High Selectivity Fuel from Efficient CO2 Conversion by Zn-Modified rGO and Amine-Functionalized CuO as a Photocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Process of rGO
2.3. Synthesis Process of CuO
2.4. Synthesis of rGO/CuO
2.5. Characterization
2.6. Experimental Setup for CO2 Conversion
3. Results and Discussions
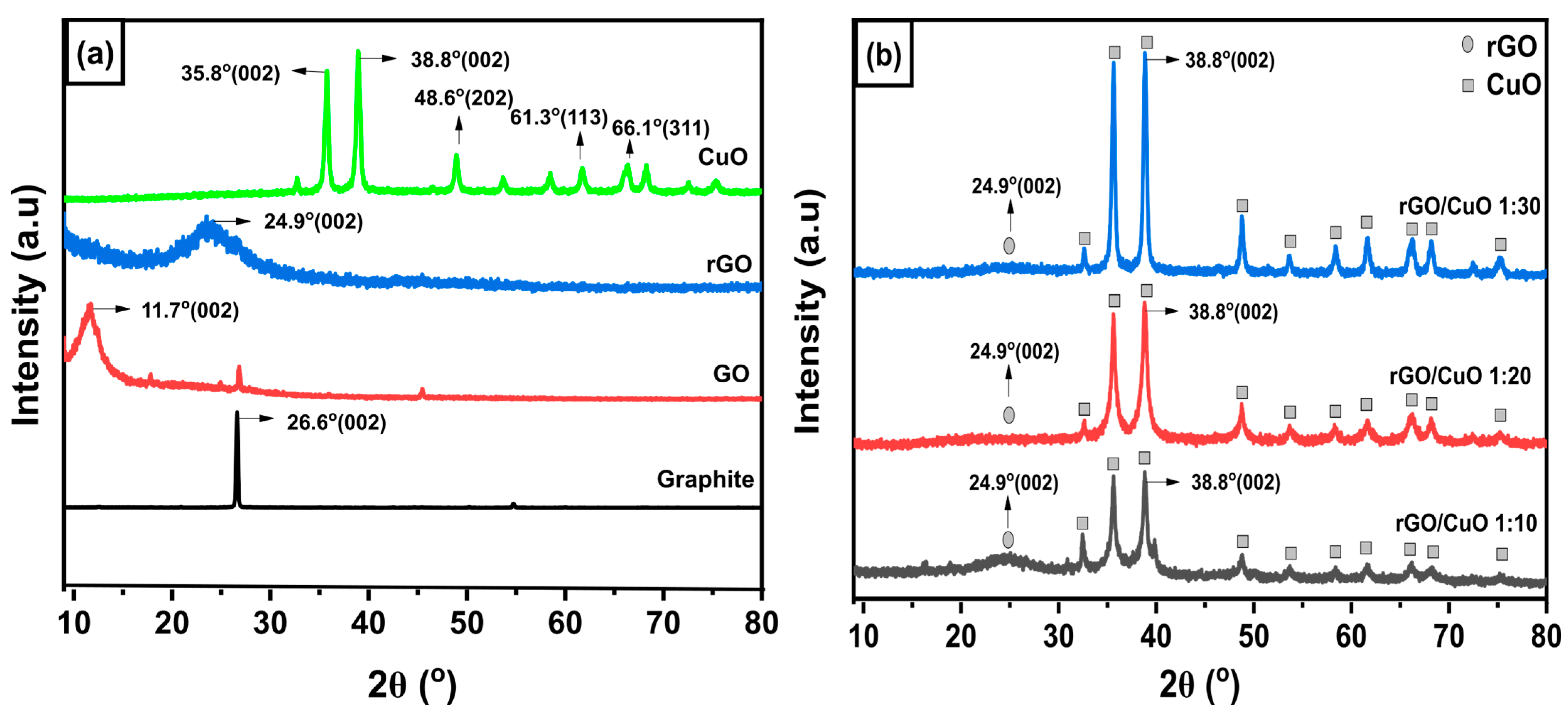
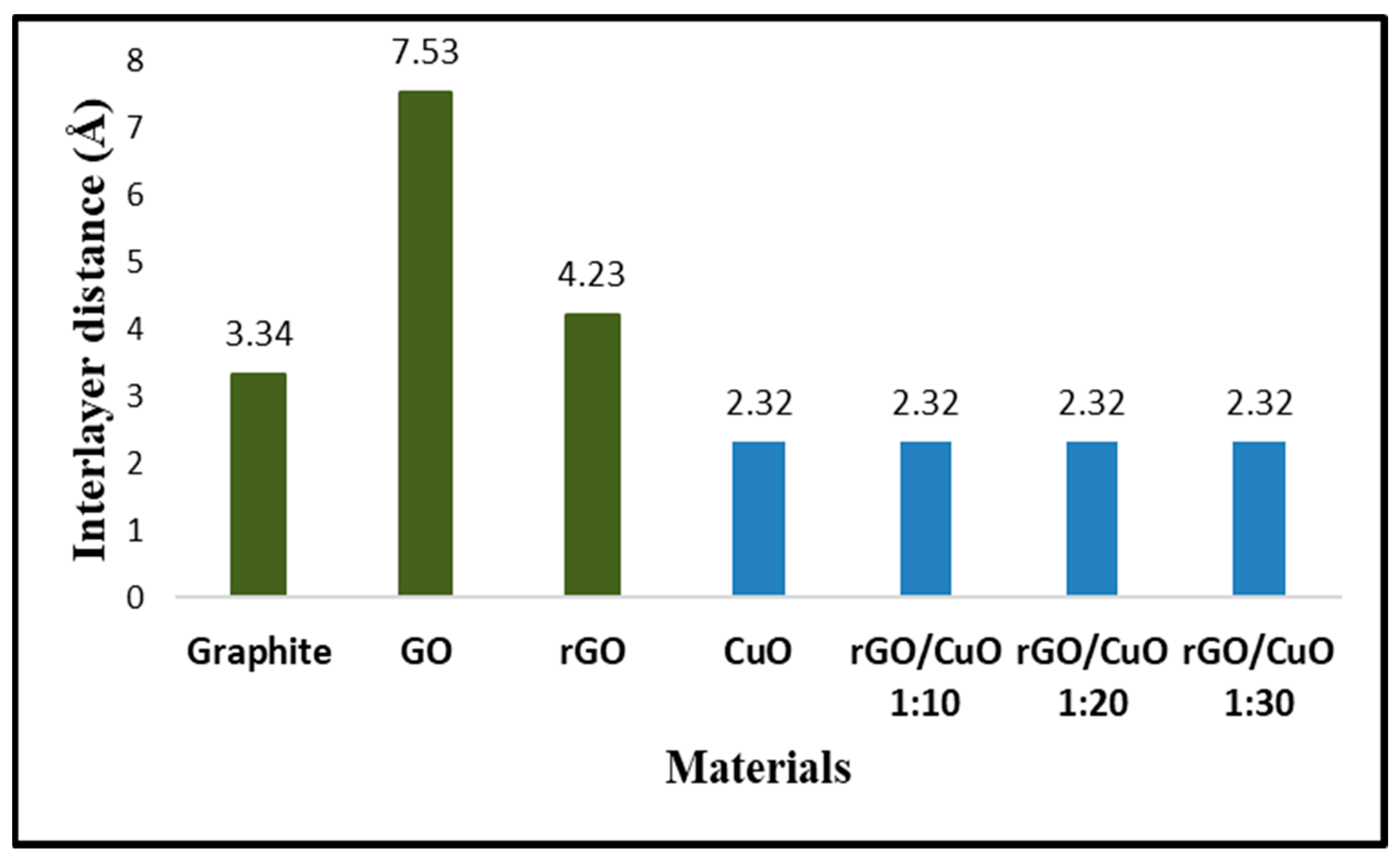
3.1. Formation of rGO/CuO Composite
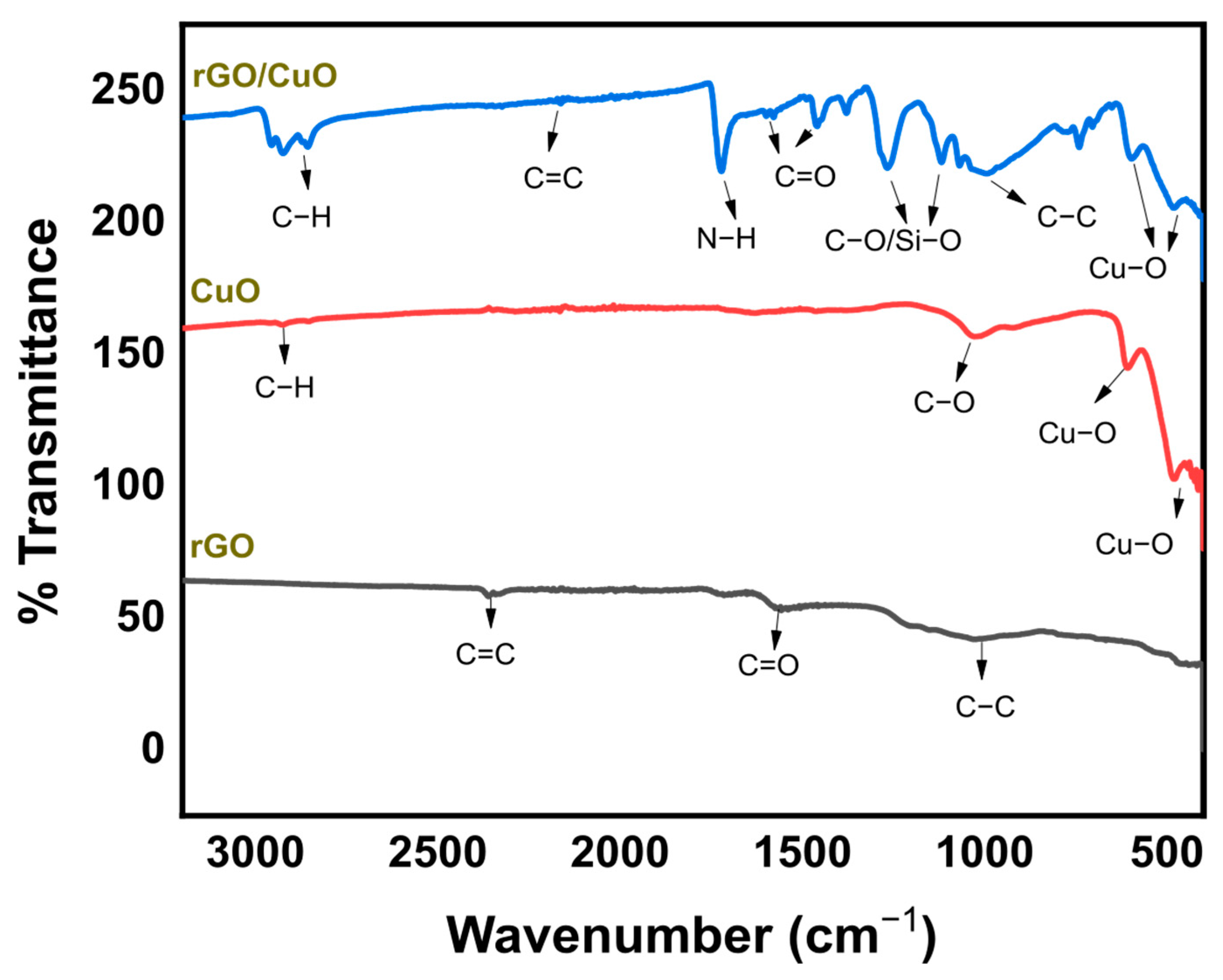
3.2. The Formation of Interaction in the rGO/CuO Composite
3.3. The Morphology of the Formation of the rGO/CuO Composite
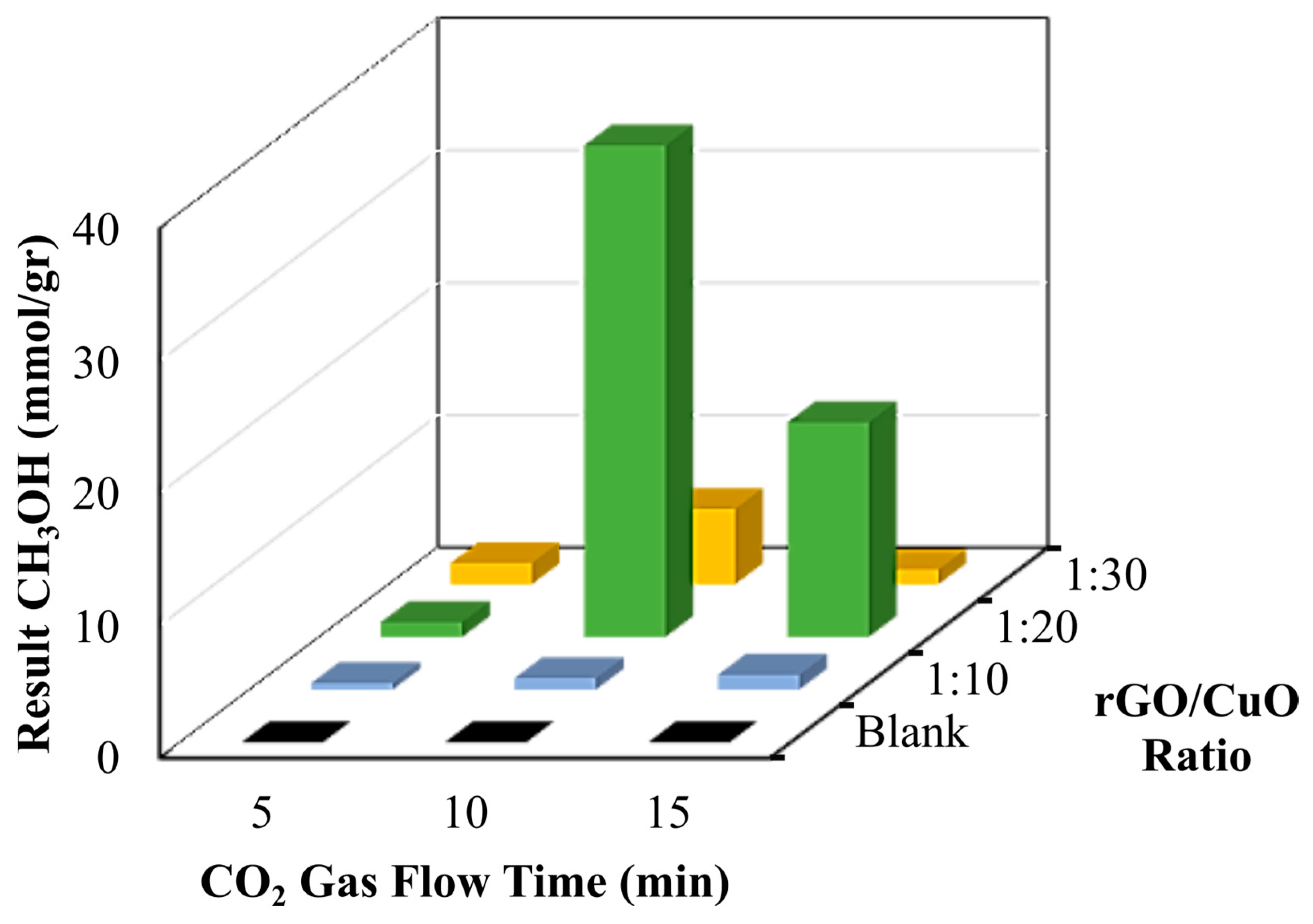
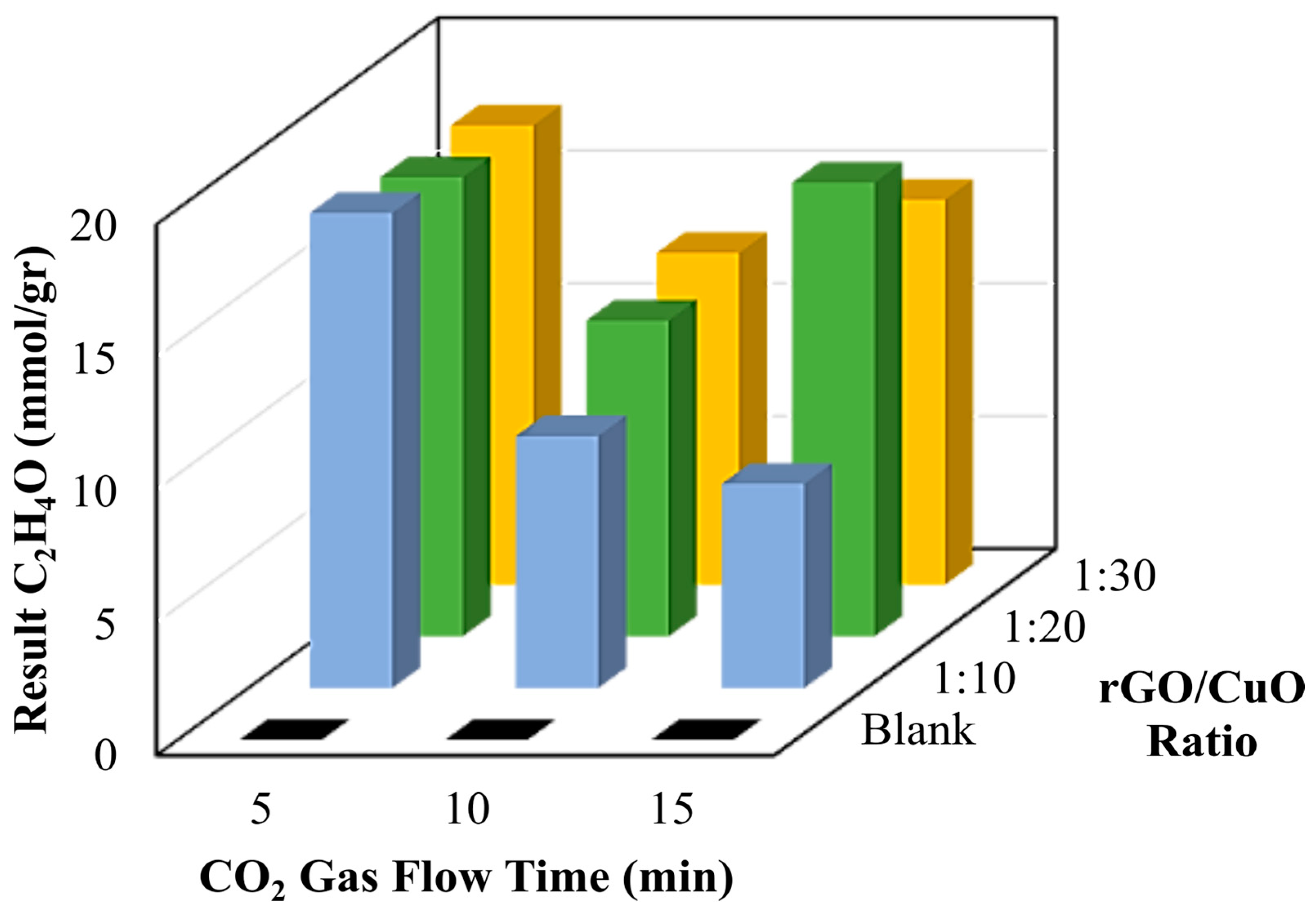
3.4. The Quantification of Fuel as Resulting from CO2 Conversion
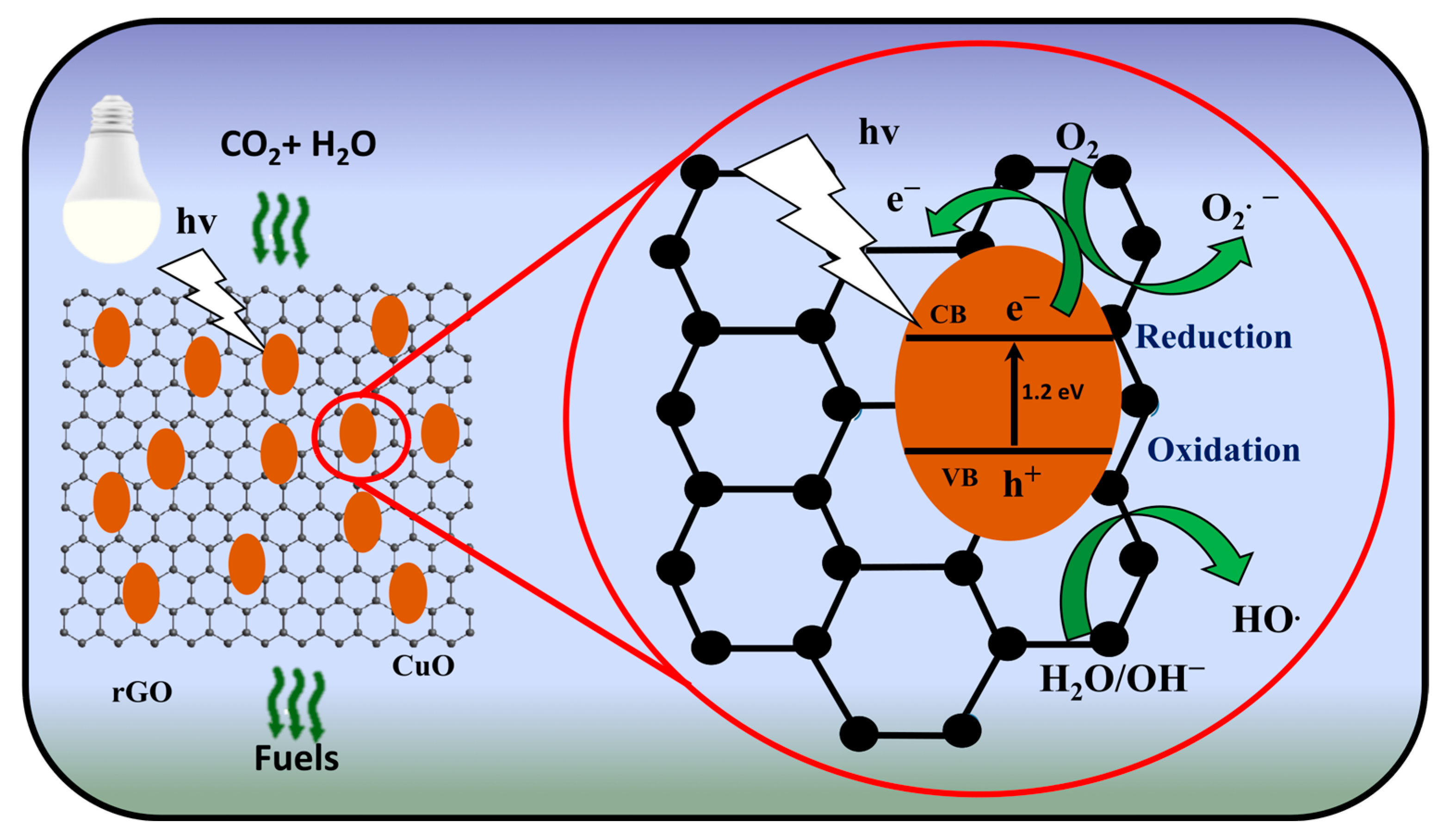
3.5. Photocatalytic Conversion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mass rGO/CuO catalyst | = 0.1 g |
| vol. Injection | = 1 µL = 10−3 mL |
| Normalization CH3OH | = 0.3 vol % (resulted from GC–MS) |
| CH3OH | = 0.792 g/cm3 |
| Mr CH3OH | = 32 |
| vol. CH3OH | = Normalization CH3OH × vol. Injection |
| = 3% × 1 µL = 0.003 µL = 0.003 × 10−3 cm3 | |
| Mass CH3OH | = CH3OH × vol. CH3OH |
| = 0.792 g/cm3 × 0.003 × 10−3 cm3 | |
| = 2.376 × 10−6 g | |
| n CH3OH in solution | = Mass CH3OH/Mr CH3OH |
| = 2.376 × 10−6 g/32 | |
| = 7.425 × 10−8 mol = 0.07425 µmol | |
| vol. Solution | = 50 mL |
| n CH3OH in injector | = (n CH3OH in solution × vol. solution)/vol. Injection |
| = 0.07425 µmol × 50 mL/10−3 mL | |
| = 3712.5 µmol | |
| n CH3OH/g catalyst | = n CH3OH in injector/Mass rGO/CuO catalyst |
| = 3712.5 µmol/0.1 g | |
| = 37125 µmol/g = 37.125 mmol/g |
References
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar]
- Guo, K.; Zhu, X.; Peng, L.; Fu, Y.; Ma, R.; Lu, X.; Zhang, F.; Zhu, W.; Fan, M. Boosting photocatalytic CO2 reduction over a covalent organic framework decorated with ruthenium nanoparticles. Chem. Eng. J. 2021, 405, 127011. [Google Scholar] [CrossRef]
- Verma, R.; Belgamwar, R.; Polshettiwar, V. Plasmonic photocatalysis for CO2 conversion to chemicals and fuels. Mater. Lett. 2021, 3, 574–598. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Wang, P.; Yu, J. Enhanced photoinduced-stability and photocatalytic activity of CdS by dual amorphous cocatalysts: Synergistic effect of Ti (IV)-hole cocatalyst and Ni (II)-electron cocatalyst. J. Phys. Chem. C 2016, 120, 3722–3730. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. A new breakthrough in photocatalytic hydrogen evolution by amorphous and chalcogenide enriched cocatalysts. Chem. Eng. J. 2023, 455, 140601. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Dutta, S.; Chowdhury, B.; Bhaumik, A. Catalytic reduction of CO2 into fuels and fine chemicals. Green Chem. 2020, 22, 4002–4033. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Nanostructured metal sulfides: Classification, modification strategy, and solar-driven CO2 reduction application. Adv. Funct. Mater. 2021, 31, 2008008. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Minale, M.; Gu, Z.; Guadie, A.; Kabtamu, D.M.; Li, Y.; Wang, X. Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: A review. J. Environ. Manag. 2020, 276, 111310. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Raizada, P.; Thakur, V.K.; Saini, V.; Khan, A.A.P.; Singh, N.; Singh, P. An overview on polymeric carbon nitride assisted photocatalytic CO2 reduction: Strategically manoeuvring solar to fuel conversion efficiency. Chem. Eng. Sci 2021, 230, 116219. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Min, S.; Irfan, R.M.; Zhu, Q.; Zhou, L.; Lei, J.; Zhang, J. Simultaneous hydrogen production with the selective oxidation of benzyl alcohol to benzaldehyde by a noble-metal-free photocatalyst VC/CdS nanowires. Chin. J. Catal. 2022, 43, 1165–1175. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Pan, L.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. One-pot in-situ hydrothermal synthesis of ternary In2S3/Nb2O5/Nb2C Schottky/S-scheme integrated heterojunction for efficient photocatalytic hydrogen production. J. Colloid Interface Sci 2022, 628, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, S.; Huang, D.; Liu, Z.; Shao, B.; Liang, Q.; Wu, T.; Pan, Y.; Huang, J.; Liu, Y. Recent advances of Zr based metal organic frameworks photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2021, 448, 214177. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, P.; Cui, Y.; Fu, X.; Wang, Y. Recent Progress in Copper-based Inorganic Nanostructure Photocatalysts: Properties, Synthesis and Photocatalysis Applications. Mater. Today Sustain. 2022, 21, 100276. [Google Scholar] [CrossRef]
- Ikram, M.; Rashid, M.; Haider, A.; Naz, S.; Haider, J.; Raza, A.; Ansar, M.; Uddin, M.K.; Ali, N.M.; Ahmed, S.S. A review of photocatalytic characterization, and environmental cleaning, of metal oxide nanostructured materials. Sustain. Mater. Technol. 2021, 30, e00343. [Google Scholar] [CrossRef]
- Said, M.I.; Othman, A. Structural, optical and photocatalytic properties of mesoporous CuO nanoparticles with tunable size and different morphologies. RSC Adv. 2021, 11, 37801–37813. [Google Scholar] [CrossRef]
- Singha, B.; Ray, K. Density functional theory insights on photocatalytic ability of CuO/TiO2 and CuO/ZnO. Mater. Today Proc. 2023, 72, 451–458. [Google Scholar] [CrossRef]
- Belay Getahun, M.; Budi Santiko, E.; Imae, T.; Chiang, C.-L.; Lin, Y.-G. Photocatalytic conversion of gaseous carbon dioxide to methanol on CuO/ZnO-embedded carbohydrate polymer films. Appl. Surf. Sci. 2022, 604, 154515. [Google Scholar] [CrossRef]
- Sun, Z.; Talreja, N.; Tao, H.; Texter, J.; Muhler, M.; Strunk, J.; Chen, J. Catalysis of carbon dioxide photoreduction on nanosheets: Fundamentals and challenges. Angewan. Chem. Intern. Ed. 2018, 57, 7610–7627. [Google Scholar] [CrossRef]
- Quan, H.; Gao, Y.; Wang, W. Tungsten oxide-based visible light-driven photocatalysts: Crystal and electronic structures and strategies for photocatalytic efficiency enhancement. Inorg. Chem. Front. 2020, 7, 817–838. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Wang, J.; Lu, Y.; Liu, Y.; Yu, J.; Chen, S.; Zhou, Y. Construction of plasmonic Ag and nitrogen-doped graphene quantum dots codecorated ultrathin graphitic carbon nitride nanosheet composites with enhanced photocatalytic activity: Full-spectrum response ability and mechanism insight. ACS Appl. Mater. Interfaces 2017, 9, 42816–42828. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Gnanavel, B.; Shkir, M. Enhanced visible light photocatalytic degradation of bisphenol A (BPA) by reduced graphene oxide (RGO)–metal oxide (TiO2, ZnO and WO3) based nanocomposites. Diamond Relat. Mater. 2021, 118, 108514. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Fabrication of a ternary BiOCl/CQDs/rGO photocatalyst: The roles of CQDs and rGO in adsorption-photocatalytic removal of ciprofloxacin. Colloids Surf. A 2020, 597, 124758. [Google Scholar] [CrossRef]
- Romeiro, F.C.; Silva, B.C.; Martins, A.S.; Zanoni, M.V.B.; Orlandi, M.O. Superior performance of rGO-tin oxide nanocomposite for selective reduction of CO2 to methanol. J. CO2 Util. 2021, 46, 101460. [Google Scholar] [CrossRef]
- Choi, S.; Kim, C.; Suh, J.M.; Jang, H.W. Reduced graphene oxide-based materials for electrochemical energy conversion reactions. Carbon Energy 2019, 1, 85–108. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Seeharaj, P.; Kongmun, P.; Paiplod, P.; Prakobmit, S.; Sriwong, C.; Kim-Lohsoontorn, P.; Vittayakorn, N. Ultrasonically-assisted surface modified TiO2/rGO/CeO2 heterojunction photocatalysts for conversion of CO2 to methanol and ethanol. Ultrason. Sonochem. 2019, 58, 104657. [Google Scholar] [CrossRef]
- Mahmudzadeh, M.; Yari, H.; Ramezanzadeh, B.; Mahdavian, M. Highly potent radical scavenging-anti-oxidant activity of biologically reduced graphene oxide using Nettle extract as a green bio-genic amines-based reductants source instead of hazardous hydrazine hydrate. J. Hazard. Mater. 2019, 371, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yue, W.; Huang, D.; Chen, C.; Lin, H.; Yang, X. A facile green strategy for rapid reduction of graphene oxide by metallic zinc. RSC Adv. 2012, 2, 8827–8832. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Shi, S.J.; Zhou, S.S.; Liu, S.Q.; Chen, Z.G. Photocatalytic activity of erbium-doped CeO2 enhanced by reduced graphene Oxide/CuO cocatalyst for the reduction of CO2 to methanol. Environ. Prog. Sustain. 2018, 37, 655–662. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. Chem Eur.J. 2014, 7, 1086–1093. [Google Scholar] [CrossRef]
- Sheelam, A.; Muneeb, A.; Talukdar, B.; Ravindranath, R.; Huang, S.-J.; Kuo, C.-H.; Sankar, R. Flexible and free-standing polyvinyl alcohol-reduced graphene oxide-Cu 2 O/CuO thin films for electrochemical reduction of carbon dioxide. J. Appl. Electrochem. 2020, 50, 979–991. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Oxide-derived Cu-Zn nanoparticles supported on N-doped graphene for electrochemical reduction of CO2 to ethanol. Appl. Suf. Sci. 2021, 556, 149790. [Google Scholar] [CrossRef]
- Momeni, S.; Sedaghati, F. CuO/Cu2O nanoparticles: A simple and green synthesis, characterization and their electrocatalytic performance toward formaldehyde oxidation. Microchem. J. 2018, 143, 64–71. [Google Scholar] [CrossRef]
- Phukan, P.; Narzary, R.; Sahu, P.P. A green approach to fast synthesis of reduced graphene oxide using alcohol for tuning semiconductor property. Mater. Sci. Semicon. Process. 2019, 104, 104670. [Google Scholar] [CrossRef]
- Velsankar, K.; Aswin Kumar, R.M.; Preethi, R.; Muthulakshmi, V.; Sudhahar, S. Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J. Environ. Chem. Eng. 2020, 8, 104123. [Google Scholar] [CrossRef]
- Shakeel, N. Optimization of rGO-PEI/Naph-SH/AgNWs/Frt/GOx nanocomposite anode for biofuel cell applications. Sci. Rep. 2020, 10, 8919. [Google Scholar]
- Das, S.; Srivastava, V.C.J.N.R. An overview of the synthesis of CuO-ZnO nanocomposite for environmental and other applications. Nanotechnol. Rev. 2018, 7, 267–282. [Google Scholar] [CrossRef]
- Wu, J.; Wen, C.; Zou, X.; Jimenez, J.; Sun, J.; Xia, Y.; Fonseca Rodrigues, M.-T.; Vinod, S.; Zhong, J.; Chopra, N. Carbon dioxide hydrogenation over a metal-free carbon-based catalyst. ACS Catal. 2017, 7, 4497–4503. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, K.; Zhang, L.; Zhang, Y.; Lei, Y.; Sun, X. Recent development of electrocatalytic CO2 reduction application to energy conversion. Small 2021, 17, 2100323. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Cao, H.; Han, D.; Li, M.; Yao, S.; Xie, J.; Zhan, J.; Zhang, Q.; Wang, W.; He, M. Theoretical insight into the degradation of p-nitrophenol by OH radicals synergized with other active oxidants in aqueous solution. J. Hazard. Mater. 2020, 389, 121901. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Hammad, A.; Tahir, M.; Rafique, M.; Iqbal, T.; Nabi, G.; Hussain, M. Enhanced photocatalytic activity of Al and Fe co-doped ZnO nanorods for methylene blue degradation. Ceram. Int. 2019, 45, 21430–21435. [Google Scholar] [CrossRef]
- Hasija, V.; Kumar, A.; Sudhaik, A.; Raizada, P.; Singh, P.; Van Le, Q.; Le, T.T.; Nguyen, V.-H. Step-scheme heterojunction photocatalysts for solar energy, water splitting, CO2 conversion, and bacterial inactivation: A review. Environ. Chem. Lett. 2021, 19, 2941–2966. [Google Scholar] [CrossRef]
- Ekuma, C.; Anisimov, V.; Moreno, J.; Jarrell, M. Electronic structure and spectra of CuO. Eur. Phys. J. B 2014, 87, 23. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Zhou, F.; Yang, X.; Zhang, D.; Chen, Y. Synergistic effect of RGO/TiO2 nanosheets with exposed (0 0 1) facets for boosting visible light photocatalytic activity. Appl. Surf. Sci. 2020, 510, 145451. [Google Scholar] [CrossRef]
- Hasani, A.; Teklagne, M.A.; Do, H.H.; Hong, S.H.; Van Le, Q.; Ahn, S.H.; Kim, S.Y. Graphene-based catalysts for electrochemical carbon dioxide reduction. Carbon Energy 2020, 2, 158–175. [Google Scholar] [CrossRef]




| Composite | Stability | Application | Result | Ref. |
|---|---|---|---|---|
| rGO/CuO | 6 cycle | CO2 conversion | Methanol 1.2 mmol/g | [31] |
| CeO2/rGO/CuO | 5 cycle | CO2 conversion | Methanol 135.6 μmol g−1 | [32] |
| Cu2O/RGO | 6 cycle | CO2 conversion | CO 0.344% | [33] |
| PVA/rGO/(Cu2O/CuO) | 4 cycle | CO2 conversion | Methanol 19.5 µmol cm−2 h−1 | [34] |
| Cu-Zn N-dop rGO | 6 cycle | CO2 conversion | Ethanol 195 µmol L−1h−1 | [35] |
| CuO/Cu2O/CILE | 5 cycle | CO2 conversion | Formaldehyde 110 mmol L−1 | [36] |
| Ratio rGO/CuO | Time Flow CO2 (min) | Normalization (%) | Methanol Result (mmol/gr Catalyst) |
|---|---|---|---|
| Blank | 5 | 0 ± 0 | 0 ± 0 |
| 10 | 0 ± 0 | 0 ± 0 | |
| 15 | 0 ± 0 | 0 ± 0 | |
| 1:10 | 5 | 0.004 ± 0.0005 | 0.49 ± 0.062 |
| 10 | 0.007 ± 0.0008 | 0.86 ± 0.107 | |
| 15 | 0.009 ± 0.0012 | 1.11 ± 0.157 | |
| 1:20 | 5 | 0.009 ± 0.0016 | 1.11 ± 0.201 |
| 10 | 0.3 ± 0.036 | 37.12 ± 4.572 | |
| 15 | 0.131 ± 0.0264 | 16.21 ± 3.267 | |
| 1:30 | 5 | 0.013 ± 0.0021 | 1.6 ± 0.324 |
| 10 | 0.046 ± 0.0047 | 5.69 ± 0.765 | |
| 15 | 0.009 ± 0.0009 | 1.11 ± 0.175 |
| Ratio rGO/CuO | Time Flow CO2 (min) | Normalization (%) | Ethanolamine Result (mmol/gr Catalyst) |
|---|---|---|---|
| Blank | 5 | 0 ± 0 | 0 ± 0 |
| 10 | 0 ± 0 | 0 ± 0 | |
| 15 | 0 ± 0 | 0 ± 0 | |
| 1:10 | 5 | 99.859 ± 12 | 8754 ± 1563.245 |
| 10 | 99.894 ± 14 | 8757 ± 1458.92 | |
| 15 | 85.568 ± 9 | 7501 ± 1109.834 | |
| 1:20 | 5 | 99.704 ± 10 | 8740 ± 1323.312 |
| 10 | 99.591 ± 9 | 8730 ± 1450.056 | |
| 15 | 99.589 ± 15 | 8730 ± 1220.033 | |
| 1:30 | 5 | 99.827 ± 12 | 8751 ± 987.536 |
| 10 | 99.683 ± 12 | 8738 ± 1325.55 | |
| 15 | 99.823 ± 11 | 8751 ± 989.976 |
| Ratio rGO/CuO | Time Flow CO2 (min) | Normalization (%) | Formaldehyde Result (mmol/gr Catalyst) |
|---|---|---|---|
| Blank | 5 | 0 ± 0 | 0 ± 0 |
| 10 | 0 ± 0 | 0 ± 0 | |
| 15 | 0 ± 0 | 0 ± 0 | |
| 1:10 | 5 | 0.2 ± 0.01 | 17.9 ± 3.12 |
| 10 | 0.107 ± 0.0155 | 9.5 ± 1.5 | |
| 15 | 0.087 ± 0.0088 | 7.7 ± 1.205 | |
| 1:20 | 5 | 0.194 ± 0.0176 | 17.3 ± 2.54 |
| 10 | 0.133 ± 0.0155 | 11.9 ± 2.105 | |
| 15 | 0.192 ± 0.0177 | 17.1 ± 2.502 | |
| 1:30 | 5 | 0.194 ± 0.0165 | 17.3 ± 2.534 |
| 10 | 0.14 ± 0.017 | 12.5 ± 2.143 | |
| 15 | 0.163 ± 0.0183 | 14.5 ± 2.103 |
| Thermodynamic Electrochemical Reactions | E° (V vs. SHE) | Product |
|---|---|---|
| CO2(g) + 2H2O(l) + 2e− = HCOO− (aq) + OH− | −1.078 | Formic Acid |
| CO2(g) + 2H2O(l) + 2e− = CO(g) + 2OH− | −0.934 | Carbon Monoxide |
| CO2(g) + 3H2O(l) + 4e− = CH2O(l) + 4OH− | −0.898 | Formaldehyde |
| CO2(g) + 5H2O(l) + 6e− = CH3OH(l) + 6OH− | −0.812 | Methanol |
| CO2(g) + 6H2O(l) + 8e− = CH4(g) + 8OH− | −0.659 | Methane |
| 2CO2(g) + 9H2O(l) + 12e− = CH3CH2OH(l) + 12OH− | −0.744 | Ethanol |
| H2O(l) + 2h+ = ½ O2 + 2H+ | 0.82 | Oxygen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damastuti, R.; Susanti, D.; Prasannan, A.; Hsiao, W.W.-W.; Hong, P.-D. High Selectivity Fuel from Efficient CO2 Conversion by Zn-Modified rGO and Amine-Functionalized CuO as a Photocatalyst. Materials 2023, 16, 4314. https://doi.org/10.3390/ma16124314
Damastuti R, Susanti D, Prasannan A, Hsiao WW-W, Hong P-D. High Selectivity Fuel from Efficient CO2 Conversion by Zn-Modified rGO and Amine-Functionalized CuO as a Photocatalyst. Materials. 2023; 16(12):4314. https://doi.org/10.3390/ma16124314
Chicago/Turabian StyleDamastuti, Retno, Diah Susanti, Adhimoorthy Prasannan, Wesley Wei-Wen Hsiao, and Po-Da Hong. 2023. "High Selectivity Fuel from Efficient CO2 Conversion by Zn-Modified rGO and Amine-Functionalized CuO as a Photocatalyst" Materials 16, no. 12: 4314. https://doi.org/10.3390/ma16124314
APA StyleDamastuti, R., Susanti, D., Prasannan, A., Hsiao, W. W.-W., & Hong, P.-D. (2023). High Selectivity Fuel from Efficient CO2 Conversion by Zn-Modified rGO and Amine-Functionalized CuO as a Photocatalyst. Materials, 16(12), 4314. https://doi.org/10.3390/ma16124314








