Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Control of n(Si/Al) of Coal Gangue via Medium-Temperature Chlorinated Roasting and Pressurized Acid Leaching
2.1.2. Hydrothermal Synthesis of the ZSM-5 Molecular Sieve
2.2. Material Characterization
3. Results and Discussion
3.1. Effect of Acid Leaching Process Conditions on the n(Si/Al) of Coal Gangue
3.2. Effect of n(Si/Al) on the Preparation of ZSM-5 Molecular Sieve
3.2.1. XRD Analysis of the ZSM-5 Molecular Sieve
3.2.2. FT-IR Analysis of the ZSM-5 Molecular Sieve
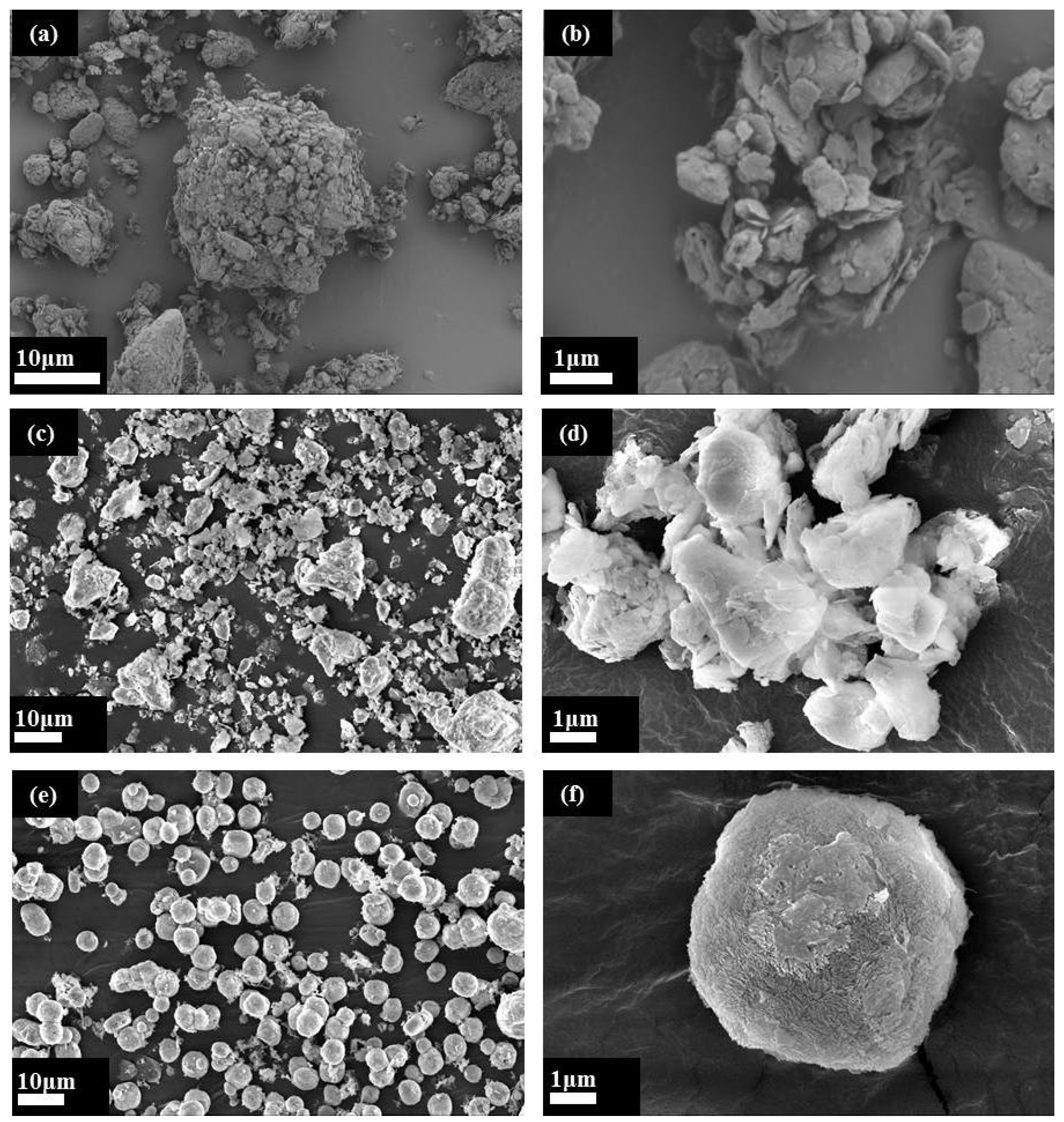
3.2.3. Morphological Analysis of the ZSM-5 Molecular Sieve
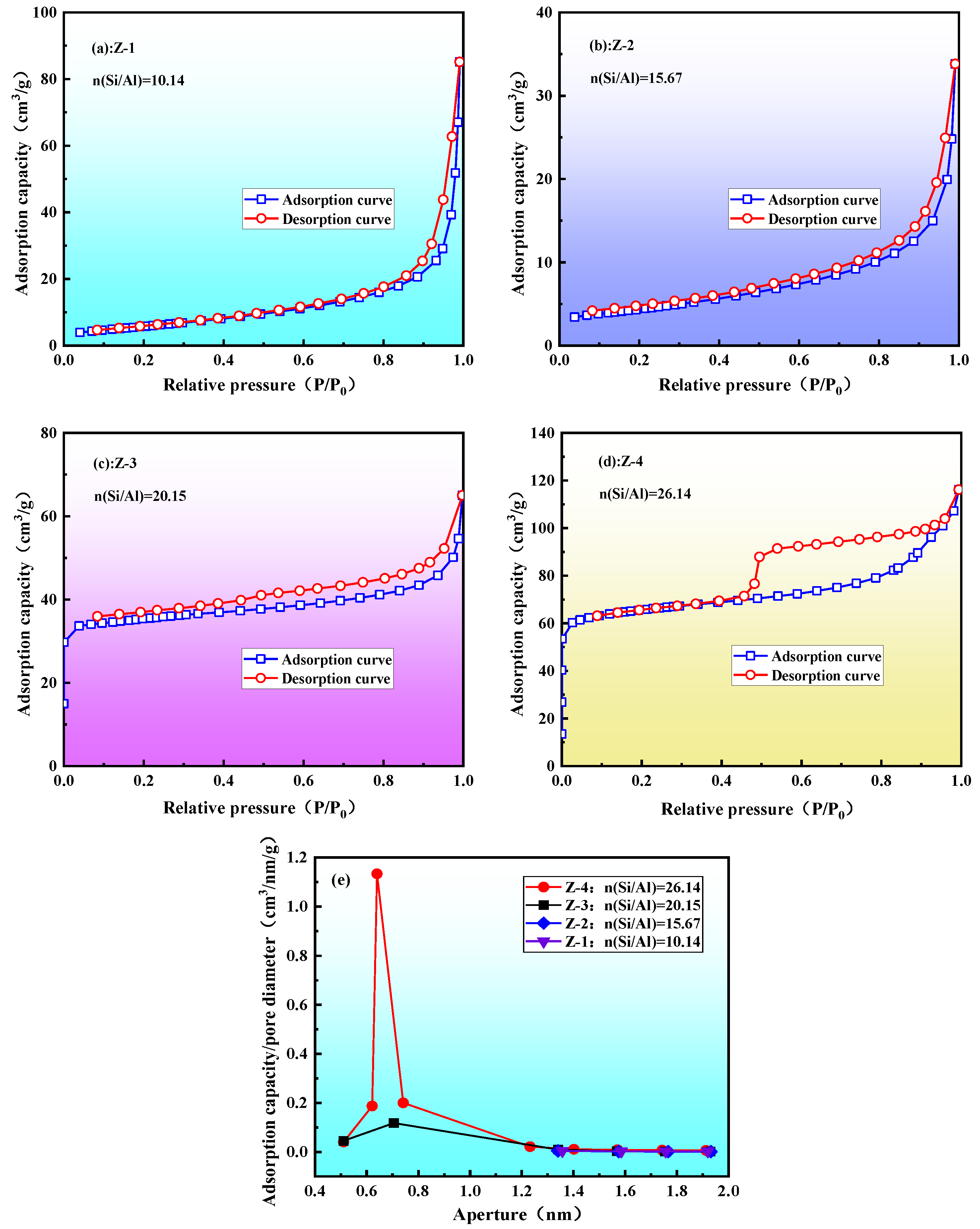
3.2.4. Pore Structure Analysis of ZSM-5 Molecular Sieve
| Sample Number | Specific Surface Area (m2/g) | Aperture (nm) | Pore Volume (cm3/g) | |
|---|---|---|---|---|
| T-Plot | BET | H-K | H-K | |
| Coal gangue | 3.0519 | 20.5090 | 1.2798 | 0.0076 |
| Z-1 | 0.0000 | 21.6663 | — | — |
| Z-2 | 0.0000 | 15.2170 | — | — |
| Z-3 | 90.9119 | 106.4974 | 0.5413 | 0.0535 |
| Z-4 | 169.6329 | 203.6859 | 0.6285 | 0.0988 |
| ZSM-5 [54] | 195.2600 | 407.8100 | — | 0.1003 |
| HZSM-5 [55] | 81.4100 | 137.8000 | — | 0.0400 |
4. Conclusions
- (1)
- Medium-temperature chlorination roasting and the pressure acid leaching process were used to effectively remove elements, such as Fe and Al, from coal gangue, increasing the n(Si/Al) of the coal gangue from 6.13 to 10.14, 15.67, 20.15, and 26.14. The pressure acid leaching process solved the limitation that mica and kaolinite in coal gangue cannot be simultaneously activated. Under optimal conditions, the n(Si/Al) of the coal gangue increased to 26.14, which met the requirements for the preparation of a ZSM-5 molecular sieve.
- (2)
- Using only coal gangue as the silicon and aluminum source, a ZSM-5 molecular sieve was successfully prepared. The minimum n(Si/Al) for the preparation was determined to be 20.15. The microstructure of the ZSM-5 molecular sieve prepared under optimal conditions showed spherical particles, which were formed by the aggregation of ZSM-5 grains. The sample had a microporous specific surface area of 169.6329 m2/g, an average pore diameter of 0.6285 nm, and a pore volume of 0.0988 cm3/g.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faezeh, M.; Reza, K.; Ramin, K. A green approach for template free synthesis of beta zeolite incorporated in ZSM-5 zeolite to enhance catalytic activity in MTG reaction: Effect of seed nature and temperature. J. Clean. Prod. 2022, 361, 132159. [Google Scholar]
- Girolamo, G.; Massimo, M.; Giorgia, F.; Giorgianni, G.; Dalena, F.; Peng, P.; Debost, M.; Boullay, P.; Liu, Z.; Guo, H.; et al. Passivated surface of high aluminum containing ZSM-5 by silicalite-1: Synthesis and application in dehydration reaction. ACS Sustain. Chem. Eng. 2022, 10, 4839–4848. [Google Scholar]
- Demikhova, N.; Rubtsova, M.; Kireev, G.; Cherednichenko, K.; Vinokurov, V.; Glotov, A. Micro-mesoporous catalysts based on ZSM-5 zeolite synthesized from natural clay nanotubes: Preparation and application in the isomerization of C-8 aromatic fraction. Chem. Eng. J. 2022, 453, 139581. [Google Scholar] [CrossRef]
- Julian, T.; Soheil, M.; Przemyslaw, R.; Sung, S.L.; Tim, G.; Vladimir, P.; van Bokhoven, J.A. Electron diffraction enables the mapping of coke in ZSM-5 micropores formed during methanol-to-hydrocarbons conversion. Angew. Chem. Int. Ed. 2022, 61, e202205413. [Google Scholar]
- Aysel, N.; Ali, K.; Nazar, H.; Avci, V.; Pehlivan, E. Synthesis of novel metal/bimetal nanoparticle-modified ZSM-5 zeolite nanocomposite catalysts and application on toluene methylation. Res. Chem. Intermed. 2021, 48, 145–165. [Google Scholar]
- Korobitsyna, L.L.; Travkina, O.S.; Velichkina, L.M.; Vosmerikov, A.V.; Kutepov, B.I. Catalytic conversion of methanol and straight-run gasoline over granulated catalysts with different concentrations of H-form ZSM-5 zeolite. Pet. Chem. 2022, 62, 544–551. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.; Kim, D. Strain development of selective adsorption of hydrocarbons in a Cu-ZSM-5 crystal. ACS Appl. Mater. Interfaces 2021, 13, 50892–50899. [Google Scholar] [CrossRef]
- Ouayloul, L.; El Doukkali, M.; Jiao, M.; Dumeignil, F.; Aguirrezabal-Telleria, I. New mechanistic insights into the role of water in the dehydration of ethanol into ethylene over ZSM-5 catalysts at low temperature. Green Chem. 2023, 25, 3644–3659. [Google Scholar] [CrossRef]
- Hedlund, J.; Zhou, M.; Faisal, A.; Öhrman, O.G.; Finelli, V.; Signorile, M.; Crocellà, V.; Grahn, M. Controlling diffusion resistance, selectivity and deactivation of ZSM-5 catalysts by crystal thickness and defects. J. Catal. 2022, 410, 320–332. [Google Scholar] [CrossRef]
- Sanhoob, M.; Khan, A.; Ummer, A. ZSM-5 Catalysts for MTO: Effect and optimization of the tetrapropylammonium hydroxide concentration on synthesis and performance. ACS Omega 2022, 7, 21654–21663. [Google Scholar] [CrossRef]
- Makshina, E.; Canadell, J.; van Krieken, J.; Sels, B.F. Potassium-modified ZSM-5 catalysts for methyl acrylate formation from methyl lactate: The impact of the intrinsic properties on their stability and selectivity. ACS Sustain. Chem. Eng. 2022, 10, 6196–6204. [Google Scholar] [CrossRef]
- Margarita, P.; Ágnes, S.; Manuela, O.; Lazarova, H.; Koseva, N.; Mihályi, M.R.; Pavletta, S. Selective production of phenol on bifunctional, hierarchical ZSM-5 zeolites. Molecules 2021, 26, 3576. [Google Scholar]
- Xiao, X.; Sun, B.; Fan, X.; Kong, L.; Jiang, G.; Zhao, Z. Synthesis of hierarchical ZSM-5 zeolites by dry-gel conversion method and their catalytic performances for cracking of n-octane. Ind. Catal. 2019, 27, 9–36. [Google Scholar]
- Yamaguchi, A.; Jin, D.; Ikeda, T.; Sato, K.; Hiyoshi, N.; Hanaoka, T.; Mizukami, F.; Shirai, M. P-ZSM-5 pretreated by high-temperature calcination as durable catalysts for steam cracking of n-hexane. Catal. Lett. 2014, 144, 44–49. [Google Scholar] [CrossRef]
- Li, X.; Shen, B.; Xu, C. Interaction of titanium and iron oxide with ZSM-5 to tune the catalytic cracking of hydrocarbons. Appl. Catal. A Gen. 2010, 375, 222–229. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Q.; Wang, J.; Ding, C.; Zhang, K. Synthesis, characterization, and catalytic application of hierarchical nano-ZSM-5 zeolite. RSC Adv. 2020, 10, 29618–29626. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Zhou, H.; Li, Z. Effect of particle morphology for ZSM-5 zeolite on the catalytic conversion of methanol to gasoline-range hydrocarbons. Catal. Lett. 2016, 146, 1973–1983. [Google Scholar] [CrossRef]
- Xue, Y.; Li, J.; Wang, P.; Cui, X.; Zheng, H.; Niu, Y.; Dong, M.; Qin, Z.; Wang, J.; Fan, W. Regulating Al distribution of ZSM-5 by Sn incorporation for improving catalytic properties in methanol to olefins. Appl. Catal. B Environ. 2021, 280, 119391. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Cen, J.; Li, X. Catalytic activity of Pt modified hierarchical ZSM-5 catalysts in benzene alkylation with methanol. Catal. Lett. 2015, 145, 715–722. [Google Scholar] [CrossRef]
- Gao, B.; Ding, C.; Ding, Y.; Wang, J.W.; Zhang, Y.K.; Yu, B. Alkylation of benzene with syngas to toluene via CuO/ZnO/Al2O3 and ZSM-5 bifunctional composite catalysts. Gas Chem. Ind. 2020, 45, 22–25+44. [Google Scholar]
- Lu, Y.; Cheng, G.; Wan, Z.; Pan, M. Effect on isomerization of carbon octaaromatic hydrocarbons using ZSM-5 modified by silicon and metal oxide. Petrochem. Technol. Appl. 2020, 38, 104–107. [Google Scholar]
- Li, Y.; Liu, S.; Xie, S.; Xu, L. Promoted aromatization and isomerization performance over ZSM-5 zeolite modified by the combined alkali-steam treatment. React. Kinet. Catal. Lett. 2009, 98, 117–124. [Google Scholar] [CrossRef]
- Rasouli, M.; Atashi, H.; Mohebbi-Kalhori, D.; Yaghobi, N. Bifunctional Pt/Fe-ZSM-5 catalyst for xylene isomerization. J. Taiwan Inst. Chem. Eng. 2017, 78, 438–446. [Google Scholar] [CrossRef]
- He, P.; Ding, J.; Qin, Z.; Tang, L.; Haw, K.-G.; Zhang, Y.; Fang, Q.; Qiu, S.; Valtchev, V. Binder-free preparation of ZSM-5@silica beads and their use for organic pollutant removal. Inorg. Chem. Front. 2020, 7, 2080–2088. [Google Scholar] [CrossRef]
- Haounati, R.; Lghnih, H.; Rahime, E.; Hafid, N.; Jada, A.; Addi, A.A. Z-Scheme g-C3N4/Fe3O4/Ag3PO4 @ Sep magnetic nanocomposites as heterojunction photocatalysts for green malachite degradation and dynamic molecular studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 13159. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Yin, H.; Xue, C.; Liu, S.; Liu, J. Study on benzene adsorption properties on nitrogen modified ZSM-5 zeolites. Environ. Chem. 2021, 40, 2934–2942. [Google Scholar]
- Oliver-Tolentino, M.; Guzmán-Vargas, A.; Arce-Estrada, E.; Ramírez-Rosales, D.; Manzo-Robledo, A.; Lima, E. Understanding electrochemical stability of Cu+ on zeolite modified electrode with Cu-ZSM-5. J. Electroanal. Chem. 2013, 692, 31–39. [Google Scholar] [CrossRef]
- Lang, X.; Hu, Z.; Zhou, Y.; Zhao, C.; Huang, T.; Chen, N. Synthesis of MnO2/ ZSM-5 molecular sieve composites activated by CO2 as electrode material for supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 5782–5792. [Google Scholar] [CrossRef]
- Xi, J.; Ma, X.; Cui, M.; Huang, X.; Zheng, Z.; Tang, X. Electrochemistry study on PEO-LiClO4-ZSM5 composite polymer electrolyte. Sci. Bull. 2004, 49, 785–789. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zeng, Y.; Liu, X.; Zhang, D.; Zhang, J. Research of corrosion resistance of ZSM-5 Zeolite membrane on the surface of aluminum. J. Guangxi Acad. Sci. 2018, 34, 268–273. [Google Scholar]
- Pour, A.; Mohammadi, A. Kinetic study of the crystallization of ZSM-5 under organic template-free conditions. Inorg. Nano Met. Chem. 2021, 53, 33–38. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Yamani, Z.H. Synthesis and characterization of SnO2- modified ZSM-5 zeolite for hydrogen gas sensing. Mater. Chem. Phys. 2021, 259, 124181. [Google Scholar] [CrossRef]
- Kadja, G.T.; Rukmana, M.D.; Mukti, R.R.; Mahyuddin, M.H.; Saputro, A.G.; Wungu, T.D. Solvent-free, small organic lactam-assisted synthesis of ZSM-5 zeolites. Mater. Lett. Zhou 2021, 290, 129501. [Google Scholar] [CrossRef]
- Huerta-Muñoz, J.G.; Guzmán-Castillo, M.D.L.; Armendáriz-Herrera, H.; Valente, J.S.; Aragón-Piña, A.; Cárdenas-Galindo, M.; Handy, B.E. Upcycling of municipal glass and aluminum wastes for synthesis of hierarchical ZSM-5. Clean Soil Air Water 2022, 50, 2100209. [Google Scholar] [CrossRef]
- Nguyen, D.; Dinh, V.; Nguyen, H.Q.; Hung, N.T. Zeolite ZSM-5 synthesized from natural silica sources and its applications: A critical review. J. Chem. Technol. Biotechnol. 2023, 98, 1339–1355. [Google Scholar] [CrossRef]
- Travkina, O.S.; Kuvatova, R.Z.; Ishkildina, A.K.; Pavlova, I.N.; Sabirov, D.S. Mass transfer between liquid and solid phases in the synthesis of high-crystallinity granular ZSM-5 with hierarchical porous structure. Pet. Chem. 2022, 62, 813–819. [Google Scholar] [CrossRef]
- Jonscher, C.; Seifert, M.; Kretzschmar, N.; Marschall, M.S.; Le Anh, M.; Doert, T.; Busse, O.; Weigand, J.J. Origin of morphology change and effect of crystallization time and Si/Al ratio during synthesis of zeolite ZSM-5. ChemCatChem 2022, 14, e202101248. [Google Scholar] [CrossRef]
- Phan, H.; Nguyen, T. Dry gel synthesis of hierarchical ZSM-5 zeolite using hydroxyl propyl methyl cellulose (HPMC) as a mesoporogen and its catalytic activity in alkylation reactions. RSC Adv. 2022, 12, 24511–24517. [Google Scholar]
- Mohammadparast, F.; Halladj, R.; Askari, S. The synthesis of nano-sized ZSM-5 zeolite by dry gel conversion method and investigating the effects of experimental parameters by Taguchi experimental design. J. Exp. Nanosci. 2018, 13, 160–173. [Google Scholar] [CrossRef]
- Sungtak, K.; Jochen, L. Synthesis of ZSM-5 catalysts via microwave-assisted heating method for military jet fuel cracking into petroleum gas. Microporous Mesoporous Mater. 2021, 328, 111446. [Google Scholar]
- Ghassan, H.; Najwa, S. Effects of synthesis parameter on zeolite ZSM-5 properties. Mater. Today Proc. 2021, 2214–7853. [Google Scholar]
- Ye, T.; Chen, Z.; Chen, Y.; Xie, H.; Zhong, Q.; Qu, H. Green synthesis of ZSM-5 zeolite for selective catalytic reduction of NO via template-free method from tailing residue. J. Environ. Chem. Eng. 2022, 10, 107766. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Extraction of valuable components from coal gangue through thermal activation and HNO3 leaching. J. Ind. Eng. Chem. 2022, 113, 564–574. [Google Scholar] [CrossRef]
- Wei, X.; Meng, C.; Li, X.; Yang, H.; Zhang, P.; Huang, T.; Yuan, Y.; Tang, F. Synthesis of ZSM-5 molecular sieve from Manganese ore overflow tailings. Min. R & D 2020, 40, 162–168. [Google Scholar]
- Jin, S.; Cheng, Y.; Zhong, L. Synthesis of ZSM-5 molecular sieve from coal gangue and its adsorption properties. Chin. Ceram. 2009, 45, 17–19. [Google Scholar]
- Li, H.; Cheng, R.; Liu, Z.; Du, C. Waste control by waste: Fenton–like oxidation of phenol over Cu-modified ZSM–5 from coal gangue. Sci. Total Environ. 2019, 683, 638–647. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, L.; Guo, Y.; Cheng, F. Effect of carbon content on the activation of coal gangue and acid leaching for aluminum extraction. Clean Coal Technol. 2020, 26, 203–208. [Google Scholar]
- Zhao, X. Principle of Chemical Reaction Kinetics; Higher Education Press: Beijing, China, 1984. [Google Scholar]
- Chang, Y.; Li, X.; Hou, Z.; Min, X.; Wei, L. Study on synthetic technology of nano-sized ZSM-5 aggregates with low Si/Al ratio. Inorg. Chem. Ind. 2020, 52, 92–95. [Google Scholar]
- Zhang, L.; Wang, X.; Shi, C. Rapid solvent-free synthesis of nano-sized ZSM-5 with a low Si/Al ratio at 90 degrees C. Inorg. Chem. Front. 2022, 9, 1992–2000. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aly, H.M.; El-Shahat, M.F.; Ibrahim, I.A. Effect of the silica sources on the crystallinity of nanosized ZSM-5 zeolite. Microporous Mesoporous Mater. 2005, 79, 7–12. [Google Scholar] [CrossRef]
- Hang, T.; Zhai, Y.; Tian, L.; He, X.; Li, X.; Yang, H.; Yuan, Y.; Tang, F. Effect of acid leaching process on synthesis of ZSM-5 from tungsten tailings. Min. R & D 2021, 41, 154–161. [Google Scholar]
- Ma, Z.; Zheng, Y.; Zhou, Z.; Hou, J.; Hang, W.; Tao, H. Study on the adsorption of Cr3+ on coal gangue based analcite molecular sieve. Non Met. Mines 2022, 45, 78–82. [Google Scholar]
- Qi, J.; Wei, X.; Zhang, W.; Li, Y.; Guan, C.; Liu, Y.; Zhang, K. Synthesis optimization and characterization of ZSM-5 zeolites with various morphology. Ind. Catal. 2021, 29, 35–38. [Google Scholar]
- Gu, Y.; Cui, N.; Yu, Q.; Li, C.; Cui, Q. Study on the influence of channel structure properties in the dehydration of glycerol to acrolein over H-zeolite catalysts. Appl. Catal. A: Gen. 2012, 429–430, 9–16. [Google Scholar] [CrossRef]
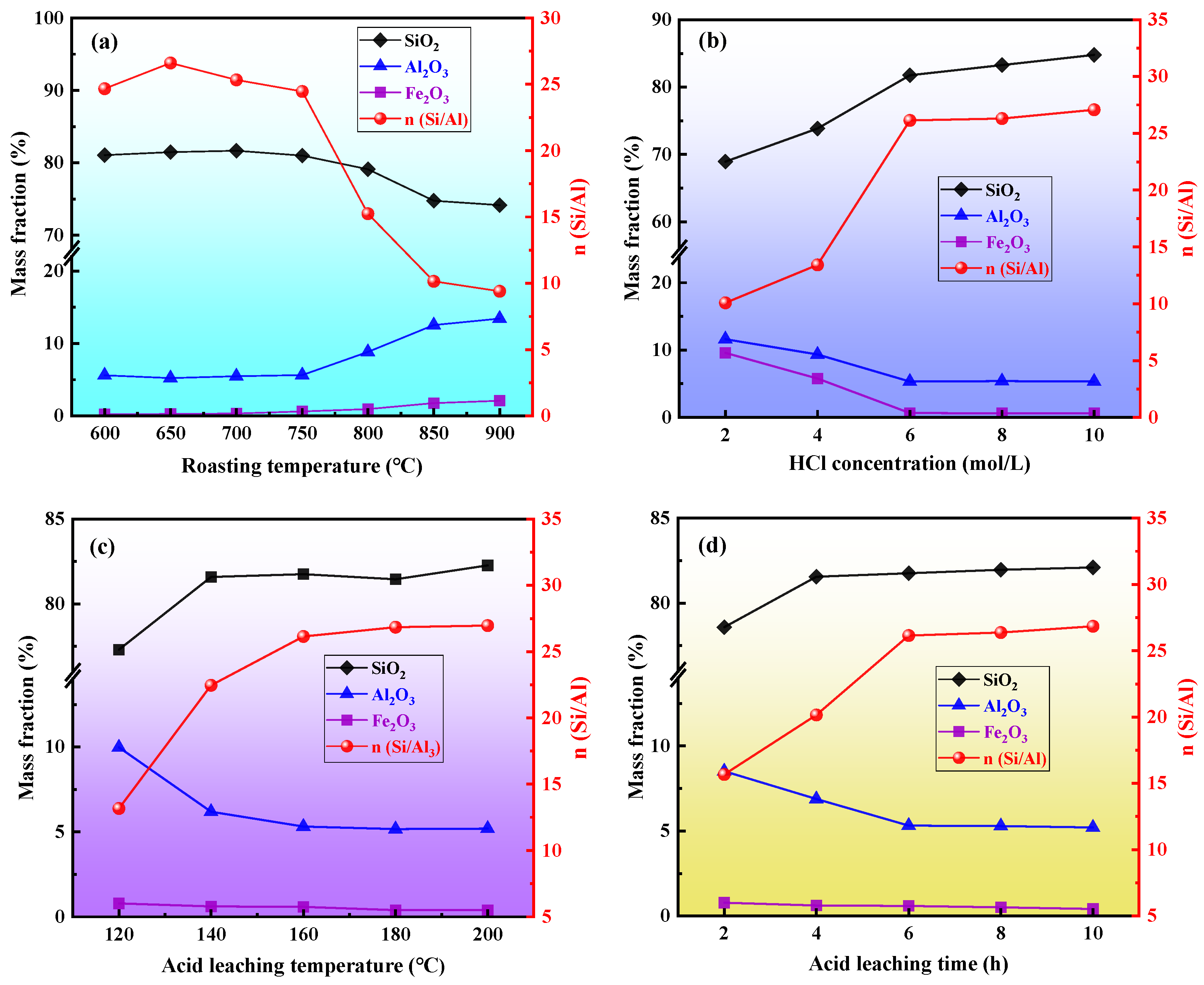


| Sample Number | Acid Leaching Process Parameters | n(Si/Al) | |||
|---|---|---|---|---|---|
| Roasting Temperature (°C) | HCl Concentration (mol/L) | Acid Leaching Temperature (°C) | Acid Leaching Time (h) | ||
| 1 | 850 | 8 | 160 | 6 | 10.14 |
| 2 | 650 | 6 | 160 | 2 | 15.67 |
| 3 | 650 | 6 | 160 | 4 | 20.15 |
| 4 | 650 | 6 | 160 | 6 | 26.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhou, J.; Ma, Z.; Weng, X.; Cheng, L.; Tang, G. Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources. Materials 2023, 16, 4338. https://doi.org/10.3390/ma16124338
Zheng Y, Zhou J, Ma Z, Weng X, Cheng L, Tang G. Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources. Materials. 2023; 16(12):4338. https://doi.org/10.3390/ma16124338
Chicago/Turabian StyleZheng, Yunsheng, Junxia Zhou, Zhijun Ma, Xingyuan Weng, Liang Cheng, and Guorong Tang. 2023. "Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources" Materials 16, no. 12: 4338. https://doi.org/10.3390/ma16124338
APA StyleZheng, Y., Zhou, J., Ma, Z., Weng, X., Cheng, L., & Tang, G. (2023). Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources. Materials, 16(12), 4338. https://doi.org/10.3390/ma16124338







