Preparation and Micromechanics of Red Sandstone–Phosphogypsum–Cement Composite Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
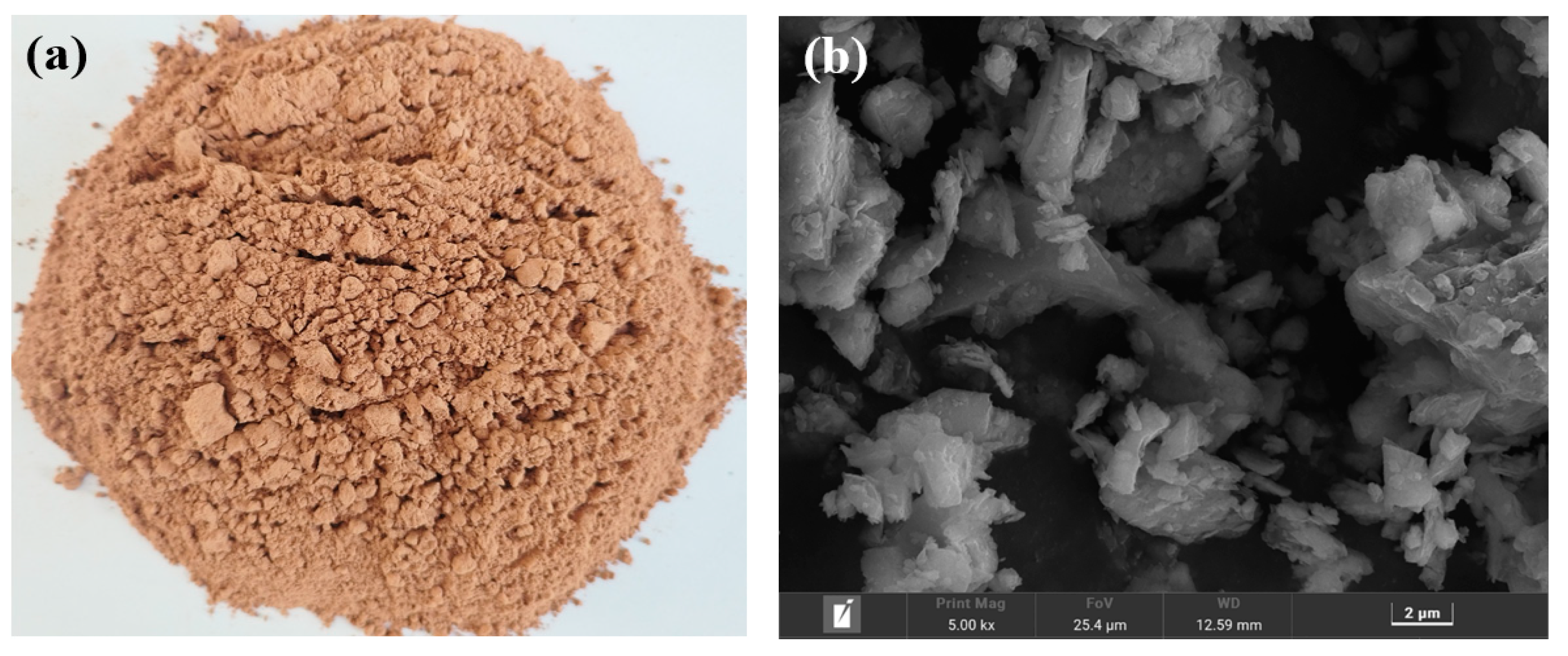
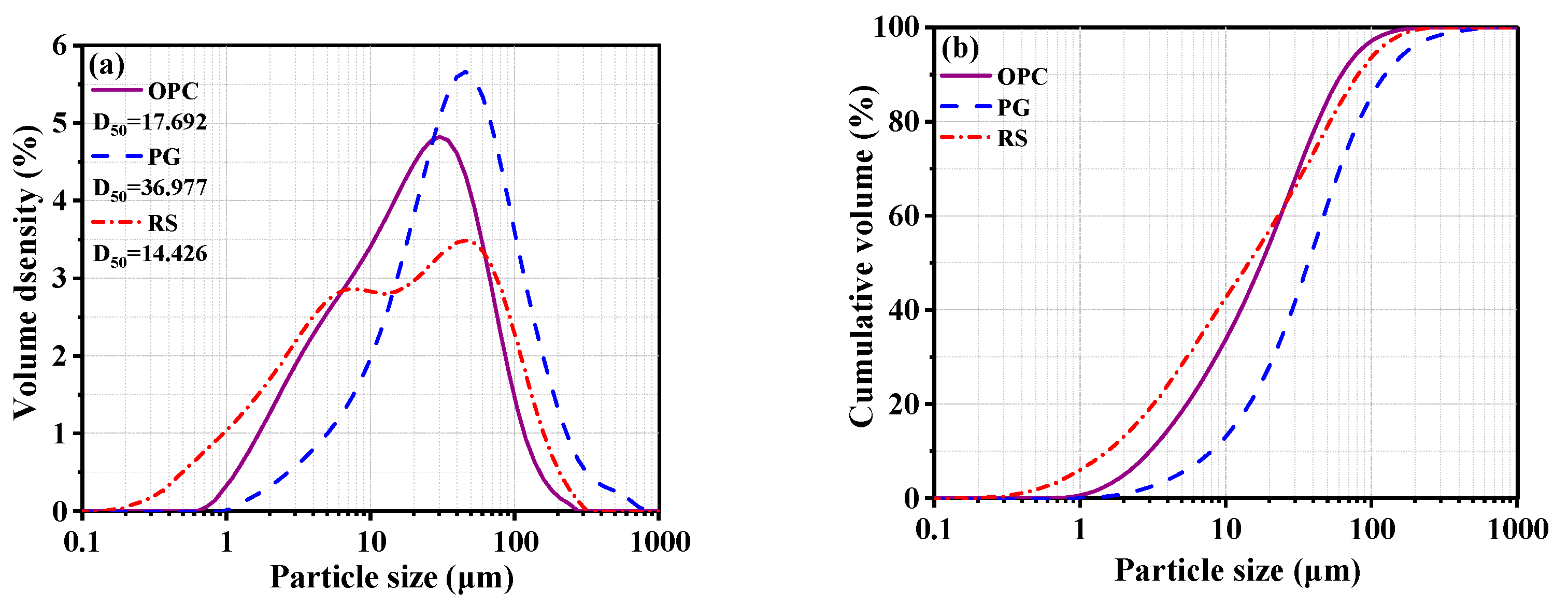
2.1. Raw Materials
2.2. Mixture Proportion
2.3. Preparation of the Samples
2.4. Testing Methods
2.4.1. Flowability
2.4.2. Mechanical Strength Test
2.5. Characterization Methods
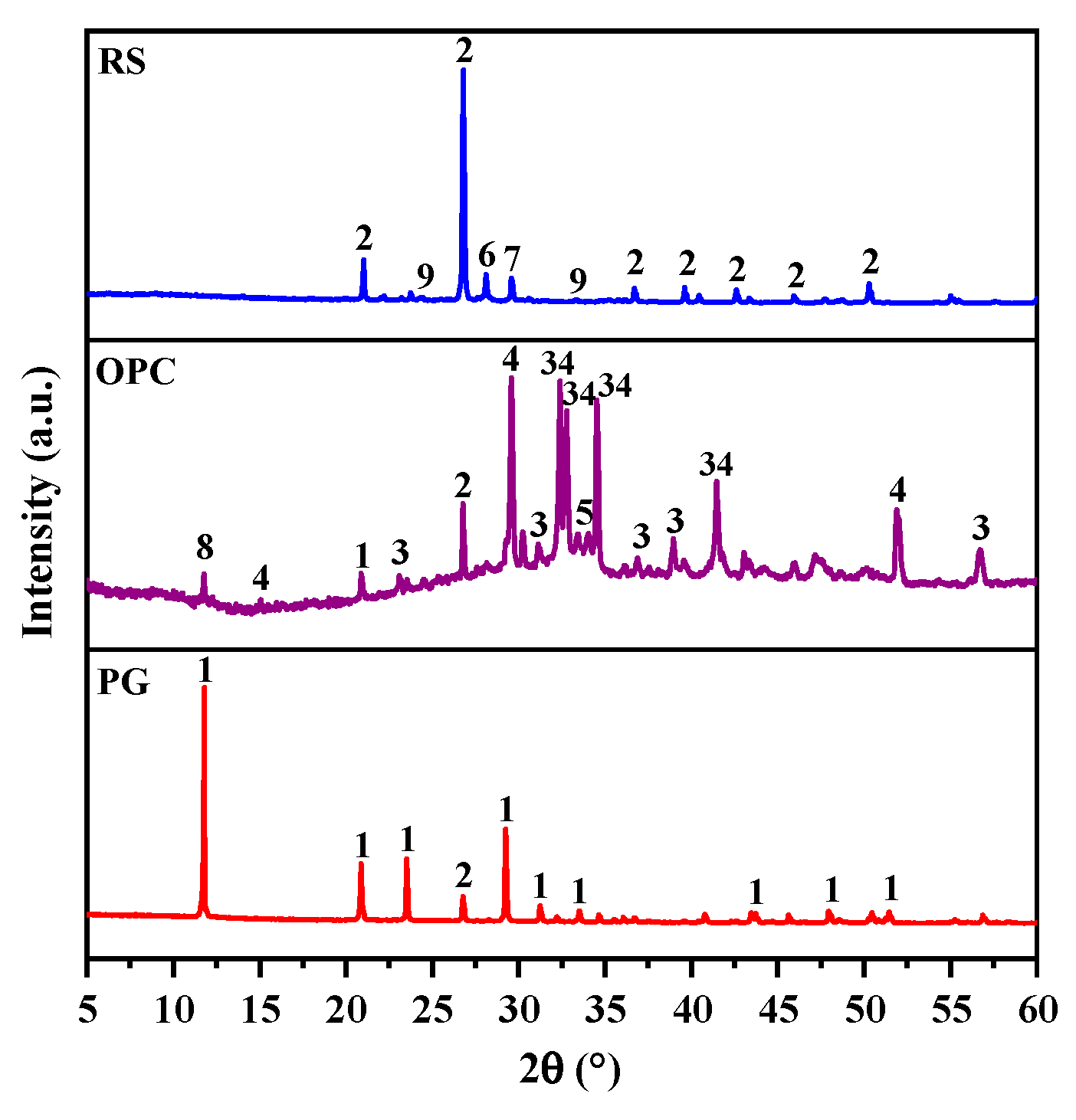
2.5.1. X-ray Diffractometry (XRD)
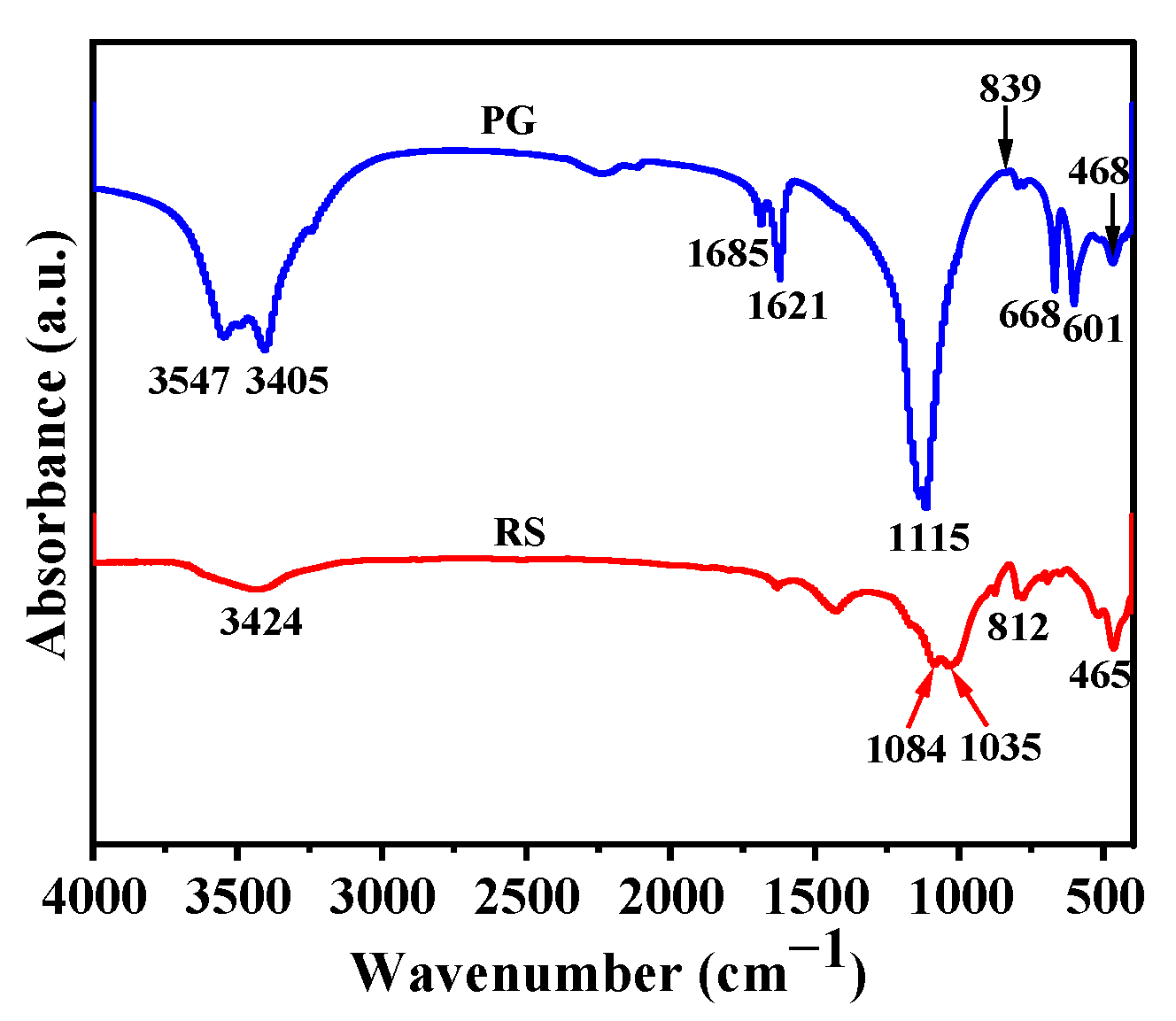
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.3. Thermal Analysis (TG-DTG)
2.5.4. Scanning Electron Microscopy (SEM)
3. Results and Discussion
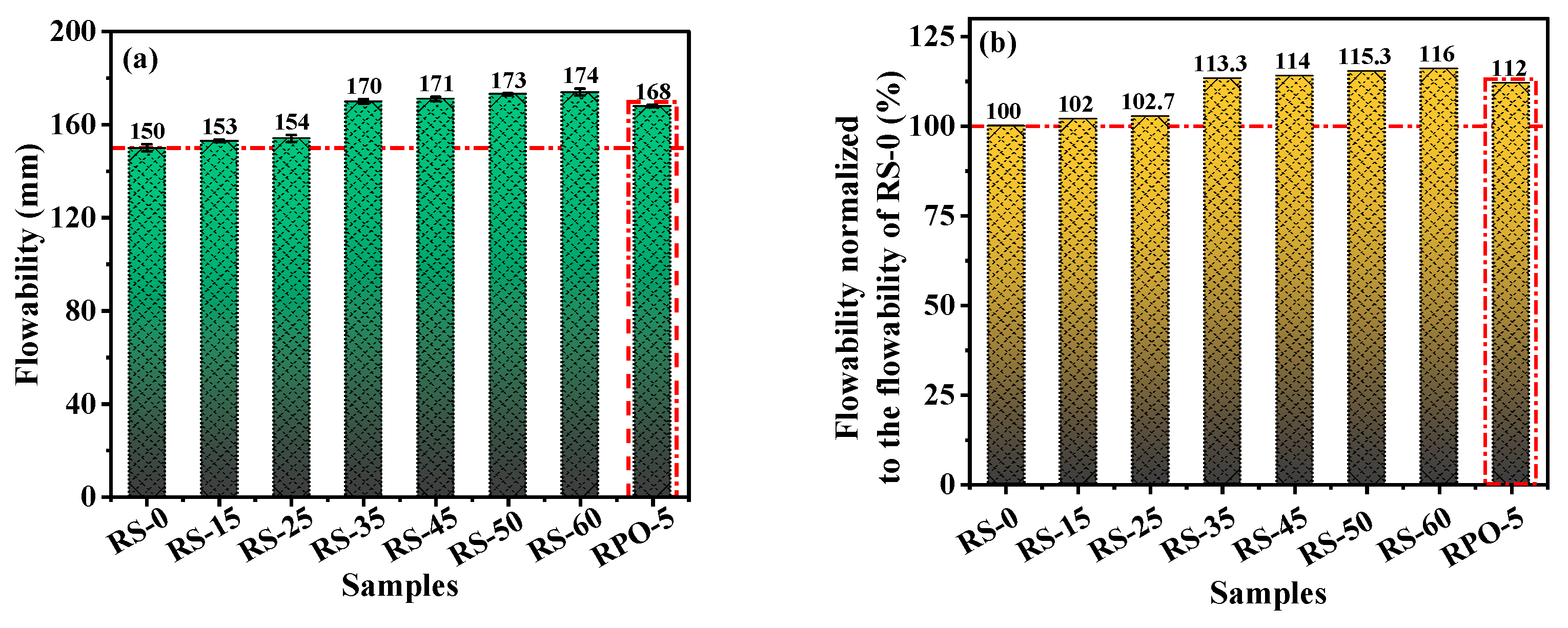
3.1. Flowability
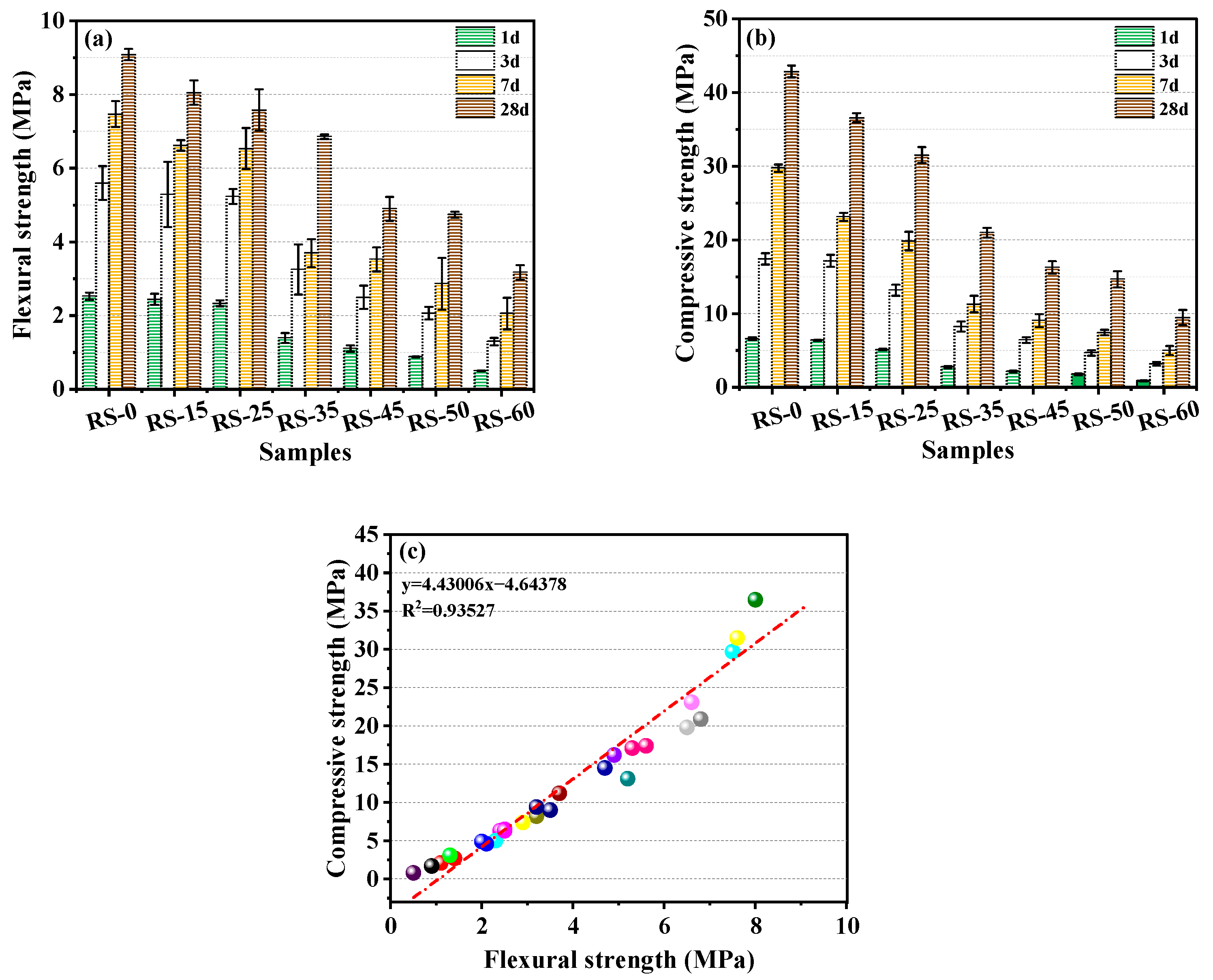
3.2. Effect of Different RS Replacement Amounts on the Mechanical Strength of RS-OPC
3.3. Microscopic Mechanisms of RS-OPC with Different Amounts of RS
3.3.1. XRD Analysis
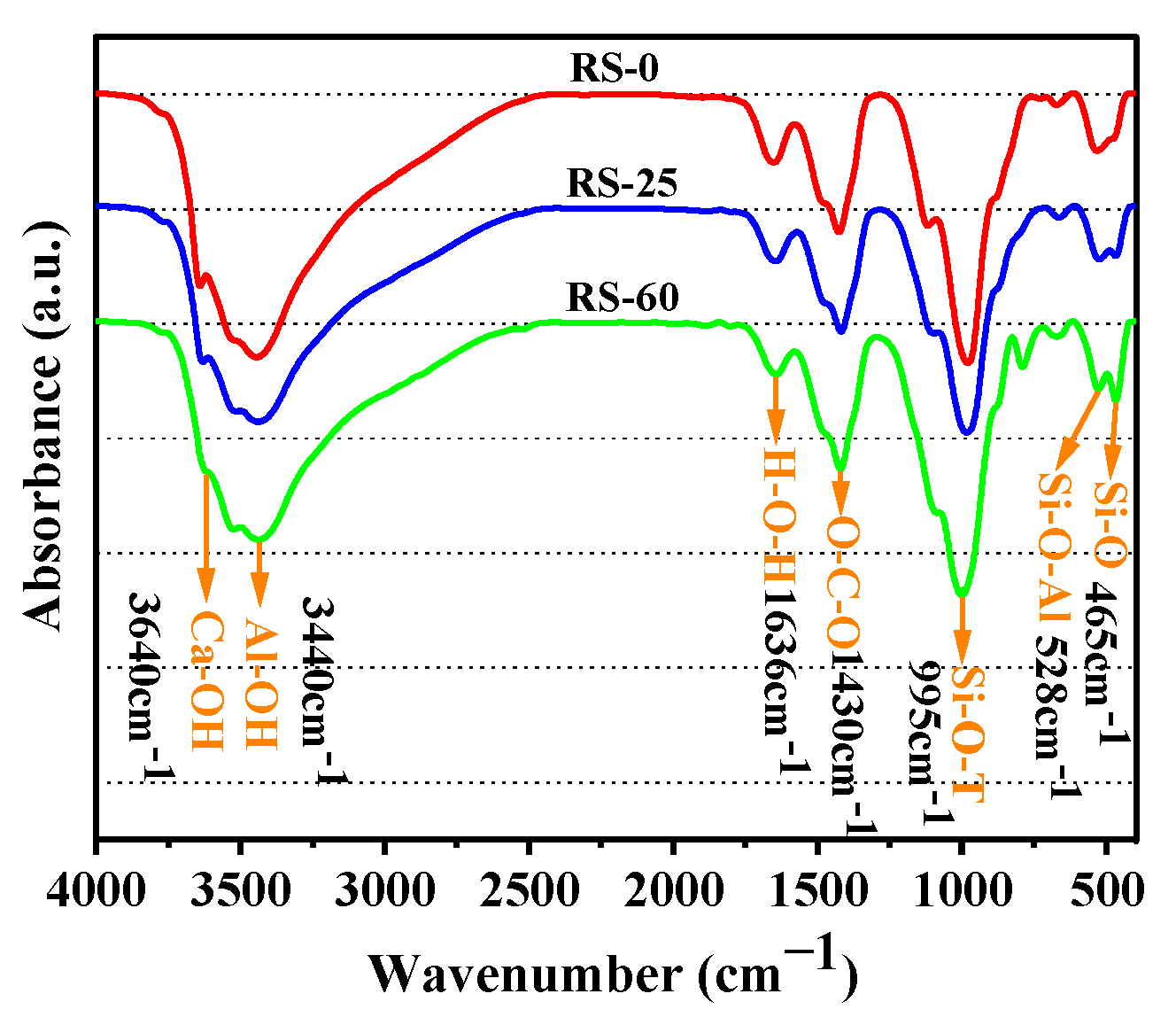
3.3.2. FTIR Analysis
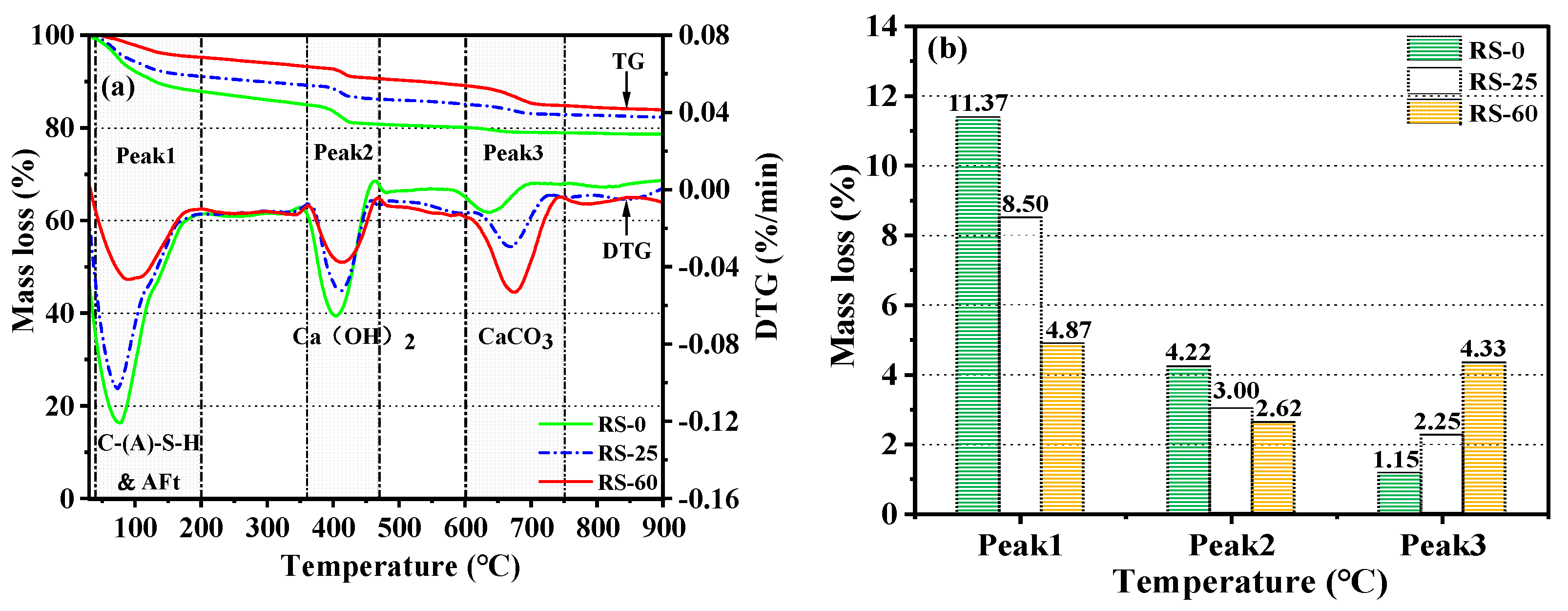
3.3.3. TG-DTG Analysis
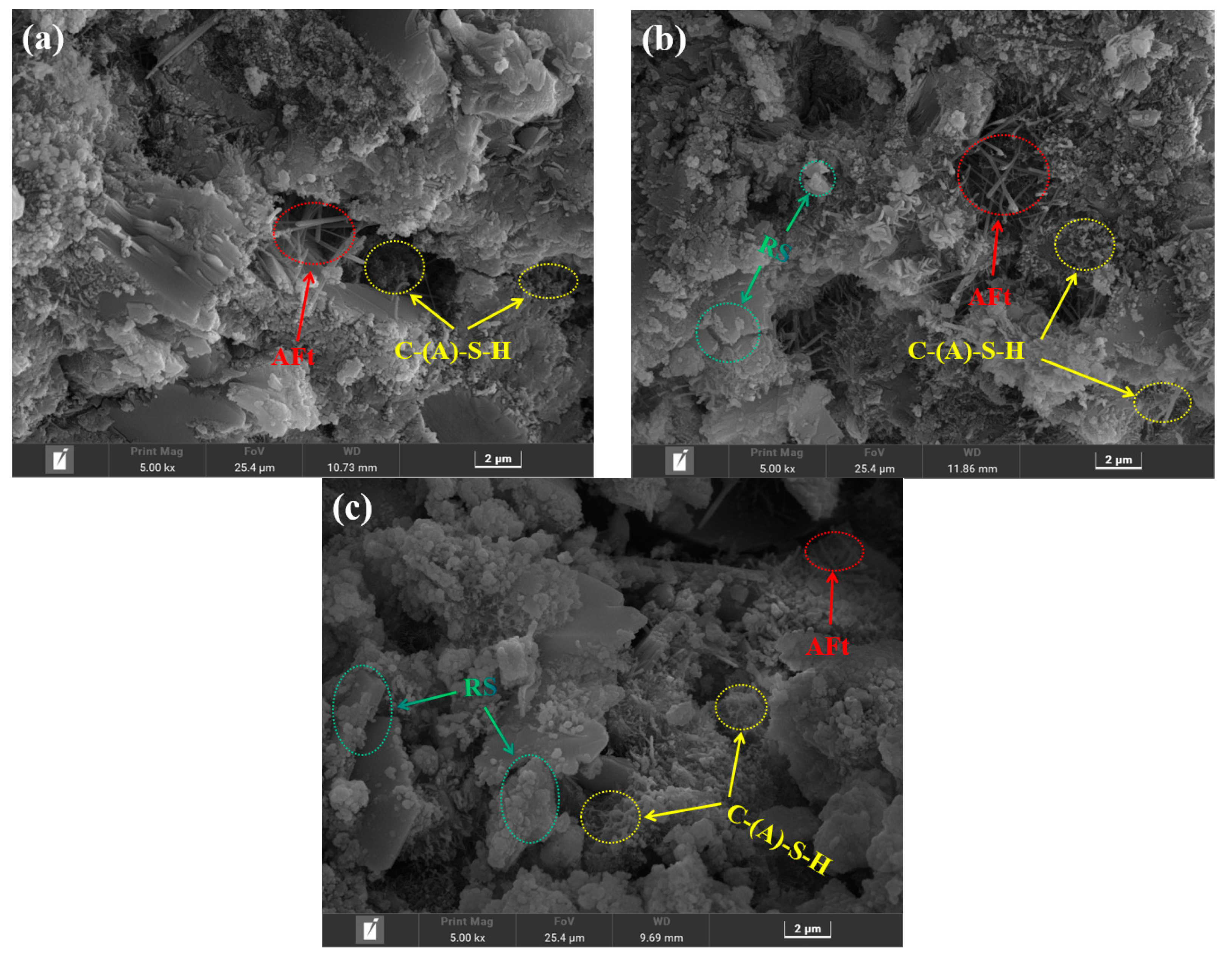
3.3.4. SEM Analysis
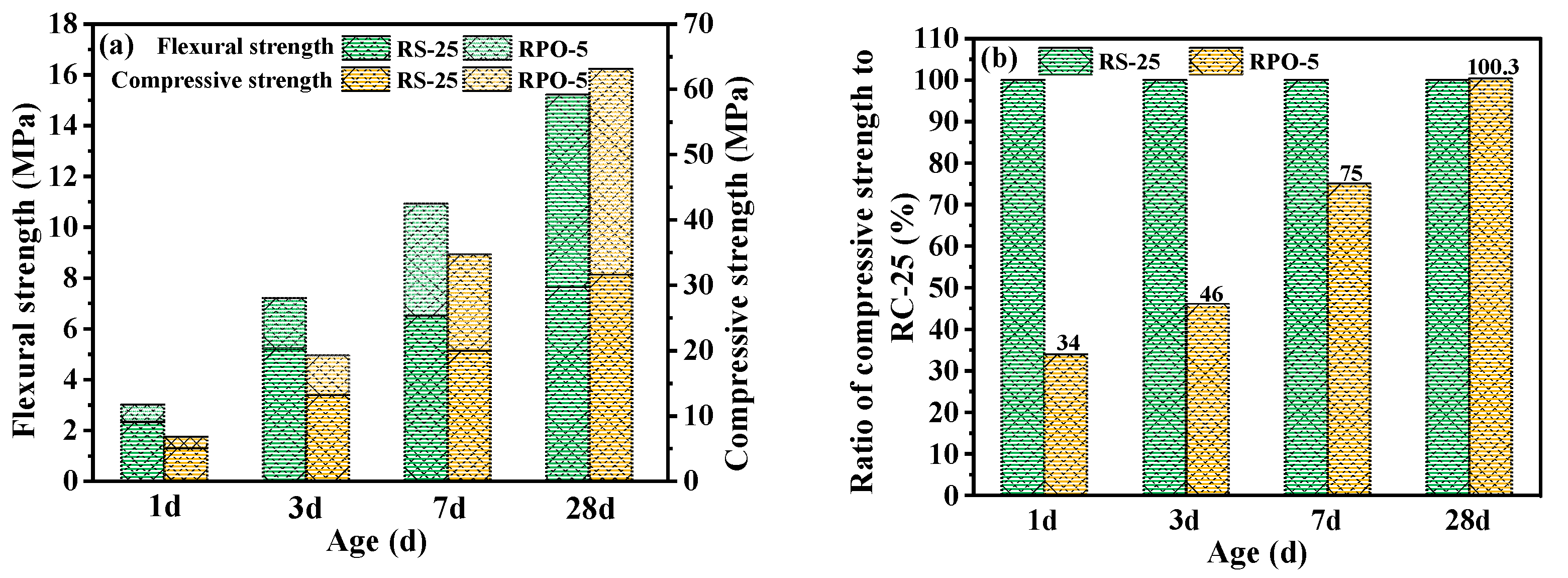
3.4. Effect of PG on the RS-PG-OPC’s Mechanical Strength
3.5. Effect of PG on the RS-PG-OPC’s Microscopic Mechanisms
3.5.1. XRD Analysis
3.5.2. FTIR Analysis
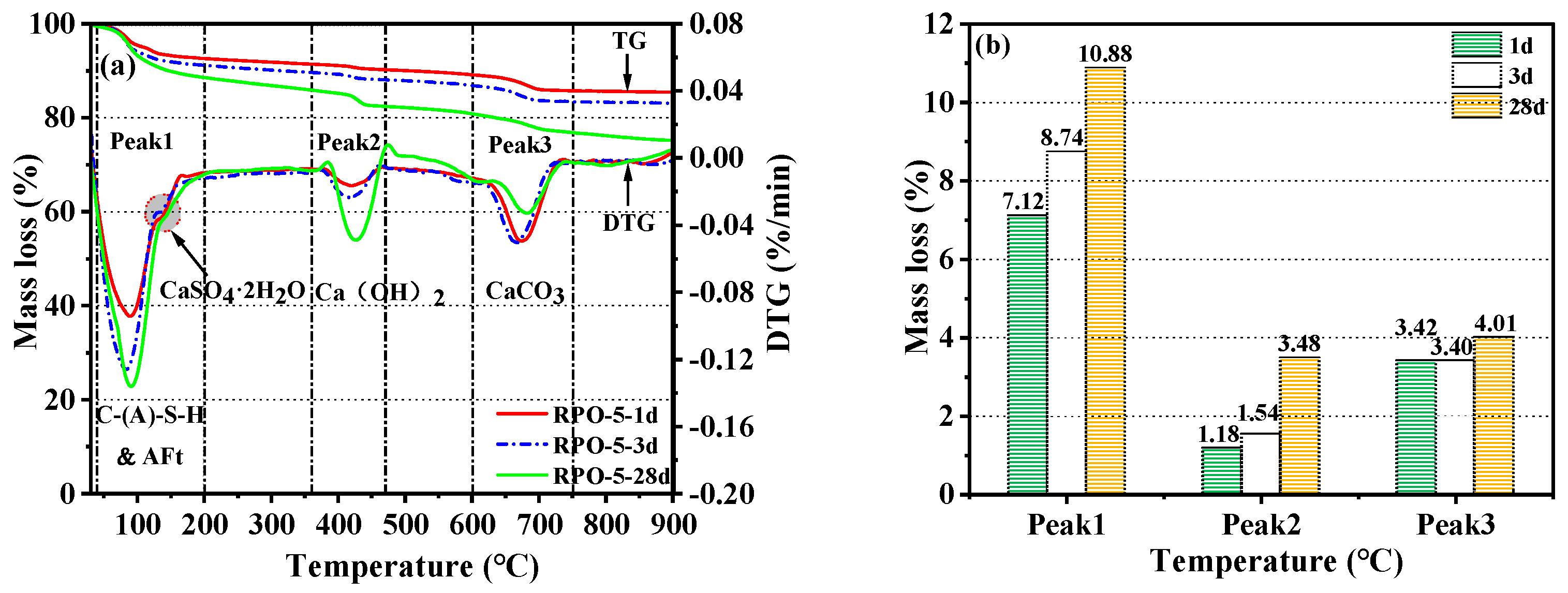
3.5.3. TG-DTG Analysis
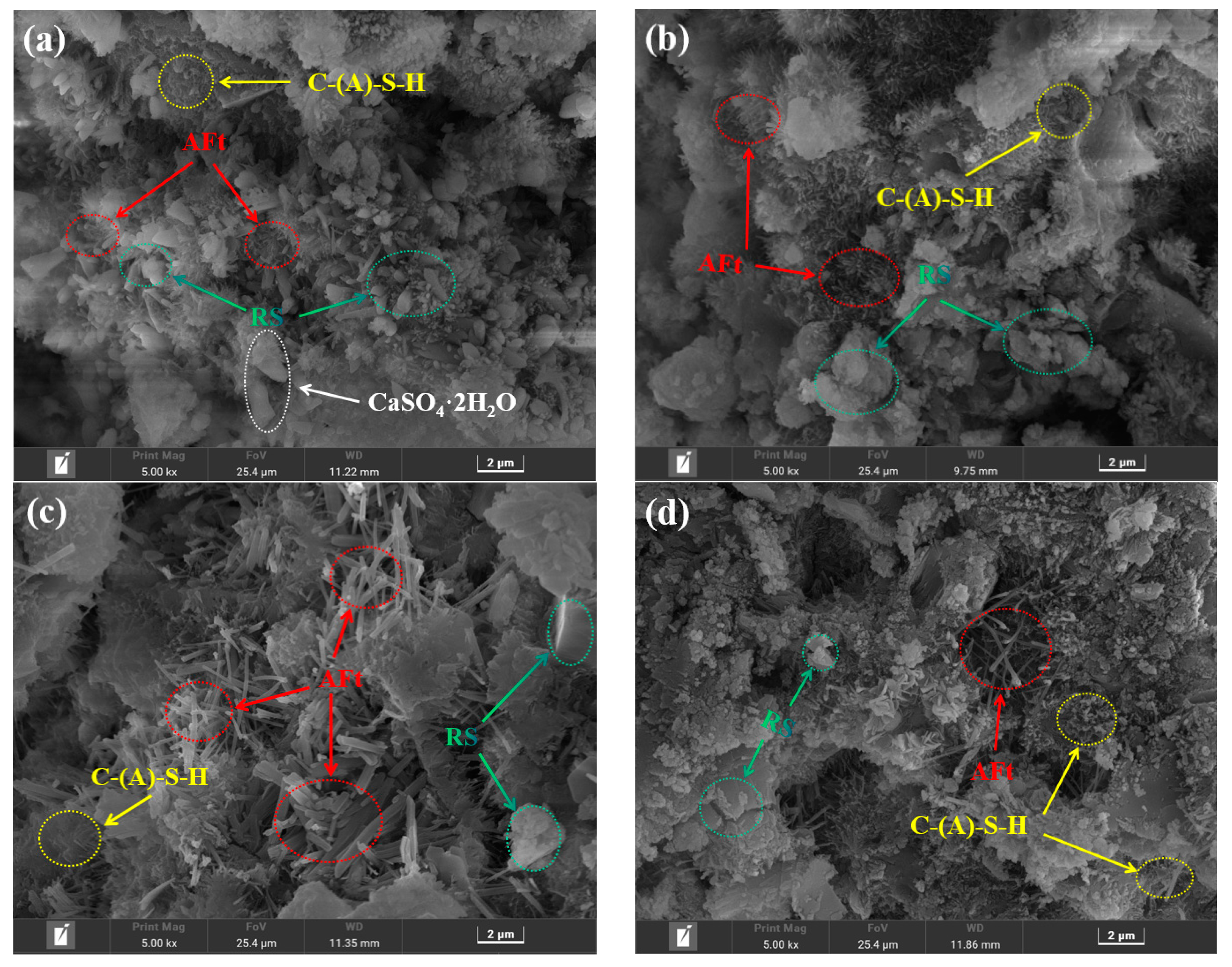
3.5.4. SEM Analysis
4. Conclusions
- RS could increase the flowability of RS-OPC but diminished its mechanical strength. When the proportion of RS exceeded 25%, the RS-OPC’s mechanical strength decreased considerably. On the other hand, 5% of PG improved the flowability and promoted the development of the mechanical strength of the RS-PG-OPC at a later stage.
- Ca(OH)2, the hydration product of OPC, was capable of secondary hydration reactions with active SiO2 in RS to form C-S-H gels, but the amount of Ca(OH)2 that could be consumed was extremely limited, indicating that RS had some volcanic ash activity, although the level of activity was low, and that RS primarily demonstrated dilution and filling effects.
- Compared with RS-OPC without PG doping, the addition of PG delayed the hydration reaction of RS-PG-OPC, but it facilitated the ensuing development of strength. Specifically, the addition of PG introduced enough Ca2+ and SO42− into the system, which could react with [Al(OH)6]3− to form Aft, and reduced the concentration of Al3+ in the system, therefore promoting the dissolution of RS and stimulating the volcanic ash activity of RS to produce more C-(A)-S-H gels and AFt, and consuming part of the Ca(OH)2, which improved the late strength of the cured slurry.
- If there is a large amount of waste RS in the area and there is a lack of other cementitious additives, a composite cementitious material can be prepared using an appropriate amount of PG to activate the RS powder that is ground finely enough to partially replace the OPC. This would reduce the extraction of raw materials for OPC while expanding the secondary use of waste RS and PG, which would be advantageous in terms of raw materials, economy, and ecology. In addition, it is necessary to continue to explore other environment-friendly and cost-effective methods of RS activation in future research in this area. In addition, the feasibility of specific applications of this cement, such as using this material as a binder for bedding concrete, sidewalk and road base materials with relatively low strength requirements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y. Revisiting the technology of red sandstone highway pavement construction. Hunan Comm. Sci. Technol. 2011, 37, 24–27+56. [Google Scholar]
- Wang, X.B.; Yang, J.H. Study on physical and mechanical properties of red sandstone and its roadbed compaction work. J. Zhejiang Univ. Sci. Technol. 2022, 34, 551–558. [Google Scholar]
- Jiang, J.Q. Research on Mechanical Behaviours and Seismic Stability of Reinforced Red-Sandstone Granular Soil Retaining Wall. Ph.D. Thesis, Central South University, Changsha, China, 2010. [Google Scholar]
- Zhou, Y. Study on Mechanical Properties of Cement Stabilized Sandstone Crushed Stone Mixture. Master’s Thesis, Chongqing Jiaotong University, Chongqing, China, 2018. [Google Scholar]
- He, Z.; Hu, L.L.; Li, Y.; Hu, J.; Shao, Y.X. Use of sandstone powder as a mineral additive for concrete. Constr. Build. Mater. 2018, 186, 276–286. [Google Scholar] [CrossRef]
- Li, C.M.; Zhang, T.T.; Wang, L.J. Preparation of geopolymeric materials by Pisha sandstone. J. Build. Mater. 2016, 19, 373–378. [Google Scholar]
- Chen, F.L.; Wen, Y.B.; Shan, J.H.; Wei, J.; Zhou, M.K.; Gao, P. Influence of red sandstone aggregate on strength and softening resistance performance of cement stabilized base. Bull. Chin. Ceram. Soc. 2022, 41, 3138–3147. [Google Scholar]
- Yang, F.Y.; Li, H.B.; Zhao, G.J.; Guo, P.; Li, W.B. Mechanical Performance and Durability Evaluation of Sandstone Concrete. Adv. Mater. Sci. Eng. 2020, 2020, 2417496. [Google Scholar] [CrossRef]
- Li, H.Y.; Luo, G.L.; Zhong, W. Exploration of the feasibility of direct filling of red sandstone in the sub-bed of highways. Anhui Archit. 2022, 29, 129–130. [Google Scholar]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioff, R.; Cappelletto, E.; Di Maggio, R. Thermally treated clay sediments as geopolymer source materials. Appl. Clay. Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Li, C.M.; Zhang, T.T.; Wang, L.J. Pozzolanic activity of pisha sandstone and mechanical properties of alkali-activated pisha sandstone materials. J. Chin. Ceram. Soc. 2015, 43, 1090–1098. [Google Scholar]
- Li, C.M.; Zhang, T.T.; Wang, L.J. Effect of dosage of fly ash and NaOH on the properties of alkali activated pisha sandstone-based mortar. Aci. Mater. J. 2016, 113, 173–183. [Google Scholar]
- Li, C.M.; Dong, J.L.; Zhao, S.B.; Liu, H.; Yao, W.Y.; Wang, L.J. Development of low cost supplementary cementitious materials utilizing thermally activated Pisha sandstone. Constr. Build. Mater. 2018, 174, 484–495. [Google Scholar] [CrossRef]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Cao, Y.B.; Wang, Y.R.; Zhang, Z.H.; Ma, Y.W.; Wang, H. Turning sandstone clay into supplementary cementitious material: Activation and pozzolanic reactivity evaluation. Compos. Part B Eng. 2021, 223, 109137. [Google Scholar] [CrossRef]
- Essaidi, N.; Samet, B.; Baklouti, S.; Rossignol, S. Feasibility of producing geopolymers from two different Tunisian clays before and after calcination at various temperatures. Appl. Clay. Sci. 2014, 88–89, 221–227. [Google Scholar] [CrossRef]
- Selmani, S.; Essaidi, N.; Gouny, F.; Bouaziz, S.; Joussein, E.; Driss, A.; Sdiri, A.; Rossignol, S. Physical–chemical characterization of Tunisian clays for the synthesis of geopolymers materials. J. Afr. Earth. Sci. 2015, 103, 113–120. [Google Scholar] [CrossRef]
- Xin, J.G.; Pei, Q.; Chang, P.P.; Tang, X.H. A Kind of Forty-Two point five Ordinary Low Alkali Silicate Cement Containing Sandstone and Its Preparation Method. China CN112250327A, 22 January 2021. [Google Scholar]
- Deng, H.H. A Kind of Sandstone Cement Formulation. China CN109851250A, 7 June 2019. [Google Scholar]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under theffects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, D.R.; You, L.Y.; Luo, H.; Xu, W. Recycling phosphogypsum in subbase of pavement: Treatment, testing, and application. Constr. Build. Mater. 2022, 342, 127948. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, L.; He, X.Y.; Su, Y.; Miao, W.J.; Strnadel, B.H. Hydration and rheology of activated ultra-fine ground granulated blast furnace slag with carbide slag and anhydrous phosphogypsum. Cem. Concr. Compos. 2022, 133, 104727. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.Y.; He, X.Y.; Zhang, Y.N.; Su, Y.; Tan, H.B. Sustainable clinker-free solid waste binder produced from wet-ground granulated blast-furnace slag, phosphogypsum and carbide slag. Constr. Build. Mater. 2022, 330, 127218. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Zhang, B.L.; Shen, W.G.; Wu, M.M.; Guan, Y.C.; Wu, J.L.; Zhang, Z.; Zhu, J.Q. High industrial solid waste road base course binder: Performance regulation, hydration characteristics and practical application. J. Clean. Prod. 2021, 313, 127879. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.S. Investigation on phosphogypsum–steel slag–granulated blast-furnace slag–limestone cement. Constr. Build. Mater. 2010, 24, 1296–1301. [Google Scholar] [CrossRef]
- Zhang, J.G.; Huang, Z.W.; Su, X.D.; Chen, Y.; Li, H.J.; Li, W. Effect of phosphogypsum addition on properties of red mud- phosphogypsum composites. China Nonferrous Metall. 2023, 52, 121–130. [Google Scholar]
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixture. China Standards Press: Beijing, China, 2012.
- GB/T 17671−2021; Test Method of Cement Mortar Strength (ISO Method). China Standards Press: Beijing, China, 2021.
- Wang, Z.Y.; Shui, Z.H.; Sun, T.; Li, X.S.; Zhang, M.Z. Recycling utilization of phosphogypsum in eco excess-sulphate cement: Synergistic effects of metakaolin and slag additives on hydration, strength and microstructure. J. Clean. Prod. 2022, 358, 131901. [Google Scholar] [CrossRef]
- Li, M.; Peng, J.H.; Zhang, H.; Zhang, J.X.; Liu, X.F. Influence of P2O5 in crystal lattice on gypsum properties and its mechanisms. Adv. Eng. Sci. 2012, 44, 200–204. [Google Scholar]
- Sun, T.; Ge, K.Y.; Wang, G.M.; Geng, H.N.; Shui, Z.H.; Cheng, S.K.; Chen, M. Comparing pozzolanic activity from thermal-activated water-washed and coal-series kaolin in Portland cement mortar. Constr. Build. Mater. 2019, 227, 117092. [Google Scholar] [CrossRef]
- Shi, C.J.; Wang, D.H.; Jia, H.F.; Liu, J.H. Role of limestone powder and its effect on durability of cement-based materials. J. Chin. Ceram. Soc. 2017, 45, 1582–1593. [Google Scholar]
- Zhu, C.F.; Tan, H.B.; Du, C.; Wang, J.; Deng, X.F.; Zheng, Z.Q.; He, X.Y. Enhancement of ultra-fine slag on compressive strength of solid waste-based cementitious materials: Towards low carbon emissions. J. Build. Eng. 2023, 63, 105475. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Guo, Y.F.; Peng, T. Correlation analysis of compressive strength and flexural strength of cement mortar. J. North China Univ. Sci. Technol. (Nat. Sci. Ed.) 2018, 40, 59–63. [Google Scholar]
- Ma, L.L.; Zhang, X.; Liu, F.; Liu, Z.F.; Li, F. Strength characteristics and mechanism of red mud-fly ash stabilized coal gangue base. J. Build. Mater. 2022, 1–15. [Google Scholar]
- Zhang, W.; Hao, X.S.; Wei, C.; Liu, X.M.; Zhang, Z.Q. Activation of low-activity calcium silicate in converter steelmaking slag based on synergy of multiple solid wastes in cementitious material. Constr. Build. Mater. 2022, 351, 128925. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.R.; Zhou, X.; Li, Y.; Wang, Y.G.; Xue, Y.; Liu, X.M. Circulating fluidized bed fly ash based multi-solid wastes road base materials: Hydration characteristics and utilization of SO3 and f -CaO. J. Clean. Prod. 2021, 316, 128355. [Google Scholar] [CrossRef]
- Li, Z.P.; Liu, X.M.; Li, Y.; Ren, Y.Y.; Wang, Y.G.; Zhang, W. Effects of sulfate on the mechanical performances and hydration characteristics of red mud based non-burnt brick. Constr. Build. Mater. 2020, 262, 120722. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhao, X.B.; Yin, H.F.; Chen, J.L.; Zhang, N. Intermediate-calcium based cementitious materials prepared by MSWI fly ash and other solid wastes: Hydration characteristics and heavy metals solidification behavior. J. Hazard. Mater. 2018, 349, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ni, W.; Huang, X.Y.; Ma, X.M.; Li, D.Z. Characteristics of hydration and hardening of red mud of bayer process in carbide slag-flue gas desulfurization gypsum system. Mater. Rep. 2016, 30, 120–124. [Google Scholar]
- Zhang, N.; Liu, X.M.; Sun, H.H.; Li, L.T. Pozzolanic behaviour of compound-activated red mud-coal gangue mixture. Cem. Concr. Res. 2010, 41, 270–278. [Google Scholar] [CrossRef]
- Huo, B.B.; Li, B.L.; Chen, C.; Zhang, Y.M. Surface etching and early age hydration mechanisms of steel slag powder with formic acid. Constr. Build. Mater. 2021, 280, 122500. [Google Scholar] [CrossRef]
- Hao, X.S.; Liu, X.M.; Zhang, Z.Q.; Zhang, W.; Lu, Y.; Wang, Y.G.; Yang, T.Y. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Clean. Prod. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Xu, C.W.; Ni, W.; Li, K.Q.; Zhang, S.Q.; Xu, D. Activation mechanisms of three types of industrial by-product gypsums on steel slag–granulated blast furnace slag-based binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.M.; Li, Z.P.; Ren, Y.Y.; Wang, Y.G.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, X.M.; Tang, B.W.; Li, Y.; Zhang, W.; Xue, Y. Effect of Ca/(Si + Al) on red mud based eco-friendly revetment block: Microstructure, durability and environmental performance. Constr. Build. Mater. 2021, 304, 124618. [Google Scholar] [CrossRef]
- Ma, F.Y.; Chen, L.L.; Lin, Z.W.; Liu, Z.; Zhang, W.C.; Guo, R.X. Microstructure and Key Properties of Phosphogypsum-Red Mud-Slag Composite Cementitious Materials. Materials 2022, 15, 6096. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.G. Research on the Modification of Phosphogypsum and Its Application as a Cement Retarder. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2005. [Google Scholar]
- Wang, C.Q.; Chen, S.; Huang, D.M.; Huang, Q.C.; Li, X.Q.; Shui, Z.H. Safe environmentally friendly reuse of red mud modified phosphogypsum composite cementitious material. Constr. Build. Mater. 2023, 368, 130348. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Qu, X.T.; Guo, Z.C. An environmental-friendly method for recovery of soluble sodium and harmless utilization of red mud: Solidification, separation, and mechanism. Resour. Conserv. Recycl. 2022, 186, 106543. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Gao, Y.; Liu, C.; Qi, Y. Effect of different gypsums on the workability and mechanical properties of red mud-slag based grouting materials. J. Clean. Prod. 2020, 245, 118759. [Google Scholar] [CrossRef]
- Li, X.; Ruhan, A.; Yan, P. Research on hydration degree of cement-fly ash complex binders. J. Build. Mater. 2010, 13, 584–588. [Google Scholar]
- Zhang, Y.L.; Liu, X.M.; Xu, Y.T.; Tang, B.W.; Wang, Y.; Mukiza, E. Synergic effects of electrolytic manganese residue-red mud-carbide slag on the road base strength and durability properties. Constr. Build. Mater. 2019, 220, 364–374. [Google Scholar] [CrossRef]



| Materials | CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | Na2O | K2O | P2O5 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| RS | 9.33 | 70.80 | 9.35 | 0.03 | 3.93 | 2.07 | 1.63 | 1.78 | 0.69 | 0.39 |
| PG | 37.88 | 10.54 | 0.45 | 49.97 | 0.18 | - | - | 0.13 | 0.12 | 0.73 |
| OPC | 61.13 | 19.88 | 5.49 | 4.23 | 3.36 | 3.11 | 0.29 | 0.80 | 0.18 | 1.53 |
| Samples | RS/% | OPC/% | PG/% | W/B |
|---|---|---|---|---|
| RS-0 | 0 | 100 | 0 | 0.5 |
| RS-15 | 15 | 85 | 0 | 0.5 |
| RS-25 | 25 | 75 | 0 | 0.5 |
| RS-35 | 35 | 65 | 0 | 0.5 |
| RS-45 | 45 | 55 | 0 | 0.5 |
| RS-50 | 50 | 50 | 0 | 0.5 |
| RS-60 | 60 | 40 | 0 | 0.5 |
| RPO-5 | 22.5 | 72.5 | 5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Zhou, B.; Guo, R.; Yan, F.; Wang, R.; Tang, C. Preparation and Micromechanics of Red Sandstone–Phosphogypsum–Cement Composite Cementitious Materials. Materials 2023, 16, 4549. https://doi.org/10.3390/ma16134549
Kong C, Zhou B, Guo R, Yan F, Wang R, Tang C. Preparation and Micromechanics of Red Sandstone–Phosphogypsum–Cement Composite Cementitious Materials. Materials. 2023; 16(13):4549. https://doi.org/10.3390/ma16134549
Chicago/Turabian StyleKong, Chuiyuan, Bin Zhou, Rongxin Guo, Feng Yan, Rui Wang, and Changxi Tang. 2023. "Preparation and Micromechanics of Red Sandstone–Phosphogypsum–Cement Composite Cementitious Materials" Materials 16, no. 13: 4549. https://doi.org/10.3390/ma16134549
APA StyleKong, C., Zhou, B., Guo, R., Yan, F., Wang, R., & Tang, C. (2023). Preparation and Micromechanics of Red Sandstone–Phosphogypsum–Cement Composite Cementitious Materials. Materials, 16(13), 4549. https://doi.org/10.3390/ma16134549






