Comparative Study of Corrosion Behaviors of WC-NiMo and WC-Co Cemented Carbides
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing the Materials and Manufacturing the Alloys
2.2. Microstructures and Chemical Characterization
2.3. Electrochemical Measurements
3. Results
3.1. Microstructural and Chemical Characterization before Corrosion
3.2. Open Circuit Potential
3.3. Potentiodynamic Polarization Curves
3.4. Electrochemical Impedance Spectroscopy
3.5. Microstructural and Chemical Characterization after Corrosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, C.M.; Senos, A.M.R. Cemented carbide phase diagrams: A review. J. Refract. Met. Hard Mater. 2011, 29, 405–418. [Google Scholar] [CrossRef]
- Phuong, D.D.; Van Trinh, P.; Van Duong, L.; Chung, L.D. Influence of sintering temperature on microstructure and mechanical properties of WC-8Ni cemented carbide produced by vacuum sintering. Ceram. Int. 2016, 42, 14937–14943. [Google Scholar] [CrossRef]
- Shi, K.H.; Zhou, K.C.; Li, Z.Y.; Zan, X.Q.; Xu, S.Z.; Min, Z.Y. Effect of adding method of Cr on microstructure and properties of WC-9Ni-2Cr cemented carbides. Int. J. Refract. Met. Hard Mater. 2013, 38, 1–6. [Google Scholar] [CrossRef]
- Alar, Ž.; Alar, V.; Fabijanić, T.A. Electrochemical corrosion behavior of near-nano and nanostructured WC-Co cemented carbides. Metals 2017, 7, 69. [Google Scholar] [CrossRef]
- Guo, S.; Bao, R.; Yang, J.; Chen, H.; Yi, J. Effect of Mo and Y2O3 additions on the microstructure and properties of fine WC-Co cemented carbides fabricated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2017, 69, 1–10. [Google Scholar] [CrossRef]
- Guo, Z.; Xiong, J.; Yang, M.; Xiong, S.; Chen, J.; Bi, S. Characterization and properties of MTCVD Ti(C,N) coated cemented carbide substrates with Fe/Ni binder. Int. J. Refract. Met. Hard Mater. 2010, 28, 238–242. [Google Scholar] [CrossRef]
- Human, A.M.; Exner, H.E. The relationship between electrochemical behaviour and in-service corrosion of WC based cemented carbides. Int. J. Refract. Met. Hard Mater. 1997, 15, 65–71. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, S.L. Characterization and properties of sintered WC-Co and WC-Ni-Fe hard metal alloys. J. Alloys Compd. 2014, 585, 407–413. [Google Scholar] [CrossRef]
- Vilhena, L.; Domingues, B.; Fernandes, C.; Senos, A.; Ramalho, A. Mechanical and Tribological Characterization of WC-Co and WC-AISI 304 Composites by a Newly Developed Equipment. Materials 2022, 15, 1187. [Google Scholar] [CrossRef]
- Imasato, S.; Tokumoto, K.; Kitada, T.; Sakaguchi, S. Properties of ultra-fine grain binderless cemented carbide “RCCFN”. Int. J. Refract. Met. Hard Mater. 1995, 13, 305–312. [Google Scholar] [CrossRef]
- Tomlinson, W.J.; Linzell, C.R. Anodic polarization and corrosion of cemented carbides with cobalt and nickel binders. J. Mater. Sci. 1988, 23, 914–918. [Google Scholar] [CrossRef]
- Tracey, V.A. Nickel in Hardmetals. Int. J. Refract. Met. Hard Mater. 1993, 11, 137–149. [Google Scholar] [CrossRef]
- Balbino, N.A.N.; Correa, E.O.; de Carvalho Valeriano, L.; Amâncio, D.A. Microstructure and mechanical properties of 90WC-8Ni-2Mo2C cemented carbide developed by conventional powder metallurgy. Int. J. Refract. Met. Hard Mater. 2017, 68, 49–53. [Google Scholar] [CrossRef]
- Correa, E.O.; Santos, J.N.; Klein, A.N. Microstructure and mechanical properties of WC Ni-Si based cemented carbides developed by powder metallurgy. Int. J. Refract. Met. Hard Mater. 2010, 28, 572–575. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Mori, G.; Kösters, R. Passivity and pseudopassivity of cemented carbides. Int. J. Refract. Met. Hard Mater. 2005, 23, 129–136. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Mori, G. Corrosion properties of Co-based cemented carbides in acidic solutions. Int. J. Refract. Met. Hard Mater. 2003, 21, 135–145. [Google Scholar] [CrossRef]
- Lin, N.; Wu, C.H.; He, Y.H.; Zhang, D.F. Effect of Mo and Co additions on the microstructure and properties of WC-TiC-Ni cemented carbides. Int. J. Refract. Met. Hard Mater. 2012, 30, 107–113. [Google Scholar] [CrossRef]
- Guo, Z.; Xiong, J.; Yang, M.; Song, X.; Jiang, C. Effect of Mo2C on the microstructure and properties of WC-TiC-Ni cemented carbide. Int. J. Refract. Met. Hard Mater. 2008, 26, 601–605. [Google Scholar] [CrossRef]
- Genga, R.M.; Cornish, L.A.; Akdogan, G. Effect of Mo2C additions on the properties of SPS manufactured WC-TiC-Ni cemented carbides. Int. J. Refract. Met. Hard Mater. 2013, 41, 12–21. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, N.; He, Y. Effects of Mo additions on the corrosion behavior of WC-TiC-Ni hardmetals in acidic solutions. Int. J. Refract. Met. Hard Mater. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Almond, E.A.; Roebuck, B. Identification of optimum binder phase compositions for improved WC hard metals. Mater. Sci. Eng. 1988, 105–106, 237–248. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Tang, H.; Ma, X.; Zhao, W. Effect of Mo addition on the microstructure and properties of WC-Ni-Fe hard alloys. J. Alloys Compd. 2015, 646, 155–160. [Google Scholar] [CrossRef]
- Nino, A.; Nakaibayashi, Y.; Sugiyama, S.; Taimatsu, H. Effect of Mo2C addition on the microstructures and mechanical properties of WC–SiC ceramics. Int. J. Refract. Met. Hard Mater. 2017, 64, 35–39. [Google Scholar] [CrossRef]
- da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, I.G.; Couto, M.; Guilemany, J.M.; Benedetti, A.V. Corrosion behavior of WC-Co coatings deposited by cold gas spray onto AA 7075-T6. Corros. Sci. 2018, 136, 231–243. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Ye, J.; Fan, H.; Qiu, Y. Effects of (Ti,Ta,Nb,W)(C,N) on the microstructure, mechanical properties and corrosion behaviors of WC-Co cemented carbides. Ceram. Int. 2017, 43, 2918–2926. [Google Scholar] [CrossRef]
- Farahmand, P.; Kovacevic, R. Corrosion and wear behavior of laser cladded Ni-WC coatings. Surf. Coat. Technol. 2015, 276, 121–135. [Google Scholar] [CrossRef]
- Guo, F.; Tian, Y.; Liu, Y.; Wang, Y. Tribological behaviors of graphite sliding against cemented carbide in CaCl2 solution. Surf. Topogr. Metrol. Prop. 2015, 3, 044003. [Google Scholar] [CrossRef]
- Human, A.M.; Exner, H.E. Electrochemical behaviour of tungsten-carbide hardmetals. Mater. Sci. Eng. A 1996, 209, 180–191. [Google Scholar] [CrossRef]
- Guo, S.; Bao, R.; Li, S.; Ye, Y.; Zhu, E.; Wang, W.; Zhang, Y.; Chen, H.; Ye, Y. The role of Y2O3, Cu, Mo and Mo2C additives on optimizing the corrosion resistance of WC-6Co cemented carbide in HCl and NaOH solutions. J. Alloys Compd. 2020, 827, 154269. [Google Scholar] [CrossRef]
- Op’tHoog, C.; Birbilis, N.; Estrin, Y. Corrosion of pure Mg as a function of grain size and processing route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Hochstrasser, S.; Mueller, Y.; Latkoczy, C.; Virtanen, S.; Schmutz, P. Analytical characterization of the corrosion mechanisms of WC-Co by electrochemical methods and inductively coupled plasma mass spectroscopy. Corros. Sci. 2007, 49, 2002–2020. [Google Scholar] [CrossRef]
- Zhang, Q.; Xi, X.; Nie, Z.; Zhang, L.; Ma, L. Electrochemical dissolution of cemented carbide scrap and electrochemical preparation of tungsten and cobalt metals. Int. J. Refract. Met. Hard Mater. 2019, 79, 145–153. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Feng, Y.P.; Chen, S.; Wan, Q.L.; Zhu, J.F. Electrochemical characterization of AlTiN, AlCrN and AlCrSiWN coatings. Int. J. Refract. Met. Hard Mater. 2015, 53, 68–73. [Google Scholar] [CrossRef]
- Han, B.; Zhu, S.; Dong, W.; Bai, Y.; Ding, H.; Luo, Y.; Di, P. Electrochemical corrosion behavior of hot-pressing sintered WC-Al2O3 composite in alkaline and acidic solutions. J. Mater. Sci. 2021, 56, 4120–4134. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrocshim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Ahmed, R.; Vourlias, G.; Algoburi, A.; Vogiatzis, C.; Chaliampalias, D.; Skolianos, S.; Berger, L.M.; Paul, S.; Faisal, N.H.; Toma, F.L.; et al. Comparative Study of Corrosion Performance of HVOF-Sprayed Coatings Produced Using Conventional and Suspension WC-Co Feedstock. J. Therm. Spray Technol. 2018, 27, 1579–1593. [Google Scholar] [CrossRef]
- Rocha, A.M.F.; Bastos, A.C.; Cardoso, J.P.; Rodrigues, F.; Fernandes, C.M.; Soares, E.; Sacramento, J.; Senos, A.M.R.; Ferreira, M.G.S. Corrosion behaviour of WC hardmetals with nickel-based binders. Corros. Sci. 2019, 147, 384–393. [Google Scholar] [CrossRef]
- Fazili, A.; Derakhshandeh, M.R.; Nejadshamsi, S.; Nikzad, L.; Razavi, M.; Ghasali, E. Improved electrochemical and mechanical performance of WC-Co cemented carbide by replacing a part of Co with Al2O3. J. Alloys Compd. 2020, 823, 153857. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Bastos, A.C.; Fernandes, C.M.; Pinho, C.M.S.; Senos, A.M.R.; Soares, E.; Sacramento, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion behaviour of WC-10% AISI 304 cemented carbides. Corros. Sci. 2015, 100, 322–331. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Fargas, G.; Isern, L.; Dorvlo, S.; Tarres, E.; Müller, C.M.; Jiménez-Piqué, E.; Llanes, L. Microstructural influence on tolerance to corrosion-induced damage in hardmetals. Mater. Des. 2016, 111, 36–43. [Google Scholar] [CrossRef]
- Derakhshandeh, M.R.; Eshraghi, M.J.; Jam, A.; Rajaei, H.; Fazili, A. Comparative studies on corrosion and tribological performance of multilayer hard coatings grown on WC-Co hardmetals. Int. J. Refract. Met. Hard Mater. 2020, 92, 105339. [Google Scholar] [CrossRef]
- Konadu, D.S.; van der Merwe, J.; Potgieter, J.H.; Potgieter-Vermaak, S.; Machio, C.N. The corrosion behaviour of WC-VC-Co hardmetals in acidic media. Corros. Sci. 2010, 52, 3118–3125. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Fargas, G.; Besharatloo, H.; Serra, M.; Roa, J.J.; Armelin, E.; Lavigne, O.; Llanes, L. Assessment of corrosion-induced changes on the mechanical integrity of cemented carbides at small length scales. Int. J. Refract. Met. Hard Mater. 2019, 84, 105033. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Fargas, G.; Lavigne, O.; Llanes, L. Indentation and scratch testing of a WC-6%wtCo cemented carbide: Corrosion effects on load-bearing capability and induced damage. Ceram. Int. 2020, 46, 17591–17598. [Google Scholar] [CrossRef]
- Fan, B.; Zhu, S.; Dong, W.; Ding, H.; Bai, Y.; Luo, Y.; Di, P. Comparative study on corrosion behavior of WC-MgO composite and WC-6Co cemented carbide in NaCl solution. Ceram. Int. 2021, 47, 7106–7116. [Google Scholar] [CrossRef]
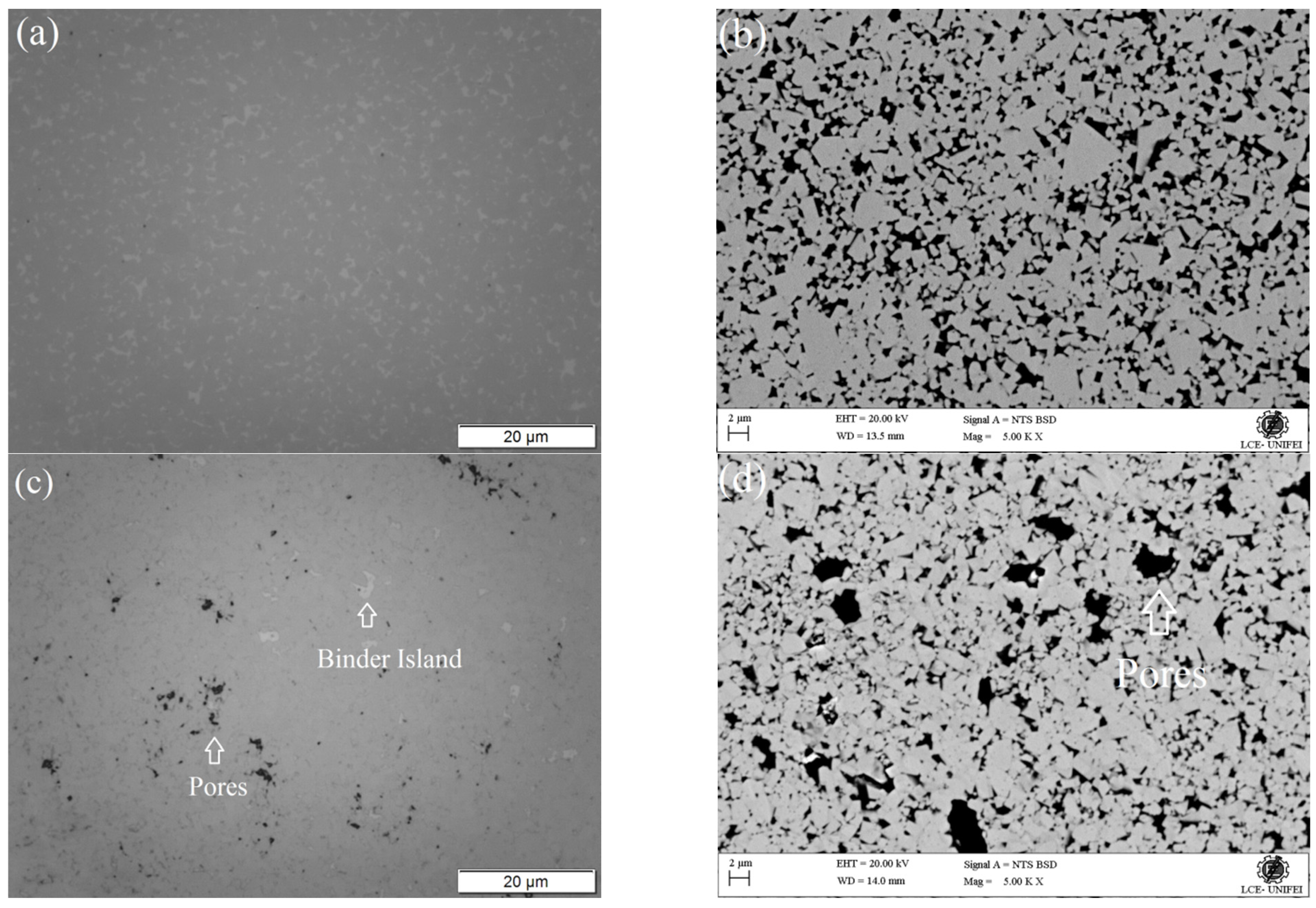
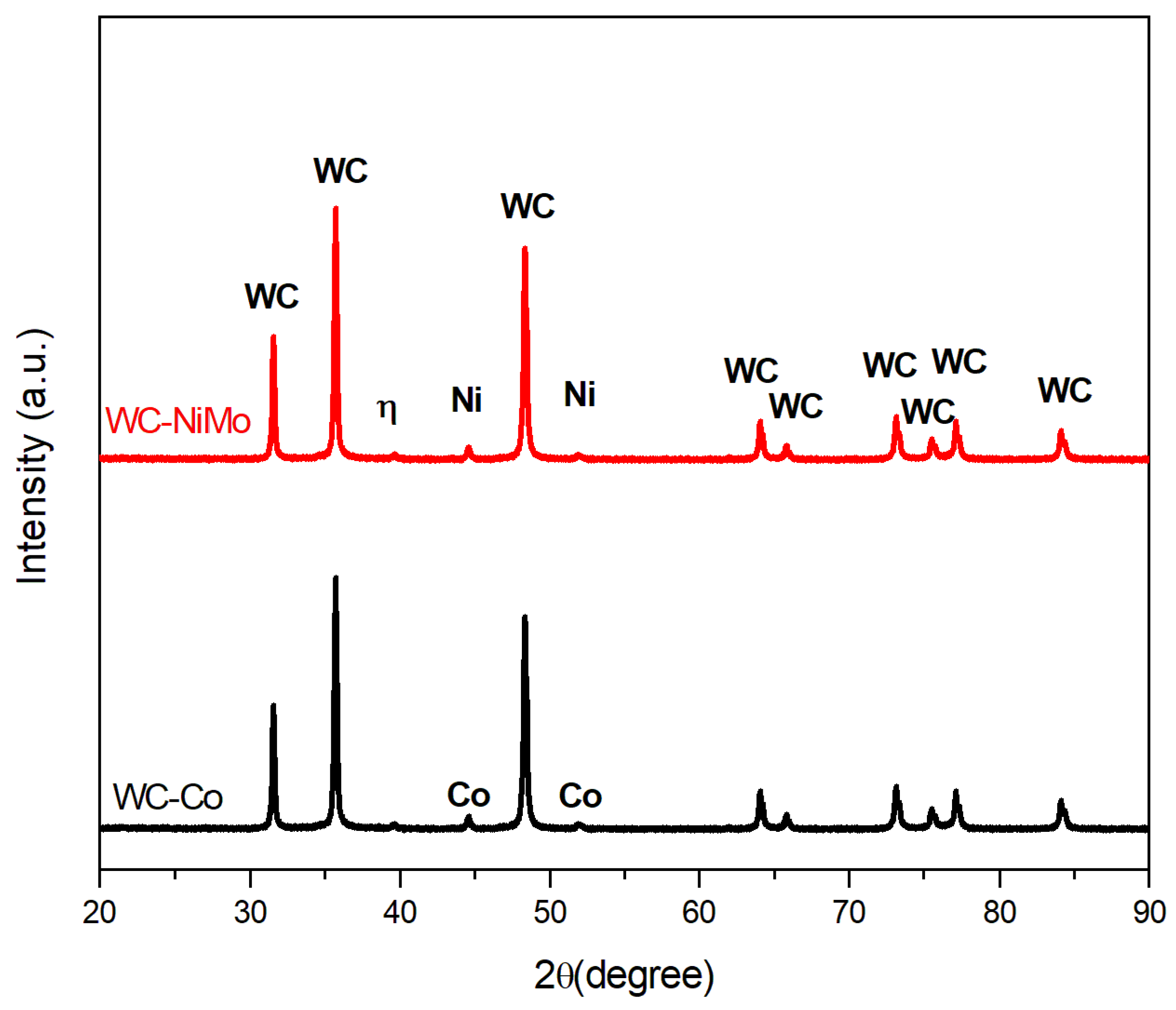

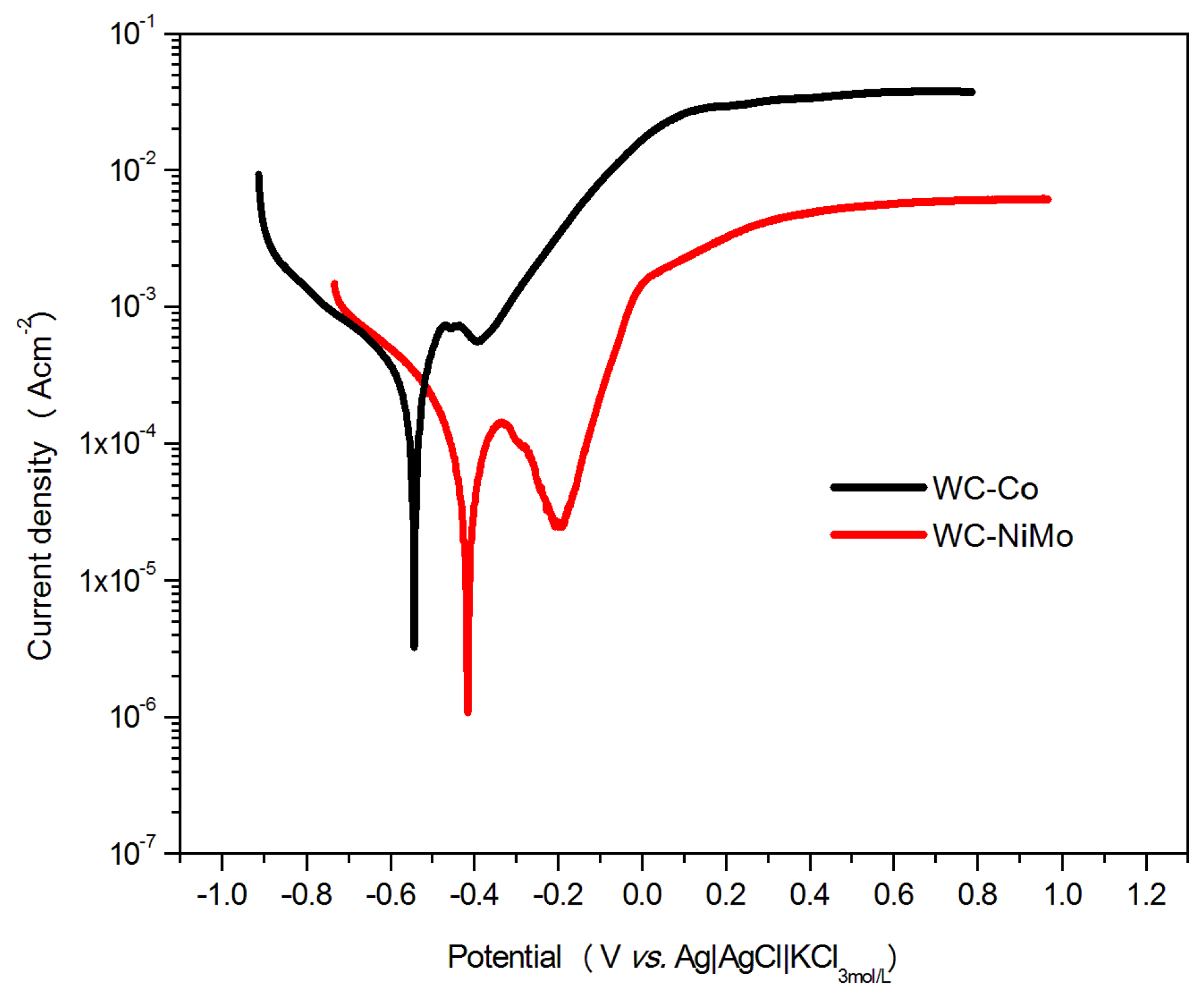



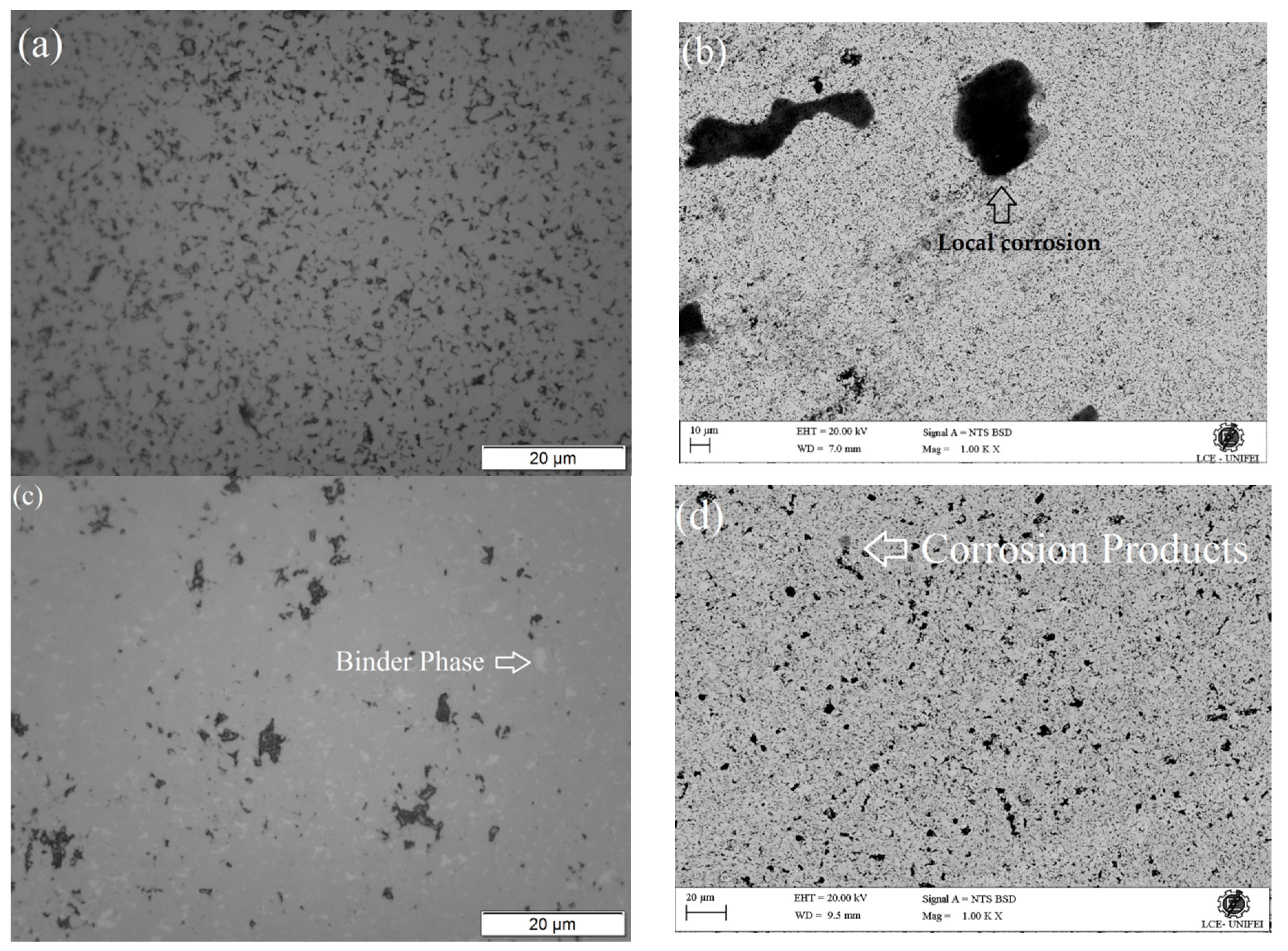

| Cemented Carbide | WC | Co | Ni | Mo2C |
|---|---|---|---|---|
| WC-Co | 90 | 10 | - | - |
| WC-NiMo | 90 | - | 8 | 2 |
| Alloy | Chemical Composition (%) | |||||
|---|---|---|---|---|---|---|
| W | C | O | Co | Ni | Mo | |
| WC-Co | 80.69 | 9.28 | 1.17 | 8.86 | - | - |
| WC-NiMo | 80.64 | 9.34 | 1.07 | - | 7.03 | 1.93 |
| Alloy | ||||
|---|---|---|---|---|
| WC-Co | −0.543 | 1190.40 | 725.74 | 556.43 |
| WC-NiMo | −0.416 | 129.68 | 142.34 | 24.80 |
| WC-Co | WC-NiMo | |
|---|---|---|
| 35.75 | 56.02 | |
| 13.10 | 53.03 | |
| 766.18 | 155.76 | |
| 0.50 | 0.61 | |
| 41.93 | 295.49 | |
| 842.17 | 97.60 | |
| 0.75 | 0.72 | |
| 1222.40 | 1970.70 | |
| 294.44 | 942.37 | |
| 0.90 | 0.21 | |
| - | 3973.30 |
| Alloy | Chemical Composition (%) | |||||
|---|---|---|---|---|---|---|
| W | C | O | Co | Ni | Mo | |
| WC-Co | 90.34 | 8.39 | 1.01 | 0.23 | - | - |
| WC-NiMo | 81.65 | 9.40 | 1.18 | - | 7.78 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balbino, N.A.N.; Corrêa, E.O.; Huanca, D.R.; de Freitas Matos, F.A.; de Carvalho Valeriano, L. Comparative Study of Corrosion Behaviors of WC-NiMo and WC-Co Cemented Carbides. Materials 2023, 16, 4480. https://doi.org/10.3390/ma16124480
Balbino NAN, Corrêa EO, Huanca DR, de Freitas Matos FA, de Carvalho Valeriano L. Comparative Study of Corrosion Behaviors of WC-NiMo and WC-Co Cemented Carbides. Materials. 2023; 16(12):4480. https://doi.org/10.3390/ma16124480
Chicago/Turabian StyleBalbino, Nádia Alves Nery, Edmilson Otoni Corrêa, Danilo Roque Huanca, Flávio Amaury de Freitas Matos, and Livio de Carvalho Valeriano. 2023. "Comparative Study of Corrosion Behaviors of WC-NiMo and WC-Co Cemented Carbides" Materials 16, no. 12: 4480. https://doi.org/10.3390/ma16124480
APA StyleBalbino, N. A. N., Corrêa, E. O., Huanca, D. R., de Freitas Matos, F. A., & de Carvalho Valeriano, L. (2023). Comparative Study of Corrosion Behaviors of WC-NiMo and WC-Co Cemented Carbides. Materials, 16(12), 4480. https://doi.org/10.3390/ma16124480







