Micro-CT Evaluation of Microgaps at Implant-Abutment Connection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microtomography Measurements
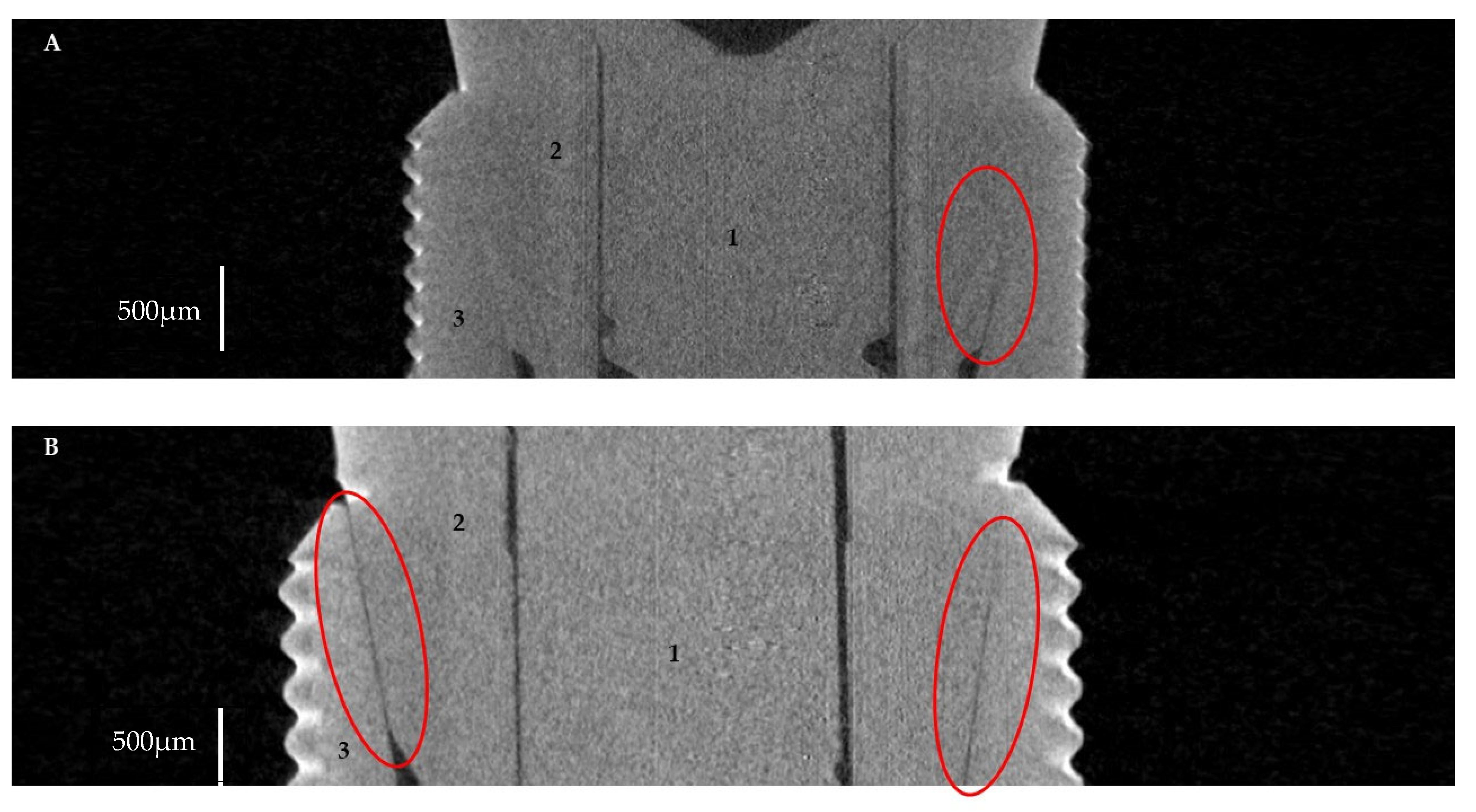
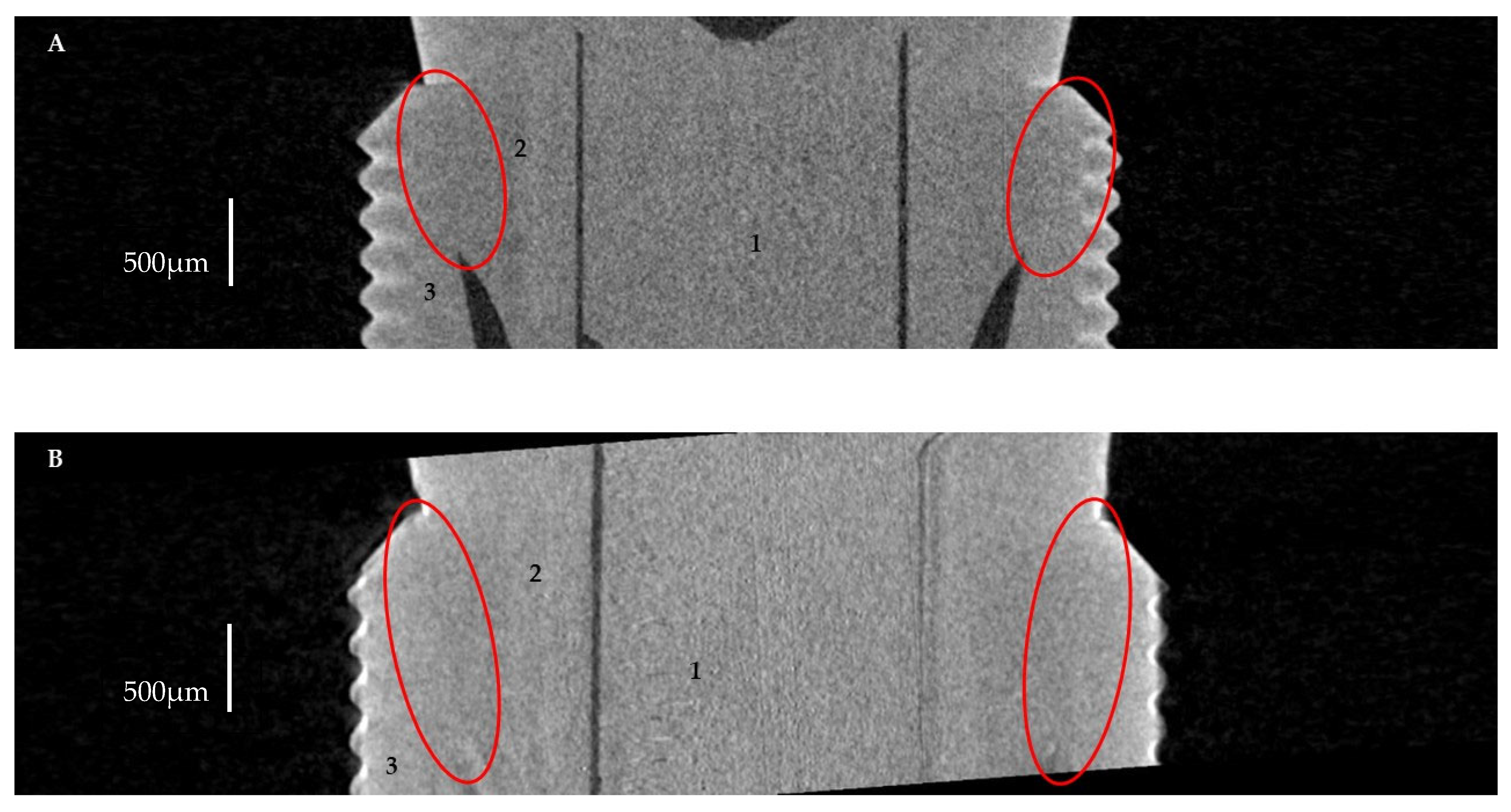
2.2.1. Horizontal Microgap (Linear Measurement)
2.2.2. Volume of Microgap
2.3. Statistical Analysis
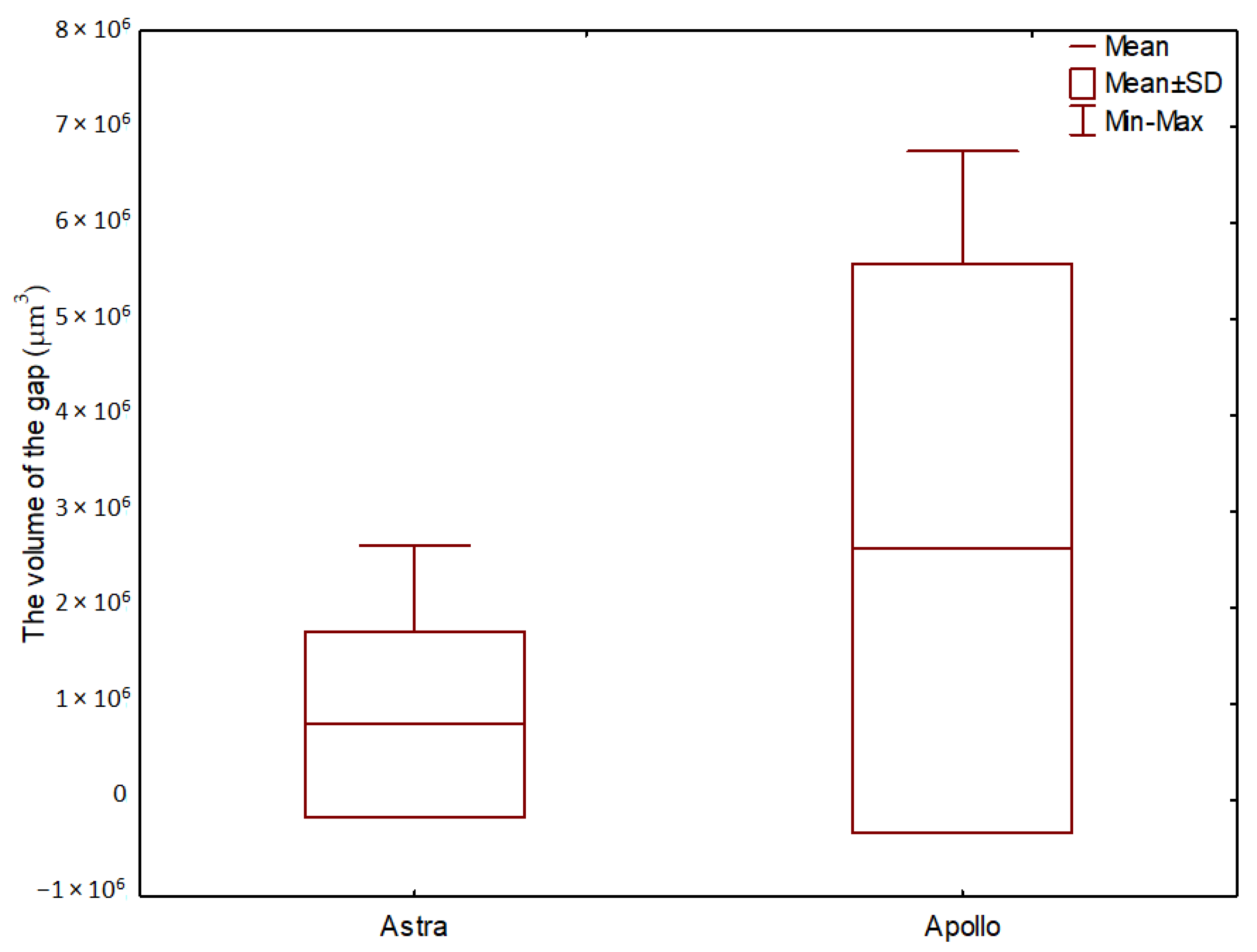
3. Results
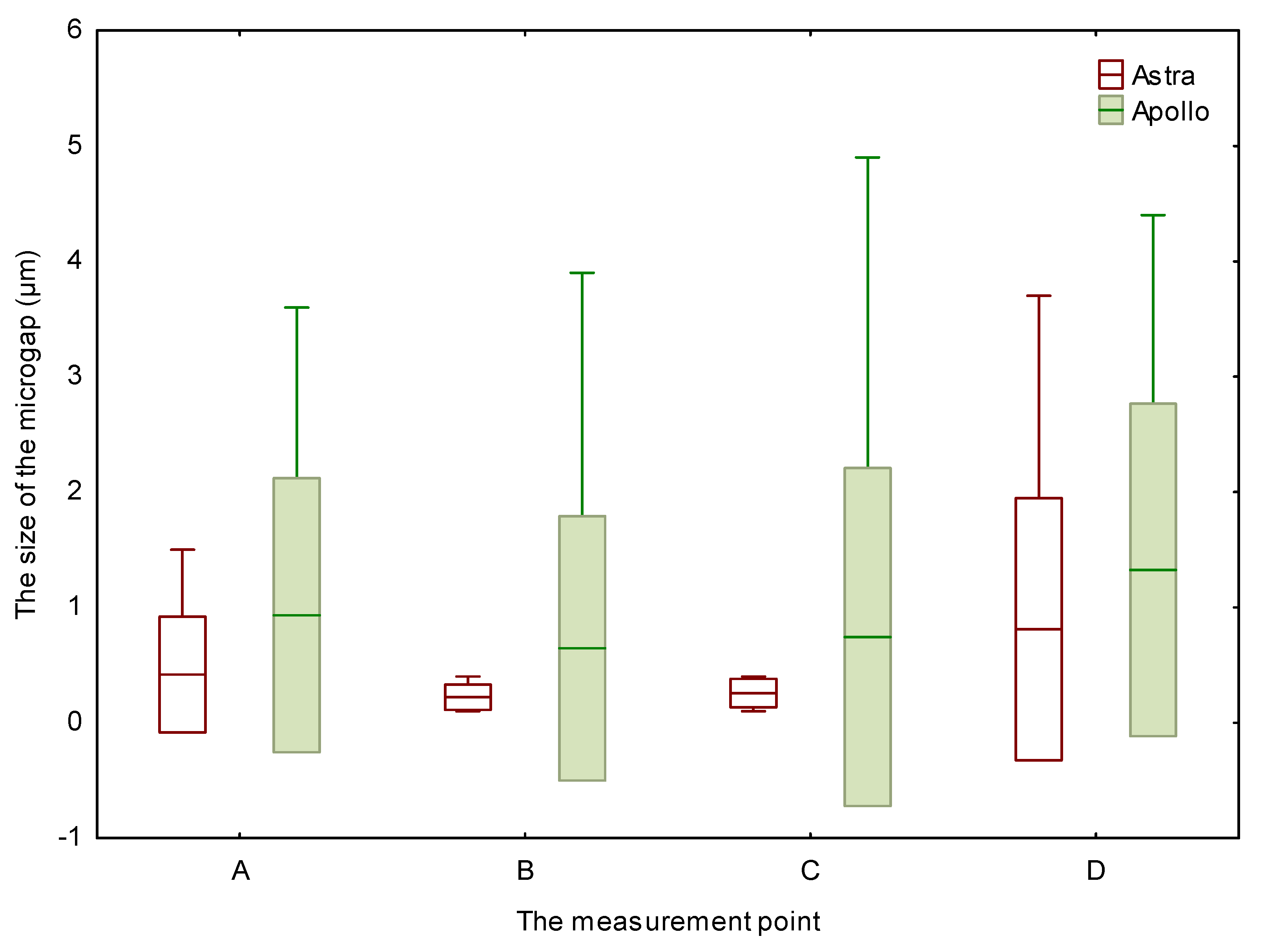
3.1. Horizontal Microgap (Linear Measurement)
3.2. The Volume of Microgaps
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study Group | Sample | Measurement Site | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Astra | 1 | − | − | − | − |
| 2 | − | − | − | − | |
| 3 | − | − | − | − | |
| 4 | + | − | − | + | |
| 5 | − | − | − | − | |
| 6 | − | − | − | − | |
| 7 | − | − | − | − | |
| 8 | − | − | − | − | |
| 9 | − | − | − | − | |
| 10 | − | − | − | + | |
| Apollo | 1 | − | − | − | − |
| 2 | + | − | − | + | |
| 3 | − | − | − | − | |
| 4 | − | − | − | − | |
| 5 | + | + | + | + | |
| 6 | − | − | − | − | |
| 7 | − | − | − | − | |
| 8 | − | − | − | + | |
| 9 | − | − | − | − | |
| 10 | − | − | − | − | |
References
- Duong, H.-Y.; Roccuzzo, A.; Stähli, A.; Salvi, G.E.; Lang, N.P.; Sculean, A. Oral Health-Related Quality of Life of Patients Rehabilitated with Fixed and Removable Implant-Supported Dental Prostheses. Periodontol. 2000 2022, 88, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Gómez-de Diego, R.; de la Rosa, M.M.; Romero-Pérez, M.-J.; Cutando-Soriano, A.; López-Valverde-Centeno, A. Indications and Contraindications of Dental Implants in Medically Compromised Patients: Update. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e483–e489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, N.; Kurt, K.; Weber, D. Dental Implants. In Bioceramics Calcium Phosphate; CRC Press: Boca Raton, FL, USA, 2022; pp. 115–130. [Google Scholar] [CrossRef]
- Kowalski, J.; Lapinska, B.; Nissan, J.; Lukomska-Szymanska, M. Factors Influencing Marginal Bone Loss around Dental Implants: A Narrative Review. Coatings 2021, 11, 865. [Google Scholar] [CrossRef]
- Rismanchian, M.; Hatami, M.; Badrian, H.; Khalighinejad, N.; Goroohi, H.; Solid, U. Evaluation of Microgap Size and Microbial Leakage in the Connection Area of 4 Abutments with Straumann (ITI) Implant. J. Oral Implantol. 2012, 38, 677–685. [Google Scholar] [CrossRef]
- Lorenzoni, F.; Coelho, P.; Bonfante, G.; Carvalho, R.; Silva, N.; Suzuki, M.; Silva, T.L.; Bonfante, E. Sealing Capability and SEM Observation of the Implant-Abutment Interface. Int. J. Dent. 2011, 2011, 864183. [Google Scholar] [CrossRef]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current Trends in Dental Implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- D’Ercole, S.; Scarano, A.; Perrotti, V.; Mulatinho, J.; Piattelli, A.; Iezzi, G.; Tripodi, D. Implants with Internal Hexagon and Conical Implant-Abutment Connections: An In Vitro Study of the Bacterial Contamination. J. Oral Implantol. 2014, 40, 30–34. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Döring, H.; Nonhoff, J. Performance of Conical Abutment (Morse Taper) Connection Implants: A Systematic Review. J. Biomed. Mater. Res. Part A 2014, 102, 552–574. [Google Scholar] [CrossRef]
- Balik, A.; Karatas, M.O.; Keskin, H. Effects of Different Abutment Connection Designs on the Stress Distribution around Five Different Implants: A 3-Dimensional Finite Element Analysis. J. Oral Implantol. 2012, 38, 491–496. [Google Scholar] [CrossRef]
- Steinebrunner, L.; Wolfart, S.; Ludwig, K.; Kern, M. Implant-Abutment Interface Design Affects Fatigue and Fracture Strength of Implants. Clin. Oral Implant. Res. 2008, 19, 1276–1284. [Google Scholar] [CrossRef]
- Souza, A.B.; Alshihri, A.M.; Kämmerer, P.W.; Araújo, M.G.; Gallucci, G.O. Histological and Micro-CT Analysis of Peri-Implant Soft and Hard Tissue Healing on Implants with Different Healing Abutments Configurations. Clin. Oral Implant. Res. 2018, 29, 1007–1015. [Google Scholar] [CrossRef]
- Lopes, P.A.; Carreiro, A.F.P.; Nascimento, R.M.; Vahey, B.R.; Henriques, B.; Souza, J.C.M. Physicochemical and Microscopic Characterization of Implant-Abutment Joints. Eur. J. Dent. 2018, 12, 100–104. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Cascos-Sanchez, R.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Del Río Highsmith, J.; Gómez-Polo, M. Effect of Fabrication Technique on the Microgap of CAD/CAM Cobalt-Chrome and Zirconia Abutments on a Conical Connection Implant: An in Vitro Study. Materials 2021, 14, 2348. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant Biomaterials: A Comprehensive Review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Pérez, R.A.; Manero, J.M.; Mur, J.G. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef]
- Palacios-Garzón, N.; Velasco-Ortega, E.; López-López, J. Bone Loss in Implants Placed at Subcrestal and Crestal Level: A Systematic Review and Meta-Analysis. Materials 2019, 12, 154. [Google Scholar] [CrossRef]
- Piattelli, A.; Vrespa, G.; Petrone, G.; Iezzi, G.; Annibali, S.; Scarano, A. Role of the Microgap Between Implant and Abutment: A Retrospective Histologic Evaluation in Monkeys. J. Periodontol. 2003, 74, 346–352. [Google Scholar] [CrossRef]
- Sasada, Y.; Cochran, D. Implant-Abutment Connections: A Review of Biologic Consequences and Peri-Implantitis Implications. Int. J. Oral Maxillofac. Implant. 2017, 32, 1296–1307. [Google Scholar] [CrossRef]
- Do Nascimento, C.; Ikeda, L.N.; Pita, M.S.; Pedroso Esilva, R.C.; Pedrazzi, V.; De Albuquerque, R.F.; Ribeiro, R.F. Marginal Fit and Microbial Leakage along the Implant-Abutment Interface of Fixed Partial Prostheses: An in Vitro Analysis Using Checkerboard DNA-DNA Hybridization. J. Prosthet. Dent. 2015, 114, 831–838. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, G.A.S.; Bernardes, S.R.; das Neves, F.D.; Fernandes Neto, A.J.; de Mattos, M.d.G.C.; Ribeiro, R.F. Relation between Implant/Abutment Vertical Misfit and Torque Loss of Abutment Screws. Braz. Dent. J. 2008, 19, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ricomini Filho, A.P.; Fernandes, F.S.d.F.; Straioto, F.G.; da Silva, W.J.; del Bel Cury, A.A. Preload Loss and Bacterial Penetration on Different Implant-Abutment Connection Systems. Braz. Dent. J. 2010, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.J.; Herrero-Climent, M.; Lázaro, P.; Rios, J.V. Implant-Abutment Connections: Influence of the Design on the Microgap and Their Fatigue and Fracture Behavior of Dental Implants. J. Mater. Sci. Mater. Med. 2014, 25, 1825–1830. [Google Scholar] [CrossRef]
- Gigandet, M.; Bigolin, G.; Faoro, F.; Bürgin, W.; Brägger, U. Implants with Original and Non-Original Abutment Connections. Clin. Implant. Dent. Relat. Res. 2014, 16, 303–311. [Google Scholar] [CrossRef]
- Keklikoglou, K.; Arvanitidis, C.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Karagiannidis, E.; Koletsa, T.; Magoulas, A.; Makris, K.; Mavrothalassitis, G.; Papanagnou, E.D.; et al. Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. J. Imaging 2021, 7, 172. [Google Scholar] [CrossRef]
- Tsuge, T.; Hagiwara, Y.; Matsumura, H. Marginal Fit and Microgaps of Implant-Abutment Interface with Internal Anti-Rotation Configuration. Dent. Mater. J. 2008, 27, 29–34. [Google Scholar] [CrossRef]
- Vélez, J.; Peláez, J.; López-Suárez, C.; Agustín-Panadero, R.; Tobar, C.; Suárez, M.J. Influence of Implant Connection, Abutment Design and Screw Insertion Torque on Implant-Abutment Misfit. J. Clin. Med. 2020, 9, 2365. [Google Scholar] [CrossRef]
- Cavusoglu, Y.; Akça, K.; Gürbüz, R.; Cavit Cehreli, M. A Pilot Study of Joint Stability at the Zirconium or Titanium Abutment/Titanium Implant Interface. Int. J. Oral Maxillofac. Implant. 2014, 29, 338–343. [Google Scholar] [CrossRef]
- Du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory X-Ray Micro-Computed Tomography: A User Guideline for Biological Samples. Gigascience 2017, 6, 1. [Google Scholar] [CrossRef]
- Kano, S.C.; Binon, P.P.; Curtis, D.A. A Classification System to Measure the Implant-Abutment Microgap. Int. J. Oral Maxillofac. Implant. 2007, 22, 879–885. [Google Scholar]
- Vásárhelyi, L.; Kónya, Z.; Kukovecz, A.; Vajtai, R. Microcomputed Tomography–Based Characterization of Advanced Materials: A Review. Mater. Today Adv. 2020, 8, 100084. [Google Scholar] [CrossRef]
- Narra, N.; Antalainen, A.-K.; Zipprich, H.; Sandor, G.K.; Wolff, J. Microcomputed Tomography–Based Assessment of Retrieved Dental Implants. Int. J. Oral Maxillofac. Implant. 2015, 30, 308–314. [Google Scholar] [CrossRef]
- Sacker, T.N.; Trentin, M.S.; dos Santos, T.M.P.; Lopes, R.T.; Rivaldo, E.G. In Vitro Microtomography Evaluation of the Implant-Abutment Interface—Gap Microtomography Evaluation/Avaliação Da Microtomografia in Vitro Da Interface Implante-Pilar—Avaliação Da Microtomografia de Gap. Braz. J. Dev. 2021, 7, 57552–57565. [Google Scholar] [CrossRef]
- Tian, K.V.; Nagy, P.M.; Chass, G.A.; Fejerdy, P.; Nicholson, J.W.; Csizmadia, I.G.; Dobó-Nagy, C. Qualitative Assessment of Microstructure and Hertzian Indentation Failure in Biocompatible Glass Ionomer Cements. J. Mater. Sci. Mater. Med. 2012, 23, 677–685. [Google Scholar] [CrossRef]
- Radwanski, M.; Leski, M.; Puszkarz, A.K.; Sokolowski, J.; Hardan, L.; Bourgi, R.; Sauro, S.; Lukomska-Szymanska, M. A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers. Biomedicines 2022, 10, 2403. [Google Scholar] [CrossRef]
- Ujiie, Y.; Todescan, R.; Davies, J.E. Peri-Implant Crestal Bone Loss: A Putative Mechanism. Int. J. Dent. 2012, 2012, 14. [Google Scholar] [CrossRef]
- Assenza, B.; Tripodi, D.; Scarano, A.; Perrotti, V.; Piattelli, A.; Iezzi, G.; D’Ercole, S. Bacterial Leakage in Implants With Different Implant–Abutment Connections: An In Vitro Study. J. Periodontol. 2012, 83, 491–497. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; Paula, L. Review of the Mechanical Behavior of Di Ff Erent Implant—Abutment Connections. Int. J. Environ. Res. Public Health Rev. 2020, 17, 8685. [Google Scholar] [CrossRef]
- Camós-Tena, R.; Escuin-Henar, T.; Torné-Duran, S. Conical Connection Adjustment in Prosthetic Abutments Obtained by Different Techniques. J. Clin. Exp. Dent. 2019, 11, e408. [Google Scholar] [CrossRef]
- Al-Thobity, A.M. Titanium Base Abutments in Implant Prosthodontics: A Literature Review. Eur. J. Dent. 2022, 16, 49–55. [Google Scholar] [CrossRef]
- Baldassarri, M.; Hjerppe, J.; Romeo, D.; Fickl, S.; Thompson, V.P.; Stappert, C.F.J. Marginal Accuracy of Three Implant-Ceramic Abutment Configurations. Int. J. Oral Maxillofac. Implant. 2012, 27, 537–543. [Google Scholar]
- Hosseini, M.; Worsaae, N.; Gotfredsen, K. A 5-Year Randomized Controlled Trial Comparing Zirconia-Based versus Metal-Based Implant-Supported Single-Tooth Restorations in the Premolar Region. Clin. Oral Implant. Res. 2022, 33, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; Relvas, M.; Braga, A.C.; Ríos-Carrasco, B.; Gil, J.; Rios-Santos, J.V.; Fernández-Palacín, A.; Herrero-Climent, M. In Vitro Study of Preload Loss in Different Implant Abutment Connection Designs. Materials 2022, 15, 1392. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.M.; Makke, A.; Elbanna, K. Radigraphic Assessment of Accuracy of Fit for Different Conical Connection Abutments on Tapered Implants. Egypt. Dent. J. 2020, 66, 2529–2539. [Google Scholar] [CrossRef]
- Lopes de Chaves e Mello Dias, E.; Sperandio, M.; Napimoga, M. Association Between Implant-Abutment Microgap and Implant Circularity to Bacterial Leakage: An In Vitro Study Using Tapered Connection Implants. Int. J. Oral Maxillofac. Implant. 2017, 33, 505–511. [Google Scholar] [CrossRef]
- Khorshidi, H.; Raoofi, S.; Moattari, A.; Bagheri, A.; Kalantari, M.H. In Vitro Evaluation of Bacterial Leakage at Implant-Abutment Connection: An 11-Degree Morse Taper Compared to a Butt Joint Connection. Int. J. Biomater. 2016, 2016, 8527849. [Google Scholar] [CrossRef]
- Tesmer, M.; Wallet, S.; Koutouzis, T.; Lundgren, T. Bacterial Colonization of the Dental Implant Fixture–Abutment Interface: An In Vitro Study. J. Periodontol. 2009, 80, 1991–1997. [Google Scholar] [CrossRef]
- Shadid, R.; Sadaqah, N.; Al-Omari, W.; Abu-Naba’a, L. Comparison between the Butt-Joint and Morse Taper Implant-Abutment Connection: A Literature Review. J. Implant. Adv. Clin. Dent. 2013, 5, 33–40. [Google Scholar]
- Do Nascimento, C.; Pita, M.S.; Santos, E.D.S.; Monesi, N.; Pedrazzi, V.; De Albuquerque Junior, R.F.; Ribeiro, R.F. Microbiome of Titanium and Zirconia Dental Implants Abutments. Dent. Mater. 2016, 32, 93–101. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Influences of Microgap and Micromotion of Implant–Abutment Interface on Marginal Bone Loss around Implant Neck. Arch. Oral Biol. 2017, 83, 153–160. [Google Scholar] [CrossRef]
- Toia, M.; Galli, S.; Cecchinato, D.; Wennerberg, A.; Jimbo, R. Clinical Evidence of OsseoSpeed EV Implants: A Retrospective Study and Characterization of the Newly Introduced System. Int. J. Periodontics Restor. Dent. 2019, 39, 863–874. [Google Scholar] [CrossRef]
- Jemt, T.; Book, K. Prosthesis Misfit and Marginal Bone Loss in Edentulous Implant Patients. Int. J. Oral Maxillofac. Implant. 1996, 11, 620–625. [Google Scholar]
- Duraisamy, R.; Krishnan, C.S.; Ramasubramanian, H.; Sampathkumar, J.; Mariappan, S.; Navarasampatti Sivaprakasam, A. Compatibility of Nonoriginal Abutments with Implants: Evaluation of Microgap at the Implant-Abutment Interface, with Original and Nonoriginal Abutments. Implant. Dent. 2019, 28, 289–295. [Google Scholar] [CrossRef]
- Solá-Ruíz, M.F.; Selva-Otaolaurruchi, E.; Senent-Vicente, G.; González-de-Cossio, I.; Amigó-Borrás, V. Accuracy Combining Different Brands of Implants and Abutments. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e332. [Google Scholar] [CrossRef]
- Canullo, L.; Penarrocha-oltra, D. Microbiological Assessment of the Implant-Abutment Interface in Different Connections: Cross-Sectional Study after 5 Years of Functional Loading. Clin. Oral Implant. Res. 2015, 26, 426–434. [Google Scholar] [CrossRef]
- Bagegni, A.; Zabler, S.; Nelson, K.; Rack, A.; Spies, B.C.; Vach, K.; Kohal, R. Synchrotron-Based Micro Computed Tomography Investigation of the Implant-Abutment Fatigue-Induced Microgap Changes. J. Mech. Behav. Biomed. Mater. 2021, 116, 104330. [Google Scholar] [CrossRef]
- Tsuruta, K.; Ayukawa, Y.; Matsuzaki, T.; Kihara, M.; Koyano, K. The Influence of Implant–Abutment Connection on the Screw Loosening and Microleakage. Int. J. Implant. Dent. 2018, 4, 11. [Google Scholar] [CrossRef]
- Ramalho, I.; Witek, L.; Coelho, P.G.; Bergamo, E.; Pegoraro, L.F.; Bonfante, E.A. Influence of Abutment Fabrication Method on 3D Fit at the Implant-Abutment Connection. Int. J. Prosthodont. 2020, 33, 641–647. [Google Scholar] [CrossRef]
- Cardoso, K.B.; Bergamo, E.T.P.; Cruz, V.D.M.; Ramalho, I.S.; Lino, L.F.D.O.; Bonfante, E.A. Three-Dimensional Misfit between Ti-Base Abutments and Implants Evaluated by Replica Technique. J. Appl. Oral Sci. 2020, 28, e20200343. [Google Scholar] [CrossRef]
- Lops, D.; Meneghello, R.; Sbricoli, L.; Savio, G.; Bressan, E.; Stellini, E. Precision of the Connection Between Implant and Standard or Computer-Aided Design/Computer-Aided Manufacturing Abutments: A Novel Evaluation Method. Int. J. Oral Maxillofac. Implant. 2018, 33, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.C.C.; de Carvalho, P.S.P.; Elias, C.N.; Vedovatto, E.; Martinez, E.F. In Vitro Analysis of the Microbiological Sealing of Tapered Implants after Mechanical Cycling. Clin. Oral Investig. 2016, 20, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Berberi, A.; Tehini, G.; Rifai, K.; Bou Nasser Eddine, F.; El Zein, N.; Badran, B.; Akl, H. In Vitro Evaluation of Leakage at Implant-Abutment Connection of Three Implant Systems Having the Same Prosthetic Interface Using Rhodamine B. Int. J. Dent. 2014, 2014, 351263. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Mortellaro, Ã.C.; Mavriqi, L.; Pecci, R.; Valbonetti, L. Evaluation of Microgap with Three-Dimensional X-Ray Microtomography: Internal Hexagon Versus Cone Morse. J. Craniofac. Surg. 2016, 27, 2015–2017. [Google Scholar] [CrossRef]
- Blum, K.; Wiest, W.; Fella, C.; Balles, A.; Dittmann, J.; Rack, A.; Maier, D.; Thomann, R.; Spies, B.C.; Kohal, R.J.; et al. Fatigue Induced Changes in Conical Implant-Abutment Connections. Dent. Mater. 2015, 31, 1415–1426. [Google Scholar] [CrossRef]
- Larrucea Verdugo, C.; Jaramillo Núñez, G.; Acevedo Avila, A.; Larrucea San Martín, C. Microleakage of the Prosthetic Abutment/Implant Interface with Internal and External Connection: In Vitro Study. Clin. Oral Implant. Res. 2014, 25, 1078–1083. [Google Scholar] [CrossRef]
- He, Y.; Fok, A.; Aparicio, C.; Teng, W. Contact Analysis of Gap Formation at Dental Implant-Abutment Interface under Oblique Loading: A Numerical-Experimental Study. Clin. Implant. Dent. Relat. Res. 2019, 21, 741–752. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R.; Kumari, S. Microleakage at the Different Implant Abutment Interface: A Systematic Review. J. Clin. Diagn. Res. 2017, 11, ZE10. [Google Scholar] [CrossRef]
- Gehrke, S.; Pereira, F. Changes in the Abutment-Implant Interface in Morse Taper Implant Connections After Mechanical Cycling: A Pilot Study. Int. J. Oral Maxillofac. Implant. 2014, 29, 791–797. [Google Scholar] [CrossRef]
- Bhering, C.L.B.; Takahashi, J.M.F.K.; Luthi, L.F.; Henriques, G.E.P.; Consani, R.L.X.; Mesquita, M.F. Influence of the Casting Technique and Dynamic Loading on Screw Detorque and Misfit of Single Unit Implant-Supported Prostheses. Acta Odontol. Scand. 2013, 71, 404–409. [Google Scholar] [CrossRef]
- De Jesus Tavarez, R.R.; Bonachela, W.C.; Xible, A.A. Effect of Cyclic Load on Vertical Misfit of Prefabricated and Cast Implant Single Abutment. J. Appl. Oral Sci. 2011, 19, 16–21. [Google Scholar] [CrossRef]
- Jorge, J.R.P.; Barao, V.A.R.; Delben, J.A.; Assuncao, W.G. The Role of Implant/Abutment System on Torque Maintenance of Retention Screws and Vertical Misfit of Implant-Supported Crowns Before and After Mechanical Cycling. Int. J. Oral Maxillofac. Implant. 2013, 28, 415–422. [Google Scholar] [CrossRef]
- Shin, H.-M.; Huh, J.-B.; Yun, M.-J.; Jeon, Y.-C.; Chang, B.M.; Jeong, C.-M. Influence of the Implant-Abutment Connection Design and Diameter on the Screw Joint Stability. J. Adv. Prosthodont. 2014, 6, 126–132. [Google Scholar] [CrossRef]
- Smith, N.A.; Turkyilmaz, I. Evaluation of the Sealing Capability of Implants to Titanium and Zirconia Abutments against PorphyromonasGingivalis, Prevotella Intermedia, and Fusobacterium Nucleatum under Different Screw Torque Values. J. Prosthet. Dent. 2014, 112, 561–567. [Google Scholar] [CrossRef]
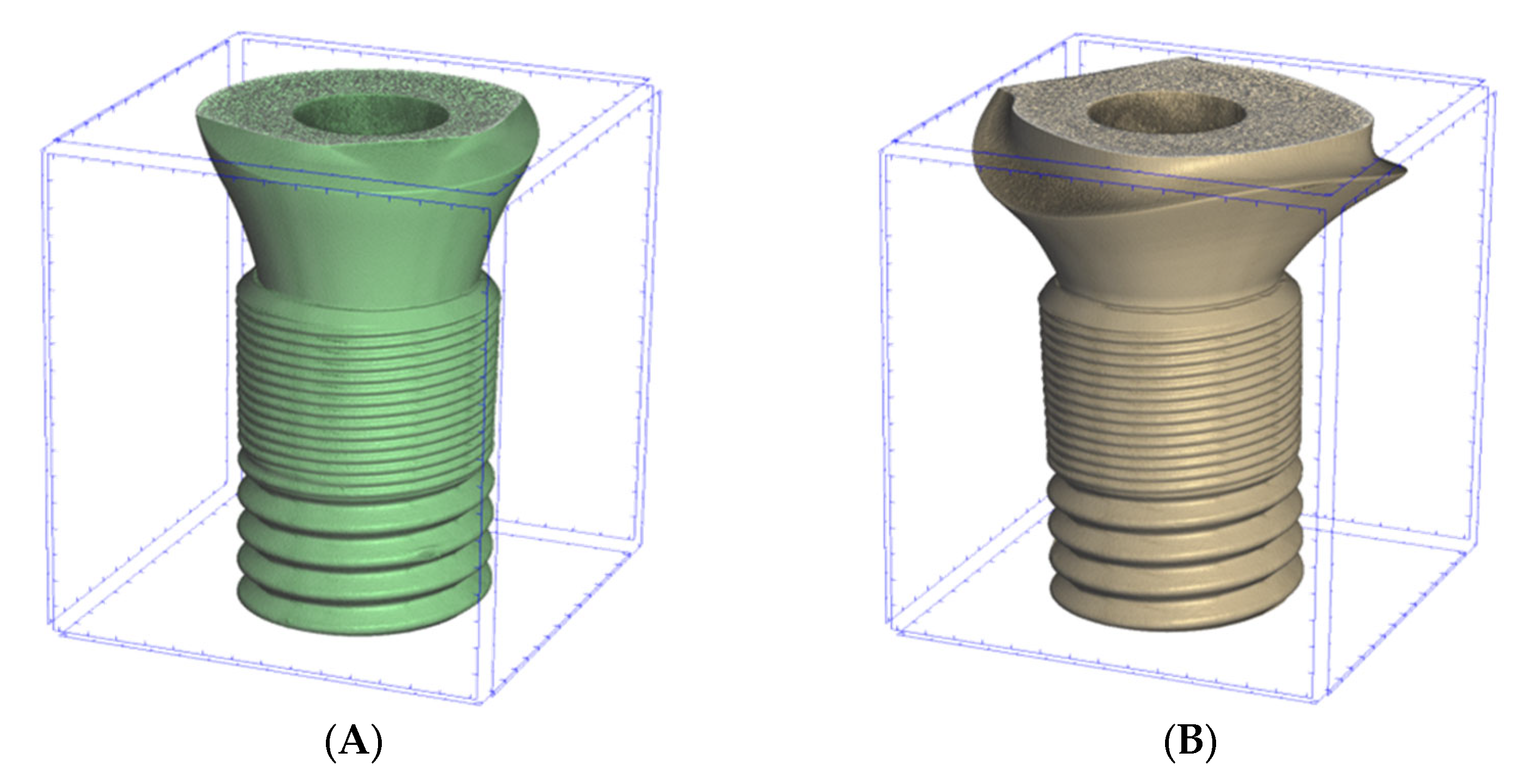

| Astra | Apollo | ||
|---|---|---|---|
| Implant | Abutment | Implant | Abutment |
OsseoSpeed EV 4.2 mm x 11 mm (Astra Tech, Dentsply, York, PA, USA)  | TiDesign EV (Astra Tech, Dentsply, York, PA, USA)  | OsseoSpeed EV 4.2 mm x 11 mm (Astra Tech, Dentsply, York, PA, USA)  | Individual Titanium Abutment (Apollo Implants Components, Pabianice, Poland)  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, J.; Puszkarz, A.K.; Radwanski, M.; Sokolowski, J.; Cichomski, M.; Bourgi, R.; Hardan, L.; Sauro, S.; Lukomska-Szymanska, M. Micro-CT Evaluation of Microgaps at Implant-Abutment Connection. Materials 2023, 16, 4491. https://doi.org/10.3390/ma16124491
Kowalski J, Puszkarz AK, Radwanski M, Sokolowski J, Cichomski M, Bourgi R, Hardan L, Sauro S, Lukomska-Szymanska M. Micro-CT Evaluation of Microgaps at Implant-Abutment Connection. Materials. 2023; 16(12):4491. https://doi.org/10.3390/ma16124491
Chicago/Turabian StyleKowalski, Jakub, Adam K. Puszkarz, Mateusz Radwanski, Jerzy Sokolowski, Michal Cichomski, Rim Bourgi, Louis Hardan, Salvatore Sauro, and Monika Lukomska-Szymanska. 2023. "Micro-CT Evaluation of Microgaps at Implant-Abutment Connection" Materials 16, no. 12: 4491. https://doi.org/10.3390/ma16124491
APA StyleKowalski, J., Puszkarz, A. K., Radwanski, M., Sokolowski, J., Cichomski, M., Bourgi, R., Hardan, L., Sauro, S., & Lukomska-Szymanska, M. (2023). Micro-CT Evaluation of Microgaps at Implant-Abutment Connection. Materials, 16(12), 4491. https://doi.org/10.3390/ma16124491












