Synthesis and Characterization of Antimicrobial Hydrophobic Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
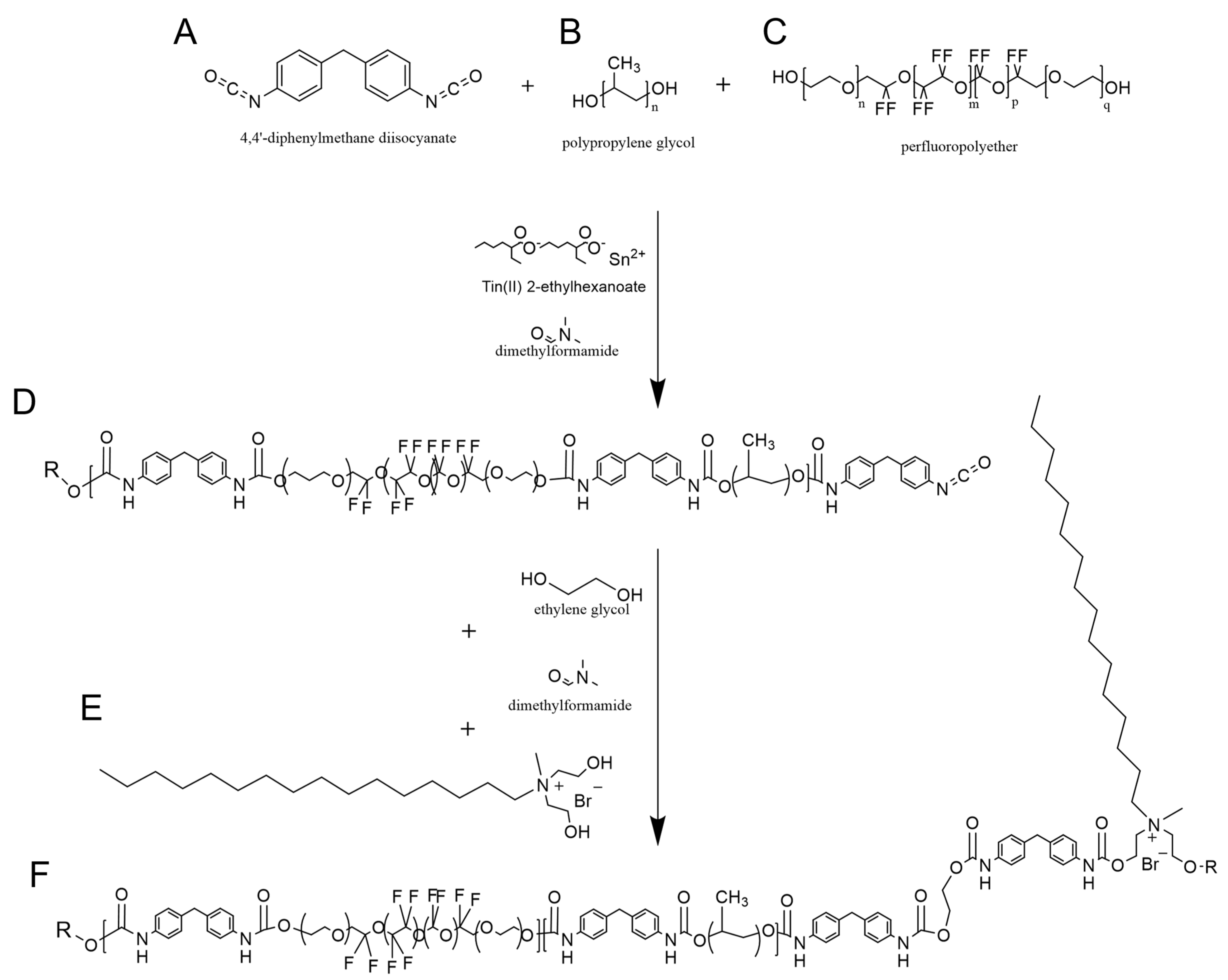
2.2. Synthesis of Polyurethanes
2.3. Material Characterization
2.4. Antimicrobial Efficacy
2.5. Statistics
3. Results and Discussion
3.1. Synthesis
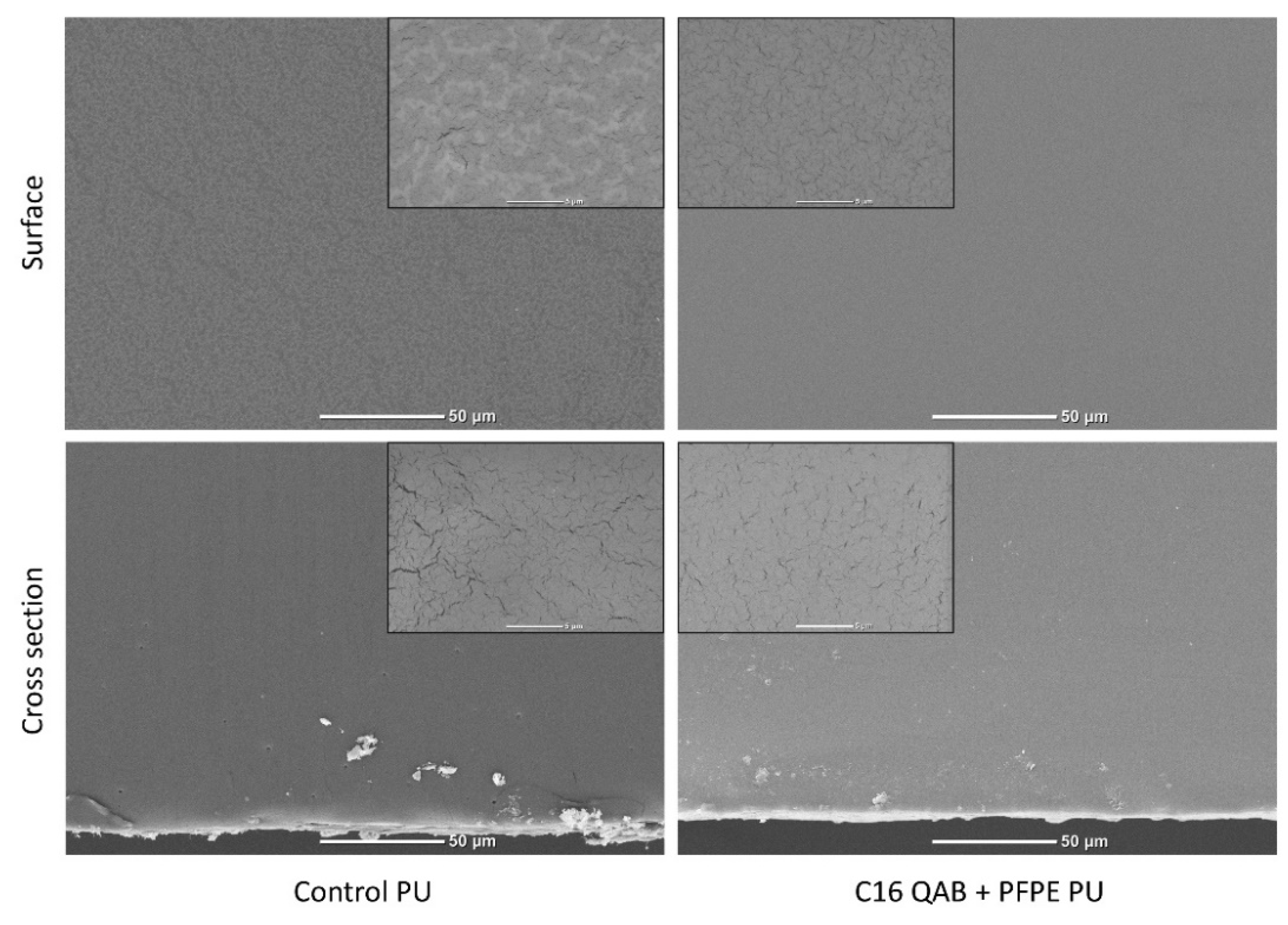
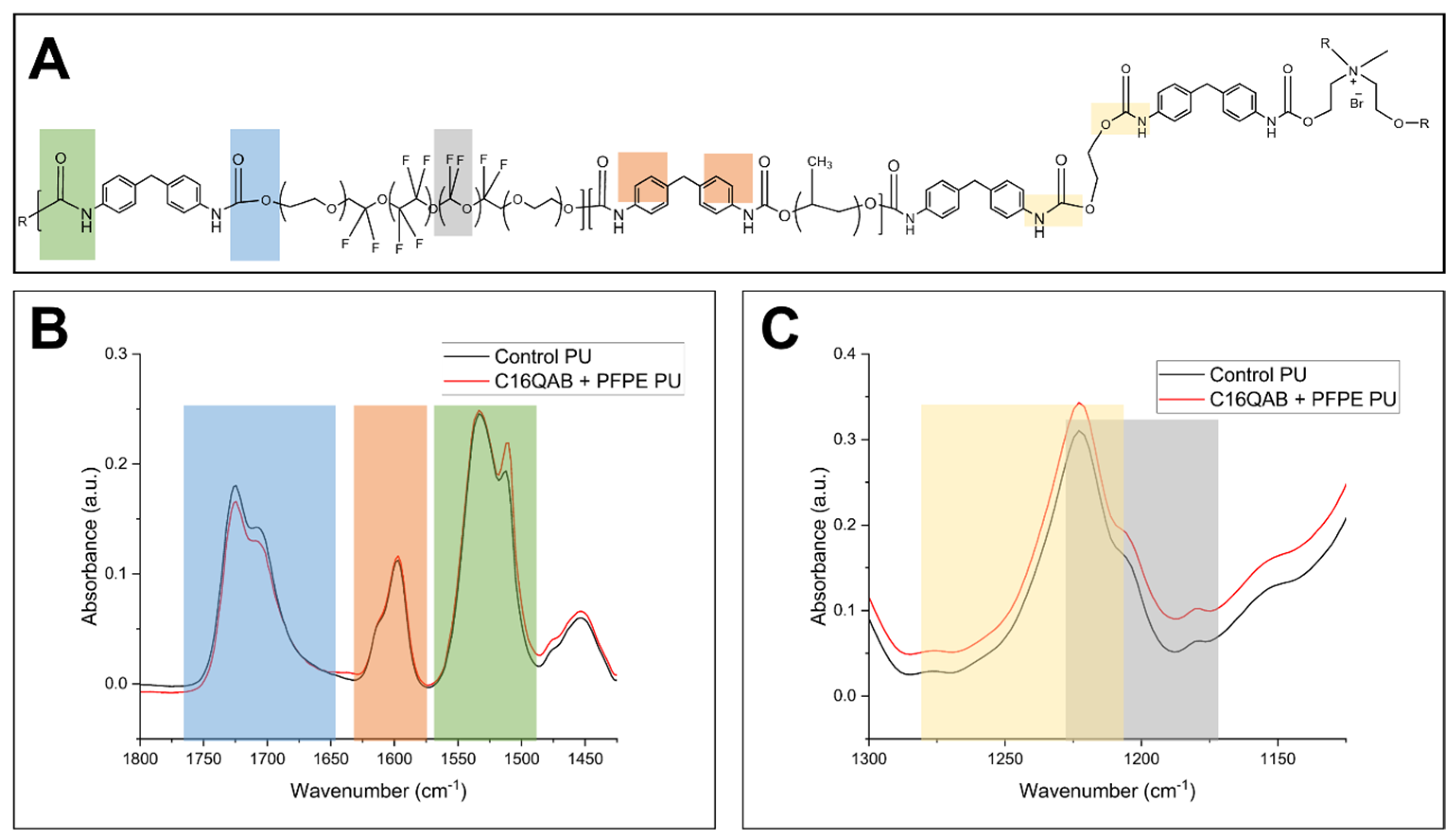
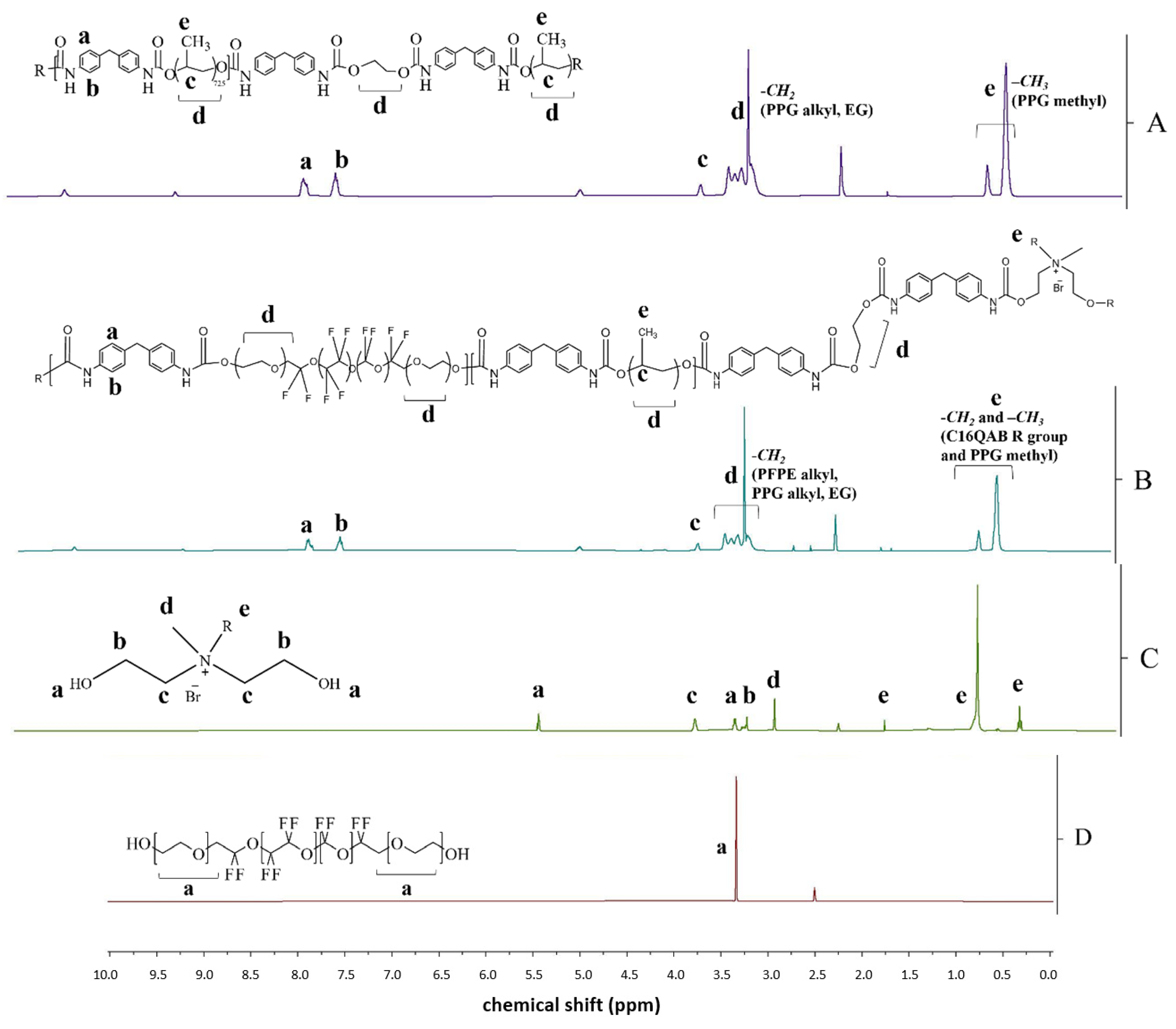
3.2. Characterization
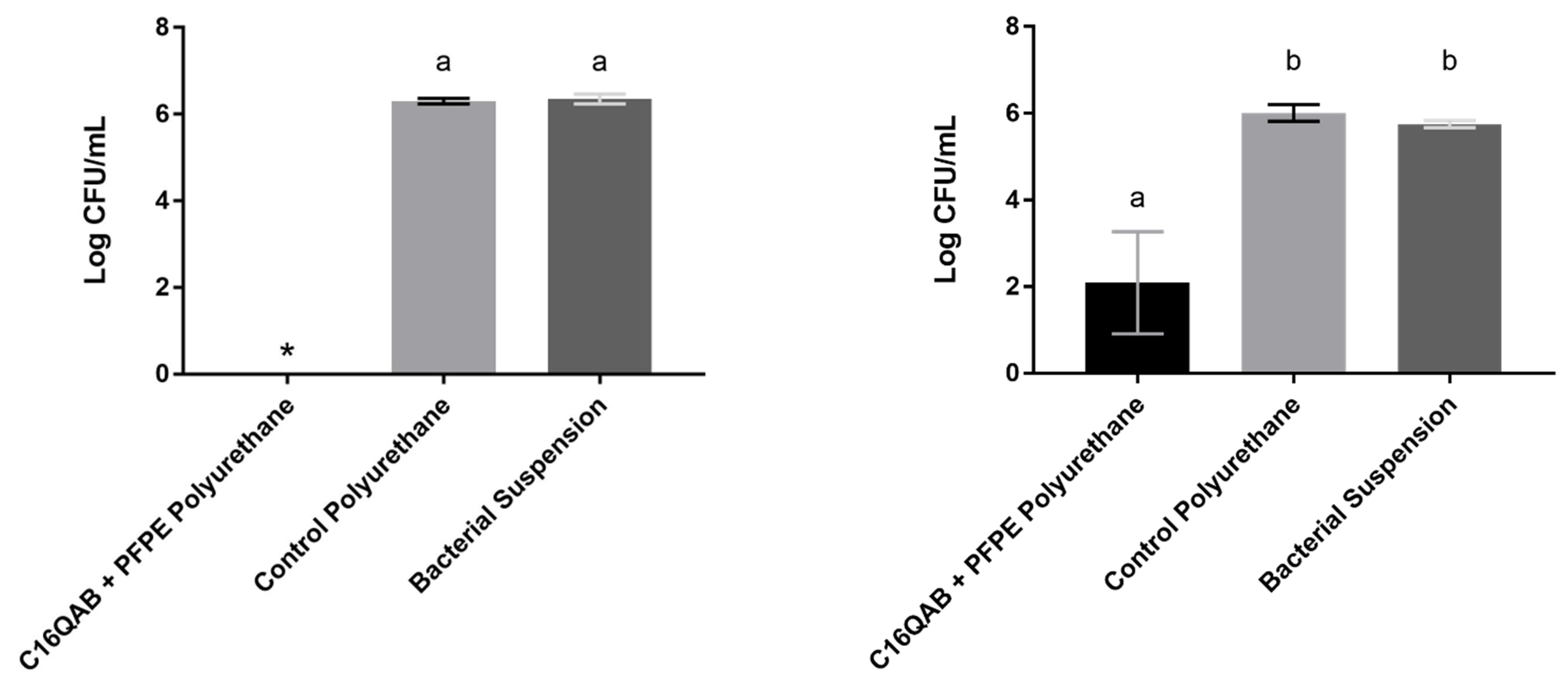
4. Antimicrobial Efficacy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, W.B.; Cutter, C.N. An ecological perspective of Listeria monocytogenes biofilms in food processing facilities. Crit. Rev. Food Sci. Nutr. 2013, 53, 801–817. [Google Scholar] [CrossRef]
- FDA. Draft Guidance for Industry: Hazard Analysis and Risk-Based Preventive Controls for Human Food- Potential Hazards Associated with the Manufacturing Processing Packing and Holding. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-hazard-analysis-and-risk-based-preventive-controls-human-food (accessed on 20 April 2023).
- FDA. Draft Guidance for Industry: Control of Listeria monocytogenes in Ready-to-Eat Foods. 2017. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-control-listeria-monocytogenes-ready-eat-foods (accessed on 20 April 2023).
- FSIS. Listeria monocytogenes Regulations. 2019. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-02/38_IM_Lm_Regs.pdf (accessed on 20 April 2023).
- FSIS. Best Practices Guidance for Controlling Listeria monocytogenes (Lm) in Retail Delicatessens. 2015. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-03/Controlling-LM-Delicatessens.pdf (accessed on 20 April 2023).
- John, J.; Joy, W.C.; Jovana, K. Prevalence of Listeria spp. in produce handling and processing facilities in the Pacific Northwest. Food Microbiol. 2020, 90, 103468. [Google Scholar] [CrossRef] [PubMed]
- Berrang, M.E.; Frank, J.F. Generation of airborne Listeria innocua from model floor drains. J. Food Prot. 2012, 75, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Wynne, J.H.; Fulmer, P.A.; McCluskey, D.M.; Mackey, N.M.; Buchanan, J.P. Synthesis and development of a multifunctional self-decontaminating polyurethane coating. ACS Appl. Mater. Interfaces 2011, 3, 2005–2011. [Google Scholar] [CrossRef]
- Kwasniewska, D.; Chen, Y.L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? ChemMedChem 2012, 7, 22–31. [Google Scholar] [CrossRef]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. Chembiochem 2020, 21, 1510–1516. [Google Scholar] [CrossRef]
- Bures, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef]
- Bakhshi, H.; Yeganeh, H.; Mehdipour-Ataei, S.; Shokrgozar, M.A.; Yari, A.; Saeedi-Eslami, S.N. Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 153–164. [Google Scholar] [CrossRef]
- Hu, P.; Greiner, A.; Agarwal, S. Synthesis and properties evaluation of quaternized polyurethanes as antibacterial adhesives. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 752–757. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hou, G.-G.; Du, Z.-Z.; Cong, W.; Sun, J.-F.; Xu, Y.-Y.; Liu, W.-S. Synthesis, characterization and antibacterial properties of polyurethane material functionalized with quaternary ammonium salt. Polym. J. 2015, 48, 259–265. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar]
- Choi, Y.-S.; Kim, N.K.; Kang, H.; Jang, H.-K.; Noh, M.; Kim, J.; Shon, D.-J.; Kim, B.-S.; Lee, J.-C. Antibacterial and biocompatible ABA-triblock copolymers containing perfluoropolyether and plant-based cardanol for versatile coating applications. RSC Adv. 2017, 7, 38091–38099. [Google Scholar] [CrossRef]
- Wang, P.C.; Lu, D.; Wang, H.; Bai, R.K. A New Strategy for the Synthesis of Fluorinated Polyurethane. Polymers 2019, 11, 1440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Lee, H.T.; Tsai, H.A.; Suen, M.C.; Chiu, C.W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. [Google Scholar] [CrossRef]
- Takakura, T.K.; Kato, M.; Yamabe, M. Fluorinated polyurethanes 1 Synthesis and characterization of fluoroine-containing segmented poly(urethane-urea)s. Macromol. Chem. Phys. 1990, 191, 625–632. [Google Scholar] [CrossRef]
- Potschke, P.; Pionteck, J.; Stutz, H. Surface tension, interfacial tension, and morphology in blends of thermoplastic polyurethanes and polyolefins. Part I. Surface tension of melts of TPU model substances and polyolefins. Polymer 2002, 43, 6965–6972. [Google Scholar] [CrossRef]
- Su, S.-K.; Gu, J.-H.; Lee, H.-T.; Wu, C.-L.; Hwang, J.-J.; Suen, M.-C. Synthesis and properties of novel biodegradable polyurethanes containing fluorinated aliphatic side chains. J. Polym. Res. 2017, 24, 142. [Google Scholar] [CrossRef]
- Liu, P.; Ye, L.; Liu, Y.; Nie, F. Preparation and properties of the main-chain-fluorinated thermoplastic polyurethane elastomer. Polym. Bull. 2010, 66, 503–515. [Google Scholar] [CrossRef]
- Tonelli, C.; Ajroldi, G. New Fluoro-Modified Thermoplastic Polyurethanes. J. Appl. Polym. Sci. 2004, 87, 2279–2294. [Google Scholar] [CrossRef]
- Chapman, T.M.B.; Marra, K.G. Determination of Low Critical Surface Tensions of Novel Fluorinated Poly(amide urethane) Block Copolymers. 2. Fluorinated Soft-Block Backbone and Side Chains. Macromolecules 1995, 28, 2081–2085. [Google Scholar] [CrossRef]
- Chapman, T.M.; Benrashid, R.; Gribbin, K.L.; Keener, J.P. Determination of Low Critical Surface Tensions of Novel Fluorinated Poly(amide urethane) Block Copolymers. 1. Fluorinated Side Chains. Macromolecules 1995, 28, 331–335. [Google Scholar] [CrossRef]
- Friesen, C.M.; Améduri, B. Outstanding telechelic perfluoropolyalkylethers and applications therefrom. Prog. Polym. Sci. 2018, 81, 238–280. [Google Scholar] [CrossRef]
- Gu, X.; Gao, T.; Meng, X.; Zhu, Y.; Wang, G. Enhanced hydrophobicity of polyurethane with the self-assembly of perfluoropolyether-based triblock copolymers. Prog. Org. Coat. 2022, 162, 106561. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Li, J.; Gao, Y.; Luo, F.; Tan, H.; Wang, K.; Fu, Q. A Novel Surface Structure Consisting of Contact-active Antibacterial Upper-layer and Antifouling Sub-layer Derived from Gemini Quaternary Ammonium Salt Polyurethanes. Sci. Rep. 2016, 6, 32140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fu, Y.; Zhang, Q.; Zhan, X.; Chen, F. Novel amphiphilic poly(dimethylsiloxane) based polyurethane networks tethered with carboxybetaine and their combined antibacterial and anti-adhesive property. Appl. Surf. Sci. 2017, 412, 1–9. [Google Scholar] [CrossRef]
- Peng, C.; Vishwakarma, A.; Li, Z.; Miyoshi, T.; Barton, H.A.; Joy, A. Modification of a conventional polyurethane composition provides significant anti-biofilm activity against Escherichia coli. Polym. Chem. 2018, 9, 3195–3198. [Google Scholar] [CrossRef]
- Yagci, M.B.; Bolca, S.; Heuts, J.P.A.; Ming, W.; de With, G. Antimicrobial polyurethane coatings based on ionic liquid quaternary ammonium compounds. Prog. Org. Coat. 2011, 72, 343–347. [Google Scholar] [CrossRef]
- Qiao, M.; Ren, T.; Huang, T.-S.; Weese, J.; Liu, Y.; Ren, X.; Farag, R. N-Halamine modified thermoplastic polyurethane with rechargeable antimicrobial function for food contact surface. RSC Adv. 2017, 7, 1233–1240. [Google Scholar] [CrossRef]
- Barish, J.A.; Goddard, J.M. Anti-fouling surface modified stainless steel for food processing. Food Bioprod. Process. 2013, 91, 352–361. [Google Scholar] [CrossRef]
- Kabza, K.G.; Gestwicki, J.E.; Mcgrath, J.L. Contact Angle Goniometry as a Tool for Surface Tension Measurements of Solids, Using Zisman Plot Method. J. Chem. Educ. 2000, 77, 63. [Google Scholar] [CrossRef]
- Combe, E.C.; Owen, B.A.; Hodges, J.S. A protocol for determining the surface free energy of dental materials. Dent. Mater. 2004, 20, 262–268. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2149-20; Standard Test Method for Determining the Antimcirobial Activity of Antimicrobial Agents under Dynamic Contact Conditions. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- Hung, Y.-T.; McLandsborough, L.A.; Goddard, J.M.; Bastarrachea, L.J. Antimicrobial polymer coatings with efficacy against pathogenic and spoilage microorganisms. LWT 2018, 97, 546–554. [Google Scholar] [CrossRef]
- Ecolab Inc. EcoLab Multi Quat Sanitizer. 2020. Available online: https://www.ecolab.com/offerings/front-of-house/oasis146-multi-quat-sanitizer (accessed on 20 April 2023).
- Li, S.; Lin, X.; Gong, S. Waterborne polyurethane assembly multifunctional coating for hydrophobic and antibacterial fabrics. Cellulose 2022, 29, 7397–7411. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer Series in Surface Sciences: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Fox, H.; Zisman, W. The spreading of liquids on low energy surfaces. I. Polytetrafluoroethylene. J. Colloid Sci. 1950, 5, 514–531. [Google Scholar] [CrossRef]
- Santos, O.; Nylander, T.; Rosmaninho, R.; Rizzo, G.; Yiantsios, S.; Andritsos, N.; Karabelas, A.; Müller-Steinhagen, H.; Melo, L.; Boulangé-Petermann, L.; et al. Modified stainless steel surfaces targeted to reduce fouling—Surface characterization. J. Food Eng. 2004, 64, 63–79. [Google Scholar] [CrossRef]
- Erceg, T.; Tanasić, J.; Banjanin, B.; Baloš, S.; Cvetinov, M.; Cakić, S.; Ristić, I. Surface, structural, and thermal properties of polydimethylsiloxane-based polyurethanes and their blends with thermoplastic polyurethane elastomer. Polym. Bull. 2022, 79, 10909–10929. [Google Scholar] [CrossRef]
- Hill, M.J.; Cheah, C.; Sarkar, D. Interfacial energetics approach for analysis of endothelial cell and segmental polyurethane interactions. Colloids Surf. B Biointerfaces 2016, 144, 46–56. [Google Scholar] [CrossRef]
- Khan, M.M.T.; Ista, L.K.; Lopez, G.P.; Schuler, A.J. Experimental and Theoretical Examination of Surface Energy and Adhesion of Nitrifying and Heterotrophic Bacteria Using Self-Assembled Monolayers. Environ. Sci. Technol. 2011, 45, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
| MDI (mmol) | PPG (mmol) | PFPE (mmol) | EG (mmol) | C16QAB (mmol) | |
|---|---|---|---|---|---|
| Control PU | 10.27 | 6.90 | - | 4.03 | - |
| C16QAB + PFPE PU | 10.35 | 6.90 | 0.09 | 3.22 | 0.12 |
| Carbon (at.%) | Oxygen (at.%) | Nitrogen (at.%) | Fluorine (at.%) | C/O | C/N | C/F | |
|---|---|---|---|---|---|---|---|
| Control PU | 72.4 | 24.7 | 2.2 | 0.8 | 2.9 | 32.6 | 91.6 |
| C16QAB + PFPE PU | 43.2 | 17.0 | 1.8 | 38.0 | 2.5 | 24.7 | 1.1 |
| Advancing Contact Angle (θa) | Receding Contact Angle (θr) | Hysteresis | |
|---|---|---|---|
| Stainless Steel | 76.3° ± 17.6 A | 18.4° ± 9.6 A | 57.9 |
| Control PU | 95.2° ± 4.6 B | 29.9° ± 6.9 B | 65.3 |
| C16QAB + PFPE PU | 129.5° ± 4.8 C | 28.3° ± 4.3 B | 101.2 |
| Critical Surface Tension γcr (mN m−1) | Goodness of Fit (r2) | |
|---|---|---|
| Stainless Steel | 19.96 | 0.99 |
| Control PU | 18.07 | 0.99 |
| C16QAB + PFPE PU | 13.14 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudlong, A.M.; Moreno Reyes, E.; Goddard, J.M. Synthesis and Characterization of Antimicrobial Hydrophobic Polyurethane. Materials 2023, 16, 4446. https://doi.org/10.3390/ma16124446
Rudlong AM, Moreno Reyes E, Goddard JM. Synthesis and Characterization of Antimicrobial Hydrophobic Polyurethane. Materials. 2023; 16(12):4446. https://doi.org/10.3390/ma16124446
Chicago/Turabian StyleRudlong, Autumn M., Elizabet Moreno Reyes, and Julie M. Goddard. 2023. "Synthesis and Characterization of Antimicrobial Hydrophobic Polyurethane" Materials 16, no. 12: 4446. https://doi.org/10.3390/ma16124446
APA StyleRudlong, A. M., Moreno Reyes, E., & Goddard, J. M. (2023). Synthesis and Characterization of Antimicrobial Hydrophobic Polyurethane. Materials, 16(12), 4446. https://doi.org/10.3390/ma16124446






