1. Introduction
Steel structures and steel-concrete composite structures have been widely used in engineering structures due to their high strength and ductile behavior [
1,
2,
3,
4]. However, steel easily suffers from environmental corrosion during its service life [
5], which is known as ‘corrosion destruction’ all over the world [
6]. The mechanical properties of steel are the key parameters to evaluate the strength of these structures and assess the remaining service life of corroded steel through its thickness in design codes [
7,
8,
9,
10]. With the increasing number of steel structures in service, it is necessary to study the degradation law of mechanical properties of steel tubes subjected to corrosion.
Corrosion media and wetting time are the two most important environmental factors affecting steel corrosion. Marine atmospheres (mainly Cl
−) and industrial atmospheres (mainly SO
2) have the most severe corrosion on steel, the widest distribution, and the largest losses. In recent years, relevant research work has been carried out on the degradation law of mechanical properties of corroded steel. In order to analyze the effect of chloride ions on the atmospheric corrosion rate of carbon steel, Ma [
11] exposed Q235 steel to a marine atmospheric environment, and evaluated the effect of chloride ions on the protective characteristics of the rust layer through an infrared spectrum, SEM-EDAX analysis, linear polarization resistance and electrochemical impedance spectroscopy (EIS). The results showed that chloride ions affected the corrosion rate and the morphology and composition of the rust layer. Krzysztof [
12] used the random field method to establish the degradation model of a corroded steel plate and used explicit dynamics to conduct nonlinear finite element analysis. The analysis showed that the irregularity of the steel plate surface after corrosion was one of the main reasons for the decline of the mechanical properties of the steel plate. Garbatov [
13] carried out tensile tests on the steel plate specimens which were cut from the corroded box girder in the real seawater environment, and then obtained the mechanical properties of the specimens, and established the regression equation of the mechanical properties degradation of the corroded steel plate. Kong [
14] carried out the neutral salt spray accelerated corrosion test on 10 groups of Q235 steel specimens, measured the three-dimensional data of the surface morphology and corrosion depth of the corroded steel plate with the three-dimensional non-contact surface topography instrument, studied the probability characteristics of the corrosion depth, analyzed the relationship between the average deviation and standard deviation of the corrosion depth and the corrosion rate, and proposed the autocorrelation function model of the corrosion depth random field model. Melchers [
15] presented a probabilistic model for “at-sea” immersion corrosion of mild and low alloy steels based on fundamental physiochemical corrosion mechanics. Jie [
16] formed a conical blind hole by mechanical drilling and milling to simulate corrosion pits. They studied the influence of the shape, depth and distribution of corrosion pits on the mechanical properties of steel. Finally, a multiple regression analysis was conducted to obtain a prediction model for elongation. Xu [
17] established a modified constitutive model of corroded Q235 steel through a salt spray accelerated corrosion test and obtained the relationship between model control parameters and corrosion rate.
In addition to corroded steel, the mechanical properties of corroded reinforcement were also studied [
18,
19,
20,
21,
22]. It was found that with increasing duration of exposure to a corrosive environment, the steel mass loss increases appreciably. In addition, a significant reduction of the tensile mechanical properties and ductility of the material was observed. It was noted that the degree of corrosion strongly affected the mechanical properties of the steel, particularly the ultimate stress and strain. Li [
23] derived a generalized equivalent stress-strain equation for the corroded steel bars, which considers the pitting corrosion using a bilinear elastoplastic constitutive equation. The bond behavior of corroded reinforcing steel bars in concrete was also studied [
24,
25,
26].
In industrial atmospheres, SO
2 is adsorbed onto the surface of steel and combined with a liquid film to form H
2SO
3, which is then oxidized to H
2SO
4. It reacts with Fe to form FeSO
4, which in turn hydrolyzes to produce trace amounts of H
2SO
4, continuing to corrode the steel substrate, forming an acid cycle locally. Yang [
27] conducted the corrosion fatigue experiment on the CT specimen of Q420B under artificial acid rain spray. Based on the Paris model, a P-da/dN-ΔK model of corrosion fatigue crack growth rate considering the randomness of the model parameters and the volatility of da/dN data is also derived. Zuo [
28] investigated the effects of pH value, chloride ion concentration and alternation of wetting and drying time in acid rain on the corrosion of 35CrMn and Q235 steel. The results showed that the corrosion rate of 35CrMn and Q235 steel increased with decreasing pH values of the simulated acid rain, whereas the corrosion potential of 35CrMn and Q235 steel became more negative.
In existing research, the main focus is on the effect of corrosion on the mechanical properties of steel, but the functional relationship between the equivalent reduction thickness of steel and corrosion time has not yet been accurately given. In addition, the composition of the atmospheric environment has a great impact on the corrosion of steel. Within the same corrosion time, the mechanical properties of corroded steel in different areas also differ. In view of the dispersion of mechanical properties of corroded steel, the evaluation of the mechanical properties of corroded steel in specific areas can only be based on local corrosion data to establish a more accurate corrosion model.
The study intends to establish an equivalent thickness model for the Q235 steel subjected to sulfate corrosion. In the work, the results of an experimental survey on artificially corroded steel are reported. The effect of corrosion on the mechanical properties of steel tensile coupons is tested and discussed. The results are interpreted also from the microstructural point of view. Simultaneously, the deterioration equations for the mechanical properties of the corroded steel are defined, which include the ultimate tensile strength reduction factor, deformability reduction factor, and energy absorption reduction factor. The equivalent reduced thickness equation considering sulfate corrosion rate is also proposed, which can be used by researchers in further studies and engineers in practice.
3. Results and Discussion
3.1. Potentiogram Analysis and Gibbs Free Energy
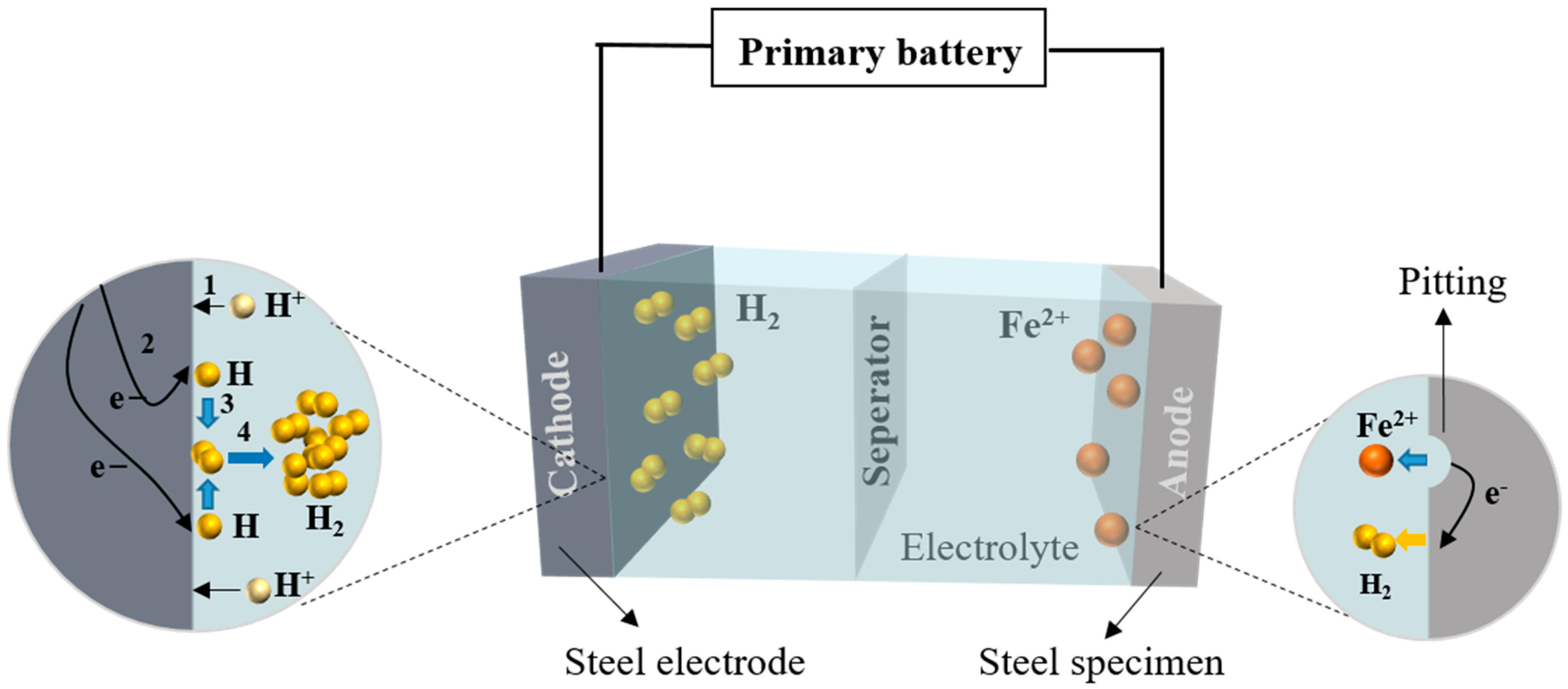
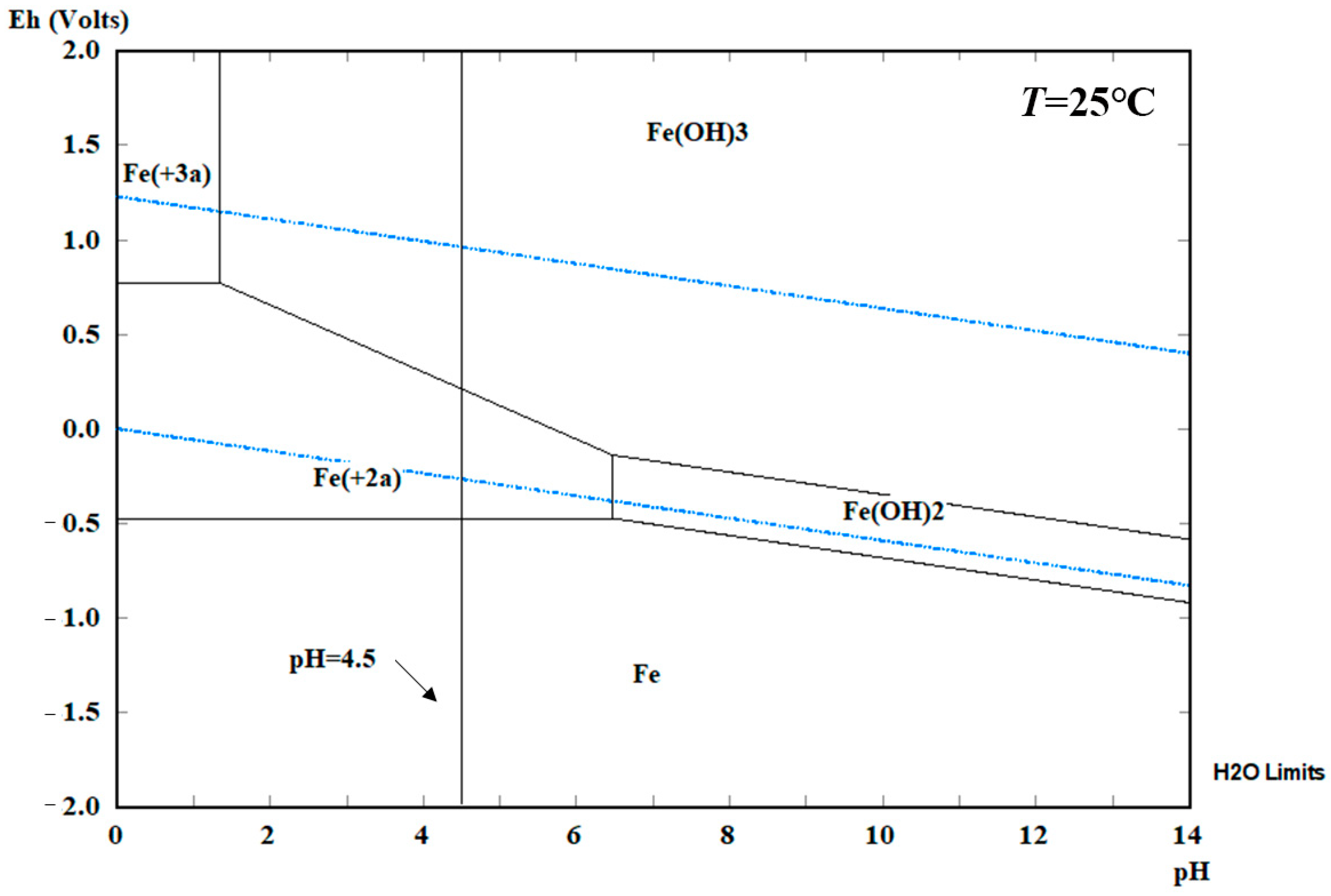
Figure 5 presents the potentiogram of steel specimens at 25 °C and one atm. It can be seen that high oxidation state substances are located above low ones. Solid substances are located on the side with a higher pH value, while dissolved substances are located at a relatively low pH.
Figure 5 shows the area within the two blue lines is defined as the water stability area, iron exists as Fe
2+ and Fe
3+, which means that steel specimens are unstable in the solution. The pH value of the electrolyte in this paper is set as 4.50, the steel specimens in this condition present corrosion. Horizontal lines in
Figure 5 represent the oxygen and proton reduction of the steel corrosion. Moreover, the steel used in structural engineering contains impurities, which will corrode due to acid rain in a lower pH value without a power supply in areas Ⅰ and Ⅱ.
According to the thermodynamic principle, the direction and limits of the corrosive reaction of steel specimens can be distinguished by the change of Gibbs free energy (
G), as expressed in Equation (3).
In the process of corrosive battery reaction of a metal, combined with Faraday’s law, Gibbs free energy could be described as Equation (4):
where the unit of Δ
G is (kJ/mol),
n denotes the stoichiometric number of electrons consumed in the electrode reaction;
F denotes the Faraday constant;
η denotes the electromotive force of a reaction (V).
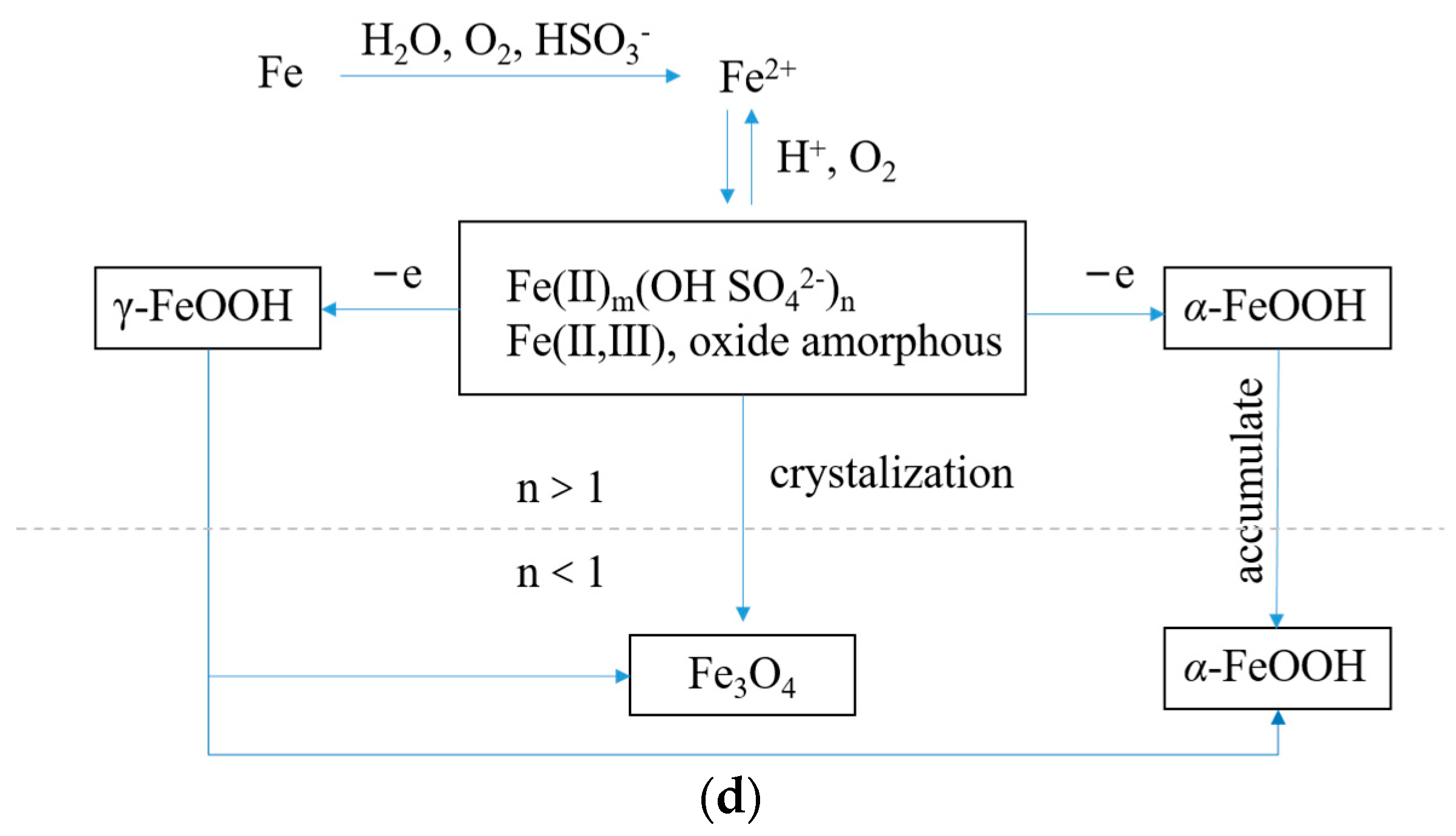
The initial corrosion products on the surface of Q235 steel are mainly Fe(II) and Fe(III) amorphous oxides with relatively high chemical activity, which uniformly cover the surface of steel specimens after reaching a certain cumulative amount. The formation of
γ-FeOOH mainly comes from the crystallization reaction of amorphous components in the rust layer, while the formation of Fe
3O
4 may come from both the reduction and crystallization of amorphous components and the transformation of
γ-FeOOH crystal form, as shown in
Figure 6.
3.2. Open Circuit Potential and Linear Polarization Curve
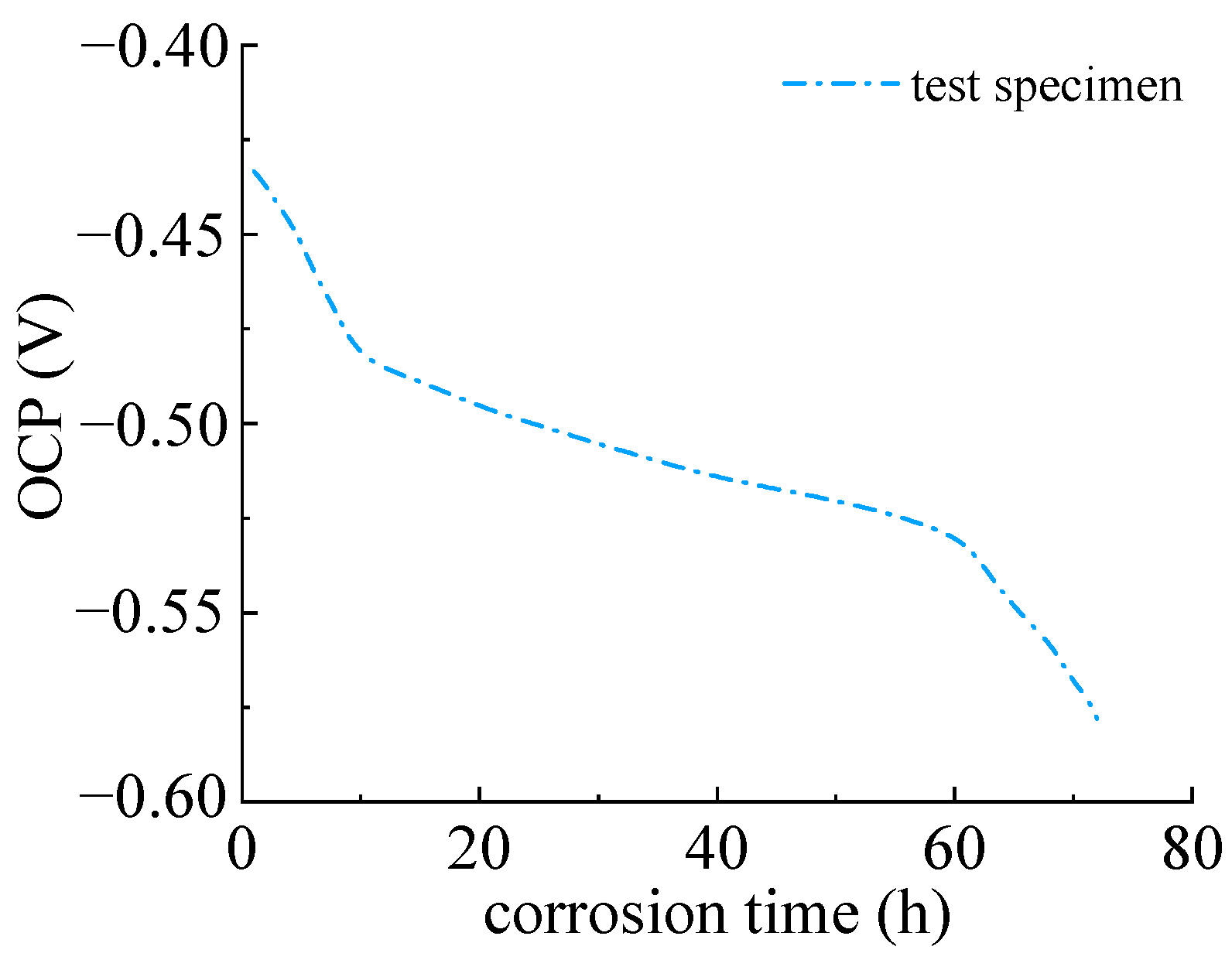
Figure 7 depicts the open circuit potential of the Q235 steel specimen within 72 h. It can be seen that open circuit potential decreased rapidly at the beginning. This is because of the initial corroded layer, which provides a formed passivation film on the surface of the steel specimen. The electrolyte solution and oxygen have not yet been reached. The corrosion potential remained at a relatively high value. The initial open circuit potential was −0.429 V and decreased to −0.578 V at 72 h. This indicated that the sulfate corrosion of Q235 steel started, the electrolyte solution and oxygen diffusion into the metal interface. The anodic reaction of steel accelerated with the decreasing of corrosion potential due to the large amount of porosity of corrosion products, which caused further electrochemical reactions.
The polarization resistance curve of Q235 steel was presented in
Figure 8. It can be seen that the polarization resistance decreased with the corrosion time, which illustrated that the corrosion was accelerated and the corroded area enlarged. The tendency of descending was obvious within the 72 h due to the fact that the corrosion products with limited impermeability since they are loose [
34].
3.3. SEM Analysis
The standard tensile coupon test of steel specimens subjected to sulfate corrosion indicates that the mechanical properties of the steel were uniformly reduced with the corrosion rate. The pits on the steel specimens are distributed randomly, and the size and form of the pits were different with different corrosion rates. Thus, the effect of corrosion rate on the microscopic properties of steel is analyzed by SEM.
In this section, the steel specimens with a thickness of 3.0 mm are analyzed as typical examples. The steel specimens after the tensile coupon test are cut into small slices below the size of 2 mm × 2 mm. It should be noted that the small slices for SEM are cut at the location where 25 mm away from the fracture on the longer side. Then SEM specimens are sanded with sandpaper, and the SEM specimens are dried with a blower. The electron microscope used was Evo18 (Zeiss, Oberkochen, Germany).
3.3.1. Surface Morphology Analysis
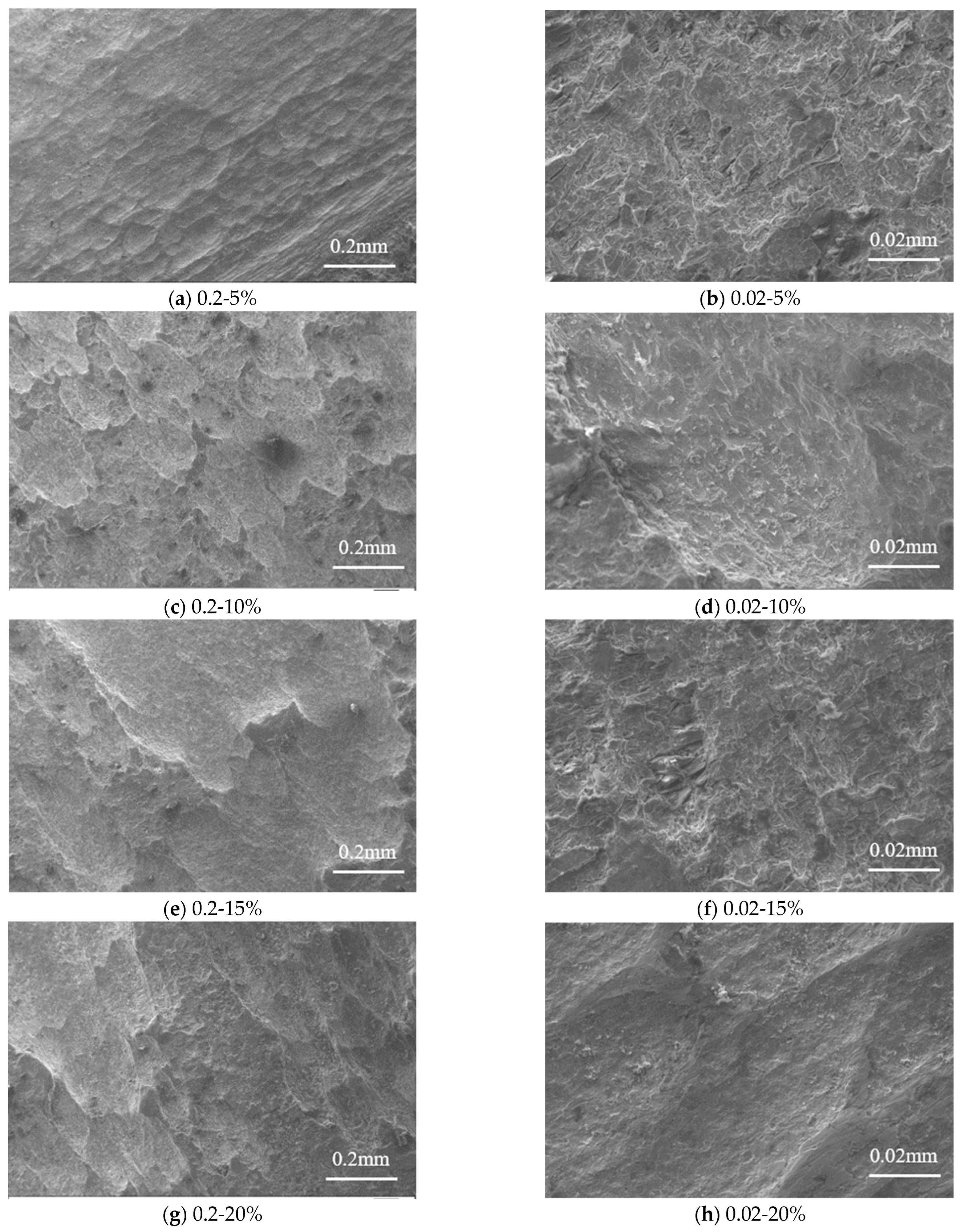
Figure 9 presents the microstructure of steel specimens at various magnifications. The comparison between these microstructures can observe that the corroded Q235 steel presented the characteristic of pitting corrosion. It can be seen that the corrosion pits were shallow quasi-circular shapes when the corrosion is 5%. Then the corrosion pits grew laterally, and the surface area of the corrosion pits increased but the numbers decreased. As the corrosion rate increased, the smaller corrosion pits merged together and became broad depressions, which presented characteristics of uniform corrosion. As the corrosion rate further increased, the characteristic of pitting corrosion occurred again, which indicated that the corrosion developed along the thickness direction of the Q235 steel. When the corrosion rate achieved 25%, the distribution range of corrosion depth increased. With the continuous increase of corrosion products, the corrode reaction gradually stops at the surface of steel due to the corroded layer causing difficulties in oxygen absorption and hydrogen evolution reaction. The corrosion in the direction of thickness is no longer developed. However, if the pH value is relatively lower, then the corrosion pits will develop on the surface of steel and form corrosion pits with larger diameters, which causes the connection of adjacent pits to become larger pits. New corrosion pits would occur and develop on those larger pits.
3.3.2. Tensile Fracture Morphology
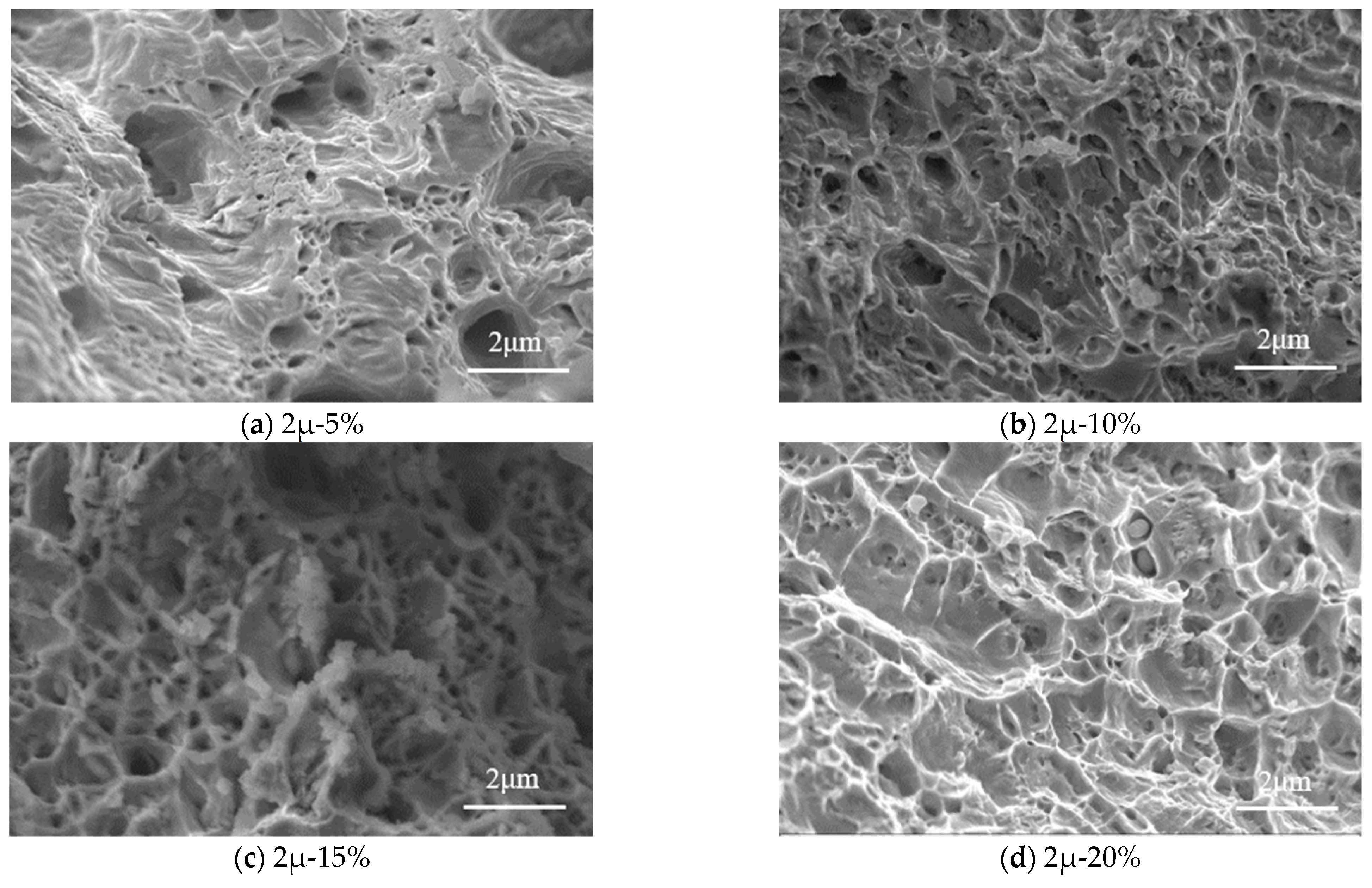
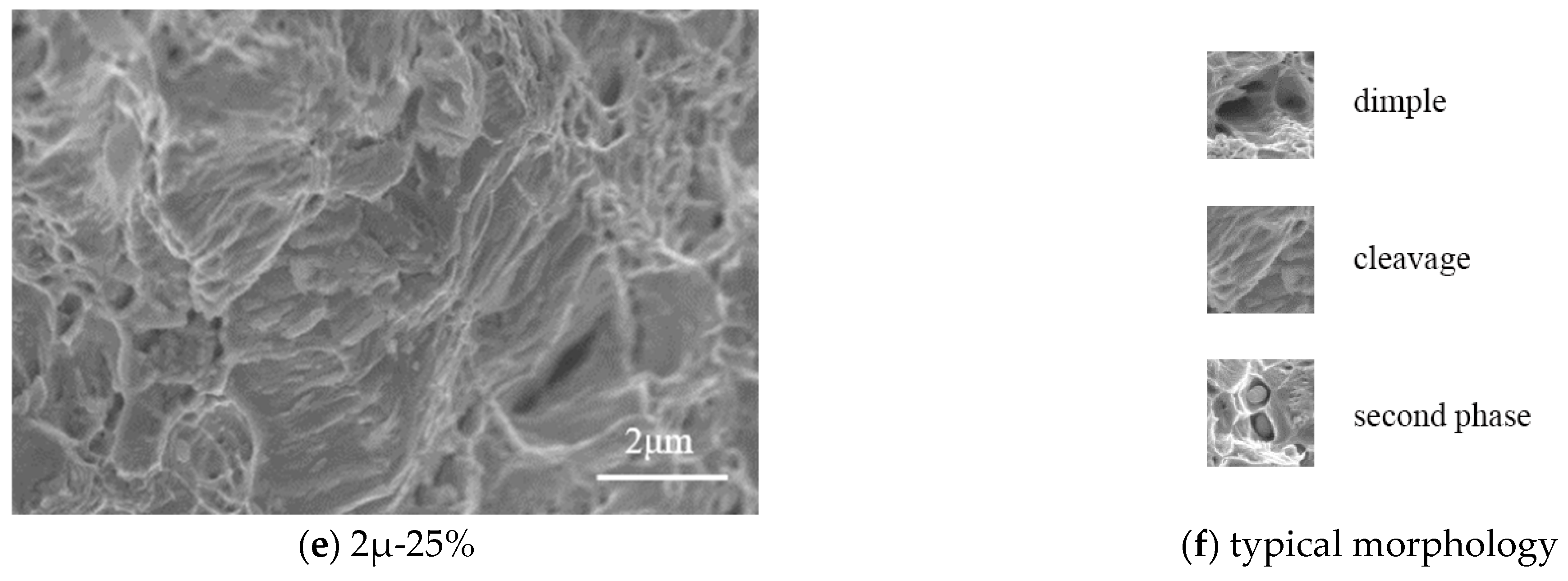
Figure 10 shows the microstructures of tensile specimens where the tensile fracture occurred. The specimens with a thickness of 3.0 mm are analyzed under the magnification of 5000. The corrosion rate ranges from 5% to 25%. It can be seen that the microstructure of the steel can be separated into two categories: (1) intercrystalline fracture, and (2) cleavage fracture.
For the corrosion rate
γ ≤ 15%, the steel shows an intercrystalline fracture, where a dimple was obvious. The number of dimples decreases with the increasing corrosion rate. Meanwhile, dimples vary from large and deep to small and shallow. It indicates that the deformability of steel becomes weaker with the increasing corrosion rate. When the corrosion rate increases to 20%, the microstructure of dimples and cleavage planes exist simultaneously. It indicates that the steel varies from ductile to brittle, and the brittle failure mode is obvious. The brittle cleavage fracture of metallic materials without plastic deformation and cleavage is caused by dislocation. The ductile becomes weaker due to the non-uniform stress concentrations. When the corrosion rate increases to 25%, the cleavage planes gradually increase but the dimples disappear. It illustrates that the steel occurs brittle failure. According to the test results of tensile coupons (
Table A1), although the elongation of the tensile coupon specimens decreases with corrosion rate, all the elongation exceeds 5%. Thus, steel specimens are ductile and subjected to sulfate corrosion. The transition from ductile to brittle in microstructure is mainly due to the non-uniform stress distribution.
Another reason for this change is hydrogen embrittlement (HE), which caused the decrease in tensile strength and elongation. Acid rain provides a source of hydrogen, as a kind of dilute sulfuric acid solution. According to
Figure 10a–e, the fracture of the steel varied from ductile fracture to brittle fracture, which was the major characteristic of HE. This transformation is due to the transgranular to intergranular fracture which is caused by hydrogen. The high-stress concentration at the tip of the pit constitutes the most favorable condition for crack initiation. The cleavage fractures are associated with locally high strains due to the high dislocation densities beneath fracture surfaces.
The deviation of the surface of the corroded steel specimen compared to its non-corroded counterparts can be considered as a stochastic process Z(x), which has self-similarity and self-affine. Therefore, fracture theory can be introduced to characterize the surface of corroded components.
The depth of corrosion pits roughly follows the normal distribution.
where
M and
N is the number of scanning points;
is the coordinate of the scanning point;
is the mean value of scanning point.
Power spectrum analysis can be used to study the surface features of corroded steel. Unitized
is used as the sample function of
ζ(
x,
y),
ζ(
x,
y) =
Z(
x,
y) +
. Discrete two-dimensional power spectral density of bilateral corrosion depth can be described as
where
a and
b is the parameter corresponding to corrosion rate.
Corrosion pits varies with the increasing corrosion rate, which presented obvious cyclic process.
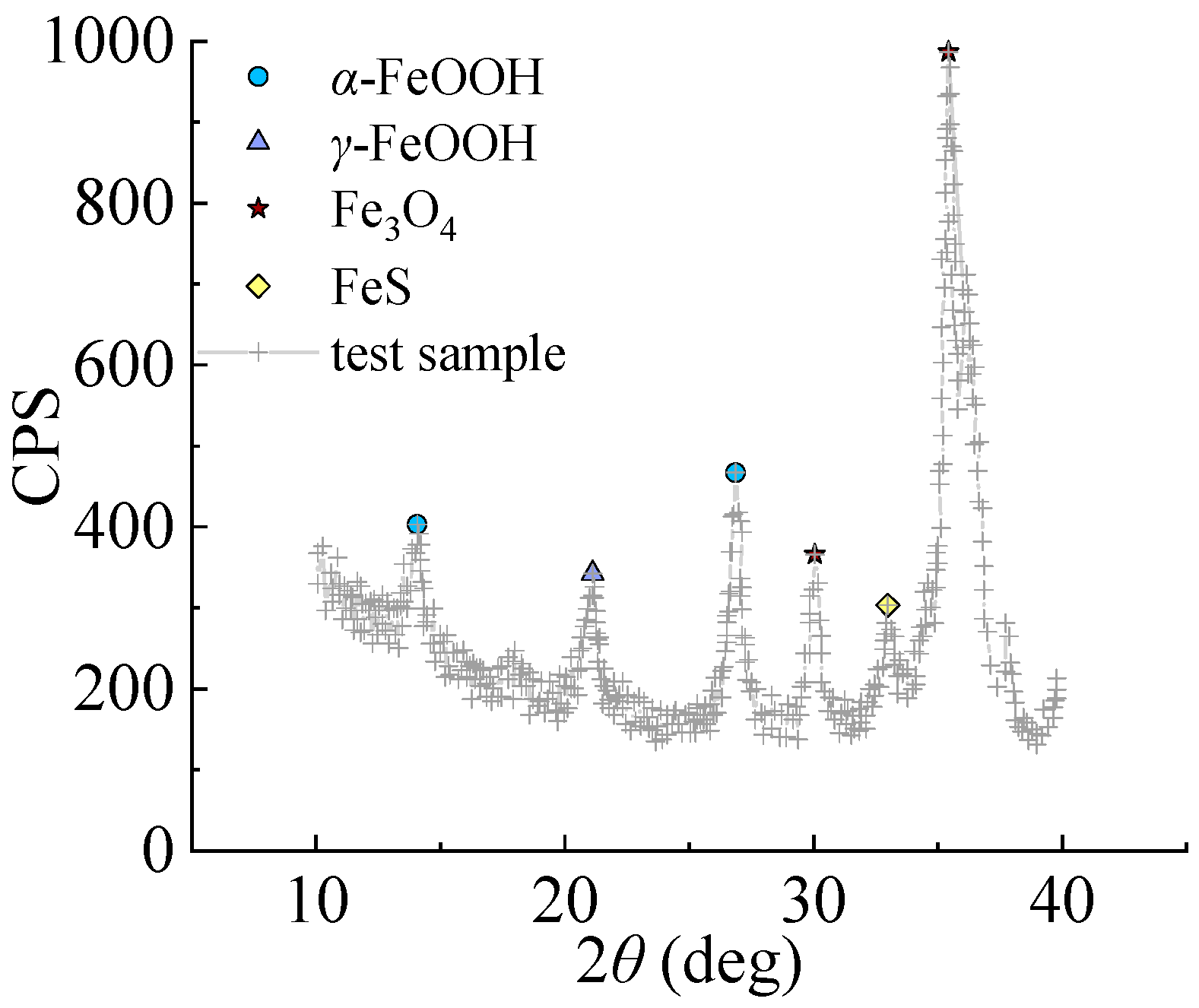
Figure 11 shows XRD of Q235 low carbon steel under sulfate corrosion. It can be seen that the final products mainly include
α-FeOOH,
γ-FeOOH, and Fe
3O
4. The total corrosion included the following process:
(1) Electrochemical corrosion begins:
(2) Formation of FeOOH:
(3) Formation of Fe3O4:
3.4. Failure Patterns of Specimens
It can be seen that the failure patterns of steel standard tensile coupon test specimens include two modes: (1) normal fault (the fracture is perpendicular to the long axis of the specimen, φ = 90°); (2) and oblique fault (the fracture intersects with the long axis, φ ≠ 90°).
Figure 12 presents the failure mode of the steel standard tensile coupon specimens with a thickness of 3.0 mm. If the corrosion rate is within 10%, the fracture of the specimen presented a normal fault; while if the corrosion rate ranges from 15% to 25%, the fracture of the specimen will be the oblique fault.
Figure 13 shows the failure patterns of steel standard tensile coupon test specimens with a thickness of 4.5 mm. It can be observed that the two types of failure modes exist and are influenced by the corrosion rate. The corrosion rate below 20% leads to a normal fault while the corrosion rate between 20% and 30% shows an oblique fault.
The failure patterns of the test specimen show that the thickness of the steel and corrosion rate affected the corrosion resistance. With the increase of the wall thickness of steel, the oblique fault occurs at a higher corrosion rate. With the increasing corrosion rate, the oblique fault occurs in the range of the high corrosion rate for all test specimens with thicknesses of 3.0 mm and 4.5 mm, respectively. It indicates that steel with a larger thickness would delay the influence of corrosion.
It can be seen that the size of the pits is different after sulfate corrosion, and the number, size, and distribution of the pits are random. When the corrosion rate
γ ≤ 10%, the number of pits increases with the corrosion rate, and the distribution of the pits became denser which caused the pits larger and deeper. When the corrosion rate is 15% ≤
γ ≤ 25%, the corrosion pits became clear and denser, and the corrosion pits are assumed as hemispherical [
35].
The test results of steel tensile coupon specimens with thicknesses of 3.0 mm and 4.5 mm are shown in
Appendix A. The following rules are utilized to distinguish the specimen: (1) The first character “T” represents the tensile coupon specimen; (2) The second Arabic number represents the thickness of the steel; (3) The third Arabic number represents the corrosion rate; (4) and the last character “a (b)” represents the different specimen in the same group. The test results of tensile coupon specimens are listed in
Figure 14.
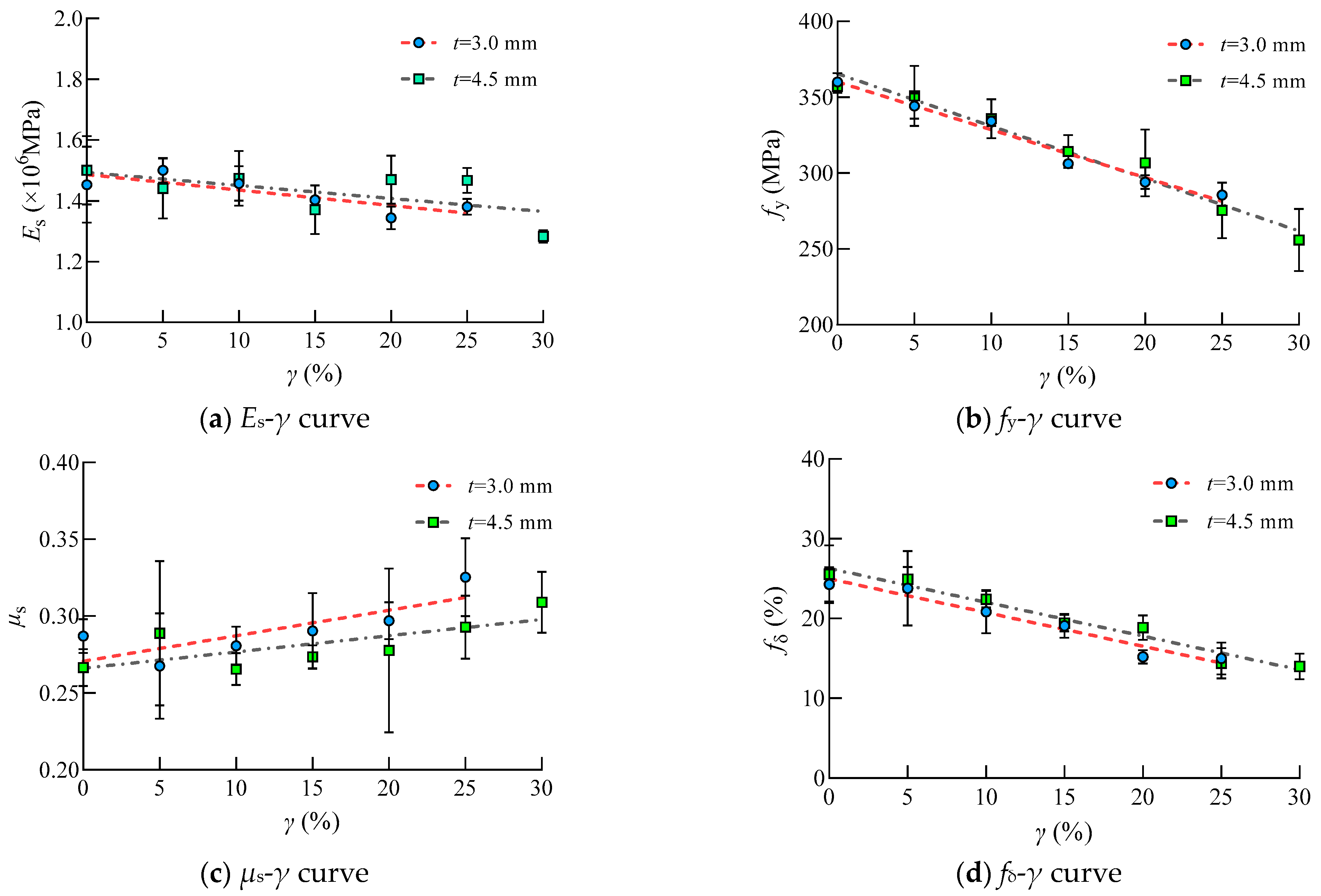
It can be seen that the value of the experimental elastic modulus, yield strength and elongation of Q235 steel decreased with the increasing sulfate corrosion rate despite the scatter of these test results. This was due to the pitting corrosion which caused the discontinuous in physical performance and local stress concentration of steel. However, the Poisson’s ratio of Q235 increased with the increasing sulfate corrosion rate, which indicated that steel changed from a plastic state to a brittle state.
It is obvious that Poisson’s ratio increased with the increasing corrosion rate. The value of Poisson’s ratio varies in the same group with relative higher SD, which is mainly due to the random distribution of corrosion pits. The increasing of Poisson’s ratio indicated that the degree of lateral shrinkage increases, and the deformation ability of the Q235 steel correspondingly decreases.
In this study, steel used in steel structures corroded at a rate of 0.06 mm/a. The corrosion rates 0%, 5%, 10%, 15%, 20%, and 25% correspond to the duration of 0, 2.5, 5, 7.5, 10, 12.5, and 15 years for the steel specimens with a thickness of 3.0 mm. The corrosion rates 0%, 5%, 10%, 15%, 20%, 25%, and 30% correspond to the duration of 0, 3.75, 7.5, 11.25, 15, 18.75, and 22.5 years for the steel specimens with the thickness of 4.5 mm. It can be seen that the Q235 steel weight loss with the increasing corrosion rate in
Figure 15. The R square of the two linear regress formula is 0.9953 and 0.9793, respectively. However, it can be observed that the corrosion speed increased rapidly in the initial stage when the corrosion rate achieved 5%, corresponding to 2.5 years and 3.75 years for the 3.0 and 4.5 mm steel, respectively, when the weight loss was the most serious. This is because the rust layer avoided the sulfate corrosion, which caused the decrease in the corrosion rate in the later corrosion stage. This regulation matched the SEM analysis.
The mechanical properties of steel subjected to sulfate corrosion changed linearly with the corrosion rate. The quantitative relationships between these mechanical parameters and corrosion rate are as follows, within the errors of ±15%.
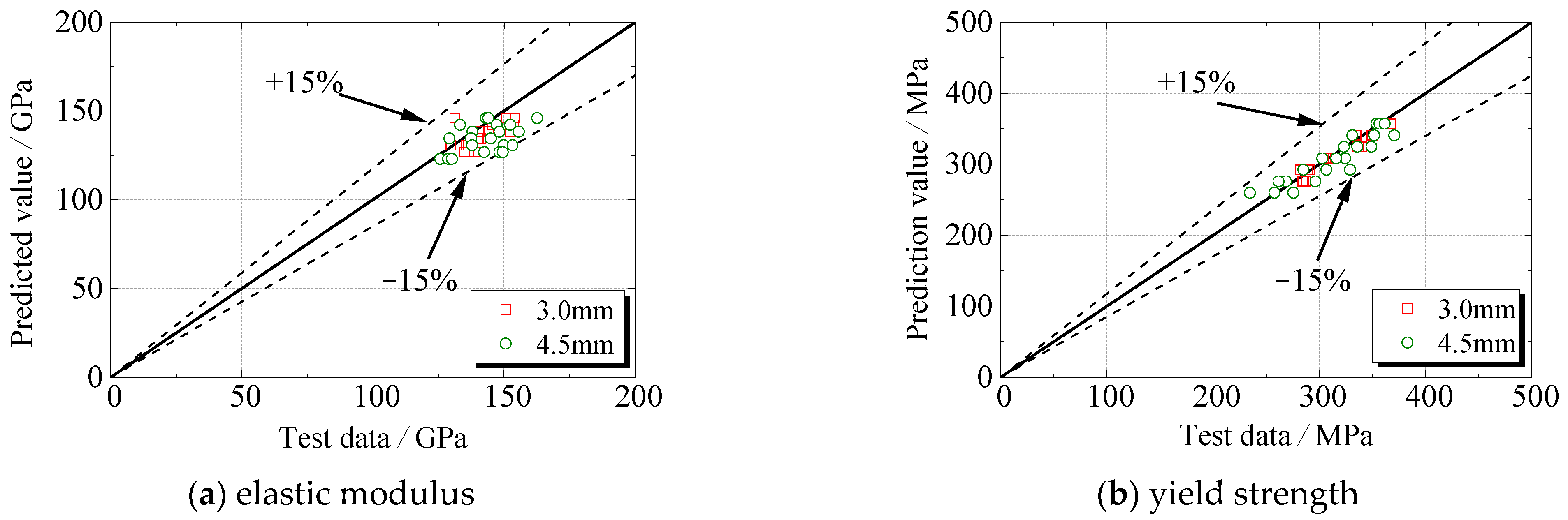
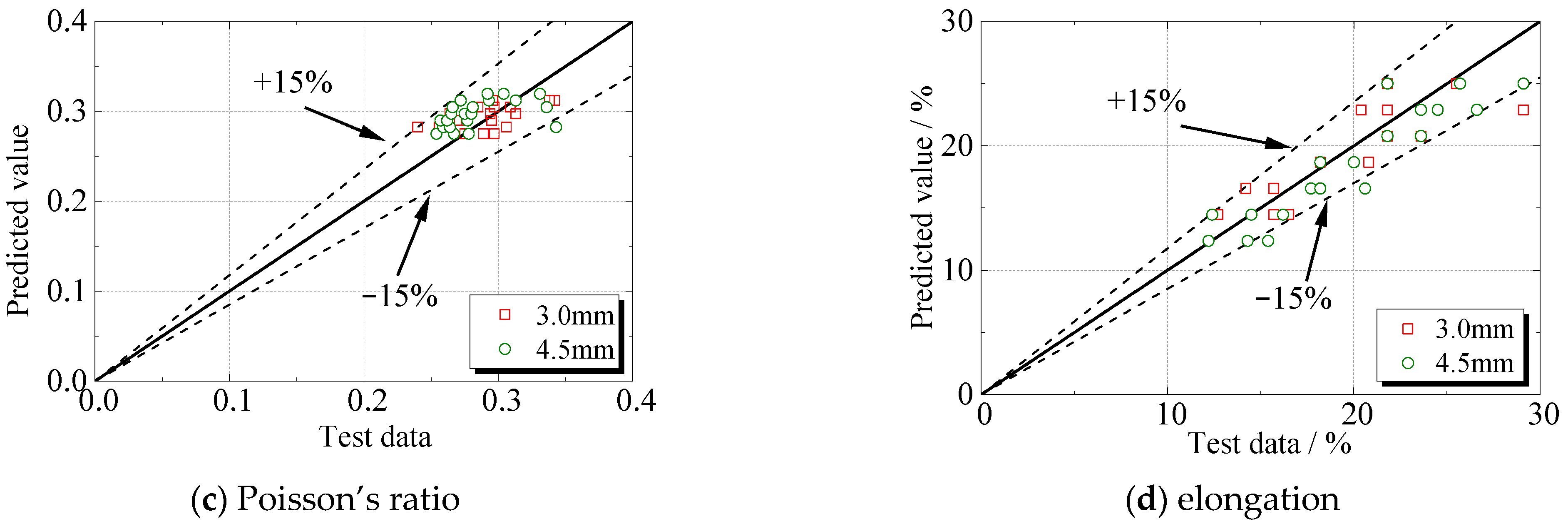
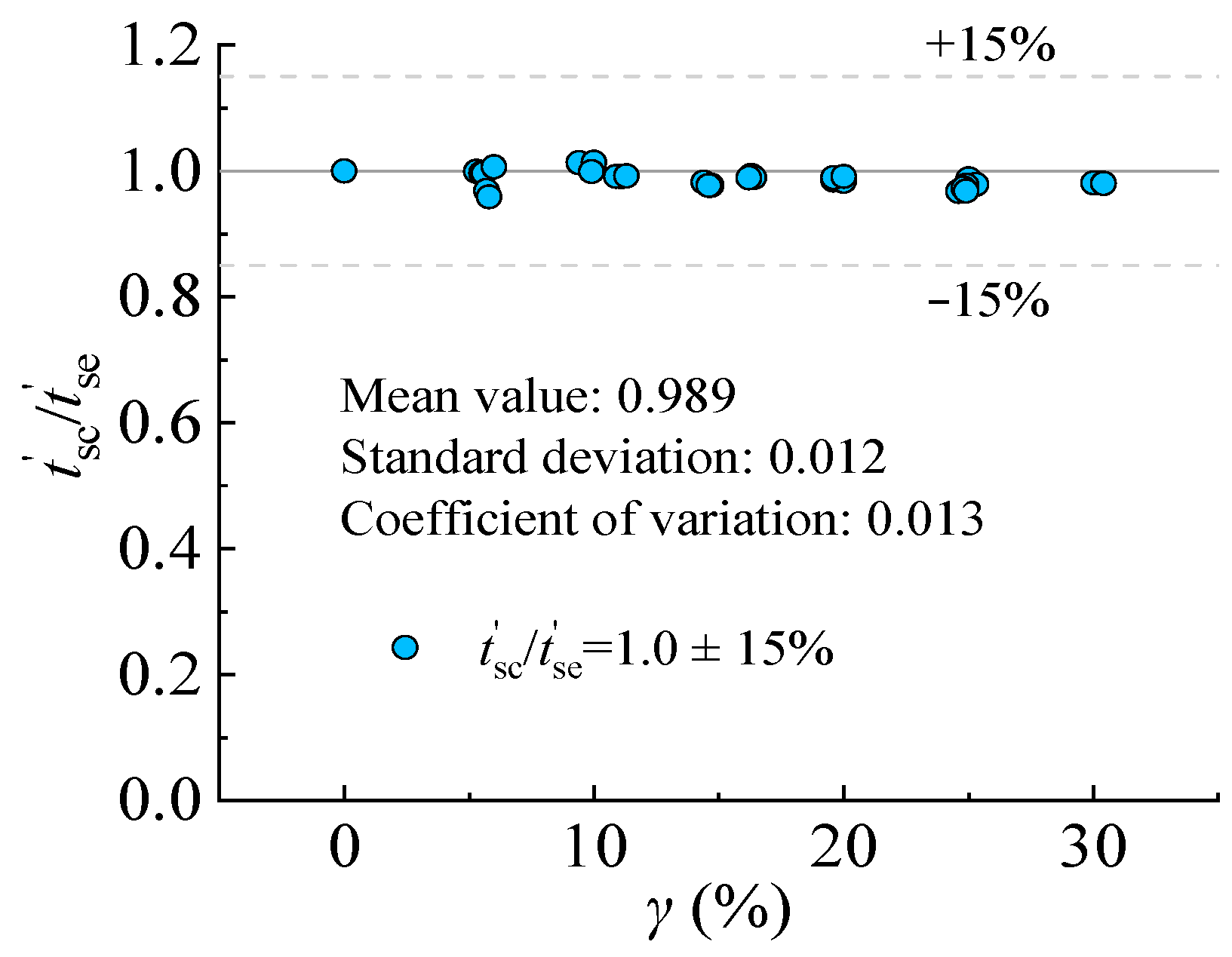
Figure 16 presented the accuracy and applicability of the model in predicting the degradation of steel specimens under sulfate corrosion. The deviation is within ±15%.
It can be seen that the elastic modulus of steel coupon specimens varies between the same group. Although the elastic modulus is not sensitive to the metallographic microstructure of steel, the test results are scattered to some degree. This is because the concentration stress occurred at the randomly distributed corrosion pits, which influenced the elastic modulus of steel.
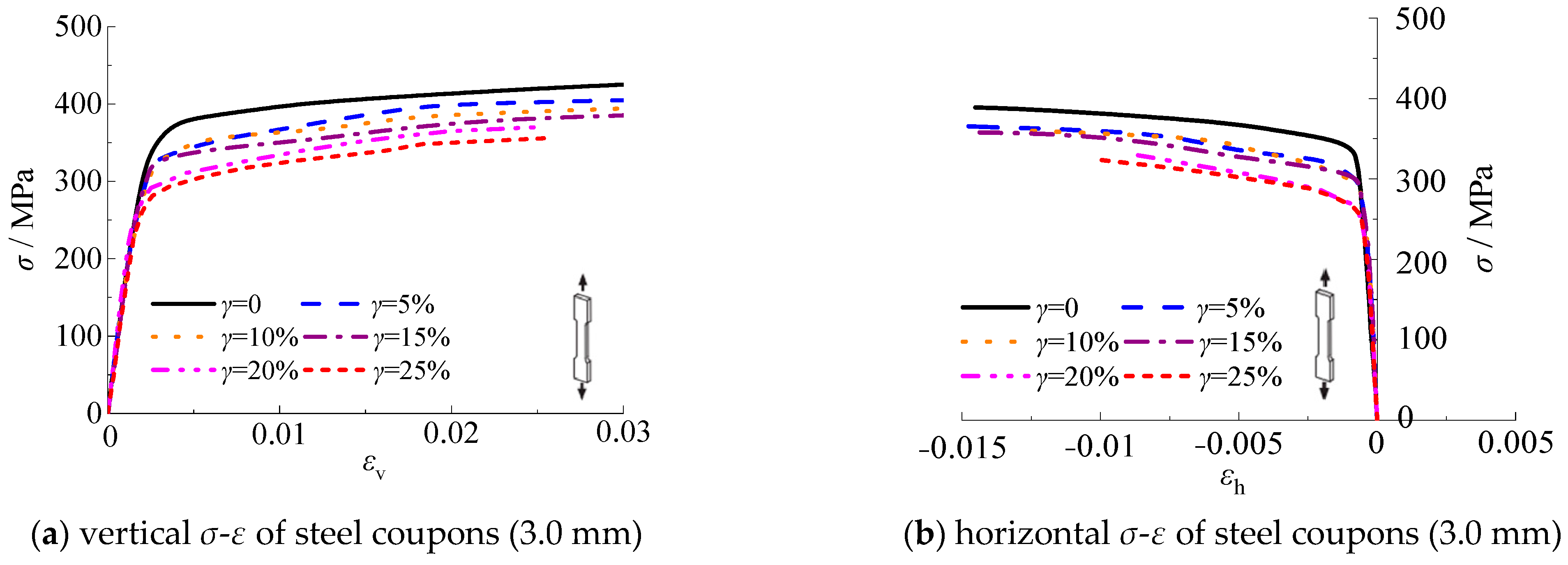
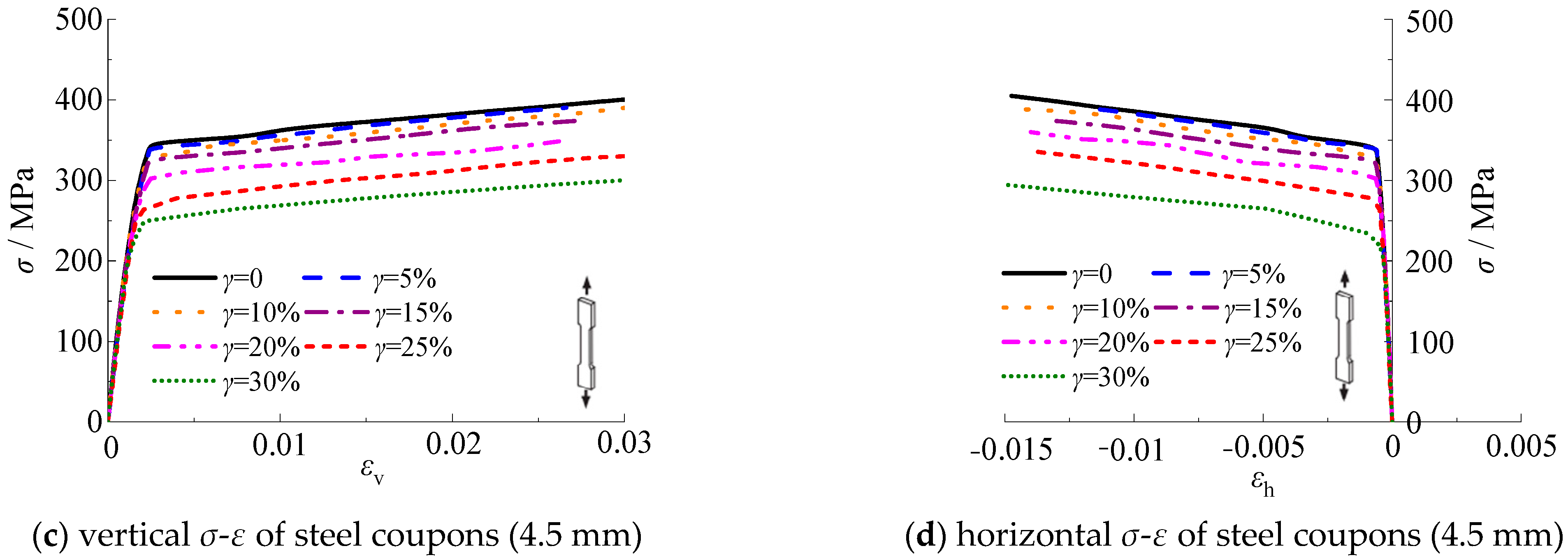
3.5. Stress-Strain Curves
Figure 17 shows the relationship of the longitudinal and transverse stress-strain for test specimens with thicknesses of 3.0 mm and 4.5 mm, respectively. The stress-strain curves are measured until the test specimens are fractured. It should be noted that the stress-strain curves are the average value of the three test specimens per group. It can be seen that the stress-strain curves of steel test specimens with thicknesses of 3.0 mm and 4.5 mm are similar. As shown in
Figure 17, the yield strength of steel decreased with the increasing corrosion rate. The slope corresponding to the elastic-plastic stage decreased with the increasing corrosion rate, which indicates that the strength and deformation ability of the specimen decreased.
3.6. Parameters of Reduce
3.6.1. Ultimate Tensile Strength Reduction Factor
Ahmmad et al. [
36] suggested that the ultimate strength reduction factor is a function of damage to the steel plate under corrosion. The equation of the ultimate strength reduction factor
Ru is as follows:
where
σup and
σu0 are the ultimate tensile strengths of steel with different sulfate corrosion rates and steel with a sulfate corrosion rate of 0%.
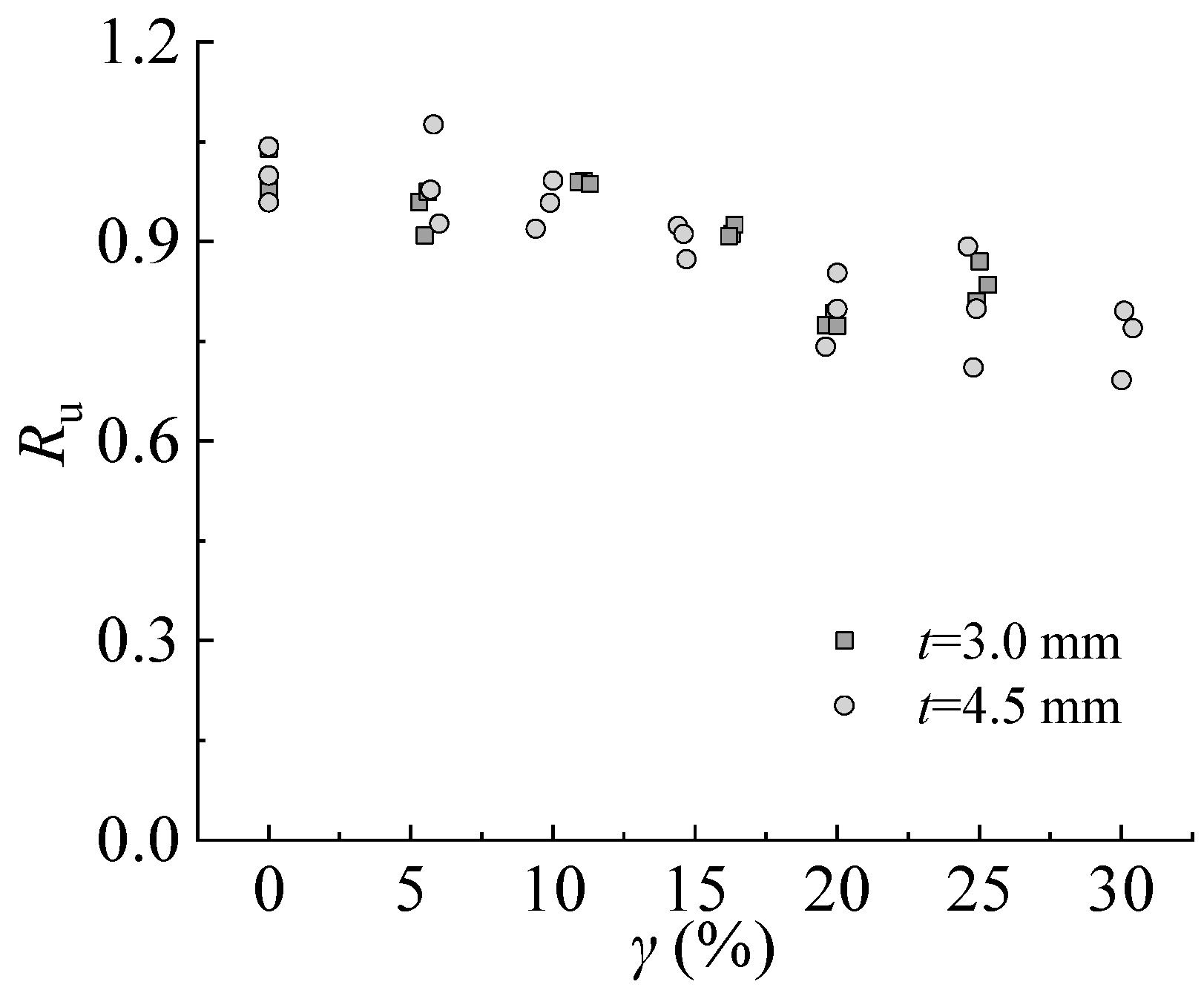
Figure 18 shows that the strength reduction factor decreases linearly with the increasing corrosion rate from 0% to 30%.
Ru decreases 13.0–19.0% when the corrosion rate increases from 0% to 25% of the steel specimen with a thickness of 3.0 mm. For the steel specimen with a thickness of 4.5 mm,
Ru decreases by 10.8–28.9% and 20.5–30.8% corresponding to the corrosion rates of 25% and 30%, respectively. The effect of sulfate corrosion is more significant on steel with larger thicknesses.
3.6.2. Deformability Reduction Factor
The deformability reduction factor
Rd is defined as Equation (13):
where
δp and
δ0 are the total elongation of steel with different sulfate corrosion rates and steel with a sulfate corrosion rate of 0%.
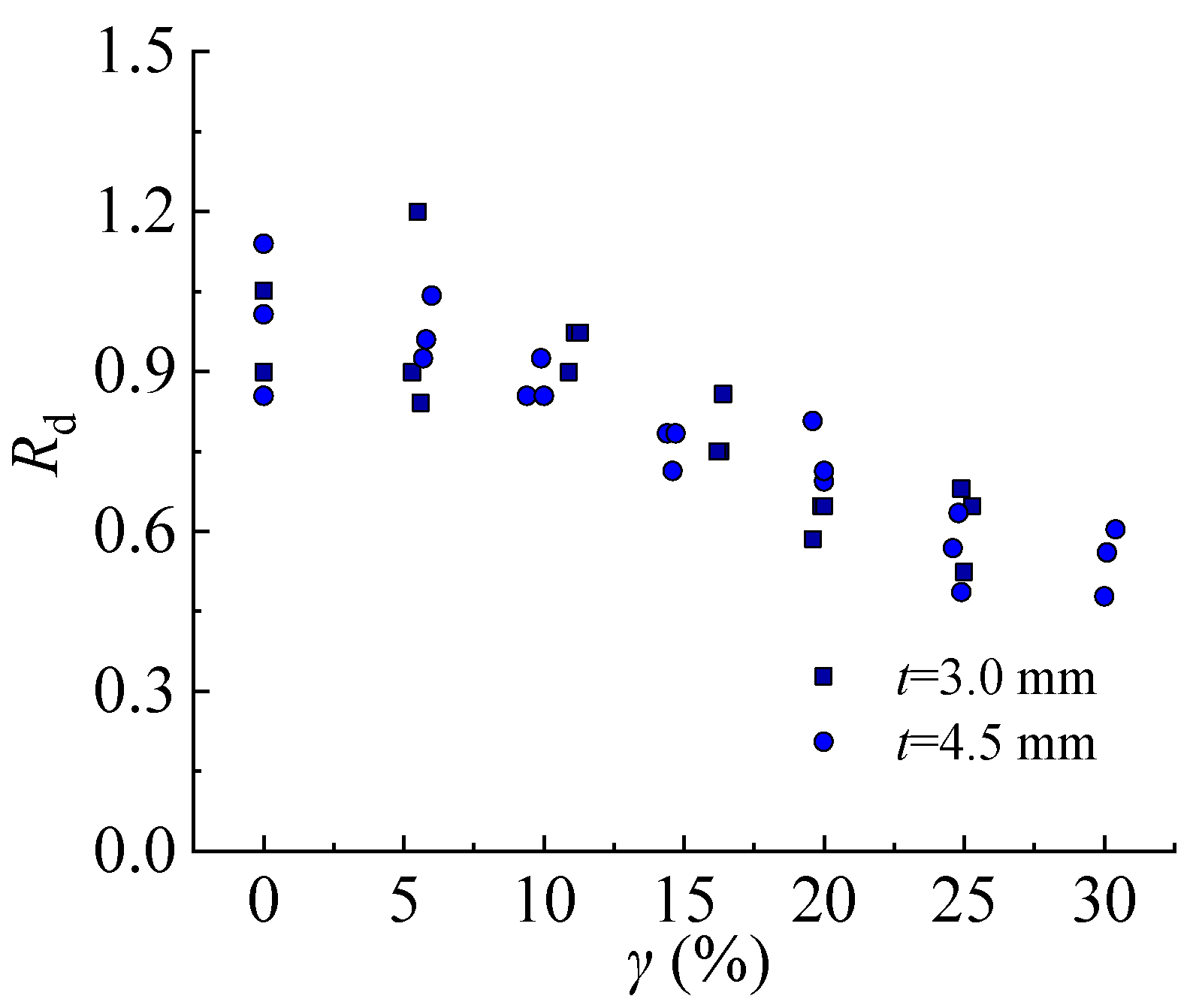
The deformability reduction factor of steel decreases with the increasing corrosion rate as shown in
Figure 19.
Rd decreases 32–47.7% of the steel specimens with a thickness of 3.0 mm when the corrosion rate increases from 0% to 25%. Moreover,
Rd decreases by 36.6–51.4% and 39.7–52.2% of the steel specimens with a thickness of 4.5 mm corresponding to the corrosion rates of 25% and 30%, respectively. It can be seen that the steel specimen with a larger thickness is more sensitive to the impact of sulfate corrosion on deformability.
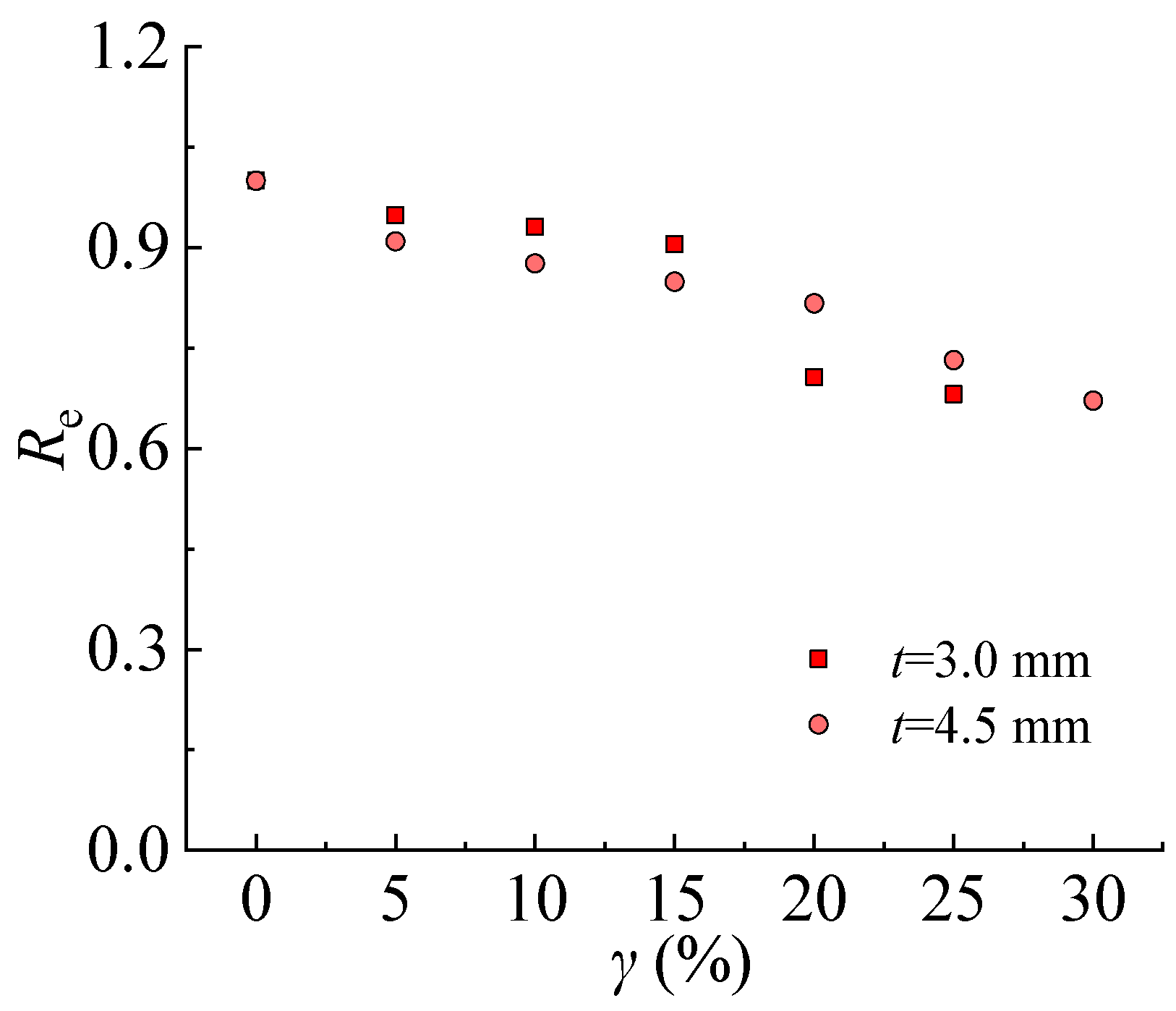
3.6.3. Energy Absorption Reduction Factor
The energy absorption reduction factor is introduced as Equation (14):
where
Enp and
En0 are the total energy absorbed by steel with different sulfate corrosion rates and steel with a sulfate corrosion rate of 0%.
The energy in this section is measured by integrating the area under the nominal stress-strain curves of the steel tensile coupon test. The energy absorption reduction factor decreases with increasing the corrosion rate, as shown in
Figure 20. For steel specimens with a thickness of 3.0 mm,
Re decreased by 31.9% corresponding to a corrosion rate of 25%. For steel specimens with a thickness of 4.5 mm,
Re decreased by 26.8% and 32.8% corresponding to the corrosion rate of 25% and 30%, respectively. It indicates that the fracture toughness of the steel specimens decreases with the sulfate corrosion. The steel is more likely to be damaged under a high corrosion rate, i.e., a longer time subjected to sulfate corrosion or a higher concentration of corrosion solution. For the steel specimen with a larger thickness, it is harder for fracture damage to occur fracture.
The elongation of the steel specimen deteriorates severely as the corrosion rate increases, and the decrease in ductility is detrimental to the seismic performance of the steel structure. When experiencing a major earthquake, steel structures are subjected to significant cyclic loads, and materials enter the plastic stage, dissipating seismic energy in the structure. At this point, there is a problem of fatigue failure at the material level [
37,
38].
3.6.4. Empirical Design Method
It can be seen that the reduction factors of deformability and energy absorption capacity are reduced by about 50% and 33% with the increasing corrosion rate increasing from 0% to 30%, respectively, while the ultimate tensile strength of steel is reduced moderately. It can be found that all the reduction parameters presented discreteness, which is due to the random distribution of the pits. However, the reduction factors of
Ru,
Rd, and
Re decreased linearly with the increasing corrosion rate. According to the least square method, reduction factor
Ru,
Rd, and
Re is described as follows:
where
γ is the corrosion rate, ranging from 0% to 30%.
In acid rain environments, the FeOOH and Fe3O4 are formed by crystallization, since the γ-FeOOH is mainly formed by amorphous oxides, which can be further converted into α-FeOOH and revert to Fe3O4. The rust layer fall off with the increasing corrosion rate, which caused the corrosion pits to develop along the thickness direction. The stress of the specimen became non-uniform. When the main stress bypassed the corrosion pit, it bent and generated a three-dimensional tensile stress; the more severe the stress concentration, the more brittle the steel tends to be. In the steel with larger thickness, the three-dimension tensile stress was greater at the corrosion pit, which was due to the significant limitation of the shrinkage deformation in the steel thickness direction at the center of the corrosion pit.
3.7. Finite Element Analysis of Axially Loaded Sulfate Corrosion CFST Stub Columns
The detail of finite element models was presented in ref. [
5].
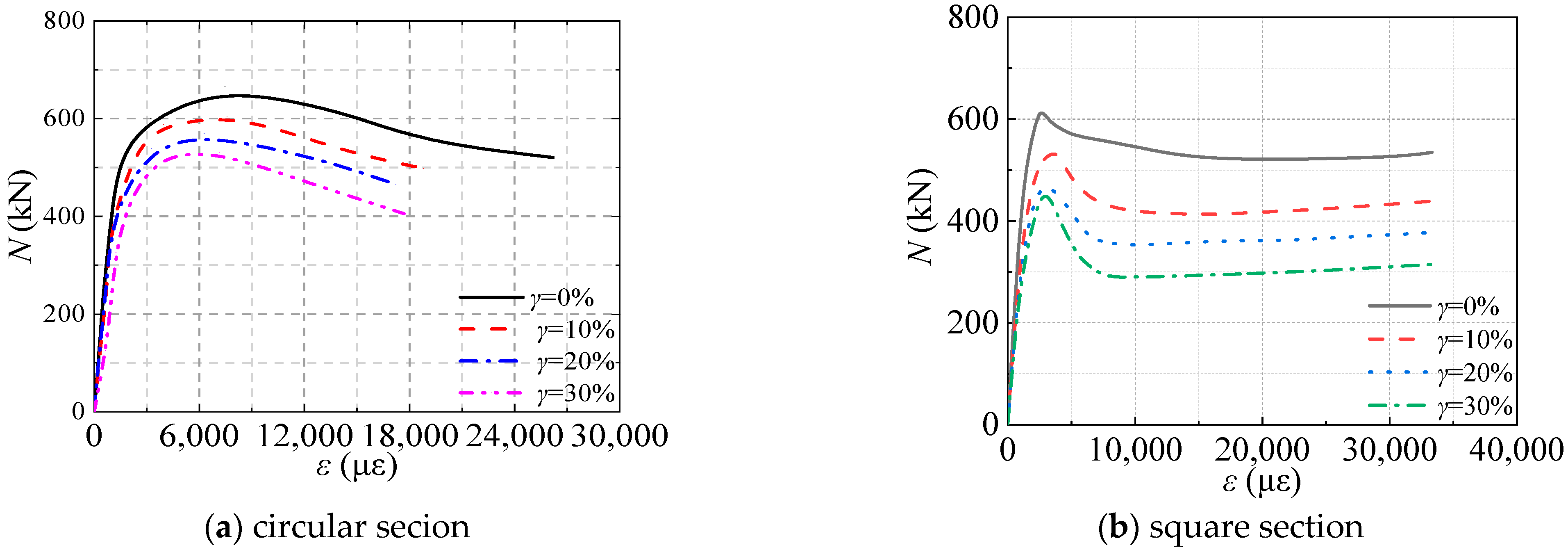
Figure 21 presented the axial load (
N)-axial strain (
ε) curve for circular and square section CFST stub columns corresponding to sulfate corrosion rates of 0%, 10%, 20%, and 30%. It can be seen that the
N-
ε curve includes the elastic stage, elastic-plastic stage, peak load, and descending stage. The elastic limit point of the CFST stub column subjected to sulfate corrosion corresponds to the smaller strain. The elastic modulus of the specimen decreased with the increasing corrosion rate. In the elastic-plastic stage, the ultimate strain corresponding to peak load decreased with the corrosion rate as well as the peak load. In the descending stage, the bearing capacity of the CFST column decreased. Compared with the reference specimen of
γ = 0%, the specimens subjected to sulfate corrosion would end the descent stage with a smaller strain.
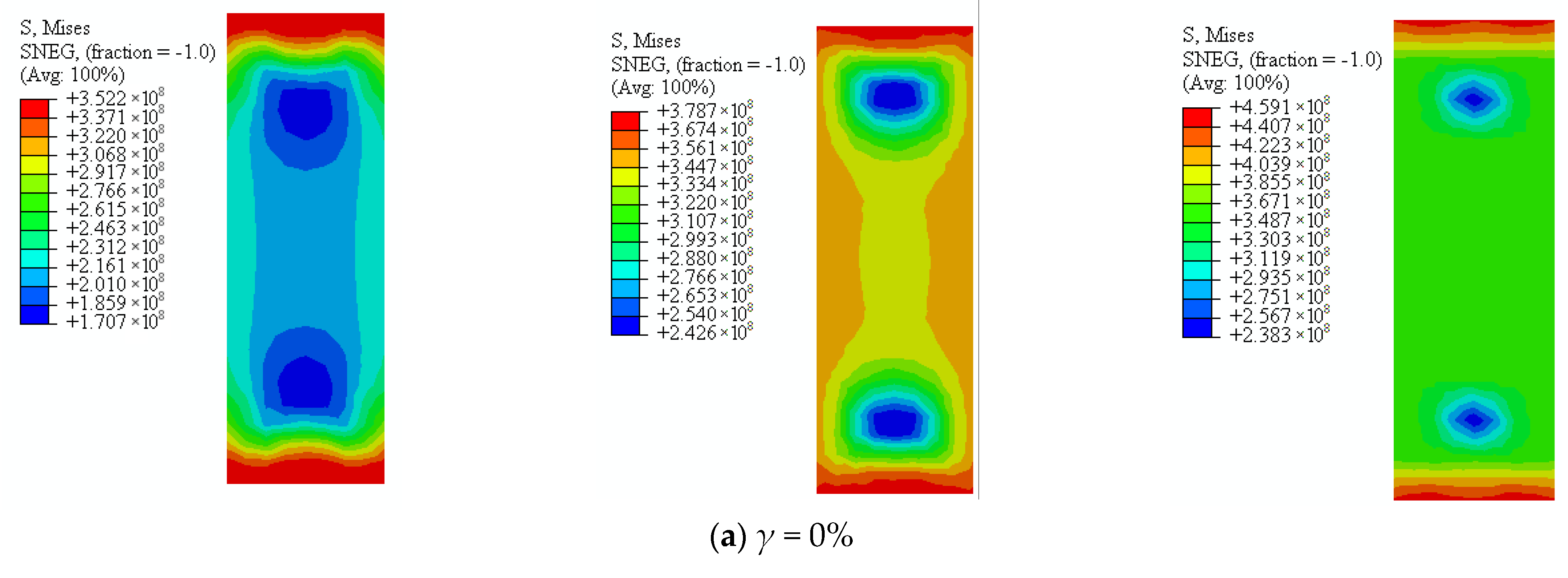
Von Mises strength of square sulfate corrosion steel tube with thickness of 4.5 mm is shown in
Figure 22. It can be seen that the stress of the steel tube increased in the process of axial compression. The stress of steel tube decreased with the increasing sulfate corrosion rate. In the elastic stage, the stress decreased by 10.39%, 21.92%, and 31.46%. In the elastic-plastic stage, the stress of steel tube decreased by 14.47%, 24.50%, and 33.22% as the corrosion rate increased from 0% to 30%. In the descending stage, the stress of steel tube decreased by 5.68%, 21.81%, and 32.03% corresponding to the corrosion rate of 10%, 20%, and 30%.
The ultimate compressive strength of the CFST stub columns subjected to sulfate corrosion can be described as follows:
(1) Circular section
where
B = 0.176
fy/213 + 0.974;
C = −0.104
fck/14.4 + 0.031;
Nus is the ultimate compressive strength subjected to sulfate corrosion;
γ represents corrosion rate;
ξ denotes confinement coefficient,
ξ =
Asfy,s/
Acfck,
As and
Ac are area of steel tube and concrete core,
fy,s is the yield strength of sulfate corroded steel, and
fck is the compressive strength of the concrete core.
(2) Square section
where
,
B = 0.131
fy/213 + 0.723,
C = −0.070
fck/14.4 + 0.026.
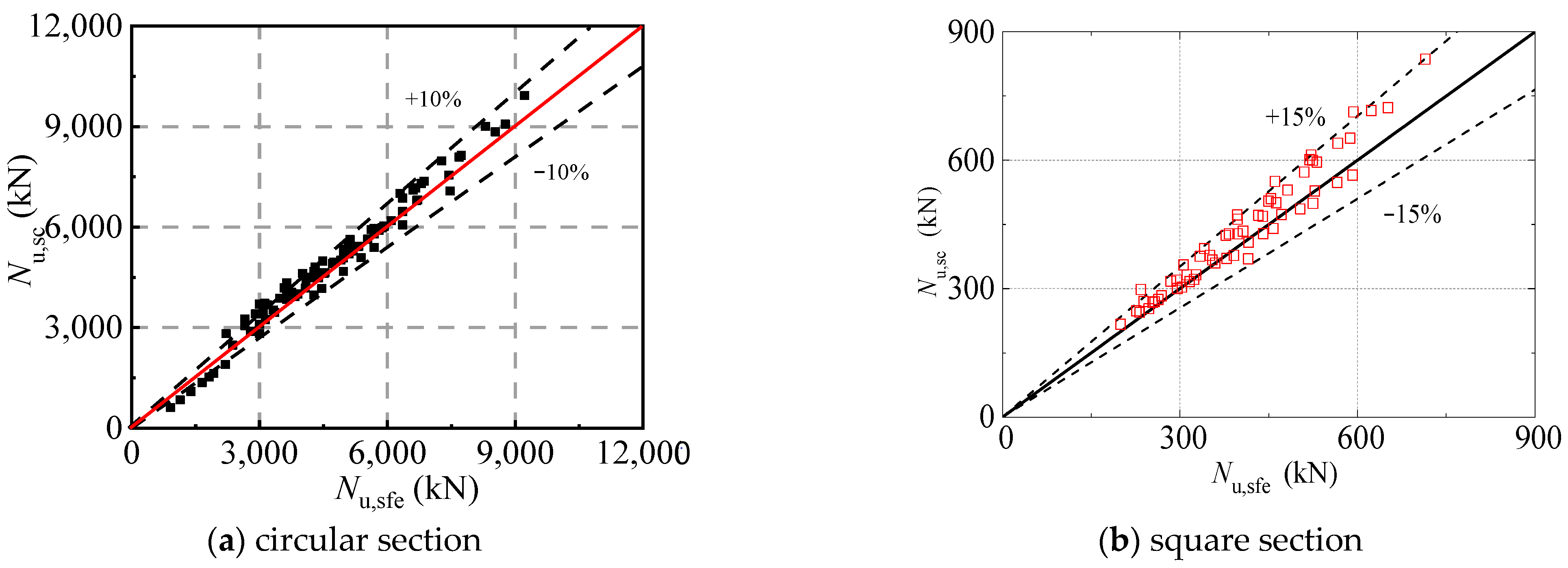
It can be seen from
Figure 23 that the proposed formula for axially loaded circular and square section CFST stub columns subjected to sulfate corrosion has high accuracy. The mean value of circular columns is 0.895 with a COV of 0.034, and the mean value of square columns is 0.937 with a COV of 0.004. It should be noted that the error interval of the square CFST columns is 15% compared to that of circular columns of 10%. This is due to the consideration of the random local buckling of the square steel tube.