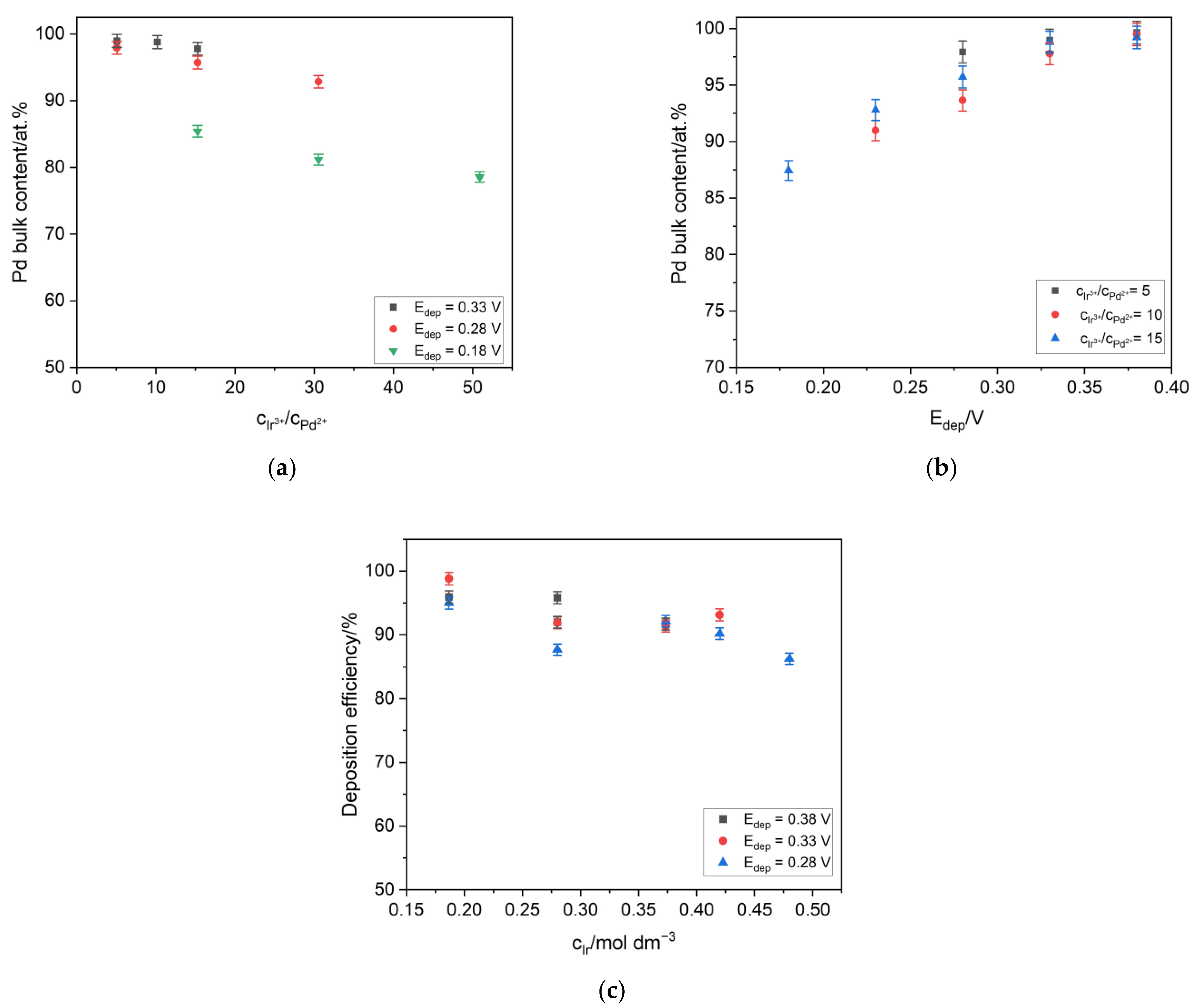
3.1. Electrodeposition of the Pd-Ir Alloys
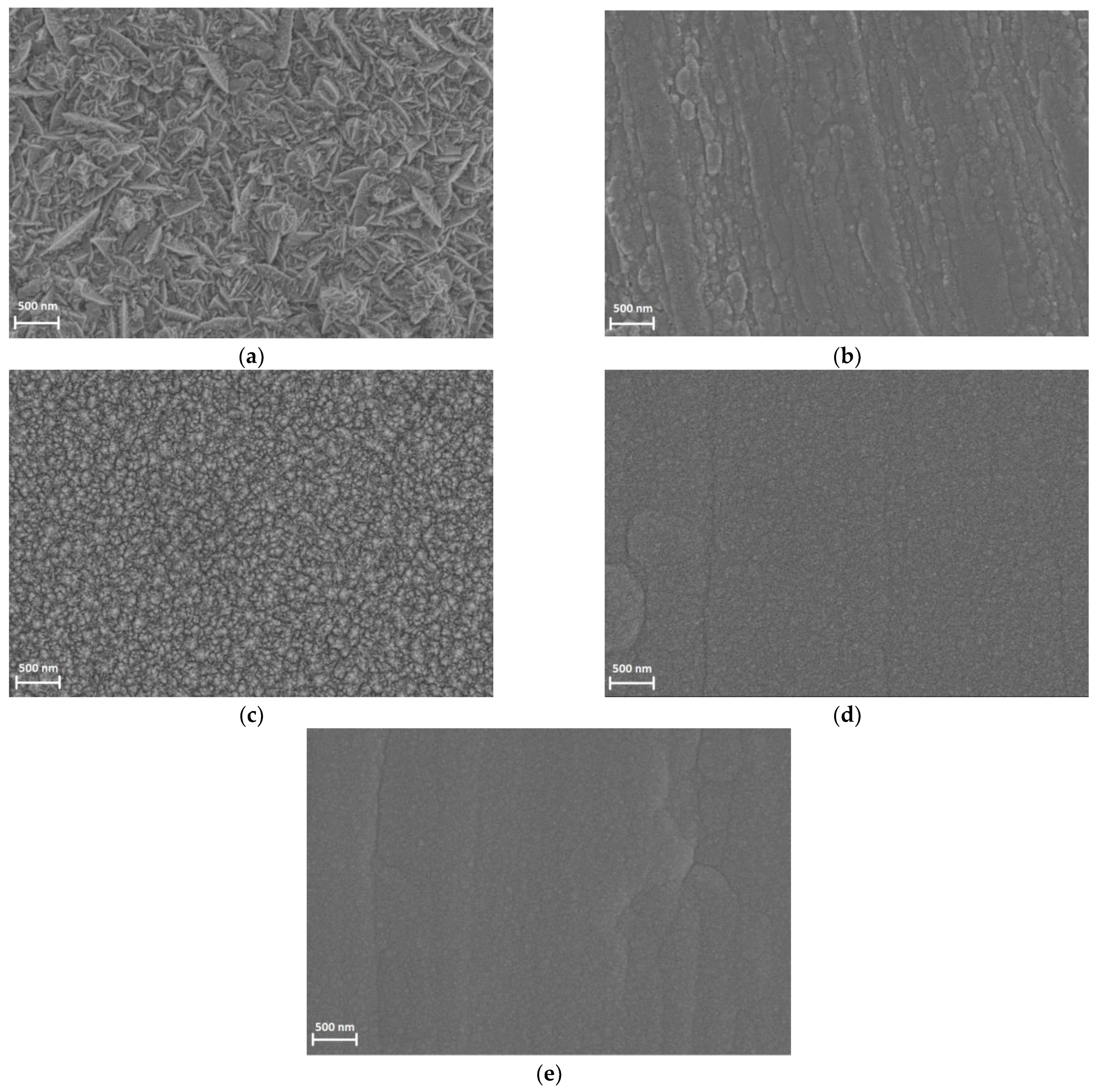
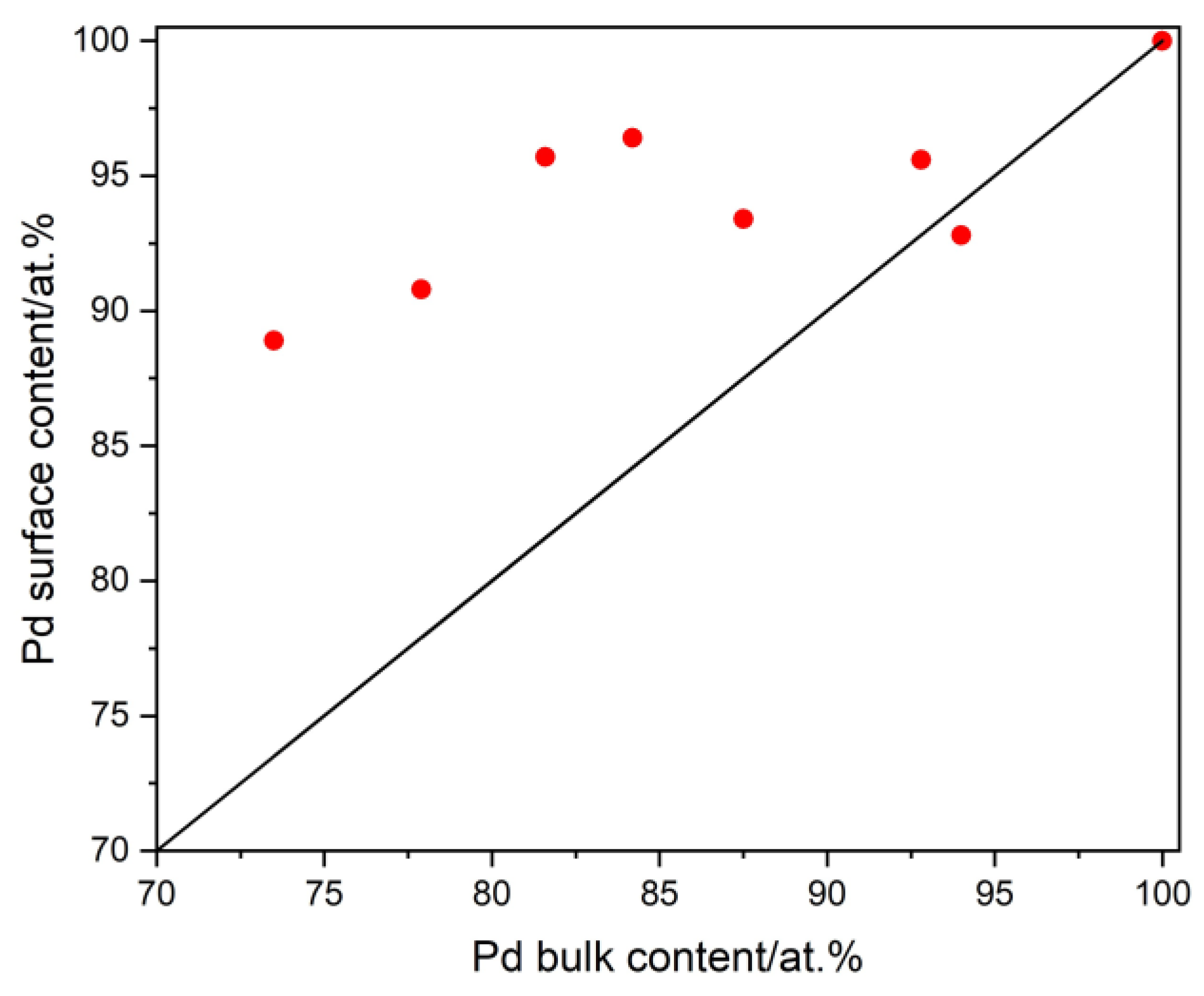
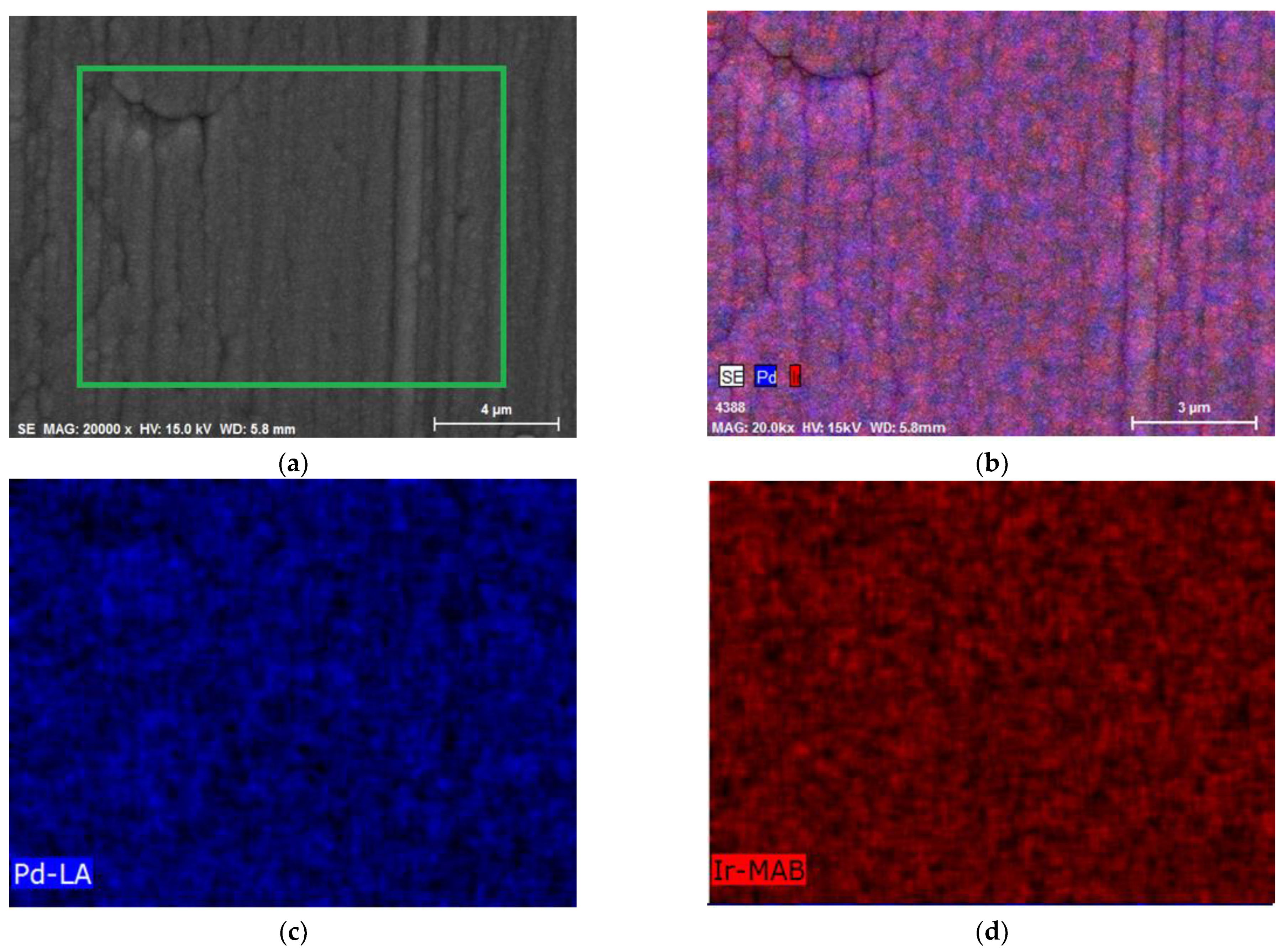
3.2. The Study of the Morphology, Composition, and the Homogeneity of Pd-Ir Alloys
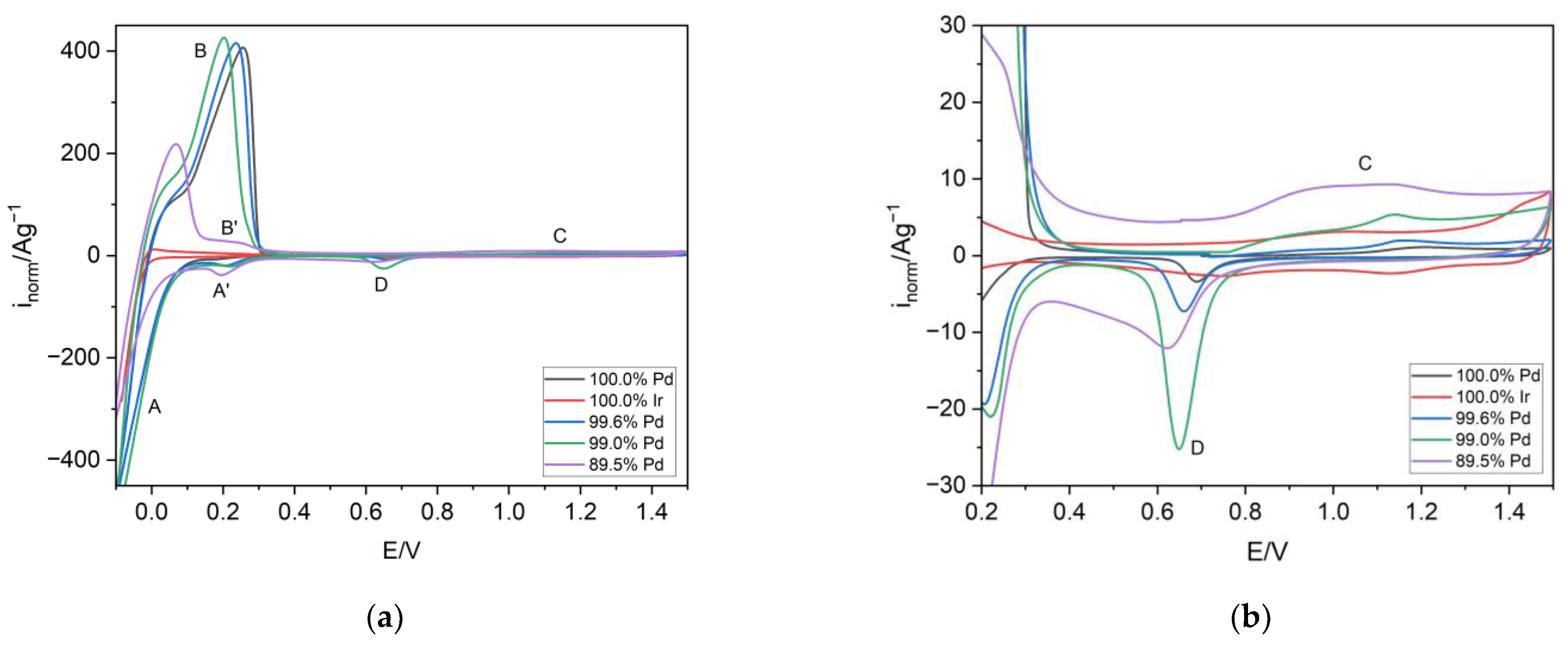
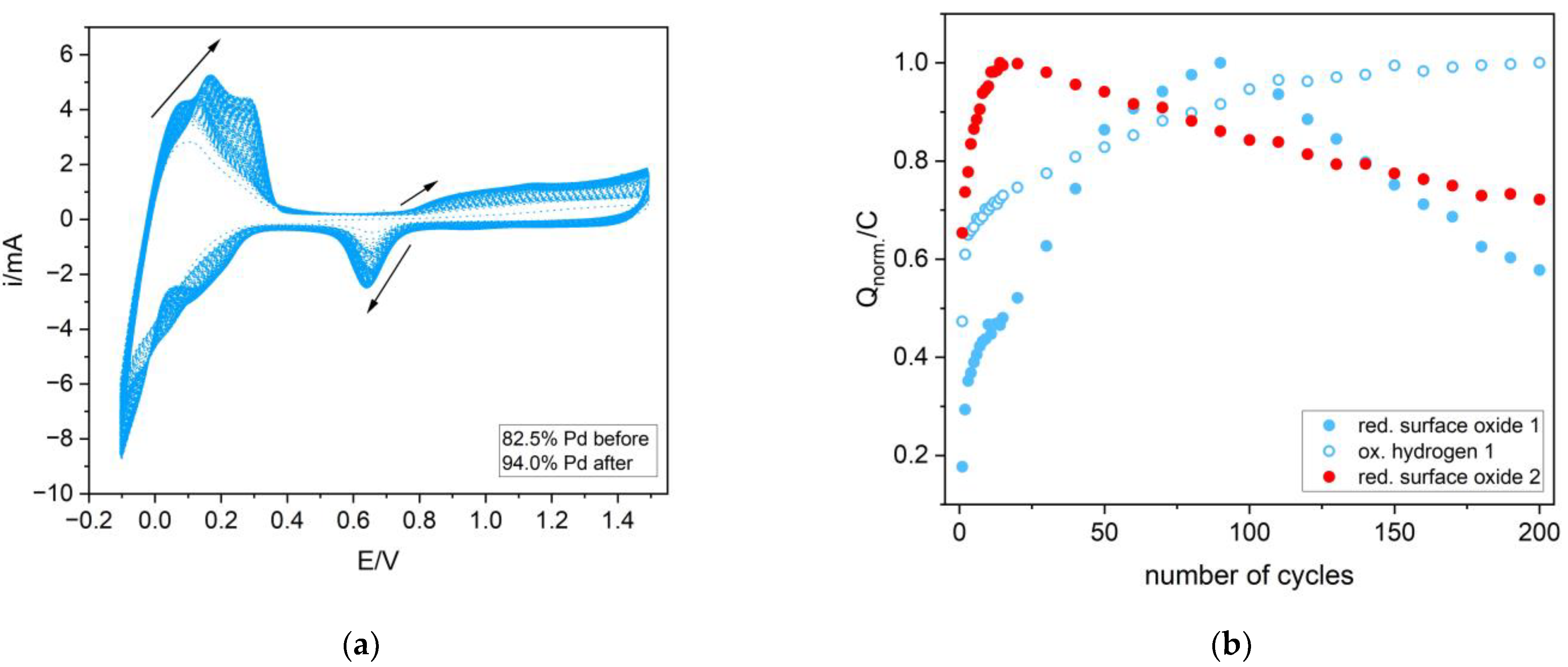
3.3. Electrochemical Dissolution of Pd-Ir Alloys
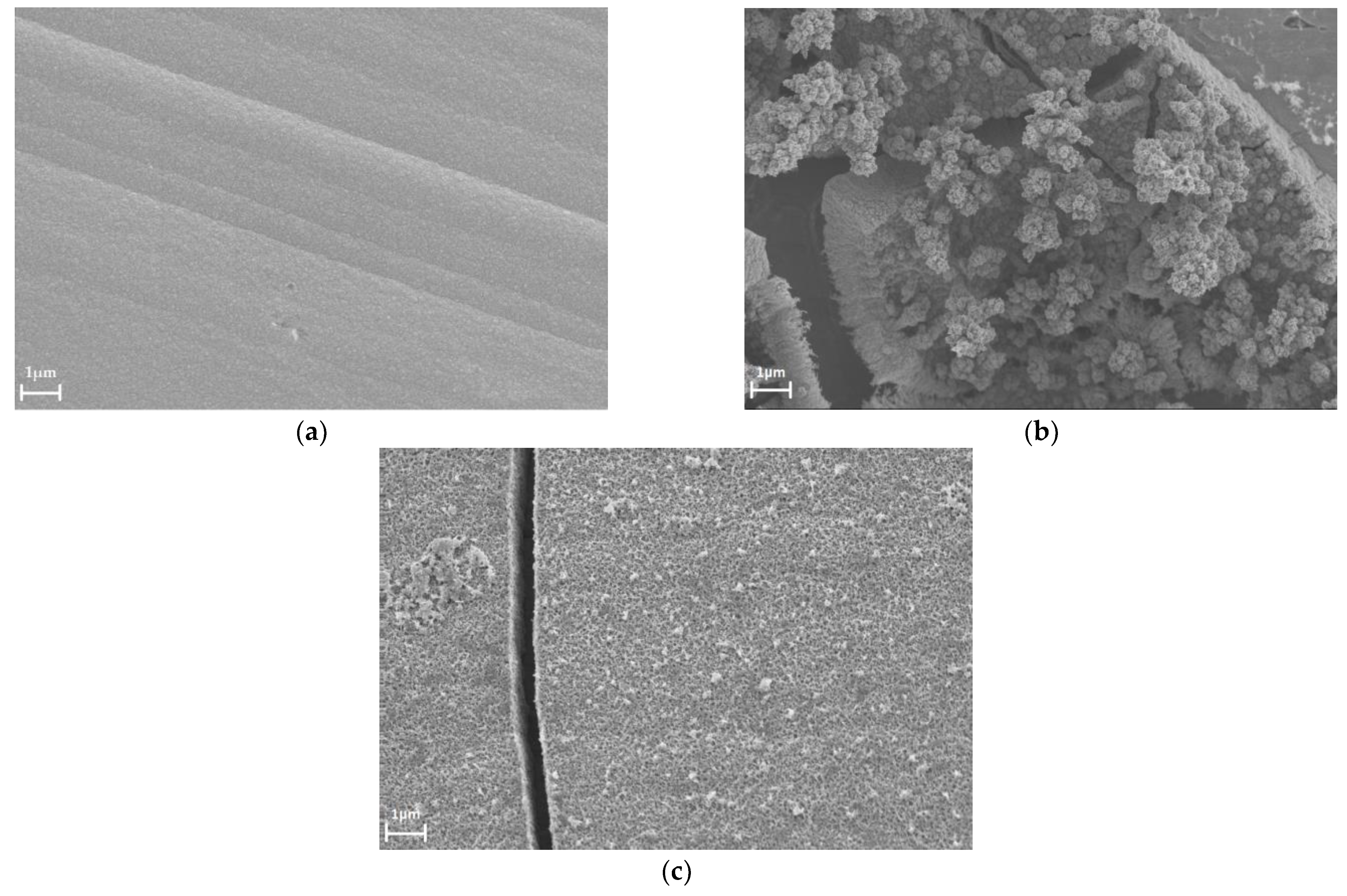
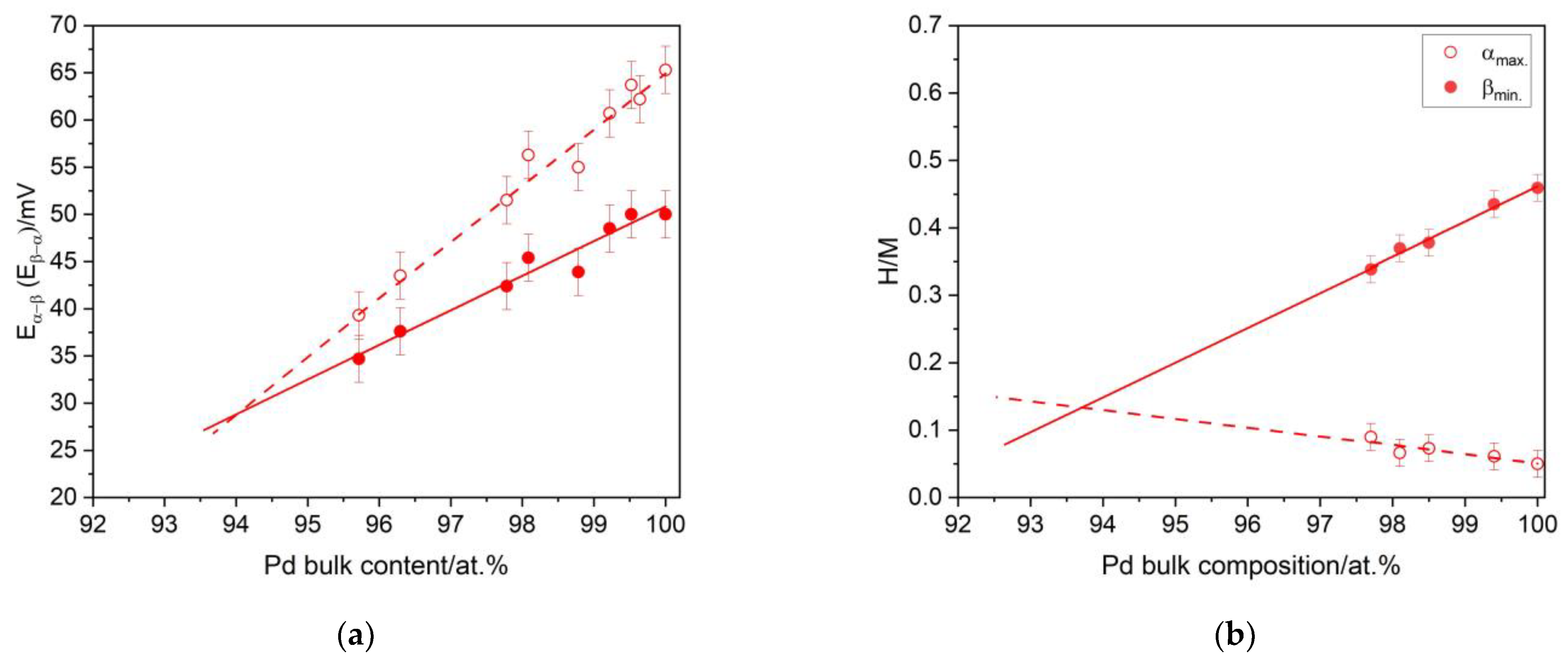
3.4. The Influence of the Hydrogen Sorption on the Surface State of the Pd-Ir Alloys
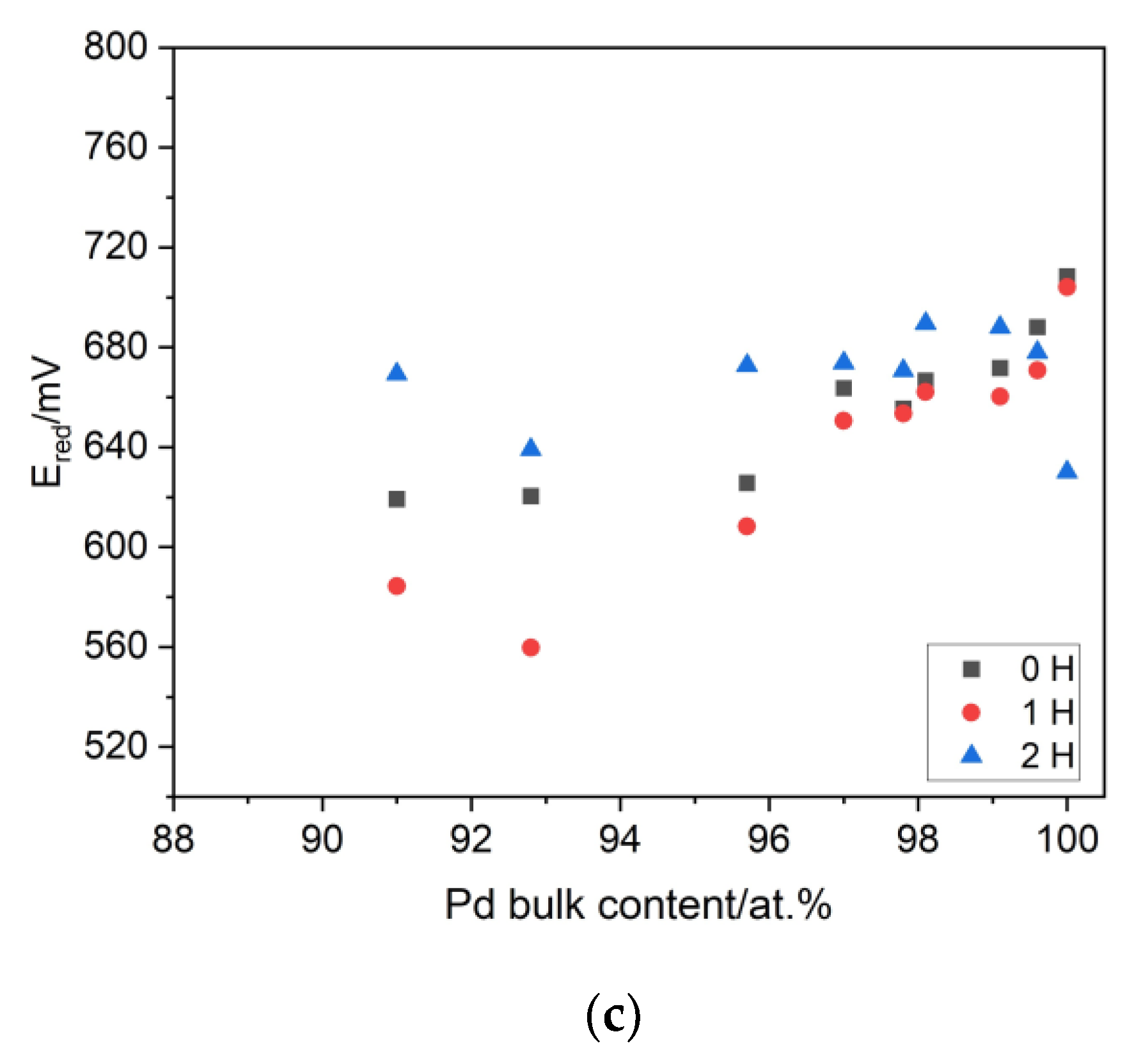
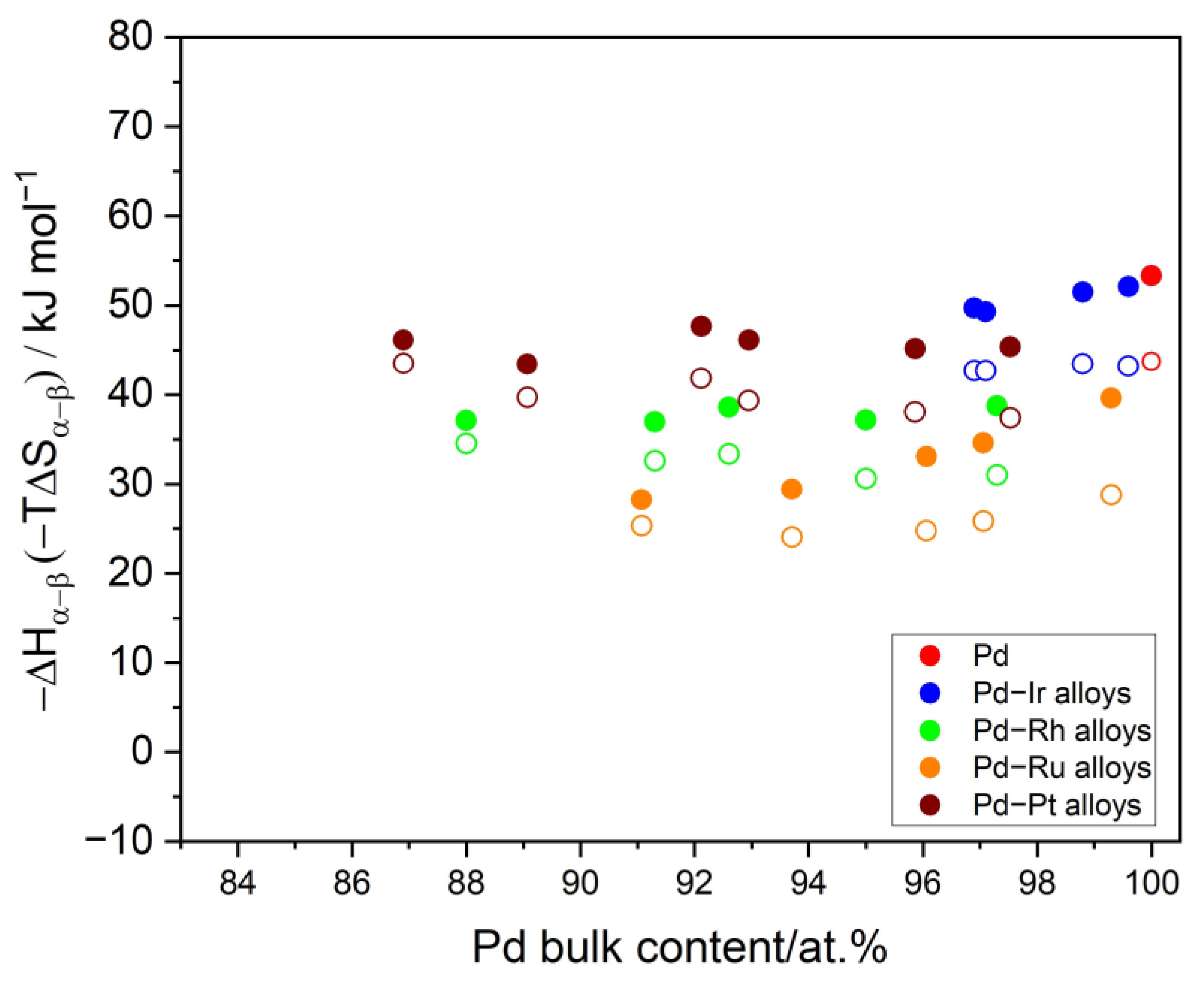
3.5. The Thermodynamics and Kinetics of the Hydrogen Absorption in Pd-Ir Alloys and the Miscibility Gap in the Pd-Ir-H System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaylock, M.L.; Klebanoff, L.E. Hydrogen Gas Dispersion Studies for Hydrogen Fuel Cell Vessels I: Vent Mast Releases. Int. J. Hydrogen Energy 2022, 47, 21506–21516. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent Development of Hydrogen and Fuel Cell Technologies: A Review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The Hydrogen-Fueled Internal Combustion Engine: A Technical Review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Wróbel, K.; Wróbel, J.; Tokarz, W.; Lach, J.; Podsadni, K.; Czerwiński, A. Hydrogen Internal Combustion Engine Vehicles: A Review. Energies 2022, 15, 8937. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal Hydride Materials for Solid Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Dematteis, E.M.; Shinzato, K.; Stevenson, S.C.; Bannenberg, L.J.; Heere, M.; Zlotea, C.; Szilágyi, P.; Bonnet, J.P.; Grochala, W.; et al. Metal Hydrides and Related Materials. Energy Carriers for Novel Hydrogen and Electrochemical Storage. J. Phys. Chem. C 2020, 124, 7599–7607. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Czerwiński, A. Electrochemical Behavior of Palladium-Gold Alloys. Electrochim. Acta 2003, 48, 2435–2445. [Google Scholar] [CrossRef]
- Piccolo, L.; Piednoir, A.; Bertolini, J.C. Absorption and Oxidation of Hydrogen at Pd and Pd-Au (1 1 1) Surfaces. Surf. Sci. 2006, 600, 4211–4215. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Olsen, R.A. Density Functional Calculations on Hydrogen in Palladium-Silver Alloys. J. Alloys Compd. 2002, 330, 332–337. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Hubkowska, K.; Czerwiński, A. Comparative Study on the Influence of Temperature, Electrode Potential and Alloy Bulk Composition on Hydrogen Electrosorption into Pd-Pt and Pd-Au Alloys. J. Electroanal. Chem. 2011, 651, 131–142. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Hubkowska, K.; Koss, U.; Czerwiński, A. On the Nature of Voltammetric Signals Originating from Hydrogen Electrosorption into Palladium-Noble Metal Alloys. Materials 2013, 6, 4817–4835. [Google Scholar] [CrossRef] [PubMed]
- Hubkowska, K.; Łukaszewski, M.; Czerwiński, A. Thermodynamics of Hydride Formation and Decomposition in Electrodeposited Pd-Rich Pd-Ru Alloys. Electrochem. Commun. 2014, 48, 40–43. [Google Scholar] [CrossRef]
- Hubkowska, K.; Łukaszewski, M.; Koss, U.; Czerwiński, A. Characterization and Electrochemical Behavior of Pd-Rich Pd-Ru Alloys. Electrochim. Acta 2014, 132, 214–222. [Google Scholar] [CrossRef]
- Wald, K.; Kubik, J.; Paciulli, D.; Talukder, M.; Nott, J.; Massicotte, F.; Rebeiz, K.; Nesbit, S.; Craft, A. Effects of Multiple Hydrogen Absorption/Desorption Cycles on the Mechanical Properties of the Alloy System Palladium/Silver (Wt% = 10–25). Scr. Mater. 2016, 117, 6–10. [Google Scholar] [CrossRef]
- Hubkowska, K.; Kubisztal, J.; Pająk, M.; Łosiewicz, B.; Czerwiński, A. Effect of the Alloying Metal on the Corrosion Resistance of Pd-Rich Binary Alloys with Pt, Rh, and Ru in Sulfuric Acid. Materials 2021, 14, 2923. [Google Scholar] [CrossRef]
- Hubkowska, K.; Pająk, M.; Czerwiński, A. Hydrogen Electrosorption Properties of Electrodeposited Pd-Ir Alloys. J. Solid State Electrochem. 2022, 26, 103–109. [Google Scholar] [CrossRef]
- Laprade, M.; Allard, K.D.; Lynch, J.F.; Flanagan, T.B. Absorption of Hydrogen by Iridium/Palladium Substitutional Alloys. J. Chem. Soc. Faraday Trans. 1974, 170, 1615–1630. [Google Scholar] [CrossRef]
- Yang, T.; Ma, Y.; Huang, Q.; Cao, G.; Wan, S.; Li, N.; Zhao, H.; Sun, X.; Yin, F. Palladium-Iridium Nanowires for Enhancement of Electro-Catalytic Activity towards Oxygen Reduction Reaction. Electrochem. Commun. 2015, 59, 95–99. [Google Scholar] [CrossRef]
- Szumełda, T.; Drelinkiewicz, A.; Kosydar, R.; Gurgul, J.; Duraczyńska, D. Synthesis of Carbon-Supported Bimetallic Palladium–Iridium Catalysts by Microemulsion: Characterization and Electrocatalytic Properties. J. Mater. Sci. 2021, 56, 392–414. [Google Scholar] [CrossRef]
- Bhalothia, D.; Wang, S.-P.; Chen, P.-Y.; Wu, H.-P.; Beniwal, A.; Yan, C.; Lee, J.-F.; Chen, T.-Y.; Chen, P.-H. Configuration-Dependent Oxygen Reduction Reaction Performance of Iridium-Decorated Ni@Pd Nanocatalysts. J. Phys. Chem. C 2023, 127, 9594–9602. [Google Scholar] [CrossRef]
- Şen, B.; Aygün, A.; Şavk, A.; Akocak, S.; Şen, F. Bimetallic Palladium–Iridium Alloy Nanoparticles as Highly Efficient and Stable Catalyst for the Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2018, 43, 20183–20191. [Google Scholar] [CrossRef]
- Davis, J.B.A.; Horswell, S.L.; Piccolo, L.; Johnston, R.L. Computational Study of the Adsorption of Benzene and Hydrogen on Palladium-Iridium Nanoalloys. J. Organomet. Chem. 2015, 792, 190–193. [Google Scholar] [CrossRef]
- Turchi, P.E.A.; Drchal, V.; Kudrnovský, J. Stability and Ordering Properties of Fcc Alloys Based on Rh, Ir, Pd, and Pt. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 064202. [Google Scholar] [CrossRef]
- Raub, E. Metals and Alloys of the Platinum Group. J. Less Common Met. 1959, 1, 3–18. [Google Scholar] [CrossRef]
- Dumont, J.; Sporken, R.; Verstraete, M.J.; Ghijsen, J.; Gonze, X. Demixing Processes in AgPd Superlattices. J. Phys. Cond. Matter 2009, 21, 315002. [Google Scholar] [CrossRef]
- Woods, R. Hydrogen Adsorption on Platinum, Iridium and Rhodium Electrodes at Reduced Temperatures and the Determination of Real Surface Area. J. Electroanal. Chem. Interfacial. Electrochem. 1974, 49, 217–226. [Google Scholar] [CrossRef]
- Hubkowska, K.; Czerwiński, A. Tuning Hydrogen Sorption Properties of Pd by Its Alloying with Ru, Rh, and Pt: The Study of Binary Alloys in Concentrated Alkaline Media. J. Solid State Electrochem. 2020, 24, 3135–3143. [Google Scholar] [CrossRef]
- Koss, U.; Łukaszewski, M.; Hubkowska, K.; Czerwiński, A. Influence of Rhodium Additive on Hydrogen Electrosorption in Palladium-Rich Pd-Rh Alloys. J. Solid State Electrochem. 2011, 15, 2477–2487. [Google Scholar] [CrossRef]
- Aas, N.; Bowker, M. The Preparation, Characterisation and Oxygen Adsorption on Ir/Pd(110) Alloys. Surf. Sci. 1994, 310, 113–127. [Google Scholar] [CrossRef]
- Schwarz, R.B.; Harms, U.; Jain, H. Elastic Stiffness of Interfaces Studied by Rayleigh Waves. Mater. Sci. Eng. A 2004, 375–377, 194–200. [Google Scholar] [CrossRef]
- Hubkowska, K.; Pająk, M.; Soszko, M.; Czerwiński, A. The Electrochemical Behavior of Unmodified and Pd-NPs Modified AB5 Hydrogen Storage Alloy in Selected Protic and Aprotic Ionic Liquids (ILs): Towards ILs-Based Electrolytes for Ni-MH Batteries. Molecules 2023, 28, 856. [Google Scholar] [CrossRef] [PubMed]


| Metal | Atomic Radius (Å) | Lattice Constant (Å) | Melting Points (°C) |
|---|---|---|---|
| Pd | 1.37 | 3.883 | 1554 |
| Ir | 1.35 | 3.831 | 2454 |
| Edep (V) | cIr/cPd | Pd Content/at.% | |||
|---|---|---|---|---|---|
| AES | XPS | EDS | AAS | ||
| 0.28 | 15.3 | 92.8 | 92.3 | 94.0 | 96.4 |
| 0.18 | 15.3 | 93.4 | 93.2 | 87.5 | 85.4 |
| 0.28 | 30.6 | 95.6 | 94.5 | 92.8 | 92.8 |
| 0.18 | 30.6 | 96.4 | 95.9 | 84.2 | 81.1 |
| 0.13 | 30.6 | 90.8 | 88.5 | 77.9 | 70.0 |
| 0.18 | 50.9 | 95.7 | 91.4 | 81.6 | 78.6 |
| 0.13 | 50.9 | 88.9 | 83.3 | 73.5 | 72.0 |
| Number of Cycles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 5′ | 15 | 15′ | 50 | 50′ | 250 | 250′ | |
| Pd bulk content/at.% | 94.0 | 95.4 | 94.9 | 96.5 | 95.2 | 97.1 | 95.4 | 100 | 95.7 |
| 86.2 | 86.7 | 86.3 | 88.0 | 86.5 | 87.8 | 86.7 | 94.1 | 86.7 | |
| 92.7 | 94.2 | 93.3 | 94.9 | 93.0 | 95.0 | 93.1 | 99.6 | 93.1 | |
| 83.4 | 84.6 | 84.2 | 85.0 | 83.9 | 86.2 | 84.3 | 99.2 | 84.3 | |
| 76.4 | 77.4 | 83.2 | 77.1 | 80.3 | 78.6 | 79.8 | 94.5 | 76.8 | |
| 80.7 | 79.6 | 80.4 | 80.6 | 78.2 | 82.5 | 78.4 | 94.0 | 81.4 | |
| 74.3 | 73.4 | 75.4 | 73.2 | 75.9 | 73.4 | 74.0 | 80.8 | 74.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubkowska, K.; Pająk, M.; Czerwiński, A. The Effect of the Iridium Alloying and Hydrogen Sorption on the Physicochemical and Electrochemical Properties of Palladium. Materials 2023, 16, 4556. https://doi.org/10.3390/ma16134556
Hubkowska K, Pająk M, Czerwiński A. The Effect of the Iridium Alloying and Hydrogen Sorption on the Physicochemical and Electrochemical Properties of Palladium. Materials. 2023; 16(13):4556. https://doi.org/10.3390/ma16134556
Chicago/Turabian StyleHubkowska, Katarzyna, Małgorzata Pająk, and Andrzej Czerwiński. 2023. "The Effect of the Iridium Alloying and Hydrogen Sorption on the Physicochemical and Electrochemical Properties of Palladium" Materials 16, no. 13: 4556. https://doi.org/10.3390/ma16134556
APA StyleHubkowska, K., Pająk, M., & Czerwiński, A. (2023). The Effect of the Iridium Alloying and Hydrogen Sorption on the Physicochemical and Electrochemical Properties of Palladium. Materials, 16(13), 4556. https://doi.org/10.3390/ma16134556






