Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review
Abstract
1. Introduction
2. Materials Used for 3D Scaffold Engineering
2.1. Fossil-Based Polymers
2.1.1. Poly(ε-caprolactone) (PCL)
2.1.2. Poly(vinyl) Alcohol (PVA)
2.1.3. Polyethylene Glycol (PEG)
2.1.4. Polypropylene Fumarate (PPF)
2.1.5. Polyurethane (PU) and Modified Polyurethanes (MPUs)
2.2. Natural and Bio-Based Polymers
2.2.1. Collagen
2.2.2. Hyaluronic Acid (HA)
2.2.3. Chitosan, Fibrin
2.2.4. Polysaccharides
2.2.5. Poly (Lactic Acid) (PLA)
2.2.6. Polyhydroxyalkanoates (PHAs)
2.2.7. Hydrogels Based on Natural Polymers
2.3. Hybrid Biomaterials
- Homogenous polymer blends (thermodynamically miscible polymers): this blend type often consists of polymers with similar chemical composition. Their mixing leads to the preparation of the blends with a single-phase structure and only one glass transition temperature is observed;
- Compatible polymer blends: this blend type consists of immiscible polymers, but thanks to their strong interphase interaction, the macroscopically consistent physical properties are observed;
- Heterogenous polymer blends (immiscible polymers): their mixing results in blends with separated phase structures, and two or more glass transition temperatures are observed [121].
2.3.1. PCL-Based Blends
2.3.2. PLA-Based Blends
2.3.3. Polymer/Bioceramic Blends
3. Interaction between Biodegradable Scaffolds and Host Immune System
3.1. Immunoengineering as an Emerging Field in the TE
3.2. Scaffolds and Foreign Body Reaction (FBR)
3.3. Immunomodulatory Scaffolds
4. Conclusions and Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bahremandi-Toloue, E.; Mohammadalizadeh, Z.; Mukherjee, S.; Karbasi, S. Incorporation of inorganic bioceramics into electrospun scaffolds for tissue engineering applications: A review. Ceram. Int. 2022, 48, 8803–8837. [Google Scholar] [CrossRef]
- Kalsi, S.; Singh, J.; Sehgal, S.S.; Sharma, N.K. Biomaterials for tissue engineered bone Scaffolds: A review. Mater. Today Proc. 2021, 81, 888–893. [Google Scholar] [CrossRef]
- Schatkoski, V.M.; Larissa do Amaral Montanheiro, T.; Canuto de Menezes, B.R.; Pereira, R.M.; Rodrigues, K.F.; Ribas, R.G.; Morais da Silva, D.; Thim, G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021, 47, 2999–3012. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Sicari, B.M.; Londono, R.; Dziki, J.L.; Badylak, S.F. Chapter 5—Extracellular matrix as a bioscaffold for tissue engineering. In Tissue Engineering, 3rd ed.; De Boer, J., Blitterswijk, C.A.V., Uquillas, J.A., Malik, N., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 137–172. [Google Scholar] [CrossRef]
- Uquillas, J.A.; Moroni, L.; de Boer, J. Chapter 1—An introduction to tissue engineering; the topic and the book. In Tissue Engineering, 3rd ed.; De Boer, J., Blitterswijk, C.A.V., Uquillas, J.A., Malik, N., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–12. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Tandon, B.; Dalton, P.D. Chapter 11—Scaffold design and fabrication. In Tissue Engineering, 3rd ed.; De Boer, J., Blitterswijk, C.A.V., Uquillas, J.A., Malik, N., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 355–385. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Zhang, Y.; Ye, Z.; Tan, W.; Lang, M. Effect of porosity on long-term degradation of poly (ε-caprolactone) scaffolds and their cellular response. Polym. Degrad. Stab. 2013, 98, 209–218. [Google Scholar] [CrossRef]
- Yang, P.B.; Davidson, M.G.; Edler, K.J.; Brown, S. Synthesis, Properties, and Applications of Bio-Based Cyclic Aliphatic Polyesters. Biomacromolecules 2021, 22, 3649–3667. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Harun, W.S.W.; Yusoff, M.M. Highly porous of hydroxyethyl cellulose biocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019, 122, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Kannan, R.; Wei, G.; Ma, P.X. Chapter 2—Synthetic polymeric biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 3rd ed.; Boccaccini, A.R., Ma, P.X., Liverani, L., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2022; pp. 41–74. [Google Scholar] [CrossRef]
- Ilha, J.; Figueiro, A.; Grando, M.C.; Macuvele, D.L.P.; Fiori, M.A.; Padoin, N.; Riella, H.G.; Soares, C. Nanosilica: Polycaprolactone ratio and heat treatment modify the wettability of nanosilica/polycaprolactone coatings for application in aqueous systems. Surf. Interfaces 2022, 31, 101997. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, J.; Inverarity, C.; Fang, Y.; Zhang, Z.; Ye, H.; Cui, Z.; Nguyen, L.; Wan, H.; Dye, J.F. Fabrication of Fibrin/Polyvinyl Alcohol Scaffolds for Skin Tissue Engineering via Emulsion Templating. Polymers 2023, 15, 1151. [Google Scholar] [CrossRef]
- Ahlawat, J.; Kumar, V.; Gopinath, P. Carica papaya loaded poly (vinyl alcohol)-gelatin nanofibrous scaffold for potential application in wound dressing. Mater. Sci. Eng. C 2019, 103, 109834. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Priyadarshini, B.; Grace, A.N.; Vijayalakshmi, U. Fabrication and characterization of Ag doped hydroxyapatite-polyvinyl alcohol composite nanofibers and its In Vitro biological evaluations for bone tissue engineering applications. J. Solgel. Sci. Technol. 2017, 81, 750–761. [Google Scholar] [CrossRef]
- Yarimitsu, S.; Sasaki, S.; Murakami, T.; Suzuki, A. Evaluation of lubrication properties of hydrogel artificial cartilage materials for joint prosthesis. Biosurf. Biotribol. 2016, 2, 40–47. [Google Scholar] [CrossRef]
- Sasaki, S.; Murakami, T.; Suzuki, A. Frictional properties of physically cross-linked PVA hydrogels as artificial cartilage. Biosurf. Biotribol. 2016, 2, 11–17. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Shao, Z.; Wang, Z.; Chang, B.; Ding, X.; Yang, Y. Biodegradable glass fiber reinforced PVA hydrogel for cartilage repair: Mechanical properties, ions release behavior and cell recruitment. J. Mater. Res. Technol. 2023, 23, 154–164. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Ahmed, I.N. The Impact of Polyethylene Glycol-Modified Chitosan Scaffolds on the Proliferation and Differentiation of Osteoblasts. Int. J. Biomater. 2023, 2023, 4864492. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.J.M.; Wong, Y.S.; Parikh, A.N.; Venkatraman, S.S.; Cao, Y.; Czarny, B. Cell-mimicking polyethylene glycol-diacrylate based nanolipogel for encapsulation and delivery of hydrophilic biomolecule. Front. Bioeng. Biotechnol. 2023, 11, 1113236. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-S.; Wang, Z.-B.; Lai, Z.-Z.; Yang, J.-W.; Song, W.-J.; Wei, Y.-B.; Mei, J.; Wang, J.-G. Polyethylene glycol crosslinked decellularized single liver lobe scaffolds with vascular endothelial growth factor promotes angiogenesis In Vivo. Hepatobiliary Pancreat. Dis. Int. 2022, 22, S1499–S3872. [Google Scholar] [CrossRef]
- Bhagyasree, K.; Mukherjee, D.; Azamthulla, M.; Debnath, S.; Sundar, L.M.; Hulikal, S.; Teja, B.V.; Bhatt, S.; Kamnoore, D. Thiolated sodium alginate/polyethylene glycol/hydroxyapatite nanohybrid for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2022, 76, 103813. [Google Scholar] [CrossRef]
- Riewruja, K.; Aguglia, A.M.; Hines, S.; Makarcyzk, M.J.; Honsawek, S.; Lin, H. PEG Reinforced Scaffold Promotes Uniform Distribution of Human MSC-Created Cartilage Matrix. Gels 2022, 8, 794. [Google Scholar] [CrossRef]
- Affar, A.; Xu, F.; Hoare, T. Poly(ethylene glycol) (PEG) and Synthetic PEG Derivatives for Tissue Engineering and Cell Delivery. Mater. Matters 2018, 13, 90–95. [Google Scholar]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Cai, Z.; Wan, Y.; Becker, M.L.; Long, Y.-Z.; Dean, D. Poly(propylene fumarate)-based materials: Synthesis, functionalization, properties, device fabrication and biomedical applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Javid-Naderi, M.J.; Behravan, J.; Karimi-Hajishohreh, N.; Toosi, S. Synthetic polymers as bone engineering scaffold. Polym. Adv. Technol. 2023, 34, 2083–2096. [Google Scholar] [CrossRef]
- Capurro, B.; Tey-Pons, M.; Carrera, A.; Marqués-López, F.; Marín-Peña, O.; Torres-Eguía, R.; Monllau, J.C.; Reina, F. Polyurethane Scaffold vs Fascia Lata Autograft for Hip Labral Reconstruction: Comparison of Femoroacetabular Biomechanics. Orthop. J. Sport. Med. 2023, 11, 23259671221150630. [Google Scholar] [CrossRef]
- Norouz, F.; Poormoghadam, D.; Halabian, R.; Ghiasi, M.; Monfaredi, M.; Salimi, A. A Novel Nanocomposite Scaffold based on Polyurethane [PU] Containing Cobalt Nanoparticles [CoNPs] for Bone Tissue Engineering Applications. Curr. Stem Cell Res. Ther. 2023, 18, 1120–1132. [Google Scholar] [CrossRef]
- Ahmadi, P.; Nazeri, N.; Derakhshan, M.A.; Ghanbari, H. Preparation and characterization of polyurethane/chitosan/CNT nanofibrous scaffold for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Naureen, B.; Haseeb, A.S.M.A.; Basirun, W.J.; Muhamad, F. Recent advances in tissue engineering scaffolds based on polyurethane and modified polyurethane. Mater. Sci. Eng. C 2021, 118, 111228. [Google Scholar] [CrossRef] [PubMed]
- Iga, C.; Pawel, S.; Marcin, L.; Justyna, K.L. Polyurethane composite scaffolds modified with the mixture of gelatin and hydroxyapatite characterized by improved calcium deposition. Polymers 2020, 12, 410. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Zou, C.Y.; Li, Q.J.; Hu, J.J.; Song, Y.T.; Zhang, Q.Y.; Nie, R.; Li-Ling, J.; Xie, H.Q. Design of biopolymer-based hemostatic material: Starting from molecular structures and forms. Mater. Today Bio 2022, 17, 100458. [Google Scholar] [CrossRef]
- Wu, Q.X.; Lin, D.Q.; Yao, S.J. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar. Drugs 2014, 12, 6236–6253. [Google Scholar] [CrossRef]
- Madihally, S.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Wang, K.; Stevens, W.F.; Kjems, J. Chitosan composites for biomedical applications: Status, challenges and perspectives. Mater. Sci. Technol. 2008, 24, 1053–1061. [Google Scholar] [CrossRef]
- Li, Z.; Leung, M.; Hopper, R.; Ellenbogen, R.; Zhang, M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials 2010, 31, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Rezaeipour, Y.; Alizadeh, P.; Keshavarz, M. Collagen scaffold impregnated with borosilicate bioactive glass for endometrial healing. Appl. Mater. Today 2023, 30, 101727. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Habermehl, J.; Vesentini, S.; Mantovani, D. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials 2005, 26, 7410–7417. [Google Scholar] [CrossRef]
- Siadat, S.M.; Ruberti, J.W. Mechanochemistry of collagen. Acta Biomater. 2023, 163, 50–62. [Google Scholar] [CrossRef]
- Liu, F.; Hu, K.; Al-Qudsy, L.H.; Wu, L.Q.; Wang, Z.; Xu, H.Y.; Yang, H.; Yang, P.F. Aging exacerbates the morphological and mechanical response of mineralized collagen fibrils in murine cortical bone to disuse. Acta Biomater. 2022, 152, 345–354. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, I.; Liu, X.; Shen, W.; Ding, P.; Zhang, Y.; Gao, T.; Mansoorianfar, M.; Gao, T.; Pei, R. Tannin-reinforced iron substituted hydroxyapatite nanorods functionalized collagen-based composite nanofibrous coating as a cell-instructive bone-implant interface scaffold. Chem. Eng. J. 2022, 438, 135611. [Google Scholar] [CrossRef]
- Gharati, G.; Shirian, S.; Sharifi, S.; Mirzaei, E.; Bakhtirimoghadam, B.; Karimi, I.; Nazari, H. Comparison Capacity of Collagen Hydrogel and Collagen/Strontium Bioglass Nanocomposite Scaffolds with and Without mesenchymal Stem Cells in Regeneration of Critical Sized Bone Defect in a Rabbit Animal Model. Biol. Trace Elem. Res. 2022, 200, 3176–3186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Lin, N.; Hou, X.; Wang, J.; Zhou, B.; Xu, P.; Xiao, Z.; Chen, B.; Dai, J.; et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials 2011, 32, 8172–8181. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Improving In Vitro biocompatibility on biomimetic mineralized collagen bone materials modified with hyaluronic acid oligosaccharide. Mater. Sci. Eng. C 2019, 104, 110008. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Guo, Z.; Li, D. Effect of molecular weight and concentration of hyaluronan on cell proliferation and osteogenic differentiation In Vitro. Biochem. Biophys. Res. Commun. 2015, 465, 569–574. [Google Scholar] [CrossRef]
- Song, W.; Lee, B.H.; Li, H.; Tan, L.P. Cardiovascular engineering materials in translational medicine. In Biomaterials in Translational Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 57–91. [Google Scholar] [CrossRef]
- Lu, D. Macroporous methacrylated hyaluronic acid hydrogel with different pore sizes for In Vitro and In Vivo evaluation of vascularization. Biomed. Mater. 2022, 17, 25006. [Google Scholar] [CrossRef]
- Dawson, J.I.; Wahl, D.A.; Lanham, S.A.; Kanczler, J.M.; Czernuszka, J.T.; Oreffo, R.O.C. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 2008, 29, 3105–3116. [Google Scholar] [CrossRef]
- Leng, Y.; Tac, V.; Calve, S.; Tepole, A.B. Predicting the mechanical properties of biopolymer gels using neural networks trained on discrete fiber network data. Comput. Methods Appl. Mech. Eng. 2021, 387, 114160. [Google Scholar] [CrossRef]
- Sawadkar, P.; Mandakhbayar, N.; Patel, K.D.; Buitrago, J.O.; Kim, T.H.; Rajasekar, P.; Lali, F.; Kyriakidis, C.; Rahmani, B.; Mohanakrishnan, J.; et al. Three dimensional porous scaffolds derived from collagen, elastin and fibrin proteins orchestrate adipose tissue regeneration. J. Tissue Eng. 2021, 12, 20417314211019238. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Schietse, J.; Zima, A.; Gorodzha, S.; Parakhonskiy, B.v.; KhaleNkow, D.; Shkarin, R.; Ivanova, A.; Baumbach, T.; Weinhardt, V.; et al. Novel self-gelling injectable hydrogel/alpha-tricalcium phosphate composites for bone regeneration: Physiochemical and microcomputer tomographical characterization. J. Biomed. Mater. Res. A 2018, 106, 822–828. [Google Scholar] [CrossRef]
- Byram, P.K.; Sunka, K.C.; Barik, A.; Kaushal, M.; Dhara, S.; Chakravorty, N. Biomimetic silk fibroin and xanthan gum blended hydrogels for connective tissue regeneration. Int. J. Biol. Macromol. 2020, 165, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A.C.; Chronakis, I.S. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. [Google Scholar] [CrossRef]
- Elizalde-Peña, E.A. (Chitosan-g-glycidyl methacrylate)-xanthan hydrogel implant in Wistar rats for spinal cord regeneration. Mater. Sci. Eng. C 2017, 78, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Adeli, H.; Dashtimoghadam, E. Fabrication and characterization of dextran/nanocrystalline β-tricalcium phosphate nanocomposite hydrogel scaffolds. Int. J. Biol. Macromol. 2020, 148, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.; Xu, K.; Zhou, H.X. Nonadditive effects of mixed crowding on protein stability. Proteins Struct. Funct. Bioinform. 2009, 77, 133–138. [Google Scholar] [CrossRef]
- Nikpour, P.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Imani, M.; Dashtimoghadam, E.; Tayebi, L. Dextran hydrogels incorporated with bioactive glass-ceramic: Nanocomposite scaffolds for bone tissue engineering. Carbohydr. Polym. 2018, 190, 281–294. [Google Scholar] [CrossRef]
- Mohamad, A.H.; Abdullah, O.G.; Saeed, S.R. Effect of very fine nanoparticle and temperature on the electric and dielectric properties of MC-PbS polymer nanocomposite films. Results Phys. 2020, 16, 102898. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Saravacos, G.D. Porosity and pore size distribution of starch materials. J. Food Eng. 1993, 18, 259–280. [Google Scholar] [CrossRef]
- Ragunathan, S.; Govindasamy, G.; Raghul, D.R.; Karuppaswamy, M.; VijayachandraTogo, R.K. Hydroxyapatite reinforced natural polymer scaffold for bone tissue regeneration. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 111–118. [Google Scholar] [CrossRef]
- Mohd Roslan, M.R.; Mohd Kamal, N.L.; Abdul Khalid, M.F.; Mohd Nasir, N.F.; Cheng, E.M.; Beh, C.Y.; Tan, J.S.; Mohamed, M.S. The state of starch/hydroxyapatite composite scaffold in bone tissue engineering with consideration for dielectric measurement as an alternative characterization technique. Materials 2021, 14, 1960. [Google Scholar] [CrossRef]
- You, B.C.; Meng, C.E.; Mohd Nasir, N.F.; Mohd Tarmizi, E.Z.; Fhan, K.S.; Kheng, E.S.; Abdul Majid, M.S.; Mohd Jamir, M.R. Dielectric and biodegradation properties of biodegradable nano-hydroxyapatite/starch bone scaffold. J. Mater. Res. Technol. 2022, 18, 3215–3226. [Google Scholar] [CrossRef]
- Mahmoud, E.M.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S.M. In Vitro and In Vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and its blends with poly(butylene succinate) (PBS): A brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Sandanamsamy, L.; Harun, W.S.W.; Ishak, I.; Romlay, F.R.M.; Kadirgama, K.; Ramasamy, D.; Idris, S.R.A.; Tsumori, F. A.; Tsumori, F. A comprehensive review on fused deposition modelling of polylactic acid. In Progress in Additive Manufacturing; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Maadani, A.M.; Salahinejad, E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments. J. Control. Release 2022, 351, 1–7. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Uzun, N. Poly(l-lactic acid) membranes: Absence of genotoxic hazard and potential for drug delivery. Toxicol. Lett. 2015, 232, 513–518. [Google Scholar] [CrossRef]
- Baptista, R.; Guedes, M. Morphological and mechanical characterization of 3D printed PLA scaffolds with controlled porosity for trabecular bone tissue replacement. Mater. Sci. Eng. C 2021, 118, 111528. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Doi, Y. Morphology and Enzymatic Degradation of Poly(L-lactic acid) Single Crystals. 1998. Available online: https://pubs.acs.org/sharingguidelines (accessed on 26 April 2023).
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen i: An In Vitro study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Bakhtiari, S.S.E.; Karbasi, S.; Toloue, E.B. Modified poly(3-hydroxybutyrate)-based scaffolds in tissue engineering applications: A review. Int. J. Biol. Macromol. 2021, 166, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Dhania, S.; Bernela, M.; Rani, R.; Parsad, M.; Kumar, R.; Thakur, R. Polyhydroxybutyrate (PHB) in nanoparticulate form improves physical and biological performance of scaffolds. Int. J. Biol. Macromol. 2023, 236, 123875. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Pereira, J.R.; Sevrin, C.; Grandfils, C.; Menda, U.D.; Fortunato, E.; Oliva, A.; Freitas, F. Preparation and characterization of porous scaffolds based on poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Life 2021, 11, 935. [Google Scholar] [CrossRef]
- Martin, D.P.; Williams, S.F. Medical applications of poly-4-hydroxybutyrate: A strong flexible absorbable biomaterial. Biochem. Eng. J. 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Ravi, S.; Qu, Z.; Chaikof, E.L. Polymeric Materials for Tissue Engineering of Arterial Substitutes. Vascular 2009, 17 (Suppl. 1), 45–54. [Google Scholar] [CrossRef]
- Pryadko, A.; Surmeneva, M.A.; Surmenev, R.A. Review of hybrid materials based on polyhydroxyalkanoates for tissue engineering applications. Polymers 2021, 13, 1738. [Google Scholar] [CrossRef]
- Mohan, A.; Girdhar, M.; Kumar, R.; Chaturvedi, H.S.; Vadhel, A.; Solanki, P.R.; Kumar, A.; Kumar, D.; Mamidi, N. Polyhydroxybutyrate-based nanocomposites for bone tissue engineering. Pharmaceuticals 2021, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.A.; Karbasi, S.; Beigi-Boroujeni, S.; Benisi, S.Z.; Saeed, M. Evaluation of the effects of starch on polyhydroxybutyrate electrospun scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2021, 191, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.-W.; Wang, M.; Lu, W.W. Electrospinning and Evaluation of PHBV-Based Tissue Engineering Scaffolds with Different Fibre Diameters, Surface Topography and Compositions. J. Biomater. Sci. Polym. Ed. 2012, 23, 779–806. [Google Scholar] [CrossRef] [PubMed]
- Carli, L.N.; Crespo, J.S.; Mauler, R.S. PHBV nanocomposites based on organomodified montmorillonite and halloysite: The effect of clay type on the morphology and thermal and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1601–1608. [Google Scholar] [CrossRef]
- Kim, H.S.; Chen, J.; Wu, L.-P.; Wu, J.; Xiang, H.; Leong, K.W.; Han, J. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Tissue Eng. 2020, 11, 2041731420949332. [Google Scholar] [CrossRef] [PubMed]
- Abdalkarim, S.Y.H.; Yu, H.-Y.; Wang, D.; Yao, J. Electrospun poly(3-hydroxybutyrate-co-3-hydroxy-valerate)/cellulose reinforced nanofibrous membranes with ZnO nanocrystals for antibacterial wound dressings. Cellulose 2017, 24, 2925–2938. [Google Scholar] [CrossRef]
- Kouhi, M.; Fathi, M.; Prabhakaran, M.P.; Shamanian, M.; Ramakrishna, S. Enhanced Proliferation and Mineralization of Human Fetal Osteoblast Cells on PHBV-Bredigite Nanofibrous Scaffolds. Mater. Today Proc. 2018, 5, 15702–15709. [Google Scholar] [CrossRef]
- Kaniuk, Ł.; Krysiak, Z.J.; Metwally, S.; Stachewicz, U. Osteoblasts and fibroblasts attachment to poly(3-hydroxybutyric acid-co-3-hydrovaleric acid) (PHBV) film and electrospun scaffolds. Mater. Sci. Eng. C 2020, 110, 110668. [Google Scholar] [CrossRef]
- Tiwari, S.; Kar, S.C.; Bhatla, R. Dynamic downscaling over western Himalayas: Impact of cloud microphysics schemes. Atmos. Res. 2018, 201, 1–16. [Google Scholar] [CrossRef]
- Freier, T. Biopolyesters in tissue engineering applications. In Polymers for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–61. [Google Scholar]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Pospisilova, A.; Melcova, V.; Figalla, S.; Mencik, P.; Prikryl, R. Techniques for increasing the thermal stability of poly[(R)-3-hydroxybutyrate] recovered by digestion methods. Polym. Degrad. Stab. 2021, 193, 109727. [Google Scholar] [CrossRef]
- Eesaee, M.; Ghassemi, P.; Nguyen, D.D.; Thomas, S.; Elkoun, S.; Nguyen-Tri, P. Morphology and crystallization behaviour of polyhydroxyalkanoates-based blends and composites: A review. Biochem. Eng. J. 2022, 187, 108588. [Google Scholar] [CrossRef]
- Furrer, P.; Panke, S.; Zinn, M. Efficient recovery of low endotoxin medium-chain-length poly([R]-3-hydroxyalkanoate) from bacterial biomass. J. Microbiol. Methods 2007, 69, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical processing of polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Mesallati, T.; Vinardell, T.; Kelly, D.J. Engineering cartilage or endochondral bone: A comparison of different naturally derived hydrogels. Acta Biomater. 2015, 13, 245–253. [Google Scholar] [CrossRef]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Hydroxyapatite-hybridized chitosan/chitin whisker bionanocomposite fibers for bone tissue engineering applications. Carbohydr. Polym. 2016, 144, 419–427. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Banerjee, S.; Szepes, M.; Dibbert, N.; Rios-Camacho, J.C.; Kirschning, A.; Gruh, I.; Dräger, G. Dextran-based scaffolds for in-situ hydrogelation: Use for next generation of bioartificial cardiac tissues. Carbohydr. Polym. 2021, 262, 117924. [Google Scholar] [CrossRef]
- Kang, D.S.; Yang, S.Y.; Lee, C.Y. Fabrication of innocuous hydrogel scaffolds based on modified dextran for biotissues. Carbohydr. Res. 2022, 522, 108699. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Qin, Y. 5—Applications of advanced technologies in the development of functional medical textile materials. In Medical Textile Materials; Qin, Y., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 55–70. [Google Scholar] [CrossRef]
- Markovic, G.; Visakh, P.M. 1—Polymer blends: State of art. In Recent Developments in Polymer Macro, Micro and Nano Blends; Visakh, P.M., Markovic, G., Pasquini, D., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K.; Yadav, K.S. 16—Fabrication and characterization of polycaprolactone-based green materials for drug delivery. In In Applications of Advanced Green Materials; Ahmed, S., Ed.; Woodhead Publishing in Materials; Woodhead Publishing: Sawston, UK, 2021; pp. 395–423. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Van de Voorde, K.M.; Korley, L.T.J.; Pokorski, J.K. Confinement and Composition Effects on the Degradation Profile of Extruded PLA/PCL Nonwoven Fiber Blends. ACS Appl. Polym. Mater. 2021, 3, 3878–3890. [Google Scholar] [CrossRef]
- Pisani, S. A Design of Experiment (DOE) approach to correlate PLA-PCL electrospun fibers diameter and mechanical properties for soft tissue regeneration purposes. J. Drug Deliv. Sci. Technol. 2022, 68, 103060. [Google Scholar] [CrossRef]
- Bhattarai, R.; Bachu, R.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhou, Y.; Zhang, Y.; Li, Q.; Shen, C. Fabrication of open-porous PCL/PLA tissue engineering scaffolds and the relationship of foaming process, morphology, and mechanical behavior. Polym. Adv Technol. 2019, 30, 2539–2548. [Google Scholar] [CrossRef]
- Sartore, L.; Inverardi, N.; Pandini, S.; Bignotti, F.; Chiellini, F. PLA/PCL-based foams as scaffolds for tissue engineering applications. Mater. Today Proc. 2019, 7, 410–417. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Wu, S.; Han, Y.; Wang, H.; Sun, H.; Kong, D.; Leng, X.; Wang, C.; Zhang, L.; et al. Micelle or polymersome formation by PCL-PEG-PCL copolymers as drug delivery systems. Chin. Chem. Lett. 2017, 28, 1905–1909. [Google Scholar] [CrossRef]
- Risangud, N. Amphiphilic polymeric photoinitiator composed of PEG-b-PCL diblock copolymer for three-dimensional printing of hydrogels. Eur. Polym. J. 2022, 168, 111094. [Google Scholar] [CrossRef]
- Piao, L.; Dai, Z.; Deng, M.; Chen, X.; Jing, X. Synthesis and characterization of PCL/PEG/PCL triblock copolymers by using calcium catalyst. Polymer 2003, 44, 2025–2031. [Google Scholar] [CrossRef]
- Dethe, M.R.; Prabakaran, A.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG copolymer based injectable thermosensitive hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Y.; Lv, C.; Han, C.; Li, Y. Enhanced wound healing activity of PEG/PCL copolymer combined with bioactive nanoparticles in wound care after anorectal surgery: Via bio-inspired methodology. J. Photochem. Photobiol. B 2018, 187, 54–60. [Google Scholar] [CrossRef] [PubMed]
- El yousfi, R.; Achalhi, N.; Mohamed, A.; Benahmed, A.; El Idrissi, A. Synthesis, characterization of multi-arm copolymers and linear blocks based on PEG and PCL: Effect of topology on dye adsorption. Mater. Today Proc. 2023, 72, 3650–3661. [Google Scholar] [CrossRef]
- Liu, M. Improved surface adhesion and wound healing effect of madecassoside liposomes modified by temperature-responsive PEG-PCL-PEG copolymers. Eur. J. Pharm. Sci. 2020, 151, 105373. [Google Scholar] [CrossRef]
- Wang, H.; Tong, D.; Wang, L.; Chen, L.; Yu, N.; Li, Z. A facile strategy for fabricating PCL/PEG block copolymer with excellent enzymatic degradation. Polym. Degrad. Stab. 2017, 140, 64–73. [Google Scholar] [CrossRef]
- Lv, F.; Mao, L.; Liu, T. Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG–PCL copolymer: Preparation, characterization and fluorescence imaging In Vivo. Mater. Sci. Eng. C 2014, 43, 221–230. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.J.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K. Fabrication of 3D Printed PCL/PEG Polyblend Scaffold Using Rapid Prototyping System for Bone Tissue Engineering Application. J. Bionic. Eng. 2018, 15, 435–442. [Google Scholar] [CrossRef]
- Ko, P.-T.; Lee, I.-C.; Chen, M.-C.; Tsai, S.-W. Polymer microneedles fabricated from PCL and PCL/PEG blends for transdermal delivery of hydrophilic compounds. J. Taiwan Inst. Chem. Eng. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Deng, H.; Dong, A.; Song, J.; Chen, X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J. Control. Release 2019, 297, 60–70. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. Effect of incorporating Elaeagnus angustifolia extract in PCL-PEG-PCL nanofibers for bone tissue engineering. Front. Chem. Sci. Eng. 2019, 13, 108–119. [Google Scholar] [CrossRef]
- Fu, S.Z.; Wang, X.H.; Guo, G.; Shi, S.; Fan, M.; Liang, H.; Luo, F.; Qian, Z.Y. Preparation and properties of nano-hydroxyapatite/PCL-PEG-PCL composite membranes for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Alizadeh, E.; Del Bakhshayesh, A.R.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and In Vitro Evaluation of Nanocomposite Hydrogel Scaffolds Based on Gelatin/PCL–PEG–PCL for Cartilage Tissue Engineering. ACS Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]
- Yedekçi, B.; Tezcaner, A.; Yılmaz, B.; Demir, T.; Evis, Z. 3D porous PCL-PEG-PCL/strontium, magnesium and boron multi-doped hydroxyapatite composite scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2022, 125, 104941. [Google Scholar] [CrossRef]
- Dolatabadi, E.N.; Asghariazar, V.; Darvish, M.; Nejati-Koshki, K. Simvastatin-loaded PCL/PEG nanofibrous scaffold: A prospective approach for suppression 5-fluorouracil resistance in MKN-45 gastric cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104104. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, H.; Wu, S.; Zhao, K.; Sun, H.; Kong, D.; Wang, C.; Leng, X.; Zhu, D. Preparation and evaluation of PCL–PEG–PCL polymeric nanoparticles for doxorubicin delivery against breast cancer. RSC Adv. 2016, 6, 54727–54737. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical processing of PCL and PCL-PEG blends to produce improved PCL-based porous scaffolds. J. Supercrit. Fluids 2022, 186, 105611. [Google Scholar] [CrossRef]
- Liu, K.; Sun, J.; Zhu, Q.; Jin, X.; Zhang, Z.; Zhao, Z.; Chen, G.; Wang, C.; Jiang, H.; Zhang, P. Microstructures and properties of polycaprolactone/tricalcium phosphate scaffolds containing polyethylene glycol fabricated by 3D printing. Ceram. Int. 2022, 48, 24032–24043. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Khodaverdi, E.; Oroojalian, F.; Rahmanian-Devin, P.; Hassan, M.; Hashemi, S.; Omidkhah, N.; Asare-Addo, K.; Nokhodchi, A.; Kamali, H. Preparation of porous PCL-PEG-PCL scaffolds using supercritical carbon dioxide. Int. J. Pharm. 2023, 631, 122507. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. A 2021, 109, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Vaez, A.; Samadian, H.; Sahrapeyma, H.; Mirzaii, M.; Ghorbani, S.; Goodarzi, A. In Vitro and In Vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018, 117, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Davoodi, P.; Vijayavenkataraman, S.; Teoh, J.H.; Thamizhchelvan, A.M.; Robinson, K.S.; Wu, B.; Fuh, J.Y.H.; DiColandrea, T.; Zhao, H.; et al. Optimized construction of a full thickness human skin equivalent using 3D bioprinting and a PCL/collagen dermal scaffold. Bioprinting 2021, 21, e00123. [Google Scholar] [CrossRef]
- Chong, C.; Wang, Y.; Fathi, A.; Parungao, R.; Maitz, P.K.; Li, Z. Skin wound repair: Results of a pre-clinical study to evaluate electropsun collagen–elastin–PCL scaffolds as dermal substitutes. Burns 2019, 45, 1639–1648. [Google Scholar] [CrossRef]
- Chang, T.; Yin, H.; Yu, X.; Wang, L.; Fan, L.; Xin, J.H.; Yu, H. 3D PCL/collagen nanofibrous medical dressing for one-time treatment of diabetic foot ulcers. Colloids Surf. B Biointerfaces 2022, 214, 112480. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Boyce, S.T. Engineered Human Skin Fabricated Using Electrospun Collagen–PCL Blends: Morphogenesis and Mechanical Properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef]
- Ma, W.; Wang, L.; Zhang, Q.; Dong, X.; Zhu, T.; Lu, S. Electrospun PCL/collagen hybrid nanofibrous tubular graft based on post-network bond processing for vascular substitute. Biomater. Adv. 2022, 139, 213031. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Lee, M.-K.; Park, C.; Jung, H.-D.; Kim, H.-E.; Jang, T.-S. Fabrication of strong, bioactive vascular grafts with PCL/collagen and PCL/silica bilayers for small-diameter vascular applications. Mater. Des. 2019, 181, 108079. [Google Scholar] [CrossRef]
- Bertram, U.; Steiner, D.; Poppitz, B.; Dippold, D.; Köhn, K.; Beier, J.P.; Detsch, R.; Boccaccini, A.R.; Schubert, D.W.; Horch, R.E.; et al. Vascular Tissue Engineering: Effects of Integrating Collagen into a PCL Based Nanofiber Material. Biomed. Res. Int. 2017, 2017, 9616939. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun Antibacterial Chitosan-Based Fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef]
- Ghavimi, S.; Ebrahimzadeh, M.; Solati-Hashjin, M.; Osman, A. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J. Biomed. Mater. Res. A 2014, 103, 2482–2498. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.C.; Carmona, V.B.; Simão, J.A.; Mattoso, L.H.C.; Marconcini, J.M. Biodegradable blends of urea plasticized thermoplastic starch (UTPS) and poly(ε-caprolactone) (PCL): Morphological, rheological, thermal and mechanical properties. Carbohydr. Polym. 2017, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure Characterization and Biodegradation Rate of Poly(ε-caprolactone)/Starch Blends. Front. Mater. 2020, 7, 141. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and disintegration of multilayer starch films with electrospun PCL fibres encapsulating carvacrol. Polym. Degrad. Stab. 2020, 173, 109100. [Google Scholar] [CrossRef]
- Deeraj, B.D.S.; Jayan, J.S.; Saritha, A.; Joseph, K. 8—PLA-based blends and composites. In Biodegradable Polymers, Blends and Composites; Rangappa, S.M., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2022; pp. 237–281. [Google Scholar] [CrossRef]
- D’Anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef]
- Nevado, P.; Lopera, A.; Bezzon, V.; Fulla, M.R.; Palacio, J.; Zaghete, M.A.; Biasotto, G.; Montoya, A.; Rivera, J.; Robledo, S.M.; et al. Preparation and In Vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mater. Sci. Eng. C 2020, 114, 111013. [Google Scholar] [CrossRef]
- Zhu, K.J.; Xiangzhou, L.; Shilin, Y. Preparation, characterization, and properties of polylactide (PLA)–poly(ethylene glycol) (PEG) copolymers: A potential drug carrier. J. Appl. Polym. Sci. 1990, 39, 1–9. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Repp, L.; Lee, H.J.; Kwon, G.S. Production of paclitaxel-loaded PEG-b-PLA micelles using PEG for drug loading and freeze-drying. J. Control. Release 2022, 350, 350–359. [Google Scholar] [CrossRef]
- Viseu, A. Nanomedicine. In Britannica; Encyclopaedia Britannica, Inc.: Chicago, IL, USA, 2020. [Google Scholar]
- Salehi, S.; Ghomi, H.; Hassanzadeh-Tabrizi, S.A.; Koupaei, N.; Khodaei, M. The effect of polyethylene glycol on printability, physical and mechanical properties and osteogenic potential of 3D-printed poly (l-lactic acid)/polyethylene glycol scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2022, 221, 1325–1334. [Google Scholar] [CrossRef]
- Serra, T.; Ortiz-Hernandez, M.; Engel, E.; Planell, J.A.; Navarro, M. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Mater. Sci. Eng. C 2014, 38, 55–62. [Google Scholar] [CrossRef]
- Asadollahi, M.; Gerashi, E.; Zohrevand, M.; Zarei, M.; Sayedain, S.S.; Alizadeh, R.; Labbaf, S.; Atari, M. Improving mechanical properties and biocompatibility of 3D printed PLA by the addition of PEG and titanium particles, using a novel incorporation method. Bioprinting 2022, 27, e00228. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Preparation of three-layered porous PLA/PEG scaffold: Relationship between morphology, mechanical behavior and cell permeability. J. Mech. Behav. Biomed. Mater. 2016, 54, 8–20. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Jing, X.; Mi, H.-Y.; Zhao, H.; Zhang, W.-H.; Peng, X.-F.; Turng, L.-S. Fabrication of polylactic acid/polyethylene glycol (PLA/PEG) porous scaffold by supercritical CO2 foaming and particle leaching. Polym. Eng. Sci. 2015, 55, 1339–1348. [Google Scholar] [CrossRef]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Wally, Z.; Rao, P.S.; Reilly, G.C. Composite porous scaffold of PEG/PLA support improved bone matrix deposition In Vitro compared to PLA-only scaffolds. J. Biomed. Mater. Res. A 2018, 106, 1334–1340. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, B.; Nie, Z.; Ding, W.; Jiang, X.; Song, Y. A novel asymmetric amphiphilic porous film of (PLA)-(PLA-b-PEG)-(PEG) with controlled gradual pore size. Results Mater. 2020, 6, 100089. [Google Scholar] [CrossRef]
- Menčík, P.; Přikryl, R.; Krobot, Š.; Melčová, V.; Kontárová, S.; Plavec, R.; Bočkaj, J.; Horváth, V.; Alexy, P. Evaluation of the Properties of PHB Composite Filled with Kaolin Particles for 3D Printing Applications Using the Design of Experiment. Int. J. Mol. Sci. 2022, 23, 4409. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Gonzalez Ausejo, J.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, J.; Adamus, G.; Janeczek, H.; Kwiecień, I.; Hercog, A.; et al. A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym. Degrad. Stab. 2018, 152, 191–207. [Google Scholar] [CrossRef]
- Sartore, L.; Pandini, S.; Dey, K.; Bignotti, F.; Chiellini, F. A versatile cell-friendly approach to produce PLA-based 3D micro-macro-porous blends for tissue engineering scaffolds. Materialia 2020, 9, 100615. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Pina, S.; Reis, R.L.; Oliveira, J.M. 4—Ceramic biomaterials for tissue engineering. In Fundamental Biomaterials: Ceramics; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 95–116. [Google Scholar] [CrossRef]
- Mala, R.; Celsia, A.S.R. 8—Bioceramics in orthopaedics: A review. In Fundamental Biomaterials: Ceramics; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 195–221. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Mathiazhagan, N.; Palaniyappan, S.; Sivakumar, N. Effect of fused filament fabrication parameters on crashworthiness studies of hydroxyapatite particle reinforced PLA composite thin-walled tubes. J. Mech. Behav. Biomed. Mater. 2023, 138, 105611. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Saberi, A.; Behnamghader, A.; Aghabarari, B.; Yousefi, A.; Majda, D.; Huerta, M.V.M.; Mozafari, M. 3D direct printing of composite bone scaffolds containing polylactic acid and spray dried mesoporous bioactive glass-ceramic microparticles. Int. J. Biol. Macromol. 2022, 207, 9–22. [Google Scholar] [CrossRef]
- Vasconcelos, E.V.; da Luz, F.B.; da Paz, S.P.A.; dos Reis, M.A.L.; da Silva, A.C.R.; Passos, M.F.; Barboza, C.A.G.; Monteiro, S.N.; Candido, V.S. Nanostructured 3D bioprinting of PLA with bioglass-CNT scaffolds for osseus tissue graft manufacturing. J. Mater. Res. Technol. 2023, 23, 5923–5938. [Google Scholar] [CrossRef]
- Melčová, V.; Svoradová, K.; Menčík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Sovková, V.; Přikryl, R.; Vojtová, L. Fdm 3d printed composites for bone tissue engineering based on plasticized poly(3-hydroxybutyrate)/poly(d,l-lactide) blends. Polymers 2020, 12, 2806. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, D.; Shen, H.; Nan, J.; Ma, N.; Guo, Z.; Wang, X.; Jin, Q. Cold sintering constructed in situ drug-loaded high strength HA-PLA composites: Potential bone substitution material. Ceram. Int. 2023, 49, 11655–11663. [Google Scholar] [CrossRef]
- Maglione, M.; Salvador, E.; Angerame, D.; Melato, M.; Ruaro, M.E.; di Lenarda, R. Bones regeneration with adult stem cells: Animal model. Dental. Mater. 2010, 26, e82–e83. [Google Scholar] [CrossRef]
- Fricain, J.C.; Schlaubitz, S.; le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite—Pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef]
- Saleh, L.S.; Bryant, S.J. The host response in tissue engineering: Crosstalk between immune cells and cell-laden scaffolds. Curr. Opin. Biomed. Eng. 2018, 6, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J. Immunoengineering has arrived. J. Biomed. Mater. Res. A 2021, 109, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.I.E.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.I.P.M.; Bouten, C.V.C. Tissue engineering meets immunoengineering: Prospective on personalized in situ tissue engineering strategies. Curr. Opin. Biomed. Eng. 2018, 6, 17–26. [Google Scholar] [CrossRef]
- Russo, V.; El Khatib, M.; Prencipe, G.; Cerveró-Varona, A.; Citeroni, M.R.; Mauro, A.; Berardinelli, P.; Faydaver, M.; Haidar-Montes, A.A.; Turriani, M.; et al. Scaffold-Mediated Immunoengineering as Innovative Strategy for Tendon Regeneration. Cells 2022, 11, 266. [Google Scholar] [CrossRef]
- Liu, M.; Nakasaki, M.; Shih, Y.-R.V.; Varghese, S. Effect of age on biomaterial-mediated in situ bone tissue regeneration. Acta Biomater. 2018, 78, 329–340. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Ravikumar, K.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef]
- Veiseh, O.; Vegas, A.J. Domesticating the foreign body response: Recent advances and applications. Adv. Drug Deliv. Rev. 2019, 144, 148–161. [Google Scholar] [CrossRef]
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef]
- Barone, D.G.; Carnicer-Lombarte, A.; Tourlomousis, P.; Hamilton, R.S.; Prater, M.; Rutz, A.L.; Dimov, I.B.; Malliaras, G.G.; Lacour, S.P.; Robertson, A.A.B.; et al. Prevention of the foreign body response to implantable medical devices by inflammasome inhibition. Proc. Natl. Acad. Sci. USA 2022, 119, e2115857119. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xie, C.; Wang, C.; Huang, J.; Yin, Z.; Heng, B.C.; Chen, X.; Shen, W. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact. Mater. 2021, 6, 4096–4109. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, Q.-Y.; Huang, L.-P.; Huang, K.; Xie, H.-Q. Decellularized scaffold and its elicited immune response towards the host: The underlying mechanism and means of immunomodulatory modification. Biomater. Sci. 2021, 9, 4803–4820. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Guo, P.; Li, X.; He, Z.; Li, Z.; Stoddart, M.J.; Grad, S.; Tian, W.; Chen, D.; et al. Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration. Bioact. Mater. 2021, 6, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger, A.D.; Tempfer, H.; Lehner, C.; Egloff, J.; Mauracher, M.; Bird, A.; Widmer, J.; Maniura-Weber, K.; Fucentese, S.F.; Traweger, A.; et al. Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon In Vitro and In Vivo. Biomaterials 2020, 249, 120034. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767. [Google Scholar] [CrossRef]
- Schoenenberger, A.D.; Foolen, J.; Moor, P.; Silvan, U.; Snedeker, J.G. Substrate fiber alignment mediates tendon cell response to inflammatory signaling. Acta Biomater. 2018, 71, 306–317. [Google Scholar] [CrossRef]
- Zhou, H.; Xue, Y.; Dong, L.; Wang, C. Biomaterial-based physical regulation of macrophage behaviour. J. Mater. Chem. B 2021, 9, 3608–3621. [Google Scholar] [CrossRef]
- Liang, R.; Fang, D.; Lin, W.; Wang, Y.; Liu, Z.; Wang, Y.; Zeng, Y.; He, M.; Li, J.; Luo, F.; et al. Macrophage Polarization in Response to Varying Pore Sizes of 3D Polyurethane Scaffolds. J. Biomed. Nanotechnol. 2018, 14, 1744–1760. [Google Scholar] [CrossRef]
- Barsch, F.; Mamilos, A.; Schmitt, V.H.; Babel, M.; Winter, L.; Wagner, W.; Winther, H.; Ottomann, C.; Niedermair, T.; Schreml, S.; et al. In Vivo Comparison of Synthetic Macroporous Filamentous and Sponge-like Skin Substitute Matrices Reveals Morphometric Features of the Foreign Body Reaction According to 3D Biomaterial Designs. Cells 2022, 11, 2834. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109927. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, R.; Deng, N.; Zhao, X.; Li, X.; Guo, C. Natural polymer-based scaffolds for soft tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 954699. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tu, T.; Yi, B.; Wang, X.; Tang, H.; Liu, W.; Zhang, Y. Electrospun acid-neutralizing fibers for the amelioration of inflammatory response. Acta Biomater. 2019, 97, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef]
- Sadtler, K.; Wolf, M.T.; Ganguly, S.; Moad, C.A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D.M.; Elisseeff, J.H. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019, 192, 405–415. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Sajadi, S.M.; Seidi, F.; Mashhadzadeh, A.H.; Rabiee, N.; Saeb, M.R.; Aminabhavi, T.; Webster, T.J. Cell-Seeded Biomaterial Scaffolds: The Urgent Need for Unanswered Accelerated Angiogenesis. Int. J. Nanomed. 2022, 17, 1035–1068. [Google Scholar] [CrossRef]
- El-Kadiry, A.E.-H.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Feng, T.; Li, R.; Wang, Z.; Zhang, L.; Yin, P.; Tang, P. Bone Engineering Scaffolds With Exosomes: A Promising Strategy for Bone Defects Repair. Front. Bioeng. Biotechnol. 2022, 10, 920378. [Google Scholar] [CrossRef]
- Phan, T.H.; Kim, S.Y.; Rudge, C.; Chrzanowski, W. Made by cells for cells—Extracellular vesicles as next-generation mainstream medicines. J. Cell Sci. 2022, 135, jcs259166. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Wang, Y.; Xiao, L.; Wu, J.; Ma, Y.; Zhang, D.; Lang, X.; Wang, X. A tailored bioactive 3D porous poly(lactic-acid)-exosome scaffold with osteo-immunomodulatory and osteogenic differentiation properties. J. Biol. Eng. 2022, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhang, Z.; Lin, Z.; Su, J.; Panayi, A.C.; Xiong, Y.; Hu, L.; Hu, Y.; Chen, L.; Yan, C.; et al. Enhanced tissue regeneration through immunomodulation of angiogenesis and osteogenesis with a multifaceted nanohybrid modified bioactive scaffold. Bioact. Mater. 2022, 18, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Liu, Z.; Tamaddon, M.; Gu, Y.; Yu, J.; Xu, N.; Gang, F.; Sun, X.; Liu, C. Cell Seeding Process Experiment and Simulation on Three-Dimensional Polyhedron and Cross-Link Design Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- De Kort, B.J.; Koch, S.E.; Wissing, T.B.; Krebber, M.M.; Bouten, C.V.C.; Smits, A.I.P.M. Immuno-regenerative biomaterials for in situ cardiovascular tissue engineering—Do patient characteristics warrant precision engineering? Adv. Drug Deliv. Rev. 2021, 178, 113960. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
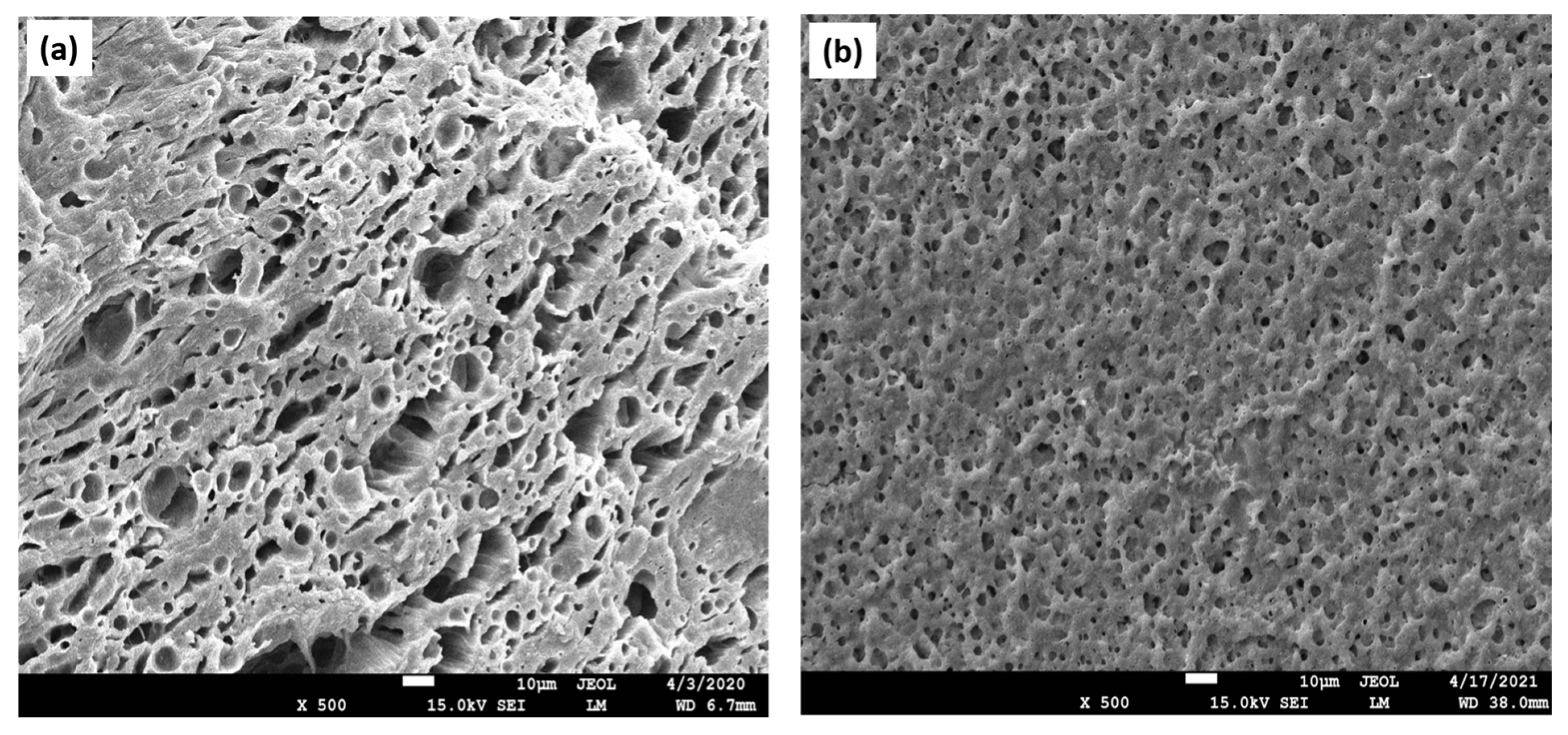
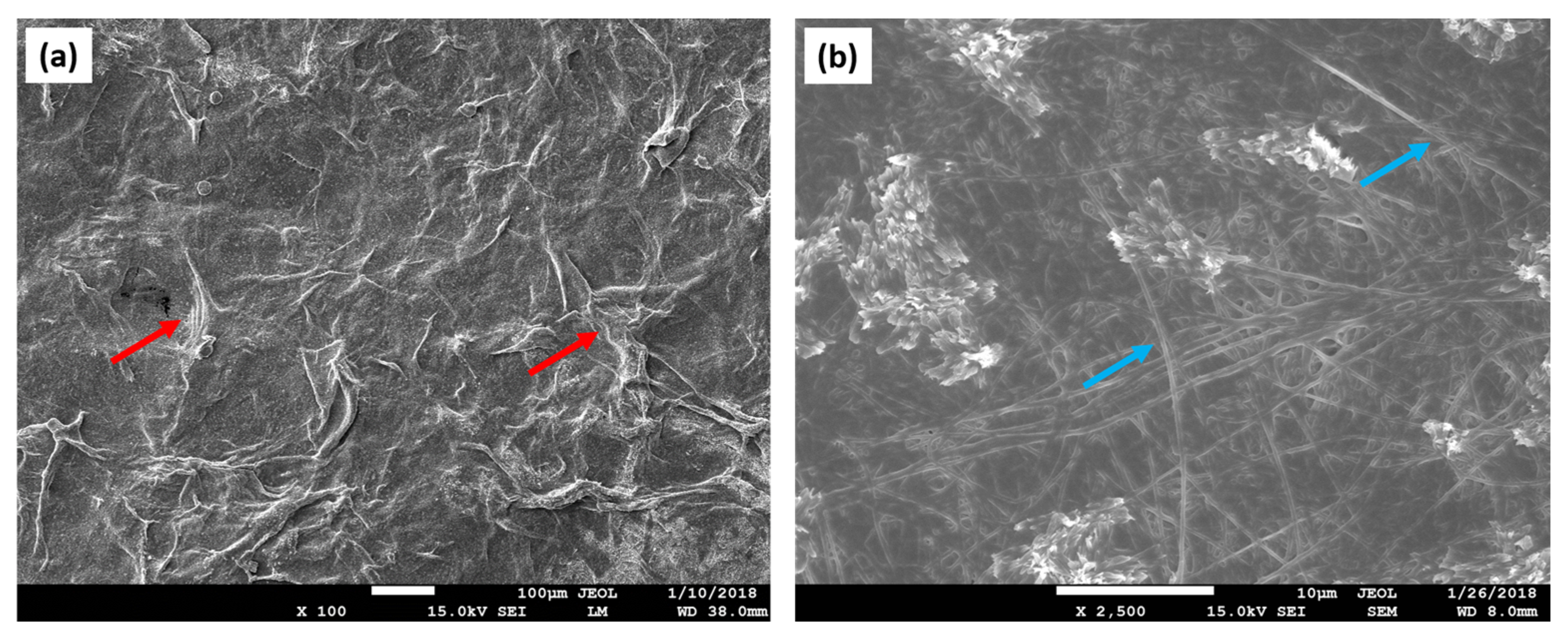
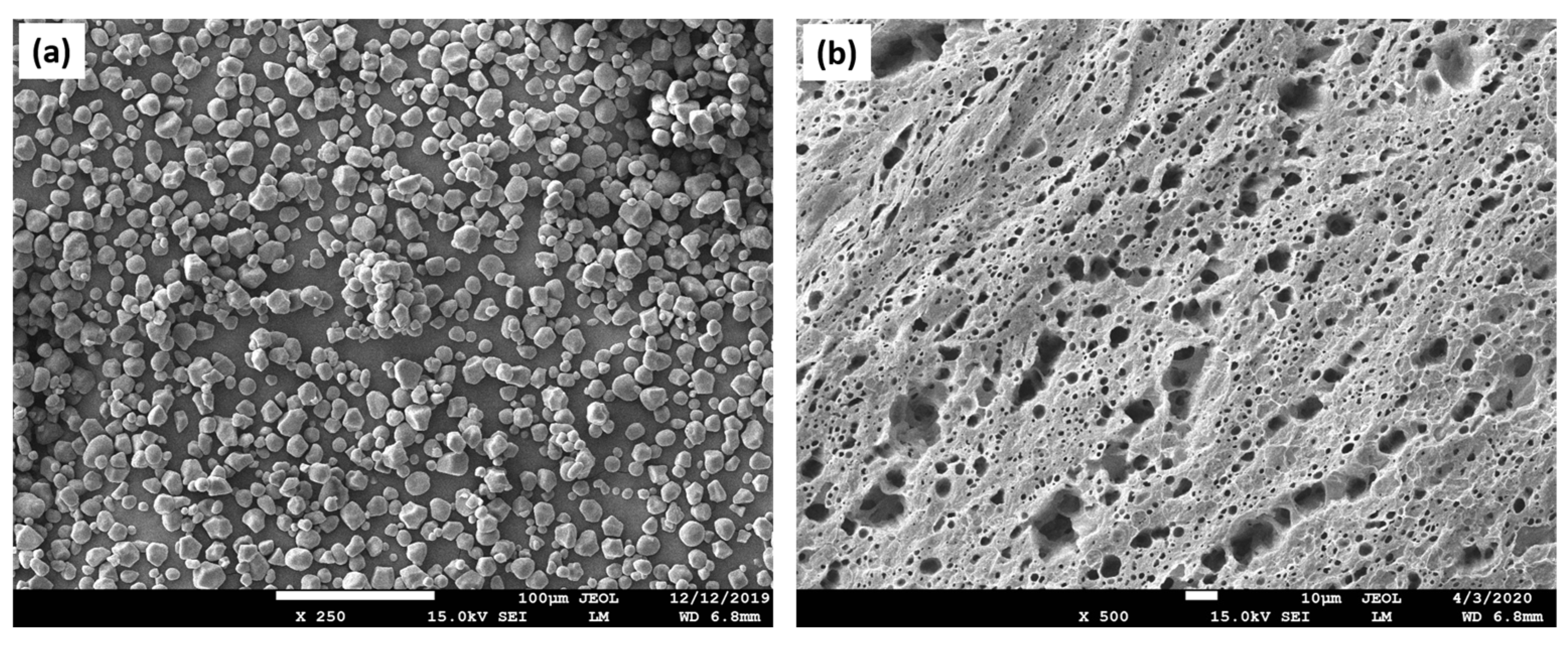
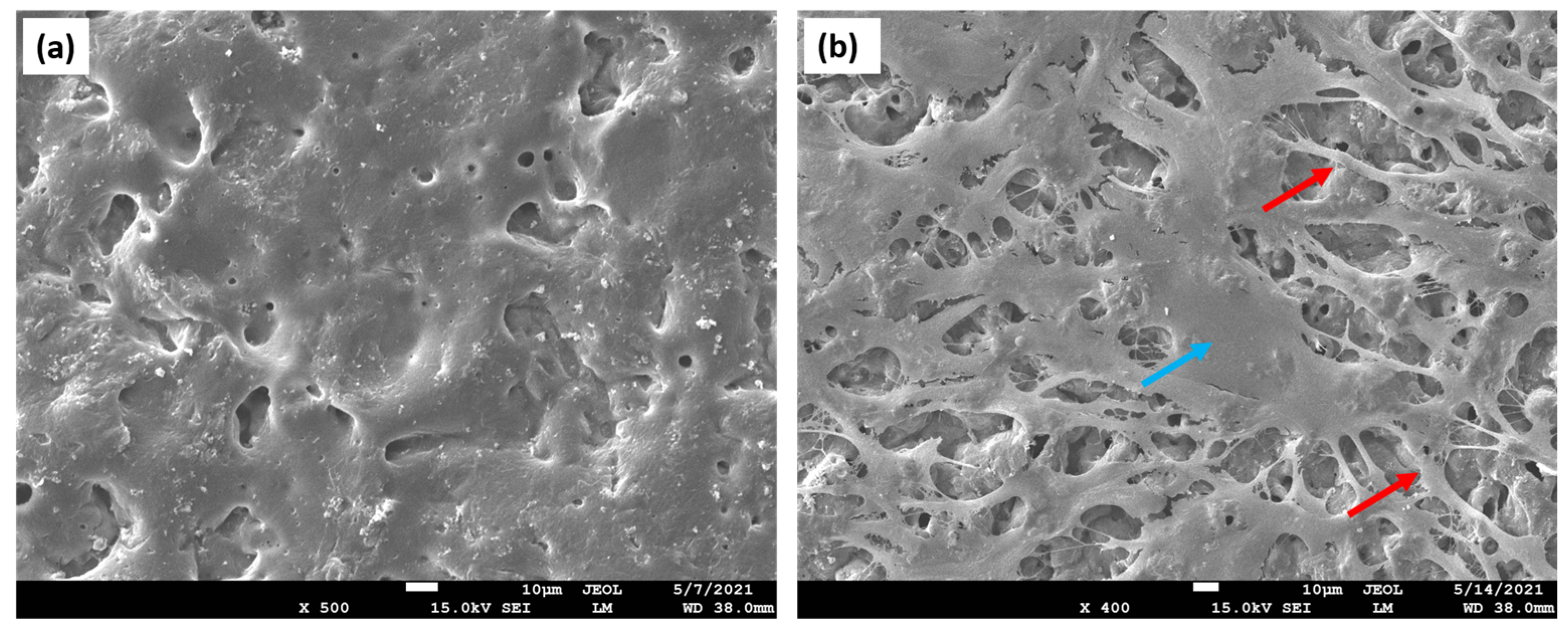
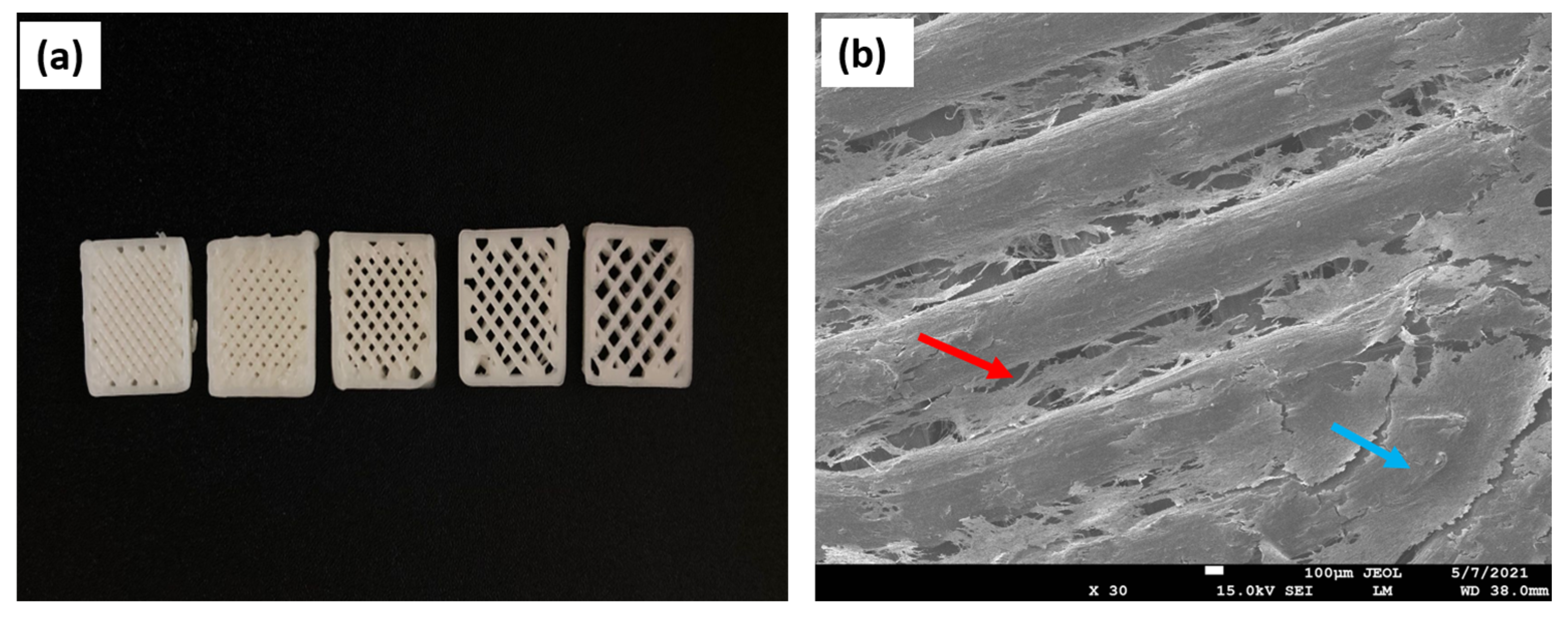
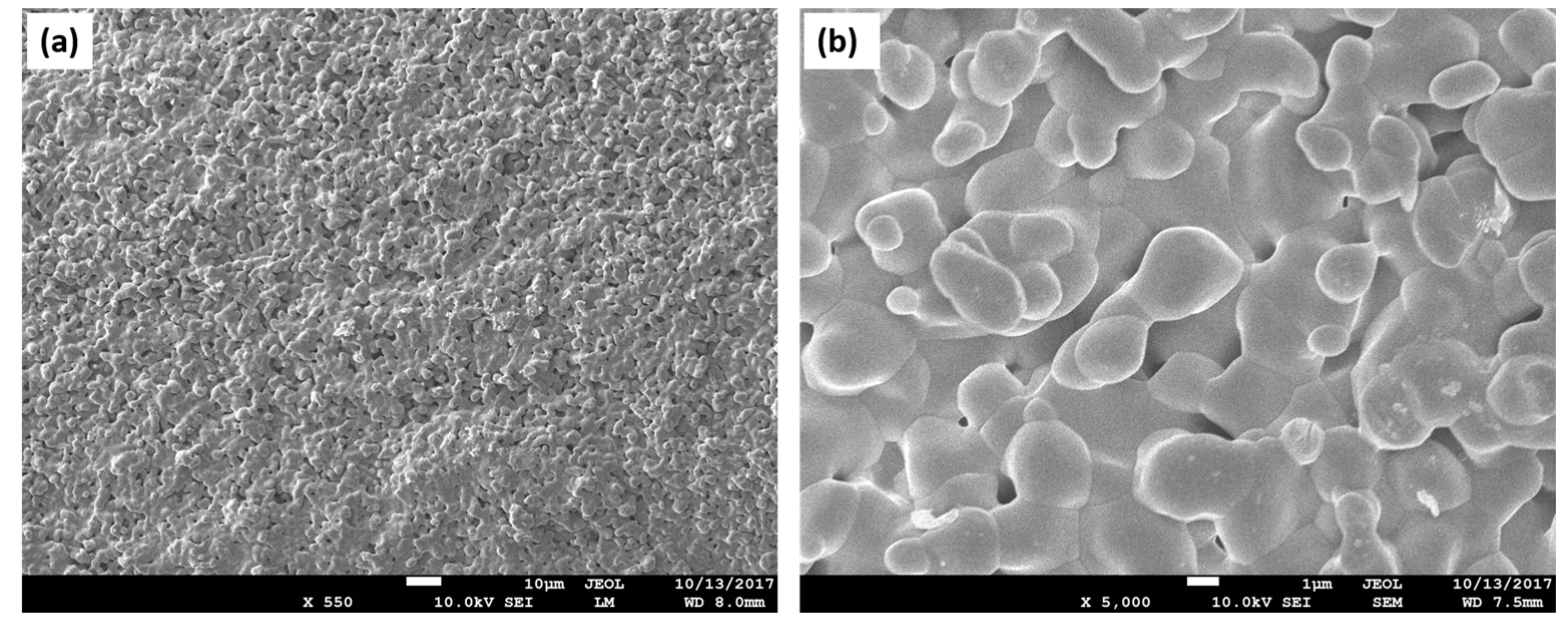

| Polymer | Advantages | Disadvantages | TE | References |
|---|---|---|---|---|
| Poly(ε-caprolactone) | bioresorbable biocompatible mechanical stiffness elasticity thermal stability low inflammability | low cell adhesion slow degradation | bone dental retina skin, vascular liver cartilage ligaments muscle, neural | [12,16,17,18] |
| Poly(vinyl) alcohol | biocompatibility hydrophilicity permeability water solubility | biodegradability after crosslinking cell adhesion | cardiac vascular bone skin cartilage neural corneal | [14,16,19,20,21,22,23,24,25] |
| Polyethylene glycol | biodegradability non-ionic water-soluble thermally stable protein repellency | cellular adhesion antigenicity | bone drug delivery | [16,26,27,28,29,30,31,32] |
| Polypropylene fumarate | biodegradability, biocompatibility excellent strength osteoconductivity | hydrophobicity | orthopedic, neural bone, ophthalmology cardiac | [33,34,35,36] |
| Polyurethane | biodegradability biocompatibility flexural endurance thrombo-resistance oxygen permeability | low interfacial tension | cardiac bone cartilage | [16,37,38,39,40,41] |
| Polymer | Advantages | Disadvantages | TE | References |
|---|---|---|---|---|
| Chitosan | biocompatibility bioresorbability physiologically degraded | mechanical properties resistance to enzymatic degradation | skin bone | [44,45,46,47,48,49] |
| Collagen | biocompatibility bioresorbability cell interaction | mechanical properties rapid degradation immunogenicity | skin bone | [50,51,52,53,54,55,56] |
| Hyaluronic acid | biocompatibility | |||
| bioresorbability | bone | |||
| enhances cell proliferation | poor cell adhesion | muscle | [57,58,59,60,61,62,63] | |
| immunosuppressive antioxidative properties | ||||
| Fibrin | biocompatibility | mechanical properties | skin | |
| bioresorbability cell interactions common natural protein | scaffold contraction | cardiovascular musculoskeletal nerve | [64,65] | |
| Xanthan gum | biocompatibility | mechanical properties | soft tissues | [66,67,68,69] |
| bioresorbability | difficult processing | bone | ||
| Dextran | biocompatibility | high cost | skin | |
| bioresorbability antithrombotic properties easily derived | low availability | vascular | [70,71,72] | |
| Starch | biocompatibility bioresorbability low cost | dimensional stability mechanical properties difficult processing high water uptake | bone | [73,74,75,76,77,78] |
| Poly (lactic acid) | biocompatibility | cardiovascular | ||
| bioresorbability mechanical strength processability | acidic degradation by-products degradation rate low cell adhesion | bone skin tendon | [79,80,81,82,83,84,85,86,87,88,89,90,91] | |
| Polyhydroxybutyrate | biocompatibility | crystallinity | skin | [92] |
| bioresorbability natural human metabolite | brittleness hydrophobic thermal stability | bone cardiovascular | [93,94,95,96,97,98,99,100] | |
| Poly(3-hydroxybutyrate-co-3-hyxdroxyvalerate) | biocompatibility | fragility | ||
| bioresorbability mechanical properties flexibility | impact resistance hydrophobicity thermal stability | bone cartilage | [101,102,103,104,105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vach Agocsova, S.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials 2023, 16, 4267. https://doi.org/10.3390/ma16124267
Vach Agocsova S, Culenova M, Birova I, Omanikova L, Moncmanova B, Danisovic L, Ziaran S, Bakos D, Alexy P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials. 2023; 16(12):4267. https://doi.org/10.3390/ma16124267
Chicago/Turabian StyleVach Agocsova, Sara, Martina Culenova, Ivana Birova, Leona Omanikova, Barbora Moncmanova, Lubos Danisovic, Stanislav Ziaran, Dusan Bakos, and Pavol Alexy. 2023. "Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review" Materials 16, no. 12: 4267. https://doi.org/10.3390/ma16124267
APA StyleVach Agocsova, S., Culenova, M., Birova, I., Omanikova, L., Moncmanova, B., Danisovic, L., Ziaran, S., Bakos, D., & Alexy, P. (2023). Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials, 16(12), 4267. https://doi.org/10.3390/ma16124267







