Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Washes
2.3. Determining Minimum Planktonic Inhibitory and Eradication Concentration, Minimum Biofilm Inhibition, and Eradication Concentration
2.4. Efficacy against 3-Day-Old Mature Biofilm at In-Use Concentration and Contact Time in the Presence of Biological Soil
2.5. Statistical Analysis
3. Results
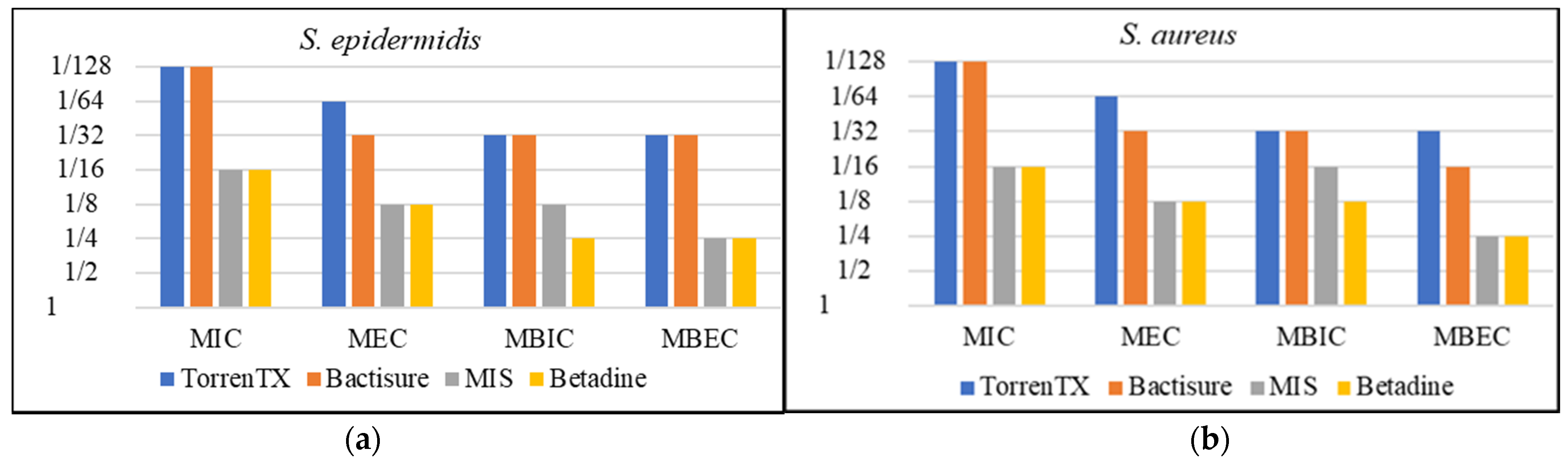
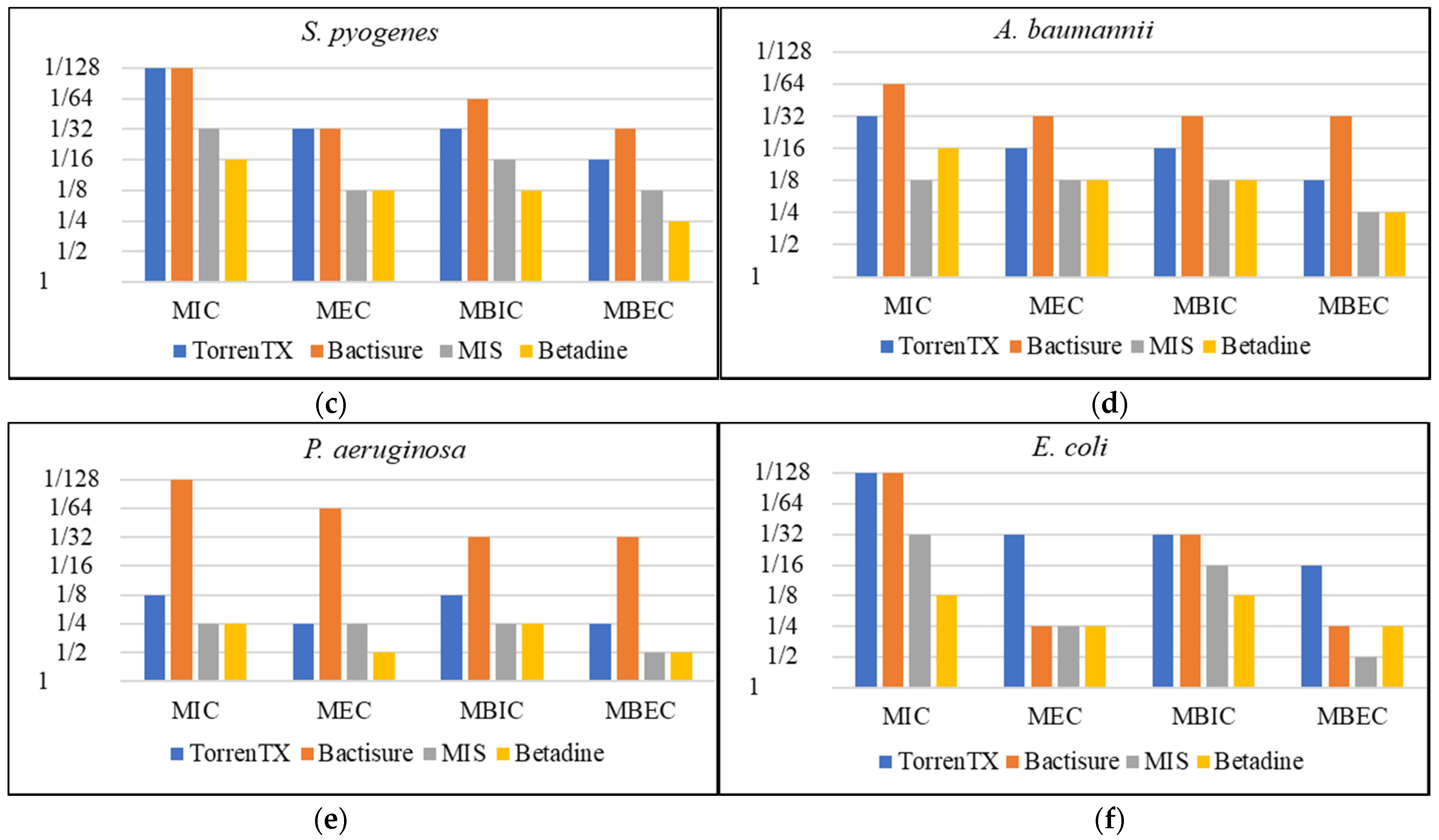
3.1. Minimum Planktonic Inhibitory and Eradication Concentration and Minimum Biofilm Inhibition and Eradication Concentration
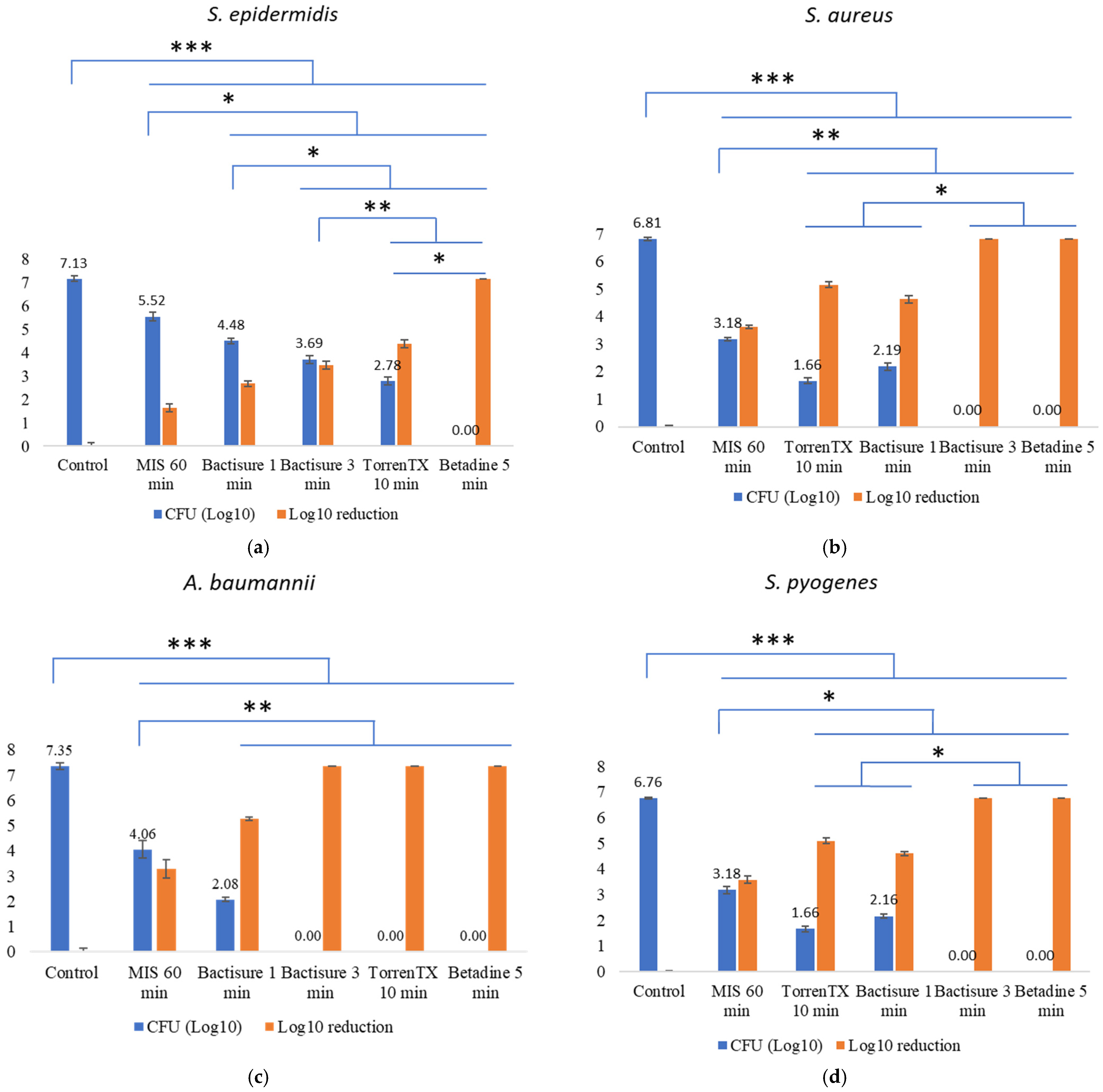
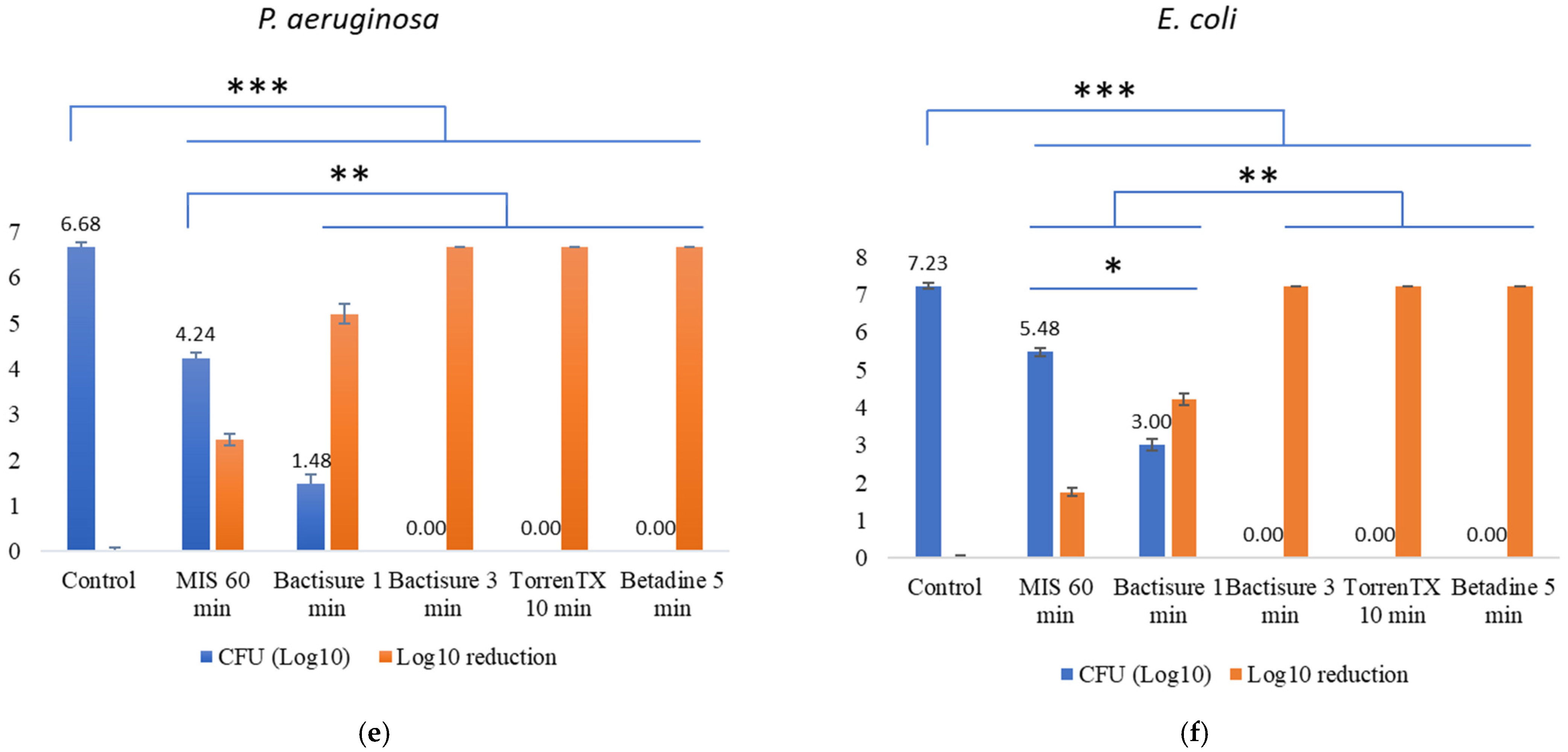
3.2. Efficacy against 3-Day-Old Mature Biofilm at In-Use Concentration and Contact Time in the Presence of Biological Soil
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Travis, J.; Malone, M.; Hu, H.; Baten, A.; Johani, K.; Huygens, F.; Vickery, K.; Benkendorff, K. The microbiome of diabetic foot ulcers: A comparison of swab and tissue biopsy wound sampling techniques using 16S rRNA gene sequencing. BMC Microbiol. 2020, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Pajkos, A.; Deva, A.K.; Vickery, K.; Cope, C.; Chang, L.; Cossart, Y.E. Detection of subclinical infection in significant breast implant capsules. Plast. Reconstr. Surg. 2003, 111, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.D.; Wolcott, R.D.; Sun, Y.; Dowd, S.E. Comparison of culture and molecular identification of bacteria in chronic wounds. Int. J. Mol. Sci. 2012, 13, 2535–2550. [Google Scholar] [CrossRef]
- Bielecki, P.; Glik, J.; Kawecki, M.; Martins dos Santos, V.A. Towards understanding Pseudomonas aeruginosa burn wound infections by profiling gene expression. Biotechnol. Lett. 2008, 30, 777–790. [Google Scholar] [CrossRef]
- Ovington, L. Bacterial toxins and wound healing. Ostomy Wound Manag. 2003, 49, 8–12. [Google Scholar]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304. [Google Scholar] [CrossRef]
- Vestby, L.K.; Nesse, L.L. Wound care antiseptics-performance differences against Staphylococcus aureus in biofilm. Acta Vet. Scand. 2015, 57, 22. [Google Scholar] [CrossRef][Green Version]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef]
- Weir, D.; Swanson, T. Ten top tips: Wound cleansing. Wounds Int. 2019, 10, 8–11. [Google Scholar]
- Ortega-Peña, S.; Hidalgo-González, C.; Robson, M.C.; Krötzsch, E. In Vitro microbicidal, anti-biofilm and cytotoxic effects of different commercial antiseptics. Int. Wound J. 2017, 14, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Vaca, D.J.; Thibau, A.; Schütz, M.; Kraiczy, P.; Happonen, L.; Malmström, J.; Kempf, V.A. Interaction with the host: The role of fibronectin and extracellular matrix proteins in the adhesion of Gram-negative bacteria. Med. Microbiol. Immunol. 2020, 209, 277–299. [Google Scholar] [CrossRef]
- Malic, S.; Hill, K.E.; Playle, R.; Thomas, D.W.; Williams, D.W. In Vitro interaction of chronic wound bacteria in biofilms. J. Wound Care 2011, 20, 569–577. [Google Scholar] [CrossRef]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef]
- Otter, J.A.; Vickery, K.; Walker, J.D.; Pulcini, E.D.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.G.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning and disinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef]
- Singla, S.; Harjai, K.; Chhibber, S. Susceptibility of different phases of biofilm of Klebsiella pneumoniae to three different antibiotics. J. Antibiot. 2013, 66, 61–66. [Google Scholar] [CrossRef]
- Chen, X.; Thomsen, T.R.; Winkler, H.; Xu, Y. Influence of biofilm growth age, media, antibiotic concentration and exposure time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol. 2020, 20, 264. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rumbaugh, K.P.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.S.; Dowd, S.E. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef]
- Coenye, T.; Goeres, D.; Van Bambeke, F.; Bjarnsholt, T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin. Microbiol. Infect. 2018, 24, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th Edition. 2018. Available online: https://clsi.org/media/2577/m11-ed9_sample.pdf (accessed on 22 September 2021).
- Schwarzer, S.; James, G.A.; Goeres, D.; Bjarnsholt, T.; Vickery, K.; Percival, S.L.; Stoodley, P.; Schultz, G.; Jensen, S.O.; Malone, M. The efficacy of topical agents used in wounds for managing chronic biofilm infections: A systematic review. J. Infect. 2020, 80, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sleiman, J.; Johani, K.; Vickery, K. Hypochlorous acid versus povidone-iodine containing irrigants: Which antiseptic is more effective for breast implant pocket irrigation? Aesthet. Surg. J. 2018, 38, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Deva, A.K.; Adams, W.P., Jr.; Vickery, K. The role of bacterial biofilms in device-associated infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef]
- ASTM E3161-18, Standard Practice for Preparing a Pseudomonas aeruginosa or Staphylococcus aureus Biofilm using the CDC Biofilm Reactor. 2018. Available online: https://www.astm.org/Standards/E3161.htm (accessed on 9 October 2021).
- Merckoll, P.; Jonassen, T.Ø.; Vad, M.E.; Jeansson, S.L.; Melby, K.K. Bacteria, biofilm and honey: A study of the effects of honey on ‘planktonic’ and biofilm-embedded chronic wound bacteria. Scand. J. Infect. Dis. 2009, 41, 341–347. [Google Scholar] [CrossRef]
- Mikhaylova, A.; Liesenfeld, B.; Moore, D.; Toreki, W.; Vella, J.; Batich, C.; Schultz, G. Preclinical evaluation of antimicrobial efficacy and biocompatibility of a novel bacterial barrier dressing. Wounds 2011, 23, 24–31. [Google Scholar]
- Percival, S.L.; Bowler, P.; Woods, E.J. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen. 2008, 16, 52–57. [Google Scholar] [CrossRef]
- Sadeghpour Heravi, F.; Zakrzewski, M.; Vickery, K.; G Armstrong, D.; Hu, H. Bacterial diversity of diabetic foot ulcers: Current status and future prospectives. J. Clin. Med. 2019, 8, 1935. [Google Scholar] [CrossRef]
- Thomsen, T.R.; Aasholm, M.S.; Rudkjøbing, V.B.; Saunders, A.M.; Bjarnsholt, T.; Givskov, M.; Kirketerp-Møller, K.; Nielsen, P.H. The bacteriology of chronic venous leg ulcer examined by culture-independent molecular methods. Wound Repair Regen. 2010, 18, 38–49. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Gregorchuk, B.S.; Reimer, S.L.; Beniac, D.R.; Hiebert, S.L.; Booth, T.F.; Wuzinski, M.; Funk, B.E.; Milner, K.A.; Cartwright, N.H.; Doucet, A.N.; et al. Antiseptic quaternary ammonium compound tolerance by gram-negative bacteria can be rapidly detected using an impermeant fluorescent dye-based assay. Sci. Rep. 2020, 10, 20543. [Google Scholar] [CrossRef] [PubMed]
- Turetgen, I.; Vatansever, C. The efficacy of nano silver sulfadiazine and nano benzalkonium chloride on heterotrophic biofilms. Microbiology 2019, 88, 94–99. [Google Scholar] [CrossRef]
- Raad, I.I.; Fang, X.; Keutgen, X.M.; Jiang, Y.; Sherertz, R.; Hachem, R. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr. Opin. Infect. Dis. 2008, 21, 385–392. [Google Scholar] [CrossRef]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Shanks, R.M.; Sargent, J.L.; Martinez, R.M.; Graber, M.L.; O’Toole, G.A. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 2006, 21, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm properties of acetic acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and-negative bacteria. FEMS Microbiol. Lett. 2016, 363, 224. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Cag, S. Use of organic acids for prevention and removal of Bacillus subtilis biofilms on food contact surfaces. Food Sci. Technol. Int. 2016, 22, 587–597. [Google Scholar] [CrossRef]
- Chowdhury, D.; Rahman, A.; Hu, H.; Jensen, S.O.; Deva, A.K.; Vickery, K. Effect of disinfectant formulation and organic soil on the efficacy of oxidizing disinfectants against biofilms. J. Hosp. Infect. 2019, 103, e33–e41. [Google Scholar] [CrossRef]
- Kawahara, T.; Takita, M.; Masunaga, A.; Morita, H.; Tsukatani, T.; Nakazawa, K.; Go, D.; Akita, S. Fatty acid potassium had beneficial bactericidal effects and removed Staphylococcus aureus biofilms while exhibiting reduced cytotoxicity towards mouse fibroblasts and human keratinocytes. Int. J. Mol. Sci. 2019, 20, 312. [Google Scholar] [CrossRef]
- Sato-Boku, A.; Nagano, K.; Hasegawa, Y.; Kamimura, Y.; Sento, Y.; So, M.; Kako, E.; Okuda, M.; Tachi, N.; Ito, H.; et al. Comparison of disinfection effect between benzalkonium chloride and povidone iodine in nasotracheal intubation: A randomized trial. BMC Anesthesiol. 2019, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.J.; Westgate, S.J.; Mueller, S. Povidone-iodine ointment demonstrates in vitro efficacy against biofilm formation. Int. Wound J. 2017, 14, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, S.J.; Gawlitta, D.; Heemstra, K.A.; Poolman, R.W.; Vogely, H.C.; Kruyt, M.C. Selection of an optimal antiseptic solution for intraoperative irrigation: An in vitro study. J. Bone Jt. Surg. 2014, 96, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, V.L. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: Prospective randomised controlled clinical trial. Int. Wound J. 2016, 13, 1129–1136. [Google Scholar] [CrossRef]
- Tawre, M.S.; Kamble, E.E.; Kumkar, S.N.; Mulani, M.S.; Pardesi, K.R. Antibiofilm and antipersister activity of acetic acid against extensively drug resistant Pseudomonas aeruginosa PAW1. PLoS ONE 2021, 16, e0246020. [Google Scholar] [CrossRef]
- Premkumar, A.; Nishtala, S.N.; Nguyen, J.T.; Bostrom, M.P.; Carli, A.V. The AAHKS best podium presentation research award: Comparing the efficacy of irrigation solutions on staphylococcal biofilm formed on arthroplasty surfaces. J. Arthroplast. 2021, 36, S26–S32. [Google Scholar] [CrossRef]
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic action of weak acid drugs on biofilms. Sci. Rep. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Brackman, G.; De Meyer, L.; Nelis, H.J.; Coenye, T. Biofilm inhibitory and eradicating activity of wound care products against Staphylococcus aureus and Staphylococcus epidermidis biofilms in an in vitro chronic wound model. J. Appl. Microbiol. 2013, 11, 1833–1842. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011, 55, 4469–4474. [Google Scholar] [CrossRef]
- Kanagalingam, J.; Feliciano, R.; Hah, J.H.; Labib, H.; Le, T.A.; Lin, J.C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int. J. Clin. Pract. 2015, 69, 1247–1256. [Google Scholar] [CrossRef]
- Stoffel, J.J.; Kohler Riedi, P.L.; Hadj Romdhane, B. A multimodel regime for evaluating effectiveness of antimicrobial wound care products in microbial biofilms. Wound Repair Regen. 2020, 28, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Johani, K.; Malone, M.; Jensen, S.O.; Dickson, H.G.; Gosbell, I.B.; Hu, H.; Yang, Q.; Schultz, G.; Vickery, K. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: From in vitro to in vivo. J. Antimicrob. Chemother. 2018, 73, 494–502. [Google Scholar] [CrossRef] [PubMed]
| Product | Ingredients | g/L | Function |
|---|---|---|---|
| Bactisure | Benzalkonium chloride | 1.30 | Surfactant/antimicrobial |
| Sodium acetate trihydrate | 30.20 | pH modifier—metal chelator (biofilm dissolution) | |
| Glacial acetic acid | 59.00 | pH modifier—metal chelator (biofilm dissolution) | |
| Ethanol | 100.00 | Solvent phase polarity modifier | |
| Water | 807.00 | Vehicle | |
| TorrenTX wound wash | Benzalkonium chloride | 1.30 | Surfactant/antimicrobial |
| Sodium citrate dihydrate | 85.00 | pH modifier—metal chelator (biofilm dissolution) | |
| Citric acid monohydrate | 81.70 | pH modifier—metal chelator (biofilm dissolution) | |
| Ethanol | 100.00 | Solvent phase polarity modifier | |
| Water | 795.00 | Vehicle | |
| Minimally invasive lavage | Sodium lauryl sulphate | 1.00 | Surfactant/antimicrobial |
| Sodium citrate dihydrate | 35.70 | pH modifier—metal chelator (biofilm dissolution) | |
| Citric acid anhydrous | 32.50 | pH modifier—metal chelator (biofilm dissolution) | |
| Water | 963.80 | Vehicle |
| Antimicrobials | Bacterial Strains | MIC | MEC | MBIC | MBEC |
|---|---|---|---|---|---|
| Bactisure | S. aureus | 1/128 | 1/32 | 1/32 | 1/16 |
| S. epidermidis | 1/128 | 1/32 | 1/32 | 1/32 | |
| S. pyogenes | 1/128 | 1/32 | 1/64 | 1/32 | |
| P. aeruginosa | 1/128 | 1/64 | 1/32 | 1/32 | |
| A. baumannii | 1/64 | 1/32 | 1/32 | 1/32 | |
| E. coli | 1/128 | 1/4 | 1/32 | 1/4 | |
| TorrenTX wound wash | S. aureus | 1/128 | 1/64 | 1/32 | 1/32 |
| S. epidermidis | 1/128 | 1/64 | 1/32 | 1/32 | |
| S. pyogenes | 1/128 | 1/32 | 1/32 | 1/16 | |
| P. aeruginosa | 1/8 | 1/4 | 1/8 | 1/4 | |
| A. baumannii | 1/32 | 1/16 | 1/16 | 1/8 | |
| E. coli | 1/128 | 1/32 | 1/32 | 1/16 | |
| Minimally invasive lavage | S. aureus | 1/16 | 1/8 | 1/16 | 1/4 |
| S. epidermidis | 1/16 | 1/8 | 1/8 | 1/4 | |
| S. pyogenes | 1/32 | 1/8 | 1/16 | 1/8 | |
| P. aeruginosa | 1/4 | 1/4 | 1/4 | 1/2 | |
| A. baumannii | 1/8 | 1/8 | 1/8 | 1/4 | |
| E. coli | 1/32 | 1/4 | 1/16 | 1/2 | |
| Betadine solution | S. aureus | 1/16 | 1/8 | 1/8 | 1/4 |
| S. epidermidis | 1/16 | 1/8 | 1/4 | 1/4 | |
| S. pyogenes | 1/16 | 1/8 | 1/8 | 1/4 | |
| P. aeruginosa | 1/4 | 1/2 | 1/4 | 1/2 | |
| A. baumannii | 1/16 | 1/8 | 1/8 | 1/4 | |
| E. coli | 1/8 | 1/4 | 1/8 | 1/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, F.; Vickery, K.; Deva, A.K.; Hu, H. Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models. Materials 2022, 15, 3630. https://doi.org/10.3390/ma15103630
Parvin F, Vickery K, Deva AK, Hu H. Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models. Materials. 2022; 15(10):3630. https://doi.org/10.3390/ma15103630
Chicago/Turabian StyleParvin, Farhana, Karen Vickery, Anand K. Deva, and Honghua Hu. 2022. "Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models" Materials 15, no. 10: 3630. https://doi.org/10.3390/ma15103630
APA StyleParvin, F., Vickery, K., Deva, A. K., & Hu, H. (2022). Efficacy of Surgical/Wound Washes against Bacteria: Effect of Different In Vitro Models. Materials, 15(10), 3630. https://doi.org/10.3390/ma15103630








