Abstract
Lead hafnate (PbHfO3) has attracted a lot of renewed interest due to its potential as antiferroelectric (AFE) material for energy storage. However, its room temperature (RT) energy-storage performance has not been well established and no reports on the energy-storage feature of its high-temperature intermediate phase (IM) are available. In this work, high-quality PbHfO3 ceramics were prepared via the solid-state synthesis route. Based on high-temperature X-ray diffraction data, the IM of PbHfO3 was found to be orthorhombic, Imma space group, with antiparallel alignment of Pb2+ ions along the [001]cubic directions. The polarization–electric field (P–E) relation of PbHfO3 is displayed at RT as well as in the temperature range of the IM. A typical AFE loop revealed an optimal recoverable energy-storage density (Wrec) of 2.7 J/cm3, which is 286% higher than the reported data with an efficiency (η) of 65% at 235 kV/cm at RT. A relatively high Wrec value of 0.7 J/cm3 was found at 190 °C with an η of 89% at 65 kV/cm. These results demonstrate that PbHfO3 is a prototypical AFE from RT up to 200 °C, making it a suitable material for energy-storage applications in a wide temperature range.
1. Introduction
While ferroelectric (FE) materials with a parallel arrangement of dipoles in the polar directions have found a wide range of applications such as in transduction sensors, actuators, non-volatile memory, photovoltaics, etc., [,,] antiferroelectric (AFE) materials with an antiparallel alignment of adjacent dipoles are promising candidates for energy-storage applications due to their high-power density, good reliability, long working lifetime, high charge-discharge rate and excellent thermal stability [,,,]. The energy-storage capability of AFE arises from the electric-field-induced phase transition from the AFE state into an FE state, which is associated with the switching of antiparallel dipoles to parallel dipoles. Despite their great potential, antiferroelectric materials generally suffer from some serious drawbacks such as limited energy density, low dielectric breakdown strength (DBS) and high critical electric fields (Ecr) needed to induce the AFE–FE transition [,]. To overcome these issues many lead-based and lead-free solid solutions have been studied in the past few decades. Among them, the most widely explored AFE material is lead zirconate (PbZrO3).
Being a prototypical antiferroelectric material, PbZrO3 has been studied in terms of structure, phase transitions and electrical properties. It adopts an orthorhombic structure of Pbam space group at room temperature, with the octahedral tilting following the a−a−c0 scheme (according to the Glazer notation) []. On the other hand, lead hafnate (PbHfO3), which is isostructural to PbZrO3, has not been studied thoroughly. The room temperature symmetry of PbHfO3 was reported to be Pbam, which is analogous to PbZrO3. However, the structure and symmetry of the intermediate phase and its AFE properties remain under debate. Based on the X-ray diffraction pattern, Kupriyanov et al. proposed that the intermediate phase is polar, but is composed of a mixture of two symmetries, C2mm and P4mm []. On the other hand, Bosak et al. suggested a centrosymmetric orthorhombic symmetry with the Imma(00γ)s00 super space group which is linked to incommensurate modulations mainly due to the ordered lead displacements [].
For PbZrO3, it is difficult to realize the electric-field-induced AFE-to-FE phase transition at room temperature due to its extremely high Ecr which is greater than its DBS. Thus, substitutions of cations such as La and Sn in PbZrO3 were carried out to help not only in decreasing its Ecr and increasing its DBS, but also in realizing AFE double hysteresis loops, so as to increase its recoverable energy-storage density (Wrec), which reached 10.4 J/cm3 when the solid solution was prepared via the rolling process [,,]. However, for PbHfO3, no clear evidence for room-temperature and high-temperature double P–E loops and thus no energy-storage performance has been reported for conventionally prepared stoichiometric PbHfO3. Earlier, similar to PbZrO3, it was difficult to detect the electric-field-induced AFE-to-FE transition in PbHfO3 since its Ecr is larger than its DBS []. Later, the substitutions of various elements into the lattice of PbHfO3 led to the observation of double hysteresis loops upon reducing the material’s Ecr. Table 1 compares the key properties including recoverable energy density (Wrec), energy-storage efficiency (η) and dielectric breakdown strength (DBS) of lead hafnium and its solid solutions, along with their respective methods of preparation.

Table 1.
Summary of key properties including recoverable energy density (Wrec), energy-storage efficiency (η), dielectric breakdown strength (DBS) and methods of preparation of lead hafnium and lead hafnium-based solid solutions.
By analyzing Table 1 above, it is apparent that the Wrec of PbHfO3 varies depending on the method of preparation used. Gao et al. were not able to display the AFE double hysteresis loop for PbHfO3 at room temperature even at an applied electric field of around 80 kV/cm []. Wei et al. only obtained a very slim AFE loop with a weak Wrec of 0.7 J/cm3 at room temperature for PbHfO3 prepared via solid-state reaction. Nevertheless, a much higher Wrec, 7.6 J/cm3, was found for the ceramic prepared via the rolling process []. Recently, Ge et al. obtained a Wrec of 7.9 J/cm3 via the conventional solid-state method but they added an excess amount of PbO powder during the sample preparation []. The abovementioned studies present inconsistent values of Wrec at room temperature for PbHfO3, especially when the ceramic samples were prepared via the conventional solid-state reaction. However, due to the high volatilization of lead oxide at high temperatures, it is challenging to prepare high-density and pure-phase PbHfO3 experimentally. Moreover, the addition of excess PbO in the preparation could raise the question of maintaining stoichiometry and the concern over lead contamination. It is thus of foremost importance to assess the room temperature energy-storage performance of PbHfO3 stoichiometrically prepared via solid-state route, so as to design better PbHfO3-based materials with high Wrec for energy-storage applications.
On the other hand, as mentioned above, it was suggested that the intermediate phase of PbHfO3 is linked to an incommensurate structure; however, Holder et al. showed a nearly ferroelectric-like hysteresis loop for PbHfO3 single crystals in the temperature range between 197 °C and 204 °C of the intermediate phase []. These contradictory results raise the following questions: (i) If the intermediate phase were a centrosymmetric orthorhombic structure with the Imma(00γ)s00 super space group, how could a nearly ferroelectric-like P–E loop be displayed? (ii) What is the true nature of the intermediate phase? (iii) How are the Pb2+ ions arranged in the intermediate phase?
To answer these questions, the structure and properties of the prototypical AFE PbHfO3 are investigated systematically in this work. Highly dense stoichiometric PbHfO3 ceramics were prepared via the solid-state synthesis and sintering route. The crystal structure of the intermediate phase was refined and proved to be AFE with the Imma space group. The microstructure, energy-storage density, electric breakdown strength and temperature-dependent dielectric properties were studied. The results reveal that PbHfO3 exhibits AFE behavior with double hysteresis loops in both the room-temperature phase and in the intermediate phase with a high recoverable energy density, making pure PbHfO3 a viable material for energy-storage applications in a wide range of temperatures.
2. Materials and Methods
2.1. Experimental Procedure
In this work, PbHfO3 was prepared via the solid-state reaction method. Reagent-grade oxides of PbO (99.9%, Alfa Aesar, Ward Hill, MA, USA) and HfO2 (98%, Aldrich Chemistry, Milwaukee, WI, USA) were used as starting materials. All the raw materials were weighed and mixed according to their stoichiometric ratios. The mixed powders were hand-ground in the presence of ethanol for two hours. The slurry was dried at room temperature and pressed into pellets of 20 mm diameter under a pressure of 200 MPa. To prevent the volatilization of PbO at high temperatures, calcination was performed at 850 °C for 4 h via a double-crucible method []. In this method the pellets were placed on an alumina plate which was covered with a small Al2O3 crucible (the diameter of the crucible was large enough to cover all the pellets). Extra PbO powder was added on top of the small alumina crucible. The small alumina crucible with the pellets inside was then covered with another alumina crucible with a larger diameter. The extra PbO helped to provide a high PbO partial pressure at high temperatures and, thereby, inhibited the volatilization of PbO from the pellets. The calcined pellets were then crushed into powder using hand-grinding for 2 h. The dried powder was mixed with 5 wt% PVA as binder and pressed into pellets with a diameter of 10 mm and a thickness of about 1 mm. To obtain high-density ceramics, the pellets were sintered at 1100 °C for 4 h. During the sintering process, the pellets were submerged into sacrificial powder of the same composition so that the volatilization of PbO at high temperatures was further minimized. The sintered ceramics were pale white in color. To study the electrical properties, the ceramics were polished on the circular faces which were then covered with silver paste as electrodes. The samples were fired at 550 °C for 30 min to provide good ohmic contact.
The crystal structure, phase purity and lattice parameters were determined using X-ray powder diffraction (high resolution Bruker (Billerica, MA, USA), D8 Advance diffractometer with a copper Kα1 X-ray tube) on the fine powder obtained by crushing the as-sintered pellets. Helium ion beam microscopy was used to examine the surface morphology and microstructure of the as-sintered pellets (Zeiss ORION NanoFab, Jena, Germany). An acceleration voltage of 25 kV and an aperture of 10 μm were used to obtain an ion current of 0.153 pA. As the samples were non-conductive in nature, the sample was tilted to an angle of 0.14° to obtain clear and good quality images, and the charge compensation was achieved using the flood gun, together with a line averaging over 16 lines and a dwell time of 500 μs.
The dielectric permittivity was measured from room temperature to 300 °C using a Novocontrol Alpha high-resolution broadband dielectric spectrometer equipped with a temperature-controlled Novotherm HT furnace over a frequency range of 100 Hz to 1 MHz. The samples used to measure the dielectric properties had a thickness of approximately 0.8 mm. Polarization vs. electric field (P–E) hysteresis loops were displayed at 10 Hz on ceramic samples of a thickness of around 0.15 mm using a standard ferroelectric analyzer (Radiant RT66A Standard Ferroelectric Testing System (Radiant Technologies inc., Albuquerque, NM)) at room temperature, and the loops at high temperatures were measured using a DELTA 9023 furnace (Delta Design Inc., CA, USA).
2.2. Principles of Energy-Storage Capacitors
A capacitor typically consists of two electrically conductive plates separated by a dielectric layer. When the capacitor is charged, electrical energy is stored within the dielectric. The ability to store energy in a dielectric capacitor is dependent upon its capacitance (C) which can be expressed as [,]
where εr is the relative permittivity, ε0 is the permittivity of the vacuum (≈8.85 × 10−12 F m−1) and A and d are the area of the conducting plate and the distance between the two parallel plates, respectively. The charging process begins as soon as the dielectric capacitor is placed under an applied electric field, and it terminates when the potential generated by the accumulated charges (±Q) on the opposite plates is equal to the applied voltage (v). The capacitance, C, can be defined as the incremental change in charge with respect to applied voltage as
and the amount of electrostatic energy stored can be calculated as
Generally, the density of charges on the surface of the plates is equal to the electrical displacement (=εrε0E) in the dielectric material. Thus, the expression for energy stored per unit volume (stored energy density Wst) can be given as
which can be further rewritten as
where the electric field E is equal to v/d and Dmax is the electric displacement under the maximum applied electric field Emax.
For ferroelectric materials D = Polarization, P, so Equation (5) can be written as
or as
and the recoverable energy density is
where Pmax and Pr are the maximum polarization and remanent polarization, respectively.
3. Results and Analysis
3.1. Structural Analysis
The room temperature powder XRD pattern of an as-sintered PbHfO3 ceramic is presented in Figure 1a. Refinement of all the major peaks (marked by the ∇ symbol) with the help of GSAS2full 4801 software [] revealed that the compound crystalizes into a perovskite structure with an orthorhombic symmetry of the Pbam space group. No pyrochlore phase was observed. The visible low-intensity peaks identified by “♣” represent the ¼ (hkl) superlattice peaks. These superlattice peaks are characteristic of the antiferroelectric structure of PbHfO3, as they appear due to the antiparallel displacements of Pb2+ ions along the pseudocubic [110]pc directions [,,]. To better represent the superlattice peaks and to study the structural change more closely, the orthorhombic lattice setting of √2apc × 2√2apc × 2apc was converted into a pseudocubic lattice setting, apc × apc × apc, by using the following transformation matrix []:
where apc is the cell dimension of the para-electric cubic phase.
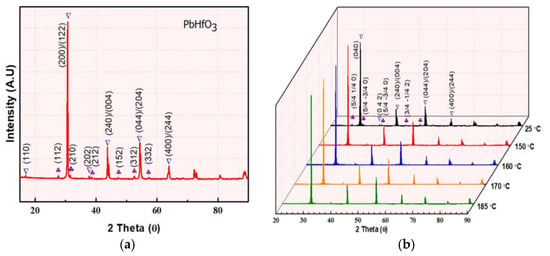
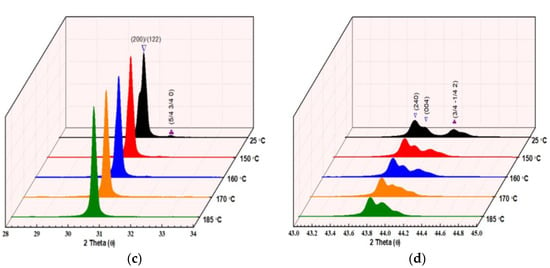
Figure 1.
(a) XRD patterns of lead hafnate PbHfO3 measured at room temperature, with the peaks labeled in the orthorhombic setting. (b) XRD patterns of PbHfO3 measured at different temperatures. (c,d) Enlarged views of the ¼ (hkl) super lattice peaks at 25 °C, 150 °C, 160 °C, 170 °C and 185 °C.
To investigate the symmetry of the intermediate phase, temperature-variable structural analysis was performed. Figure 1b depicts the powder XRD patterns of PbHfO3 measured at selected temperatures between room temperature and 185 °C (powder XRD at temperatures higher than 200 °C was not carried out as it is beyond the limit of our instrument). Distinctly, the (200)/(122) peaks merge in to a single peak (Figure 1c) at 160 °C, and, at the same time, the shape of the (240)/(004) peaks (Figure 1d) changes significantly. These indicate a structural change from the orthorhombic Pbam symmetry to an orthorhombic Imma symmetry (to be discussed later). Moreover, the diminishing intensity of the superlattice peaks with increasing temperature also supports the change in symmetry.
To investigate the abovementioned phase transition, a detailed analysis of the structure of PbHfO3 in the intermediate phase was carried out using the Rietveld refinements of the high-temperature X-ray diffraction data using the GSAS2full 4801 software. Figure 2a–e present the refinement results for the intermediate phase, with comparison of the experimental and calculated XRD patterns, along with the respective space groups and reliability factors. Since no superlattice reflections of the 1/4 (hkl)-type were found in the temperature range of the intermediate phase, the Pbam space group can be excluded, while the Imma space group fits the experimental data most satisfactorily. The assignment of the Imma space group to the intermediate phase of PbHfO3 agrees with a report by Bosak et al. []. The details of the Rietveld refinement results of PbHfO3 at different temperatures within the intermediate phase are given in Table 2. The reliability factors of the weighted patterns (Rwp), the final refinement (wR) and the goodness of fit (GOF) are in the ranges of 3.434–6.485%, 4.926–6.49% and 1.513–2.32%, respectively, indicating good fitting of the selected structural model. At high temperatures, no oxygen octahedral tilting about the a-axis was observed, as opposed to at room temperature. The tilting scheme for the Imma phase is a0b-b-, while at room temperature; the Pbam symmetry shows the a−a−c0 tilting scheme, similar to that in lead zirconate [,,,]. The antiparallel displacement of Pb2+ ions was found to be along the [001]cubic direction in the intermediate phase, as shown in Figure 2e, which is clearly different from the alignment of Pb2+ ions at room temperature, as shown in Figure 2d. This antiparallel dipolar alignment suggests an AFE nature for the intermediate phase. The change in the lattice volume with temperature is presented in Figure 2f. It can be seen that the unit cell volume was drastically decreased upon heating at about 160 °C, as PbHfO3 underwent the transition from the room temperature Pbam phase to the intermediate phase of the Imma space group.
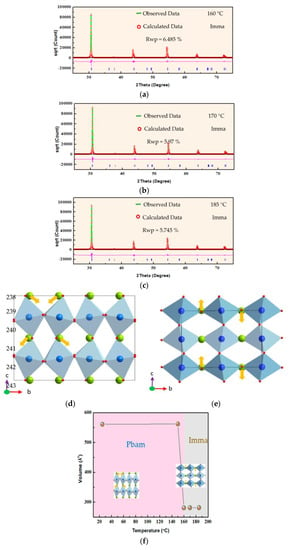
Figure 2.
(a–c) Rietveld refinement results of PbHfO3 at 160 °C, 170 °C and 185 °C. (d) Refined crystal structure of PbHfO3 at room temperature with the Pbam space group, viewed along the a-axis. (e) Refined crystal structure of PbHfO3 at 185 °C with the Imma space group, viewed along the a-axis. Light blue polyhedrons stand for the HfO6 octahedrons. Blue and green spheres denote the Pb and Hf atoms, respectively. Yellow arrows stand for the displacements of the Pb2+ ions. (f) Variation of the unit cell volume of PbHfO3 as a function of temperature between the Pbam and Imma phases.

Table 2.
Refined structural parameters of PbHfO3 at different temperatures.
3.2. Dielectric Properties
Figure 3 depicts the temperature and frequency dependencies of the dielectric permittivity of an as-sintered ceramic measured at various frequencies upon heating. Two dielectric anomalies can be clearly found at 163 °C (TC1) and 199 °C (TC2), which correspond to the phase transitions from the antiferroelectric orthorhombic Pbam phase to the antiferroelectric orthorhombic Imma phase, and from the antiferroelectric Imma phase to the paraelectric, cubic Pmm phase, respectively.
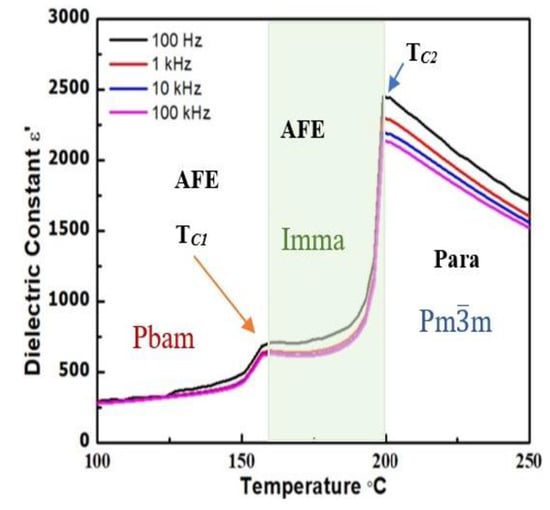
Figure 3.
Temperature dependence of the dielectric permittivity of a PbHfO3 ceramic measured at various frequencies showing the phase transitions from Pbam to Imma, and then from Imma to Pmm, upon heating.
3.3. Microstructure and Energy-Storage Performance
To reveal the AFE character of PbHfO3, which is primordial for energy-storage applications, it is necessary to obtain high-density ceramics with optimum microstructures, so as to apply a high electric field and to realize the AFE to FE switching. Figure 4a presents the HIM image of a PbHfO3 ceramic, which shows a dense microstructure achieved via the conventional sintering process described in Section 2. The average relative density of the ceramics reached 95% of the theoretical density of 10.27 g/cm3. The average grain size of the ceramic calculated with the help of ImageJ 1.8.0 software was about 0.5 μm. The graph of the average grain size distribution is presented in the inset of Figure 4a.
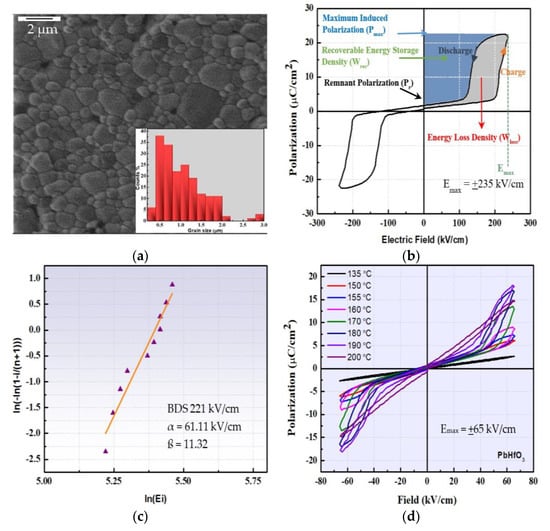
Figure 4.
(a) HIM image of a PbHfO3 ceramic (scale bar = 2 μm) with the average grain size distribution presented in the inset. (b) Polarization–electric field (P–E) relation of PbHfO3 displayed at room temperature; the blue area constitutes the recoverable energy density (Wrec) and the grey area represents the energy loss (Wloss). (c) The Weibull distribution of the PbHfO3 ceramics used for the calculation of the DBS. (d) The polarization–electric field (P–E) relations measured at an electric field of +65 kV/cm at high temperatures.
The polarization–electric field (P–E) relation of PbHfO3 measured at room temperature at 10 Hz is depicted in Figure 4b. The P–E relation exhibits a typical AFE double hysteresis loop, which results from the electric-field-induced AFE-to-FE phase transition, demonstrating the antiferroelectricity of PbHfO3 at room temperature thanks to the high quality of the ceramics. The maximum induced polarization was 23 μC/cm2 at an applied electric field of 235 kV/cm and the remanent polarization was 1.63 μC/cm2 when the applied electric field returned to zero. Theoretically, the stored energy density (Wst) and recoverable energy density (Wrec) can be calculated as discussed in Section 2.2.
The calculated stored energy density Wst, recoverable energy density Wrec, energy loss Wloss and efficiency ɳ were found to be 4.216 J/cm3, 2.7 J/cm3, 1.5 J/cm3 and 65%, respectively, with the energy-storage efficiency calculated from []:
The dielectric breakdown strength (DBS) is one of the most important factors affecting energy-storage performance. It is highly influenced by the density, grain size and microstructure of the ceramics. Dense ceramics with smaller grain size tend to exhibit higher DBS values. Figure 4c presents the Weibull distribution of the DBS for the PbHfO3 ceramics, which can be expressed as [,,,,,]
and
where Xi and Yi are the parameters in the Weibull distribution function, Ei is the specific breakdown electric field of the specimen, Pi is the polarizability, n is the sum of specimens and i is the serial number of the specimen. To obtain the average breakdown strength of PbHfO3, ten samples were examined and their data taken into consideration. The shape parameter ß is the slope of the line, and is related to the range of the DBS. The intercept on the X-axis is ln(α), where α is the scale parameter which reflects the magnitude of the DBS. The values of ß and α were found to be 11.32 and 61.11 kV/cm, respectively. All the dielectric breakdown data were found to follow the Weibull distribution and the obtained DBS value for PbHfO3 was 221 kV/cm. The values of ß and the DBS are in good agreement with previously reported data [].
Xi = ln (Ei),
Yi = ln (ln (1/(1 − Pi))),
Pi = i/(n + 1),
Figure 4d portrays the polarization–electric field (P–E) relation of PbHfO3 measured at ±65 kV/cm in the temperature range from 150 to 190 °C. Well-defined double hysteresis loops clearly demonstrate the antiferroelectric nature for the intermediate Imma phase. The display of the AFE loops is in strong support of the antiparallel alignment of Pb2+ ions along the [001]cubic direction, as depicted from the refined crystal structure of PbHfO3. The highest polarization of 18.0 μC/cm2 was obtained at 190 °C with a very small value of remanent polarization of 0.6 μC/cm2. The calculated Wst, Wrec, Wloss and ɳ were 0.774 J/cm3, 0.7 J/cm3, 0.084 J/cm3 and 89%, respectively, at 190 °C.
4. Discussion
Environmental concerns related to lead: Environmental concerns related to the safety of hazardous materials, such as lead oxide, involved in processing, distribution, transportation, and final decomposition are important issues and require careful consideration. In our case, to ensure the safety, the lead hafnate ceramics were sintered and calcined using a muffle furnace which was kept under a high-power ventilation hood. This effectively prevented the volatile materials from escaping into the air, thereby mitigating potential health hazards associated with the exposure of lead oxide during the processing. The double crucible method used in this work for calcining and sintering helped further minimize the volatilization of lead oxide. During transportation, the raw materials were properly packed, contained and labelled [,]. For the disposal of lead-based materials, they could be converted into nontoxic products or metals and recycled as solid waste [,].
Economics of scale achievement: The solid-state synthesis route developed in this work offers several advantages over other processing routes, such as wet chemical methods, by its simplicity and need for less equipment and is thus a viable technique for scaling up industrial production of lead hafnate for practical applications. Additionally, the raw materials used in the solid-state synthesis are relatively inexpensive, making it a cost-effective option for commercial manufacturing [].
In an effort to clarify the structural nature and AFE properties of lead hafnate, high-quality and dense PbHfO3 ceramics were prepared in pure perovskite phase using the solid-state synthesis method and a conventional sintering process with special caution to preserve the stoichiometry. Detailed structural analysis using the Rietveld refinements confirmed the orthorhombic symmetry with the Pbam space group at room temperature which is in agreement with earlier reports [,]. Temperature-variable structural analysis revealed an orthorhombic Imma symmetry for the intermediate phase of lead hafnate between 150 °C and 185 °C, which agrees with a report by Bosak et al. []. The refined crystal structure of the Imma phase illustrates antiparallel displacement of Pb2+ ions along the [001]cubic direction with the tilting scheme of a0b-b-, which is different from the antiparallel alignment of Pb2+ ions in the Pbam phase at room temperature.
The high quality of the ceramics allowed us to apply a high enough electric field so as to display the P–E double hysteresis loops, demonstrating the AFE nature of PbHfO3 at room temperature and also at high temperatures (up to 190 °C). The realization of the double P–E loops also allowed us to evaluate the energy-storage performance of PbHfO3. The recoverable energy density was found to be 2.7 J/cm3 with an efficiency of 65% at 235 kV/cm at room temperature. According to the data presented in Table 1, the energy-storage performance of the lead hafnate ceramics prepared in this work is 286% higher than the values reported so far for the conventionally prepared stoichiometry PbHfO3 without the addition of extra PbO. This enhanced performance is attributed to the high density and small grain size of the ceramics, which lead to a high value of dielectric breakdown strength, DBS, allowing the antiferroelectric-phase-to-ferroelectric-phase transition to be induced at room temperature.
The double P–E hysteresis loops displayed at high temperatures reveal for the first time the presence of antiferroelectricity in the intermediate phase, with a recoverable energy density of 0.7 J/cm3 and an efficiency of 89% at an electric field of 65 kV/cm at 190 °C.
5. Conclusions
To better understand the structural nature and AFE properties of lead hafnate, high-quality and dense PbHfO3 ceramics were prepared in pure perovskite phase using the solid-state synthesis method and a conventional sintering process with special caution to preserve the stoichiometry without adding excess PbO. However, due to the high volatilization of lead oxide at high temperatures, it is challenging to prepare high-density and pure-phase PbHfO3 experimentally. Rietveld refinements on high-temperature structural analysis revealed an orthorhombic Imma symmetry for the intermediate phase of lead hafnate between 150 °C and 185 °C, with antiparallel displacement of Pb2+ ions along the [001]pc direction with the tilting scheme of a0b−b−. This is different from the antiparallel alignment of Pb2+ ions in the Pbam phase at room temperature. The realization of the double P–E loops at room temperature also allowed us to evaluate the energy-storage performance of PbHfO3. At room temperature, the recoverable energy density was found to be 2.7 J/cm3, with an efficiency of 65% at 235 kV/cm at room temperature. The energy-storage performance of lead hafnate prepared in this work is 286% higher than the values reported so far for the conventionally prepared stoichiometry PbHfO3. This enhanced performance is attributed to two key factors: the high density and the small grain size of the ceramics. This led to a high value of dielectric breakdown strength, DBS, which allows the transition of antiferroelectric phase to ferroelectric phase at room temperature. The double P–E hysteresis loops displayed at high temperatures reveal, for the first time, the presence of antiferroelectricity in the intermediate phase. These results demonstrate that the prototypical AFE PbHfO3 is indeed a promising candidate for energy-storage applications [] in a wide range of temperatures from room temperature up to 190 °C.
Author Contributions
Conceptualization, V.C. and Z.-G.Y.; methodology, V.C., B.-X.W. and Z.-G.Y.; software, V.C. and B.-X.W.; validation, V.C., B.-X.W. and Z.-G.Y.; formal analysis, V.C.; investigation, V.C. and B.-X.W.; resources, Z.-G.Y.; data curation, V.C.; writing—original draft preparation, V.C.; writing—review and editing, Z.-G.Y.; visualization, V.C. and Z.-G.Y.; supervision, Z.-G.Y.; project administration, Z.-G.Y.; funding acquisition, Z.-G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery Grants Nos. RGPIN-2017-06915 and RGPIN-2023-04416) and the U.S. Office of Naval Research (ONR, Grant No. N00014-21-1-2085).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin, L.W.; Rappe, A.M. Thin-film ferroelectric materials and their applications. Nat. Rev. Mater. 2017, 2, 16087. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, Z.; Yang, B.; Huang, J. Arising applications of ferroelectric materials in photovoltaic devices. J. Mater. Chem. A 2014, 2, 6027–6041. [Google Scholar] [CrossRef]
- Blazquez-Castro, A.; Garcıa-Cabanes, A.; Carrascosa, M. Biological applications of ferroelectric materials. Appl. Phys. Rev. 2018, 5, 41101. [Google Scholar] [CrossRef]
- Tagantsev, A.K.; Vaideeswaran, K.; Vakhrushev, S.B.; Filimonov, A.V.; Burkovsky, R.G.; Shaganov, A.; Andronikova, D.; Rudskoy, A.I.; Baron, A.Q.R.; Uchiyama, H.; et al. The origin of antiferroelectricity in PbZrO3. Nat. Commun. 2013, 4, 2229. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.; Liu, H.; Li, J.F.; Zhang, S. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sci. 2019, 102, 72–108. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Ye, J.; Wang, G.; Dong, X.; Withers, R.; Liu, Y. Antiferroelectrics for energy storage applications: A review. Adv. Mater. Technol. 2018, 3, 1800111. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Xu, Z.; Zhang, S. Multilayer lead-free ceramic capacitors with ultrahigh energy density and efficiency. Adv. Mater. 2018, 30, 1802155. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.; Zhang, N.; Wu, H.; Bokov, A.A.; Ren, W.; Ye, Z.-G. New antiferroelectric perovskite system with ultrahigh energy-storage performance at low electric field. Chem. Mater. 2019, 31, 979–990. [Google Scholar] [CrossRef]
- Glazer, A.M.; Roleder, K.; Dec, J. Structure and disorder in single-crystal lead zirconate, PbZrO3. Acta Crystallogr. B 1993, 49, 846–852. [Google Scholar] [CrossRef]
- Kupriyanov, M.F.; Petrovich, E.V.; Dutova, E.V.; Kabirov, Y.V. Sequence of phase transitions in PbHfO3. Crystallogr. Rep. 2021, 57, 205–207. [Google Scholar] [CrossRef]
- Bosak, A.; Svitlyk, V.; Arakcheeva, A.; Burkovsky, R.; Diadkin, V.; Roleder, K.; Chernyshov, D. Incommensurate crystal structure of PbHfO3. Acta Crystallogr. B. 2020, 76, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Yang, T.; Zhang, S. Ultrahigh energy-storage density in antiferroelectric ceramics with field-induced multiphase transitions. Adv. Funct. Mater. 2019, 29, 1807321. [Google Scholar] [CrossRef]
- Xu, H.; Dan, Y.; Zou, K.; Chen, G.; Zhang, Q.; Lu, Y.; He, Y. Superior energy storage performance in Pb0.97La0.02(Zr0.50 Sn0.43Ti0.07)O3 antiferroelectric ceramics. J. Mater. Res. Technol. 2019, 8, 3291–3296. [Google Scholar] [CrossRef]
- Shirane, G.; Pepinsky, R. Phase transitions in antiferroelectric PbHfO3. Phys. Rev. Lett. 1953, 91, 812–815. [Google Scholar] [CrossRef]
- Ge, P.Z.; Tang, X.G.; Liu, Q.X.; Jiang, Y.P.; Guo, X.B. Superior energy and power density realized in Pb(Hf1−xTix)O3 system at low electric field. Energy Mater. Adv. 2023, 4, 25. [Google Scholar] [CrossRef]
- Ge, P.Z.; Tang, X.G.; Meng, K.; Huang, X.X.; Liu, Q.X.; Jiang, Y.P.; Gong, W.P.; Wang, T. Ultrahigh energy storage density and superior discharge power density in a novel antiferroelectric lead hafnate. Mater. Today Phys. 2022, 24, 100681. [Google Scholar] [CrossRef]
- Guo, J.; Yang, T. Giant energy storage density in Ba, La co-doped PbHfO3-based antiferroelectric ceramics by a rolling process. J. Alloys Compd. 2021, 888, 161539. [Google Scholar] [CrossRef]
- Chao, W.; Yang, T.; Li, Y. Achieving high energy efficiency and energy density in PbHfO3-based antiferroelectric ceramics. J. Mater. Chem. C 2020, 8, 17016–17024. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ge, P.G.; Tang, H.; Tang, X.G.; Zeng, S.M.; Jiang, Y.P.; Tang, Z.H.; Liu, Q.X. High temperature dielectric properties and impedance spectroscopy of PbHf1−xSnxO3 ceramics. IET Nanodielectr. 2020, 3, 131–137. [Google Scholar] [CrossRef]
- Ge, P.Z.; Tang, X.G.; Meng, K.; Huang, X.X.; Li, S.F.; Liu, Q.X.; Jiang, Y.P. Energy storage density and charge–discharge properties of PbHf1−xSnxO3 antiferroelectric ceramics. J. Chem. Eng. 2022, 429, 132540. [Google Scholar] [CrossRef]
- Wei, J.; Yang, T.; Wang, H. Excellent energy storage and charge-discharge performances in PbHfO3 antiferroelectric ceramics. J. Eur. Ceram. Soc. 2019, 39, 624–630. [Google Scholar] [CrossRef]
- Bussmann-Holder, A.; Kim, T.H.; Lee, B.W.; Ko, J.H.; Majchrowski, A.; Soszyński, A.; Roleder, K. Phase transitions and interrelated instabilities in PbHfO3 single crystals. J. Phys. Cond. Matters 2015, 27, 105901. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S. Low-temperature synthesis route. Am. Ceram. Soc. 1988, 71, 250–251. [Google Scholar] [CrossRef]
- Yao, Z.; Song, Z.; Hao, H.; Yu, Z.; Cao, M.; Zhang, S.; Lanagan, M.T.; Liu, H. Homogeneous/inhomogeneous-structured dielectrics and their energy-storage performances. Adv. Mater. 2017, 29, 1601727. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, Z.; Li, Y.; Li, L.; Ji, H.; Feteira, A.; Zhou, D.; Wang, D.; Zhang, S.; Reaney, I.M. Electroceramics for high-energy density capacitors: Current status and future perspectives. Chem. Rev. 2021, 121, 6124–6172. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Sawaguchi, E.; Kittaka, T. Antiferroelectricity and ferroelectricity in lead zirconate. J. Phys. Soc. Jpn. 1952, 7, 336–337. [Google Scholar] [CrossRef]
- Corker, D.L.; Glazer, A.M.; Kaminsky, W.; Whatmore, R.W.; Dec, J.; Roleder, K. Investigation into the crystal structure of the perovskite lead hafnate, PbHfO3. Acta Crystallogr. B 1998, 54, 18–28. [Google Scholar] [CrossRef]
- Woodward, P.M. Octahedral tilting in perovskites. I. geometrical considerations. Acta Cryst. 1997, 53, 32–43. [Google Scholar] [CrossRef]
- Glazer, A.M. The classification of tilted octahedra in perovskites. Acta Crystall. B-Stru. 1972, 28, 3384–3392. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Z.; Xu, K.; Cao, Y.; Tian, Y.; Guo, L.; Tian, J.; Tian, H.; Liu, X.; Lin, L.; et al. Modulated band structure and phase transitions in calcium hafnate titanate modified silver niobate ceramics for energy storage. J. Chem. Eng. 2021, 426, 131047. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Deng, C.; Dai, X.; Li, L. Effect of the Ba/Ti ratio on the microstructures and dielectric properties of barium titanate-based glass-ceramics. J. Am. Ceram. Soc. 2009, 92, 1350–1353. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Ma, T.; Li, H.; Zhang, L. Correlation between dielectric breakdown strength and interface polarization in barium strontium titanate glass ceramics. Appl. Phys. Lett. 2010, 96, 42902. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, J.; Zhang, S.J.; Li, J.F. Lead-free antiferroelectric silver niobate tantalate with high energy storage performance. Adv. Mater. 2017, 29, 1701824. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.; Zhou, Z.; Dong, X. Superior energy storage properties and excellent stability of novel NaNbO3-based lead-free ceramics with A-site vacancy obtained: Via a Bi2O3 substitution strategy. J. Mater. Chem. A 2018, 6, 17896–17904. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, J.; Liu, Q.; Zhang, S.J.; Li, J.F. Silver niobate lead-free antiferroelectric ceramics: Enhancing energy storage density by B–site doping. ACS Appl. Mater. Interfaces 2018, 10, 819–826. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Swaringen, F.B.; Gawlik, E.; Kamenov, G.D.; McTigue, N.E.; Cornwell, D.A.; Claude, J.; Bonzongo, J. Children’s exposure to environmental lead: A review of potential sources, blood levels, and methods used to reduce exposure. Environ. Res. 2022, 204, 112025. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Han, W. Recycling and management of waste lead-acid batteries: A mini-review. Waste Manag. Res. 2016, 34, 298–306. [Google Scholar] [CrossRef]
- Kavourasa, P.; Kaimakamisa, G.; Ioannidisb, T.A.; Kehagiasa, T.; Komninoua, P.; Kokkoua, S.; Pavlidoua, E.; Antonopoulosa, I.; Sofonioub, M.; Zouboulisb, A.; et al. Vitrification of lead-rich solid ashes from incineration of hazardous industrial wastes. Waste Manag. 2003, 23, 361–371. [Google Scholar] [CrossRef]
- Dragan, M.; Enache, S.; Varlam, M.; Petrov, K. Perovskite-type lanthanum cobaltite LaCoO3: Aspects of processing route toward practical applications. In Cobalt Compounds and Applications, 2nd ed.; Yildiz, Y., Manzak, A., Eds.; IntechOpen: London, UK, 2019; Chapter 6; pp. 1–120. [Google Scholar]
- Kyriakopoulos, G.L.; Arabatzis, G. Electrical energy storage systems in electricity generation: Energy policies, innovative technologies, and regulatory regimes. Renew. Sustain. Energy Rev. 2016, 56, 1044–1067. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).