Study on the Performance and Mechanism of Cement Solidified Desulfurization Manganese Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
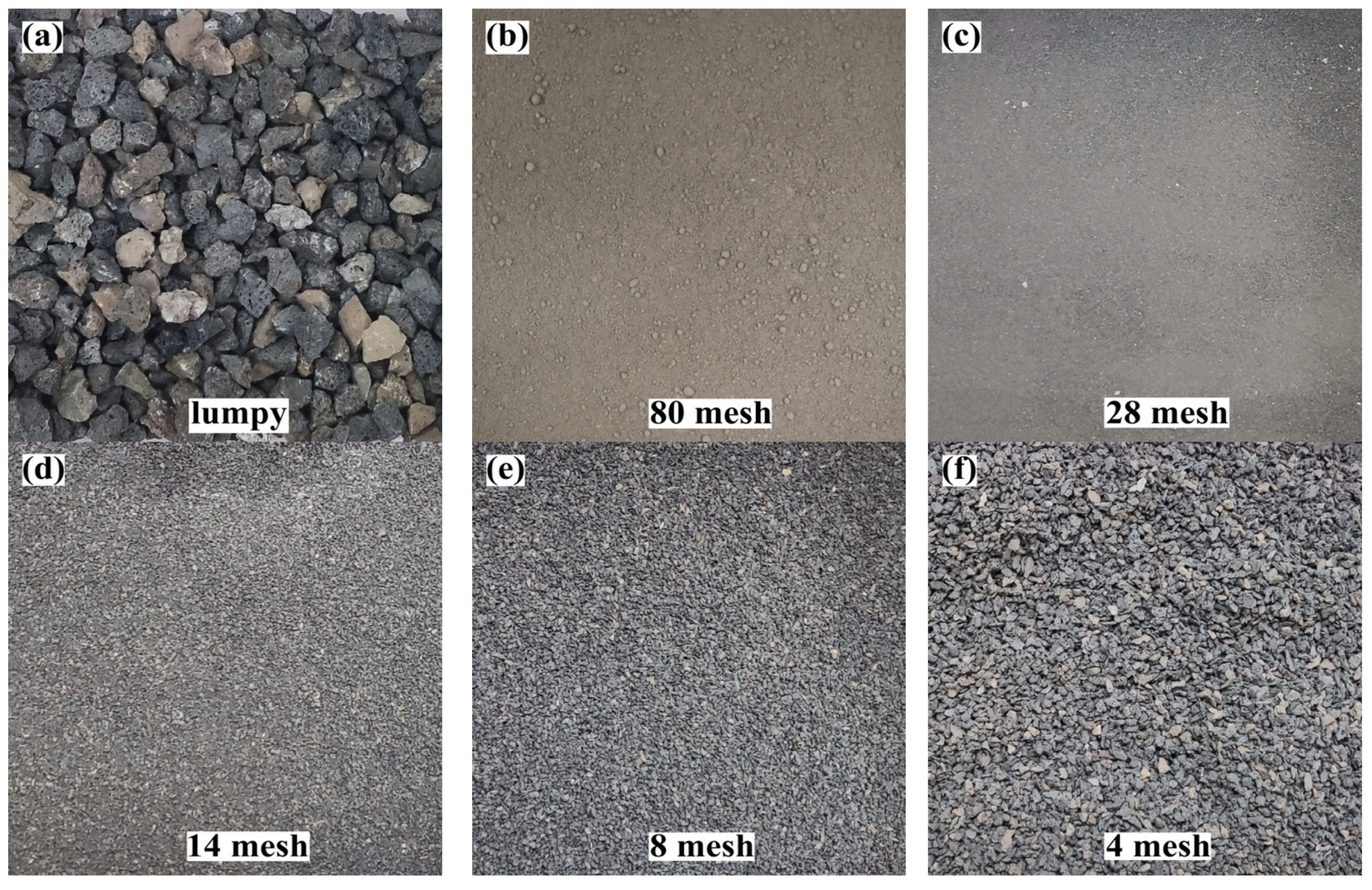
2.2. Pretreatment of DMR
2.3. Preparation of Cement Solidified DMR Solidified Body
- (1)
- The influence of different cement content: the ratio of cement to 80 mesh DMR is 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, and the water cement ratio is 0.44;
- (2)
- Put the cement and DMR into a mixer and mix them at low speed for 1 min, then add water to the mixture slowly and stir for 3 min, and then inject them into a test mold of 40 mm × 40 mm × 160 mm after mixing evenly. Vibrate the cement mortar on the shaking table for 120 times until it is compact, and smooth it with a scraper after vibrating. After curing at 22 °C and 96% humidity for 24 h, demoulding was carried out, and curing was continued to the specified curing ages of 3d, 7d, 14d, and 28d under the above conditions, and the cement-DMR solidified specimens to be tested were obtained.
2.4. Flexural Strength and Compressive Strength
2.5. Characterization of Solidified Body
2.6. Leaching Toxicity Test
3. Results and Discussion
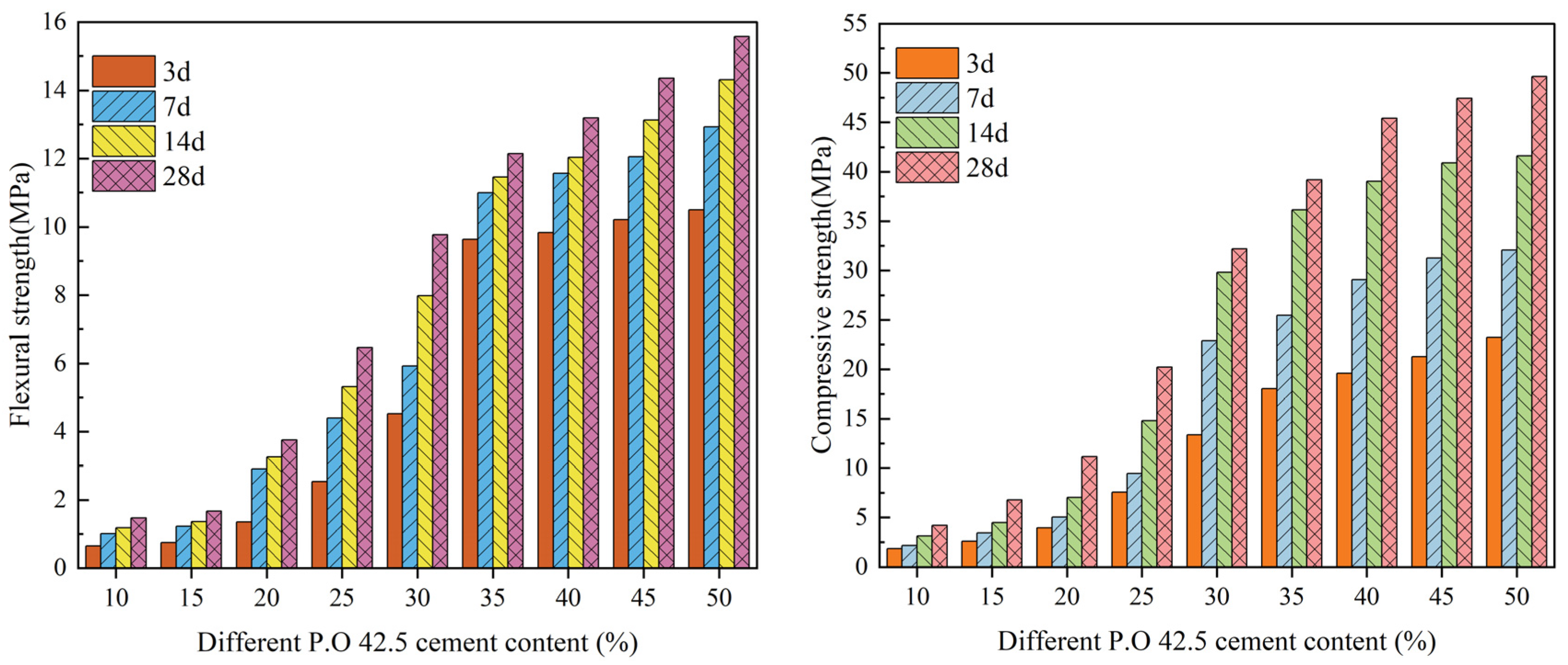
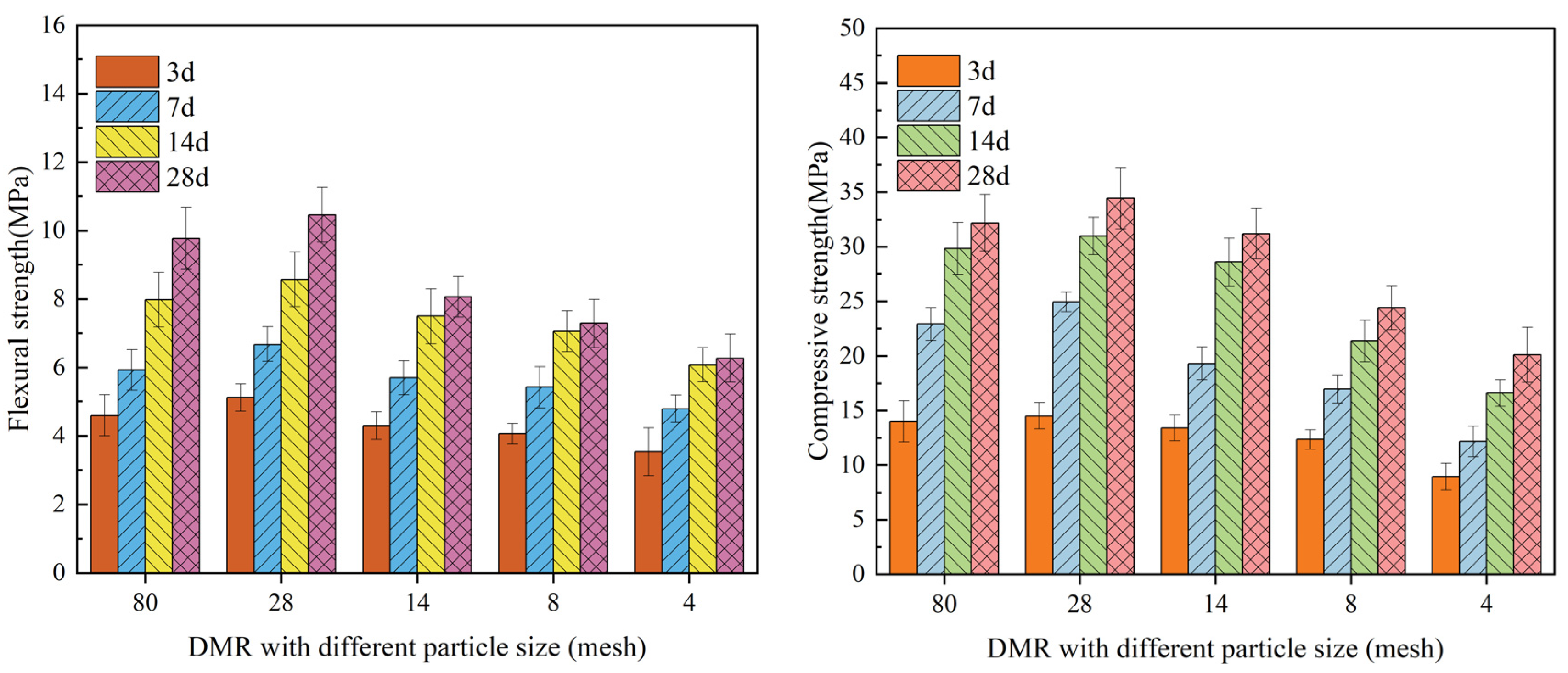
3.1. Flexural Strength and Compressive Strength
3.2. Toxicity Leaching Test of Cement Solidified Desulfurization Manganese Residue
3.3. Study on Hydration Products and Micro-Morphology Analysis of Cement Solidified Desulfurization Manganese Residue Solidified Body
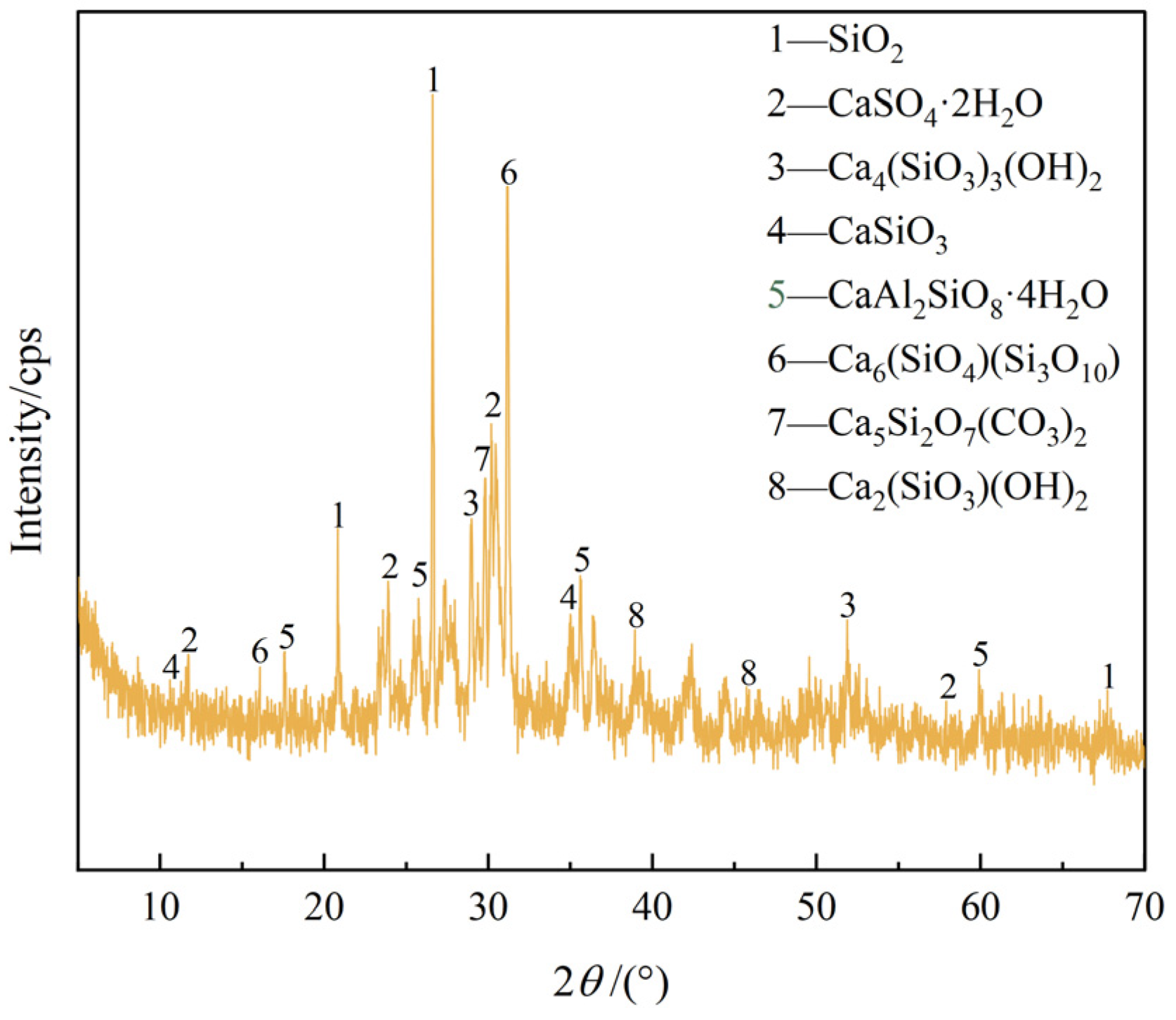
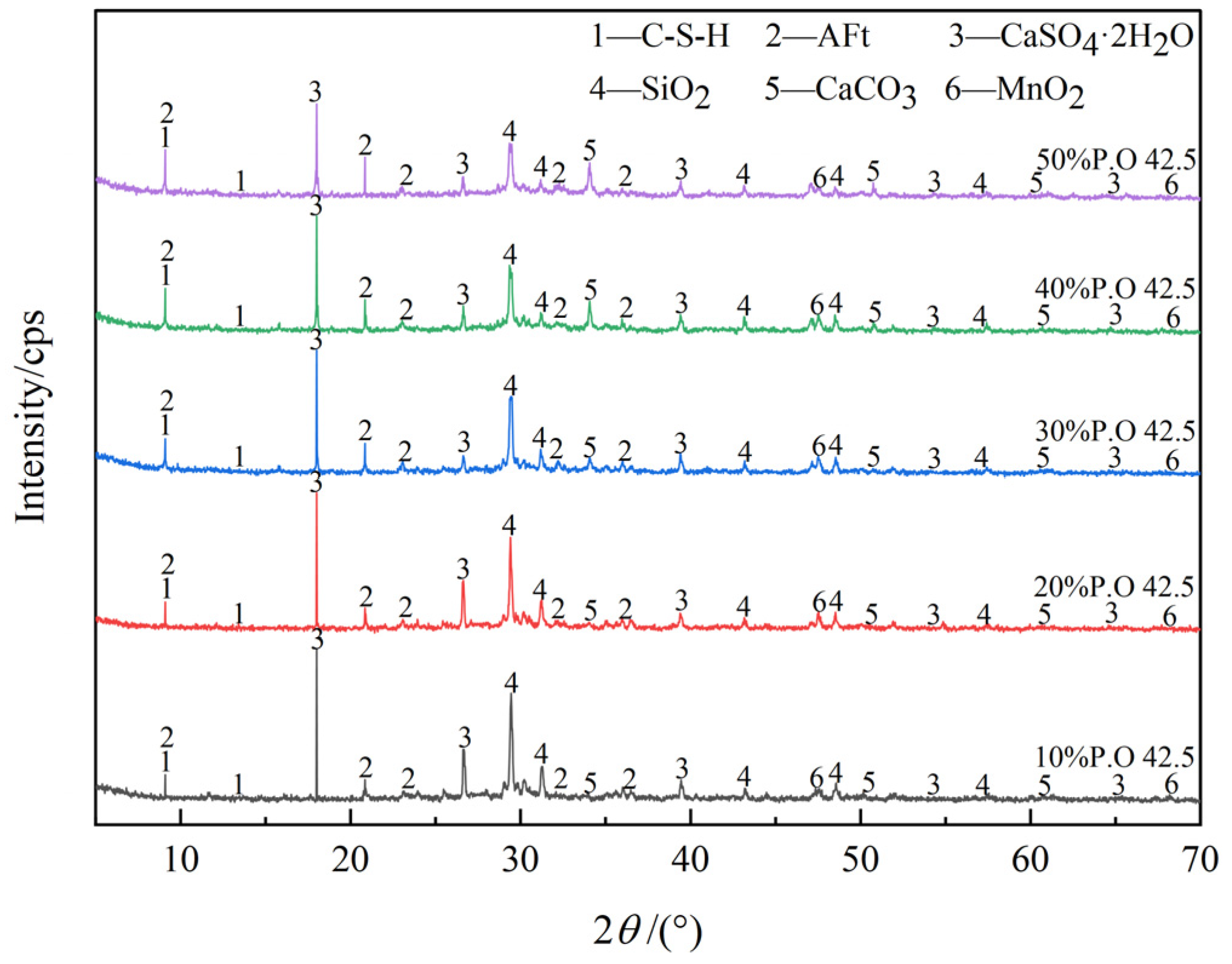
3.3.1. XRD Analysis
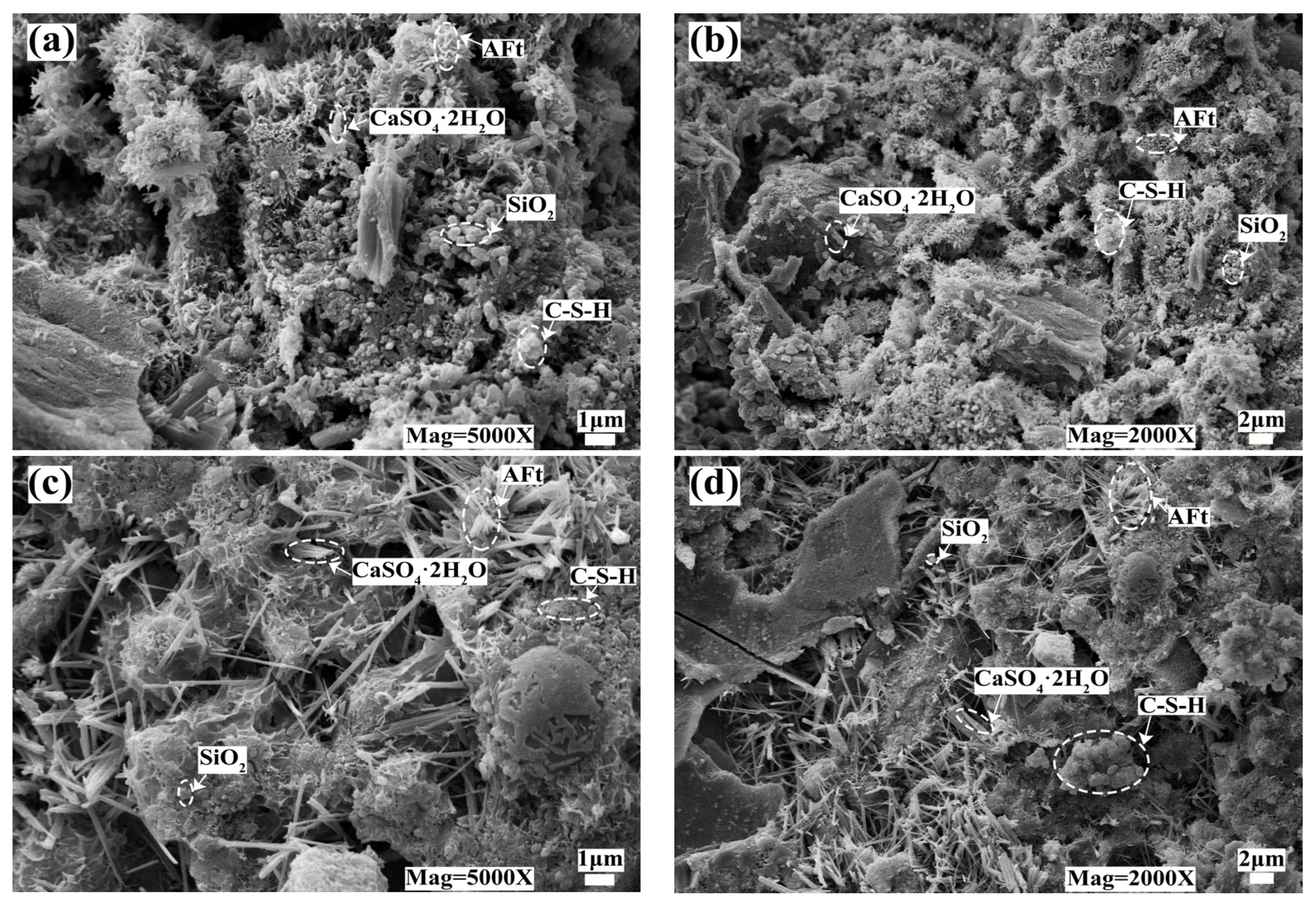
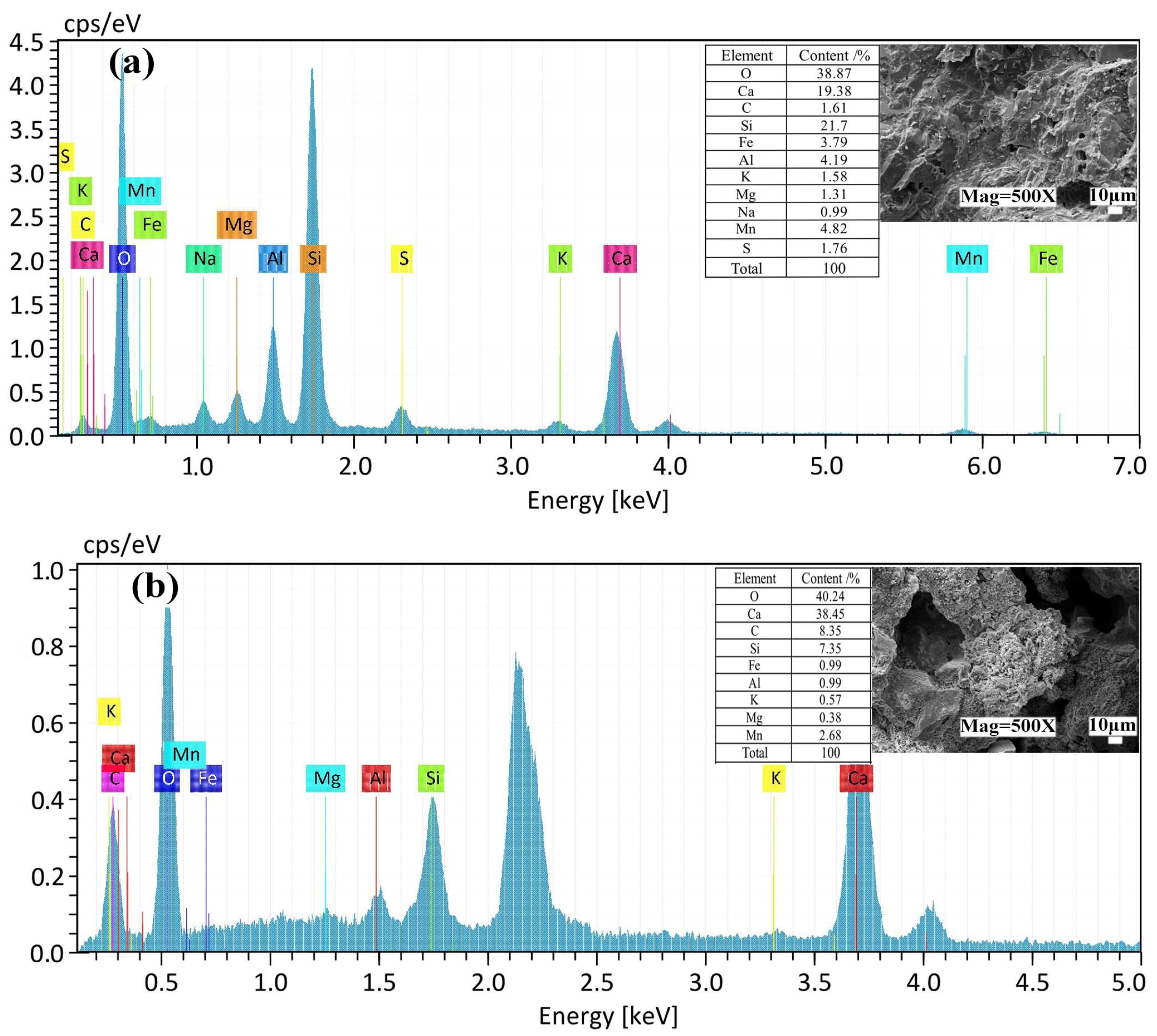
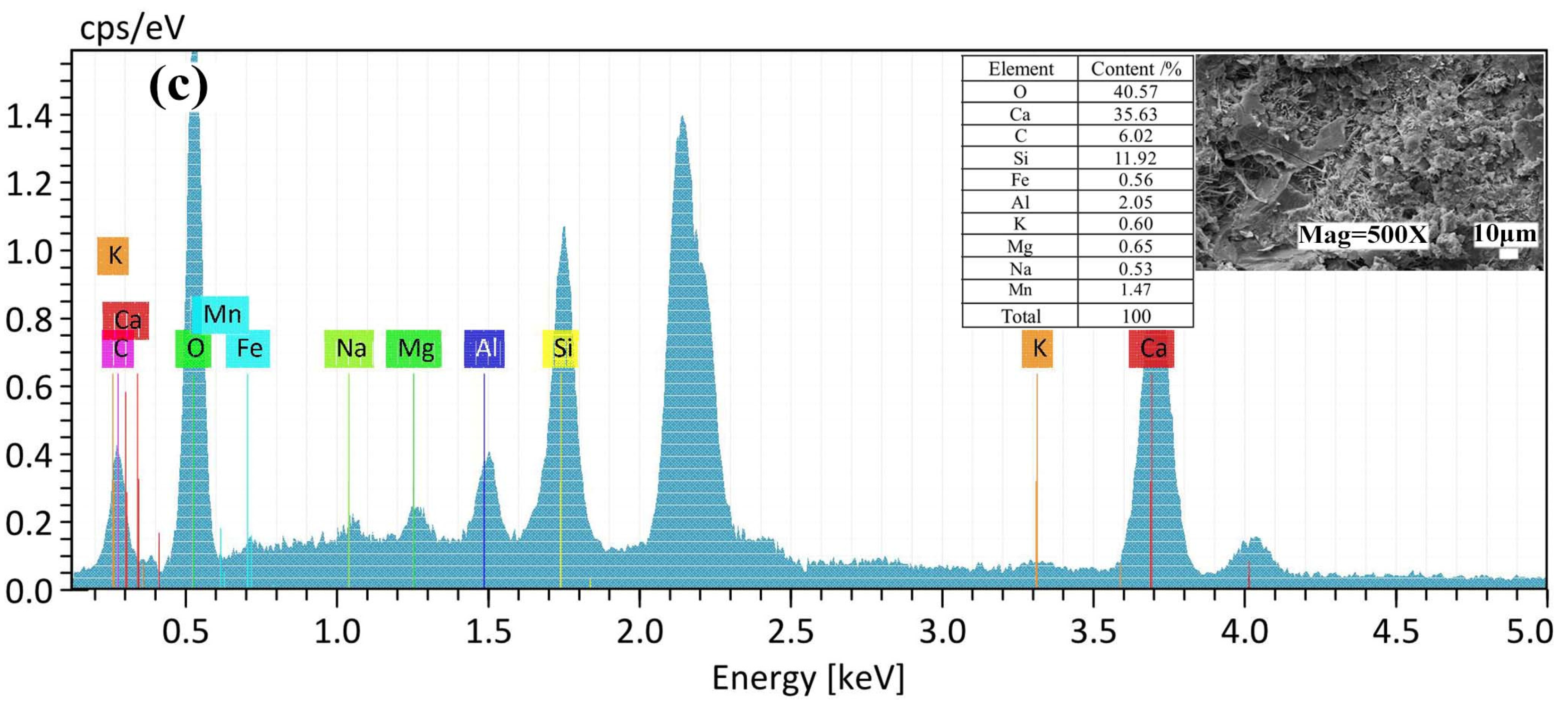
3.3.2. SEM-EDS Analysis
4. Conclusions
- (1)
- DMR, prepared by high-temperature and high-pressure desulfurization and deamination calcination, has a solidified body mixed with cement that has good flexural and compressive strength. Under 80 mesh particle size, the increase in cement content can significantly improve the flexural strength and compressive strength of the solidified body at different ages. Under the condition of 30% cement content, DMR with different particle sizes has a great influence on the strength of the solidified body, and the solidified body formed by 30% cement with four mesh particle sizes will form stress concentration points, thus reducing the strength of the solidified body.
- (2)
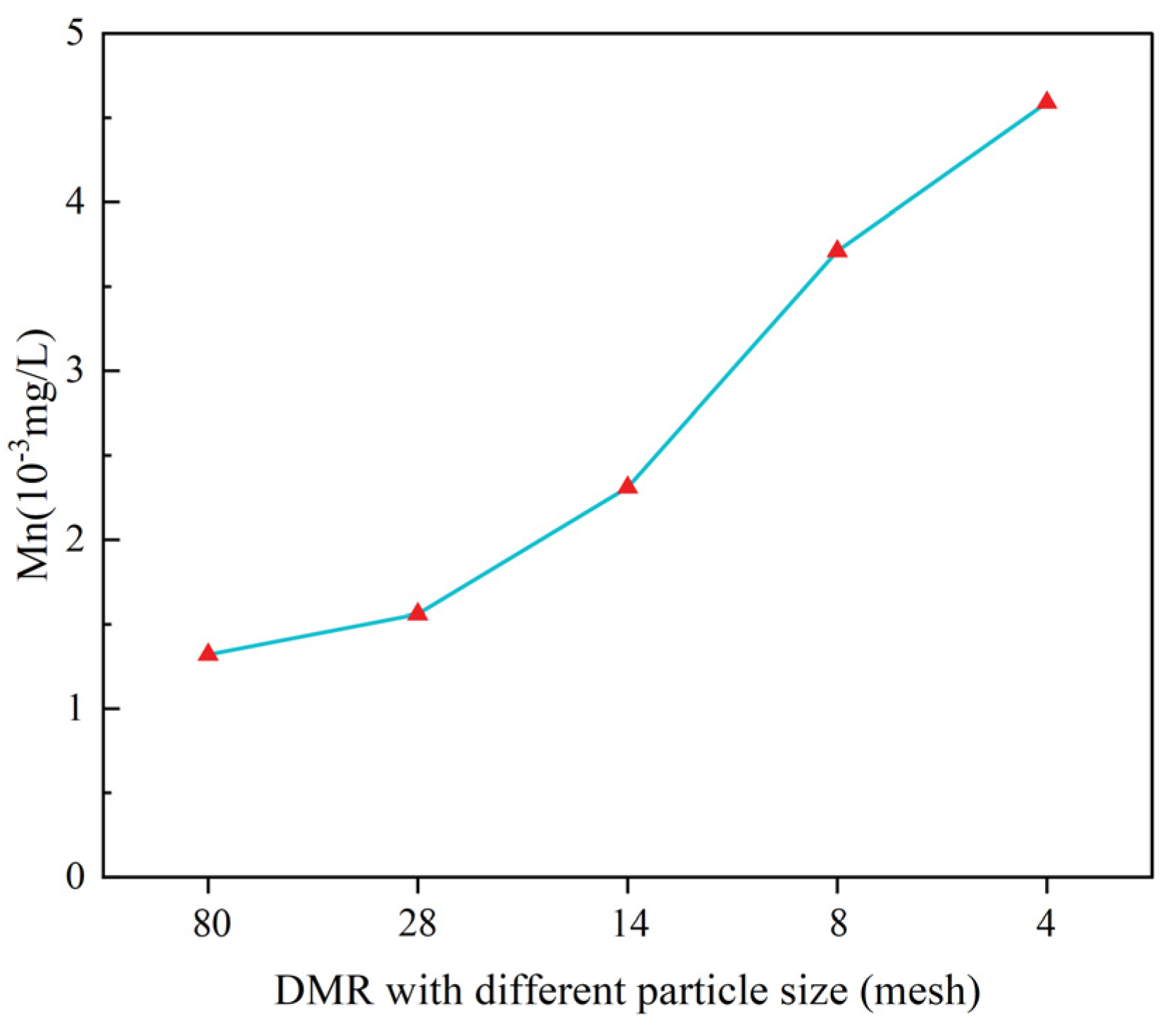
- Cement can effectively solidify the soluble Mn in DMR. With the increase in cement content, the Mn in DMR can be further removed. The solidification rate of Mn in a cement-DMR-solidified body with 10% cement content is 99.8%. The increase in particle size has little negative impact on the solidification of Mn, but it can still meet the Class I emission standard in GB8978-1996.
- (3)
- C-S-H gel is formed by the cement hydration reaction, and DMR and cement can provide enough Al elements. Under the alkaline environment provided by the cement hydration reaction, the main substances of DMR, dihydrate gypsum (CaSO4·2H2O) and quartz (SiO2), react to form ettringite (AFt), and C-S-H gel and AFt cooperate to form a dense network structure, which improves the strength of the solidified body. In the alkaline environment formed by cement hydration, a small amount of soluble Mn in DMR finally forms MnO2, and some manganese is solidified in C-S-H gel through isomorphic transformation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.M.; Dreisinger, D.; Glück, T. Manganese electrodeposition—A literature review. Hydrometallurgy 2014, 141, 105–116. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Wang, L.A.; Wang, L.; Gong, J.; Wang, X.; Song, X.; Xu, T.T. Co-sintering MSWI fly ash with electrolytic manganese residue and coal fly ash for lightweight ceramisite. Chemosphere 2021, 263, 127914. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.C.; Chen, M.J.; Wu, H.P.; Li, B.B.; Wang, B.; Li, B.; Liu, R.L.; Liu, Z.H. An innovative method for synergistic stabilization/solidification of Mn2+, NH4+-N, PO43− and F− in electrolytic manganese residue and phosphogypsum. J. Hazard. Mater. 2019, 376, 212–222. [Google Scholar] [CrossRef]
- Shu, J.C.; Liu, R.L.; Liu, Z.H.; Chen, H.L.; Tao, C.Y. Simultaneous removal of ammonia and manganese from electrolytic metal manganese residue leachate using phosphate salt. J. Clean. Prod. 2016, 135, 468–475. [Google Scholar] [CrossRef]
- He, D.J.; Shu, J.C.; Zeng, X.F.; Wei, Y.F.; Chen, M.J.; Tan, D.Y.; Liang, Q. Synergistic solidification/stabilization of electrolytic manganese residue and carbide slag. Sci. Total. Environ. 2022, 810, 152175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, P.; Li, J.X.; Lv, Y.; Ye, H.P.; Li, S.; Du, D.Y. Synthesis of electrolytic manganese residue-fly ash based geopolymers with high compressive strength. Constr. Build. Mater. 2020, 248, 118489. [Google Scholar] [CrossRef]
- He, D.J.; Shu, J.C.; Wang, R.; Chen, M.J.; Wang, R.; Gao, Y.S.; Liu, R.L.; Liu, Z.H.; Xu, Z.H.; Tan, D.Y.; et al. A critical review on approaches for electrolytic manganese residue treatment and disposal technology: Reduction, pretreatment, and reuse. J. Hazard. Mater. 2021, 418, 126235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X. Reusing electrolytic manganese residue as an activator: The effect of calcination on its mineralogy and activity. Constr. Build. Mater. 2021, 294, 123533. [Google Scholar] [CrossRef]
- Wang, Y.G.; Gao, S.; Liu, X.M.; Tang, B.W.; Mukiza, E.; Zhang, N.A. Preparation of non-sintered permeable bricks using electrolytic manganese residue: Environmental and NH3-N recovery benefits. J. Hazard. Mater. 2019, 378, 120768. [Google Scholar] [CrossRef]
- Lan, J.R.; Sun, Y.; Huang, P.; Du, Y.G.; Zhan, W.; Zhang, T.C.; Du, D.Y. Using Electrolytic Manganese Residue to prepare novel nanocomposite catalysts for efficient degradation of Azo Dyes in Fenton-like processes. Chemosphere 2020, 252, 126487. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, H.; Luo, T.; Wang, H.J.; Hou, H.B. Pure water leaching soluble manganese from electrolytic manganese residue: Leaching kinetics model analysis and characterization. J. Environ. Chem. Eng. 2020, 8, 103916. [Google Scholar] [CrossRef]
- Shu, J.C.; Cai, L.H.; Zhao, J.J.; Feng, H.; Chen, M.; Zhang, X.R.; Wu, H.P.; Yang, Y.; Liu, R.L. A low cost of phosphate-based binder for Mn2+ and NH4+-N simultaneous stabilization in electrolytic manganese residue. Ecotox. Environ. Saf. 2020, 205, 111317. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Wang, D.Q.; Wang, H.; An, M.Z.; Wang, Q. Occurrence and leaching behaviors of heavy-metal elements in metallurgical slags. Constr. Build. Mater. 2022, 330, 127268. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, H.; Wang, S.; Xue, J.R.; Zhang, Z.Y. A novel conversion process for waste residue: Synthesis of zeolite from electrolytic manganese residue and its application to the removal of heavy metals. Colloid. Surface. A 2015, 470, 258–267. [Google Scholar] [CrossRef]
- Li, J.; Du, D.Y.; Peng, Q.J.; Wu, C.J.; Lv, K.L.; Ye, H.P.; Chen, S.H.; Zhan, W. Activation of silicon in the electrolytic manganese residue by mechanical grinding-roasting. J. Clean. Prod. 2018, 192, 347–353. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ren, Y.Y.; Zhang, Z.Q.; Liu, X.M.; Wang, Y.G. Harmless treatment of electrolytic manganese residue: Ammonia nitrogen recovery, preparation of struvite and nonsintered bricks. Chem. Eng. J. 2023, 455, 140739. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, X.W.; Long, K.Q.; Shen, C.N.; Yang, Y.; Liu, J.L. Using the Biocarbonization of Reactive Magnesia to Cure Electrolytic Manganese Residue. Geomicrobiol. J. 2021, 38, 709–718. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Wang, L.A.; Wang, L.; Wang, X.; Gong, J.; Yang, L.; Bai, J.S. Enhanced geopolymeric co-disposal efficiency of heavy metals from MSWI fly ash and electrolytic manganese residue using complex alkaline and calcining pre-treatment. Waste Manag. 2019, 98, 135–143. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Liu, X.M.; Xu, Y.T.; Tang, B.W.; Wang, Y.G. Preparation of road base material by utilizing electrolytic manganese residue based on Si-Al structure: Mechanical properties and Mn2+ stabilization/solidification characterization. J. Hazard. Mater. 2020, 390, 122188. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.C.; Bai, M.; Wang, J.L.; Yang, Z.H.; Zhou, X.; Zhou, J.L. Cleaner and safer disposal of electrolytic manganese residues in cement-based materials using direct electric curing. J. Clean. Prod. 2022, 356, 131842. [Google Scholar] [CrossRef]
- Zhang, S.H.; Li, X.D.; Zhang, W.; Fan, F.; Wang, Y.Z. Study on the leaching toxicity and performance of manganese slag-based cementitious materials. Mater. Res. Express. 2021, 8, 125308. [Google Scholar] [CrossRef]
- Chen, M.; Wei, J.M.; Jia, L.P.; Yao, Q.Q.; Chen, Y.S. Study on Solidification Treatment of Electrolytic Manganese Slag and Numerical Simulation of Slope Stability. Geotech. Geol. Eng. 2021, 40, 1201–1212. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Zhang, Y.; He, W.L.; Yang, J.J.; Wang, Y. Study on mutual harmless treatment of electrolytic manganese residue and red mud. Environ. Sci. Pollut. Res. 2023, 30, 59660–59675. [Google Scholar] [CrossRef]
- Shu, J.C.; Wu, H.P.; Liu, R.L.; Liu, Z.H.; Li, B.; Chen, M.J.; Tao, C.Y. Simultaneous stabilization/solidification of Mn2+ and NH4+-N from electrolytic manganese residue using MgO and different phosphate resource. Ecotox. Environ. Saf. 2018, 148, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.R.; Zhang, S.S.; Mei, T.; Dong, Y.Q.; Hou, H.B. Mechanochemical modification of electrolytic manganese residue: Ammonium nitrogen recycling, heavy metal solidification, and baking-free brick preparation. J. Clean. Prod. 2021, 329, 129727. [Google Scholar] [CrossRef]
- Shu, J.C.; Li, B.; Chen, M.J.; Sun, D.Y.; Wei, L.; Wang, Y.; Wang, J.Y. An innovative method for manganese (Mn2+) and ammonia nitrogen (NH4+-N) stabilization/solidification in electrolytic manganese residue by basic burning raw material. Chemosphere 2020, 253, 126896. [Google Scholar] [CrossRef]
- Shu, J.C.; Liu, R.L.; Liu, Z.H.; Chen, H.L.; Du, J.; Tao, C.Y. Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO. J. Hazard. Mater. 2016, 317, 267–274. [Google Scholar] [CrossRef]
- Wang, D.Q.; Wang, Q.; Xue, J.F. Reuse of hazardous electrolytic manganese residue: Detailed leaching characterization and novel application as a cementitious material. Resour. Conserv. Recycl. 2020, 154, 104645. [Google Scholar] [CrossRef]
- Wang, D.Q.; Wang, Q. Clarifying and quantifying the immobilization capacity of cement pastes on heavy metals. Cement. Concrete. Rec. 2022, 161, 106945. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Liu, X.M.; Xu, Y.T.; Tang, B.W.; Wang, Y.G.; Mukiza, E. Synergic effects of electrolytic manganese residue-red mud-carbide slag on the road base strength and durability properties. Constr. Build. Mater. 2019, 220, 364–374. [Google Scholar] [CrossRef]
- Fang, X.J.; Wang, Z.; Qian, J.S.; Jiang, X.H.; Hou, P.K. On the solidification of electrolytic manganese residue with cement and its leaching toxicity. J. Saf. Environ. 2010, 10, 46–49. (In Chinese) [Google Scholar]
- GB/T 17671-2021. Test Method of Cement Mortar Strength (ISO Method). National Standardization Management Committee, People’s Republic of China: Beijing, China, 2021. (In Chinese)
- HJ 557-2010. Solid Waste—Extraction Procedure for Leaching Toxicity—Horizontal Vibration Method. Ministry of Environmental Protection, People’s Republic of China: Beijing, China, 2010. (In Chinese)
- Zhang, L.F.; Yang, L.; Hao, Z.H. Study on the effect of manganese slag admixture on the properties of cement mortar. Appl. Chem. Ind. 2021, 50, 2164–2167. (In Chinese) [Google Scholar] [CrossRef]
- Wang, F.; Long, G.C.; Bai, M.; Wang, J.L.; Zhou, J.L.; Zhou, X. Application of electrolytic manganese residues in cement products through pozzolanic activity motivation and calcination. J. Clean. Prod. 2022, 338, 130629. [Google Scholar] [CrossRef]
- Wang, D.Q.; Fang, J.R.; Wang, Q.; Liu, Y.J. Utilizing desulphurized electrolytic-manganese residue as a mineral admixture: A feasibility study. Cement. Concrete. Comp. 2022, 134, 104822. [Google Scholar] [CrossRef]
- GB 8978-1996. Integrated Wastewater Discharge Standard. National Environmental Protection Agency, People’s Republic of China: Beijing, China, 1996. (In Chinese)
- Singh, M.; Garg, M. Activation of gypsum anhydrite-slag mixtures. Cement. Concrete. Res. 1995, 25, 332–338. [Google Scholar] [CrossRef]
- Ren, J.; Hu, L.; Dong, Z.J.; Tang, L.P.; Xing, F.; Liu, J. Effect of silica fume on the mechanical property and hydration characteristic of alkali-activated municipal solid waste incinerator (MSWI) fly ash. J. Clean. Prod. 2021, 295, 126317. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, N.; Yao, Y.; Sun, H.H.; Feng, H. Micro-structural characterization of the hydration products of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2013, 262, 428–438. [Google Scholar] [CrossRef]
- Liu, S.H.; Ouyang, J.Y.; Ren, J. Mechanism of calcination modification of phosphogypsum and its effect on the hydration properties of phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Montañés, M.T.; Sánchez-Tovar, R.; Roux, M.S. The effectiveness of the stabilization/solidification process on the leachability and toxicity of the tannery sludge chromium. J. Environ. Manag. 2014, 143, 71–79. [Google Scholar] [CrossRef]


| Composition | SiO2 | CaO | Al2O3 | MnO | Fe2O3 | MgO | SO3 | Na2O | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| DMR | 40.22 | 31.14 | 7.98 | 6.63 | 6.41 | 1.89 | 1.36 | 1.31 | 1.25 | 1.81 |
| P.O 42.5 | 22.35 | 59.22 | 6.49 | - | 2.73 | 4.01 | 2.22 | - | - | 1.78 |
| Test Elements | Leaching Mass Concentration/(mg·L−1) | GB8978-1996/(mg·L−1) |
|---|---|---|
| Mn | 2.8 | 2.0 |
| Pb | 0.003 | 1.0 |
| Ni | 0.015 | 1.0 |
| Cu | 0.004 | 1.0 |
| Zn | 0.013 | 5.0 |
| Se | 0.009 | 0.2 |
| As | 0.175 | 0.5 |
| P | 0.026 | 0.3 |
| Cd | 0.0001 | 0.1 |
| Cr | 0.0009 | 1.5 |
| NH4-N | N.D 1 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, F.; Che, J.; Ma, L. Study on the Performance and Mechanism of Cement Solidified Desulfurization Manganese Residue. Materials 2023, 16, 4184. https://doi.org/10.3390/ma16114184
Wang S, Wang F, Che J, Ma L. Study on the Performance and Mechanism of Cement Solidified Desulfurization Manganese Residue. Materials. 2023; 16(11):4184. https://doi.org/10.3390/ma16114184
Chicago/Turabian StyleWang, Shicheng, Fang Wang, Jialing Che, and Lihua Ma. 2023. "Study on the Performance and Mechanism of Cement Solidified Desulfurization Manganese Residue" Materials 16, no. 11: 4184. https://doi.org/10.3390/ma16114184
APA StyleWang, S., Wang, F., Che, J., & Ma, L. (2023). Study on the Performance and Mechanism of Cement Solidified Desulfurization Manganese Residue. Materials, 16(11), 4184. https://doi.org/10.3390/ma16114184









