Microstructure and Recrystallization Behavior of Heating Rate-Controlled Electrolytic Capacitor Aluminum Foil under Cold Forming and Annealing
Abstract
:1. Introduction
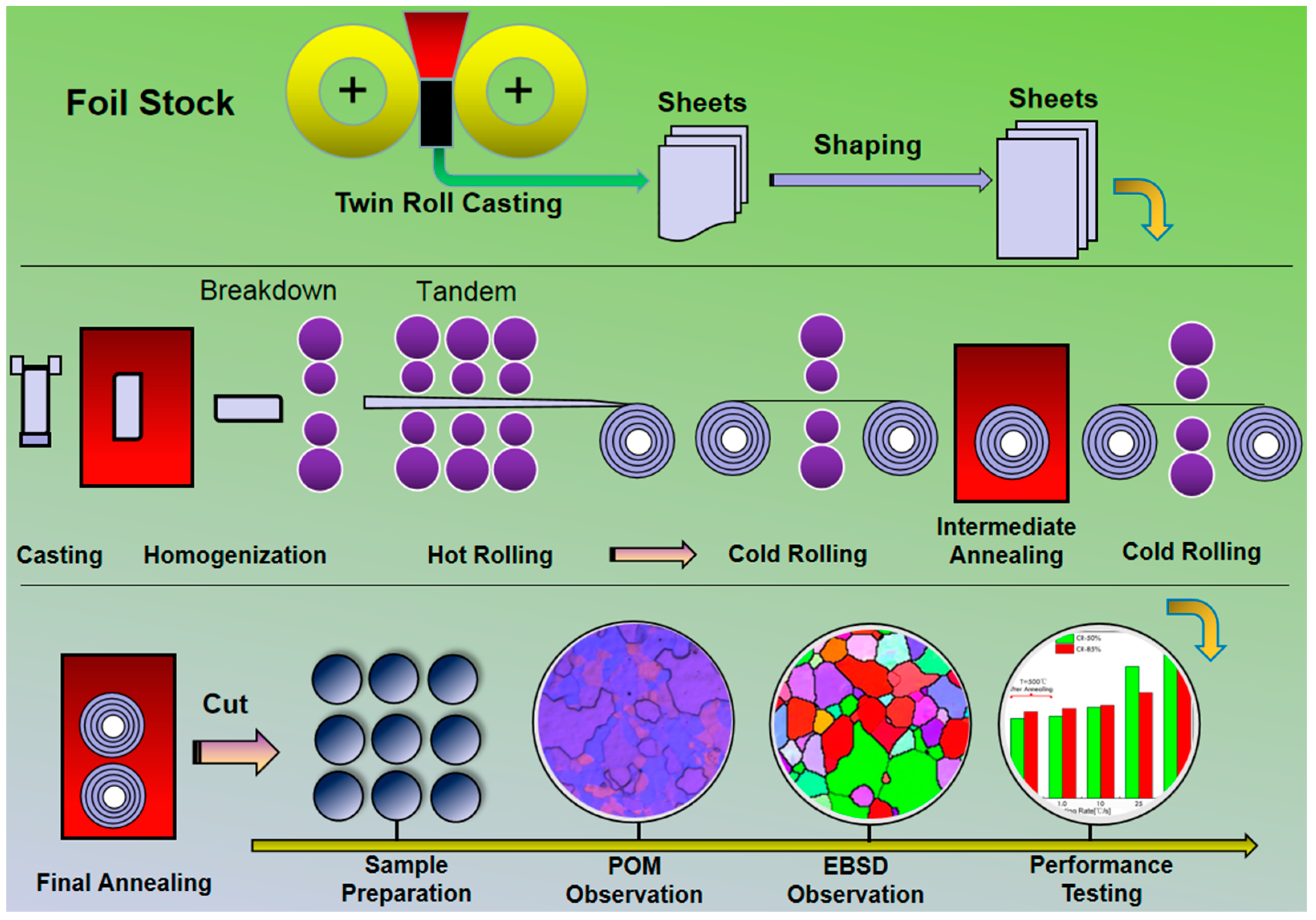
2. Materials and Methods
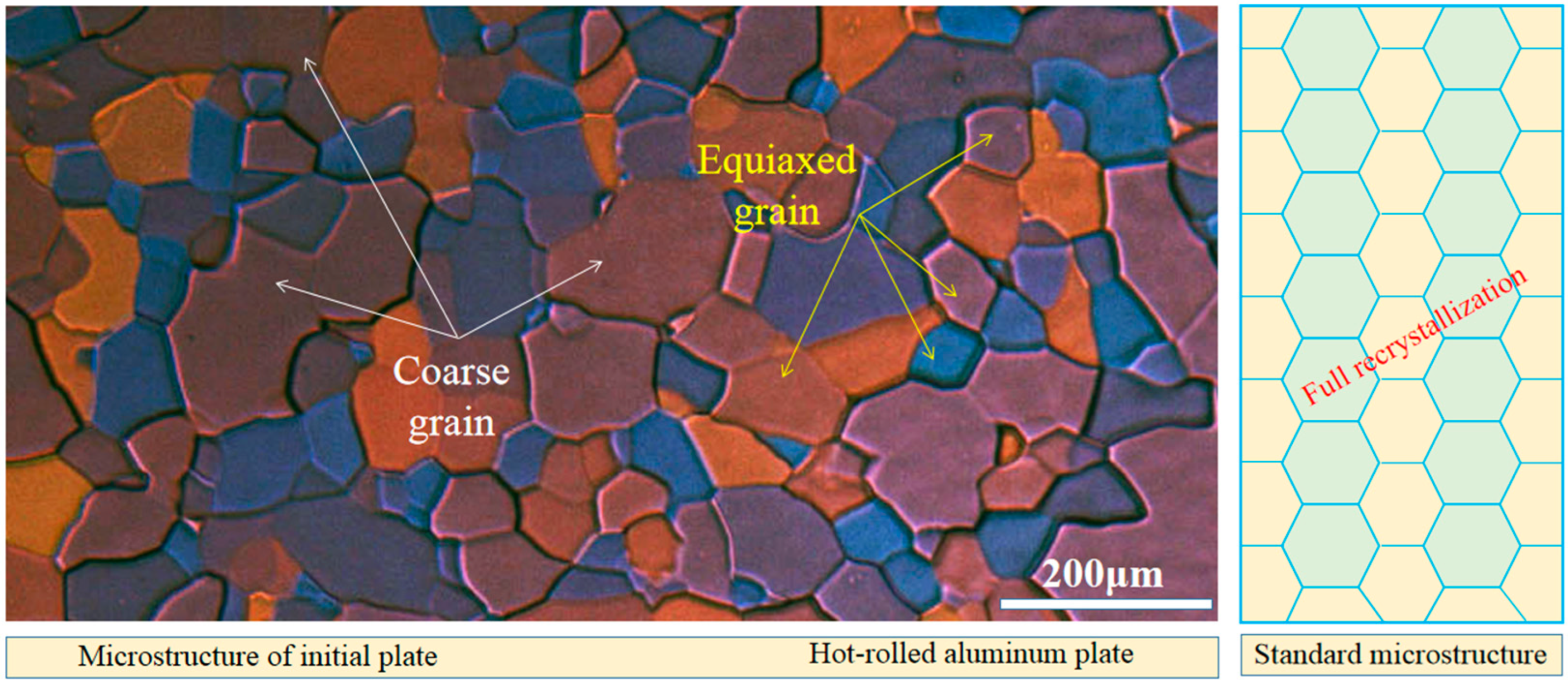
2.1. Materials
2.2. Methods
3. Results and Analysis
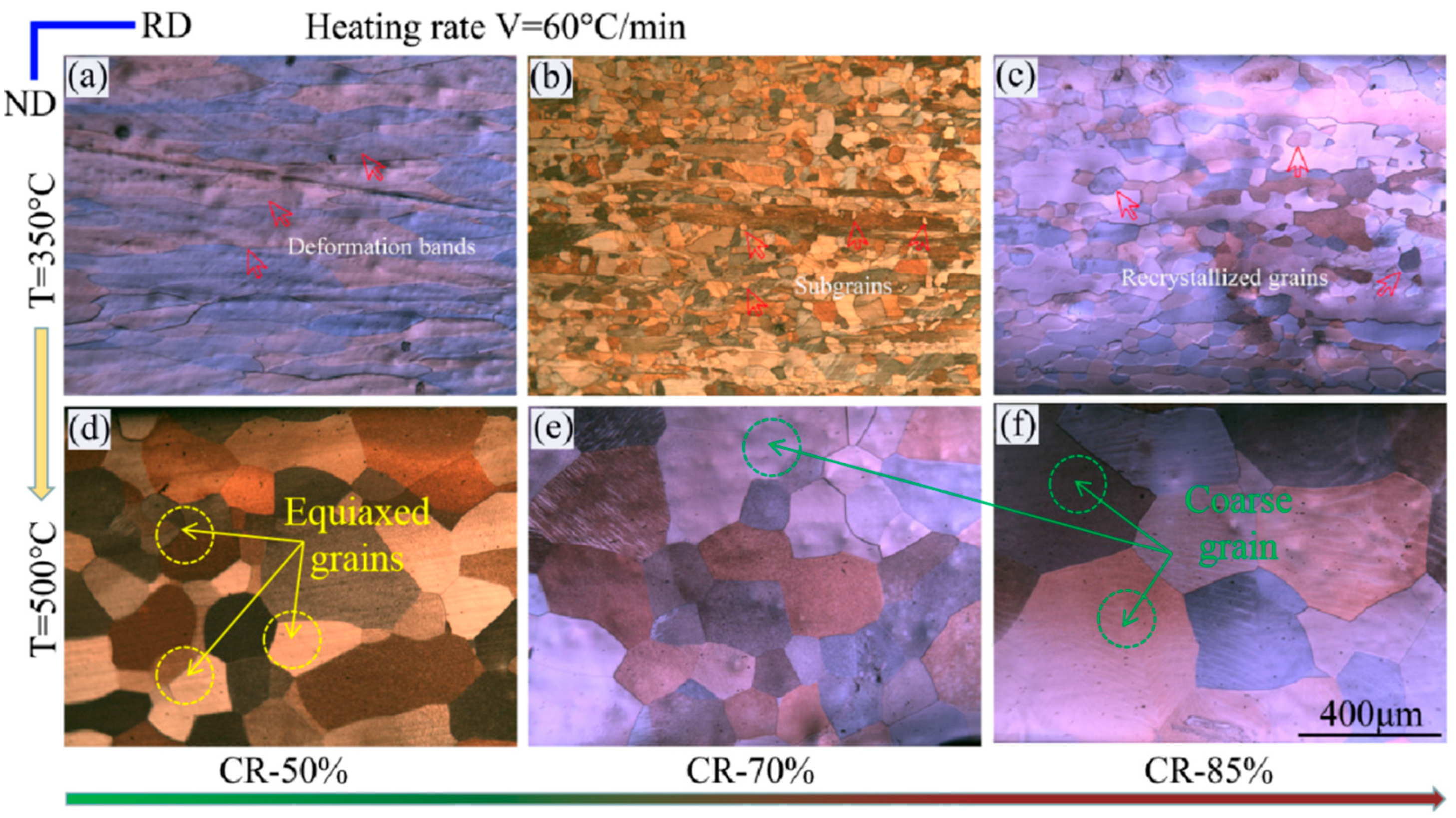
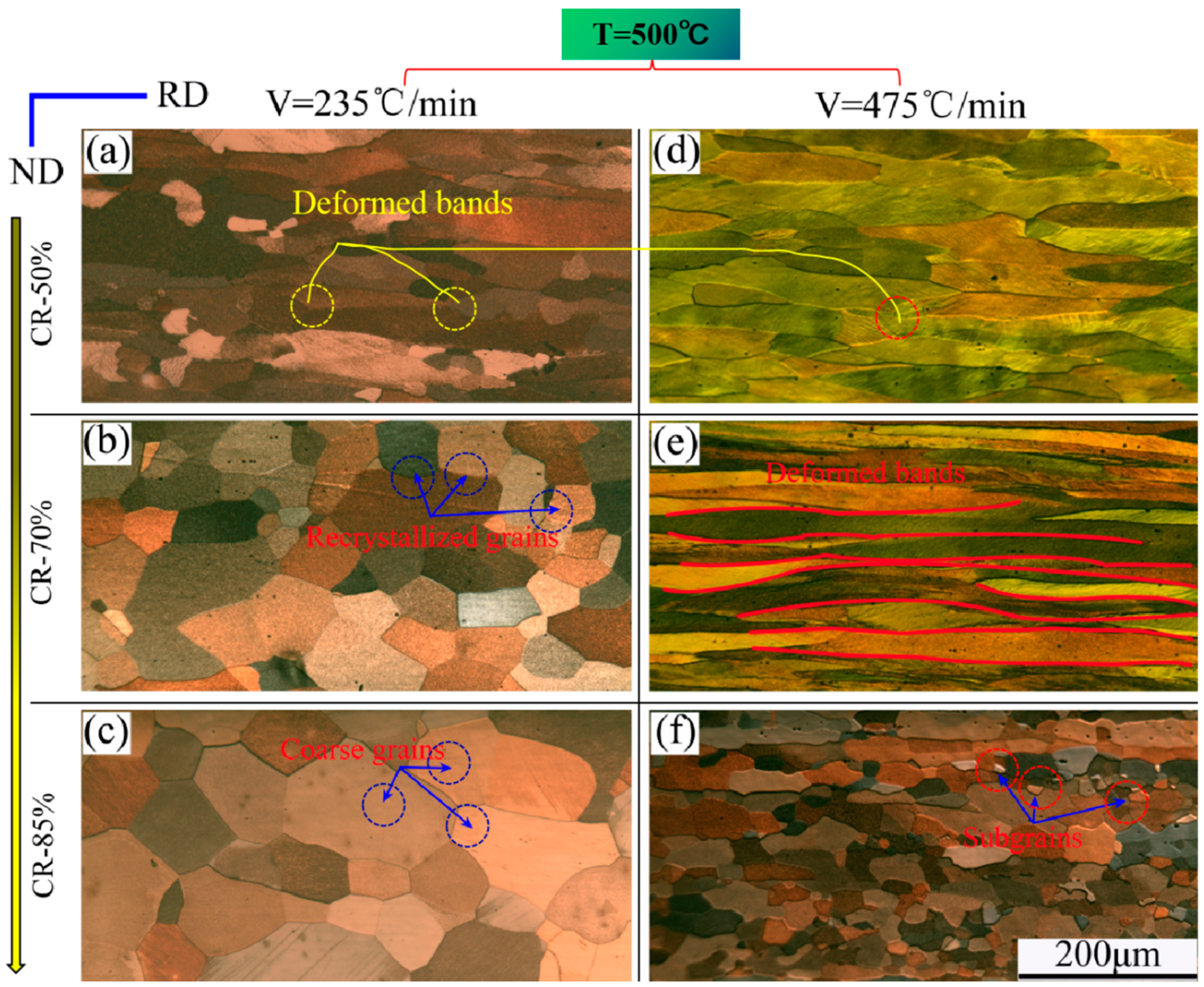
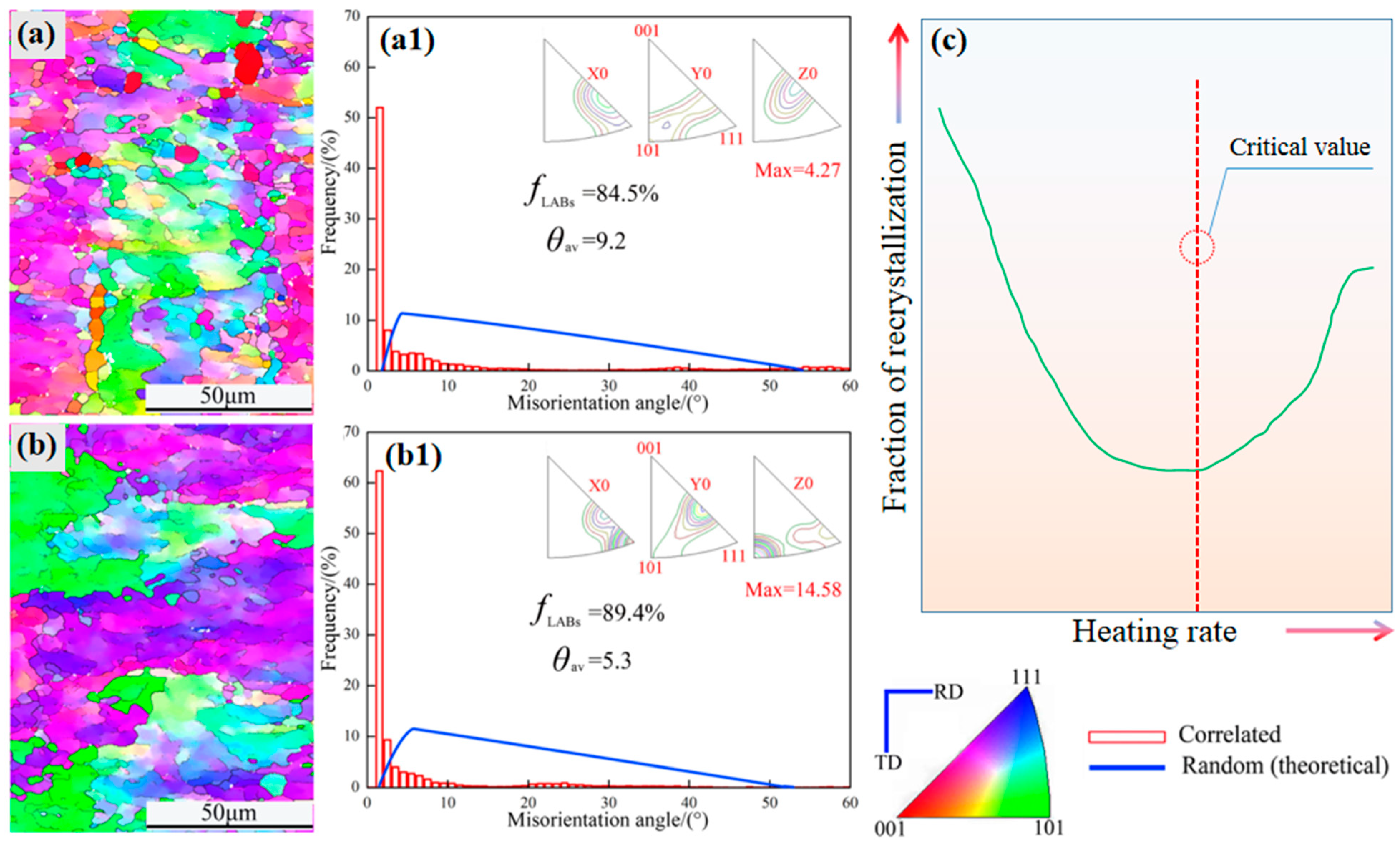
3.1. Microstructure Observation
3.2. Crystal Orientation Analysis
3.3. Hardness Testing
4. Discussion
5. Conclusions
- (1)
- The cold rolling reduction rate, annealing temperature, and heating rate have a common impact on the microstructure, recrystallization behavior, and secondary grain growth during the annealing process.
- (2)
- The recrystallization temperature increases with an increase in the heating rate, while the recrystallization fraction decreases. Additionally, a decrease in the recrystallization temperature is observed, which means that the heating rate is a very important factor affecting the recrystallization.
- (3)
- In order to ensure the uniformity of microstructure and obtain a high cubic texture content, it is necessary to determine a reasonable cold rolling reduction rate, appropriate annealing temperature, and appropriate heating rate for this study. It is a very important reference to guide the actual production of enterprises in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osawa, N.; Fukuoka, K. Pit nucleation behavior of aluminium foil for electrolytic capacitors during early stage of DC etching. Corros. Sci. 2000, 42, 585–597. [Google Scholar] [CrossRef]
- Romero, J.; Azarian, M.H.; Pecht, M. Reliability analysis of multilayer polymer aluminum electrolytic capacitors. Microelectron. Reliab. 2020, 112, 113725. [Google Scholar] [CrossRef]
- Ye, X.R.; Hu, Y.F.; Zheng, B.K.; Chen, C.; Feng, R.; Liu, S.R.; Zhai, G.F. Reliability assessment of film capacitors oriented by dependent and nonlinear degradation considering three-source uncertainties. Microelectron. Reliab. 2021, 126, 114277. [Google Scholar] [CrossRef]
- Cousseau, R.; Patin, N.; Forgez, C.; Monmasson, E.; Idkhajine, L. Improved electrical model of aluminum electrolytic capacitor with anomalous diffusion for health monitoring. Math. Comput. Simulat. 2017, 131, 268–282. [Google Scholar] [CrossRef]
- Vicentini, R.; Nunes, W.G.; Costa, L.H.; Da Silva, L.M.; Pascon, A.; Jackson, P.; Doubek, G.; Zanin, H. Highly stable nickel-aluminum alloy current collectors and highly defective multi-walled carbon nanotubes active material for neutral aqueous-based electrochemical capacitors. J. Energy Storage 2019, 23, 116–127. [Google Scholar] [CrossRef]
- Xue, R.; Qian, Y.Y.; Liu, K.K.; Jiang, X.X.; Zhu, J.J.; Zhang, J.R. Nano-pit corrosion of the tabs in aluminum electrolytic capacitor: Polarization characteristics of the tabs in ethyleneglycol-borate solution with chloride ions. Corros. Sci. 2008, 50, 2779–2784. [Google Scholar] [CrossRef]
- Peng, N.; Li, Y.Z.; Yang, X.F.; Liang, L.B. Effects of porous hydrous oxide layer on the microstructure and dielectric properties of TiO2-coated aluminum foil. Mater. Charact. 2021, 173, 110931. [Google Scholar] [CrossRef]
- Wang, J.B.; Sun, C.X.; Guo, J.; Xu, Z.M. Integrated process for recycling aluminum electrolytic capacitors from waste printed circuit boards: Disassembly, heat treatment and magnetic-eddy current-electrostatic separating. J. Clean. Prod. 2017, 165, 334–345. [Google Scholar] [CrossRef]
- Nogami, K.; Sakamoto, K.; Hayakawa, T.; Kakimoto, M. The effects of hyperbranched poly(siloxysilane)s on conductive polymer aluminum solid electrolytic capacitors. J. Power Sources 2007, 166, 584–589. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, J.; Im, H.; Kim, J. The effects of different oxidants on the characteristics of conductive polymer aluminum solid electrolyte capacitors. J. Power Sources 2013, 230, 1–9. [Google Scholar] [CrossRef]
- Tsai, M.L.; Lu, Y.F.; Do, J.S. High-performance electrolyte in the presence of dextrose and its derivatives for aluminum electrolytic capacitors. J. Power Sources 2002, 112, 643–648. [Google Scholar] [CrossRef]
- Chi, C.-S.; Jeong, Y.; Ahn, H.-J.; Lee, J.-H.; Kim, J.-G.; Lee, J.-H.; Jang, K.-W.; Oh, H.-J. Transition of hydrated oxide layer for aluminum electrolytic capacitors. Mater. Sci. Eng. A 2007, 449–451, 314–317. [Google Scholar] [CrossRef]
- Peng, N.; He, Y.; Song, H.; Yang, X.; Cai, X. Effects of electrodeposited Zn nuclei on tunnel etching behavior of aluminum foil. Corros. Sci. 2015, 91, 213–219. [Google Scholar] [CrossRef]
- Su, Y.-H.; Shih, C.-Y.; Su, C.-H.; Lee, Y.-L.; Hsieh, C.-T.; Teng, H. Dielectric gel electrolytes for safe charge storage from −20 to 80 °C by double-layer capacitors. J. Taiwan Inst. Chem. E 2022, 134, 104309. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Zhu, W.; Huang, J. Environmental impact assessment of China’s primary aluminum based on life cycle assessment. Trans. Nonferr. Metals Soc. 2019, 29, 1784–1792. [Google Scholar] [CrossRef]
- Zhang, C.; Jing, J.; Yun, L.; Zheng, Y.; Huang, H. A cradle-to-grave life cycle assessment of high-voltage aluminum electrolytic capacitors in China. J. Clean. Prod. 2022, 370, 133244. [Google Scholar] [CrossRef]
- Querne, C.; Vignal, T.; Pinault, M.; Banet, P.; Hermite, M.M.; Aubert, P.-H. A comparative study of high density Vertically Aligned Carbon Nanotubes grown onto different grades of aluminum-Application to supercapacitors. J. Power Sources 2023, 553, 232258. [Google Scholar] [CrossRef]
- Ono, S. Passive Films for Electrolytic Capacitors. Encycl. Interfacial Chem. 2018, 376–395. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Tang, J.; Li, L.; Chen, M.; Liu, S.; Zhu, B. Element segregation on the surfaces of pure aluminum foils. Appl. Surf. Sci. 2010, 256, 7300–7304. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Chen, J.; Liu, X.; Yin, Y.; Ban, C.D.C. Etching Anode Aluminum Foil to Form Branch Tunnels By Electroless Depositing Cu. Anti-Corros. Method. Mater. 2019, 66, 697–703. [Google Scholar] [CrossRef]
- Zeng, X.; Bian, J.; Liang, L.; Cao, Q.; Liu, L.; Chen, X.; Wang, Y.; Xie, X.; Xie, G. Preparation and characterization of anode foil for aluminum electrolytic capacitors by powder additive manufacturing. Powder Technol. 2023, 27, 118602. [Google Scholar] [CrossRef]
- Pan, S.; Liang, L.; Lu, B.; Li, H. Microstructure evolution for oxide film of anodic aluminum foil used in high voltage electrolytic capacitor. J. Alloys Compd. 2020, 823, 153795. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.; Jing, J.; Liu, Y.; Huang, H. A comparative LCA study on aluminum electrolytic capacitors: From liquid-state electrolyte, solid-state polymer to their hybrid. J. Clean. Prod. 2022, 375, 134044. [Google Scholar] [CrossRef]
- Smith, L.; Ibn-Mohammed, T.; Lenny Koh, S.C.; Reaney, I.M. Life cycle assessment and environmental profile evaluations of high volumetric efficiency capacitors. Appl. Energy 2018, 220, 496–513. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, J.; Wang, J.Z. (Ba0.5Sr0.5)TiO3 modification on etched aluminum foil for electrolytic capacitor. Ceram. Int. 2008, 34, 1285–1287. [Google Scholar]
- Yoo, J.E.; Choi, J. Electrochemical surface enlargement of a niobium foil for electrolytic capacitor applications. Electrochem. Commun. 2011, 13, 298–301. [Google Scholar] [CrossRef]
- Khera, N.; Khan, S.A. Prognostics of aluminum electrolytic capacitors using artificial neural network approach. Microelectron. Reliab. 2018, 81, 328–336. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, T.; Che, L.; Huang, G. Microstructure and Recrystallization Behavior of Heating Rate-Controlled Electrolytic Capacitor Aluminum Foil under Cold Forming and Annealing. Materials 2023, 16, 4128. https://doi.org/10.3390/ma16114128
Wang Y, Wu T, Che L, Huang G. Microstructure and Recrystallization Behavior of Heating Rate-Controlled Electrolytic Capacitor Aluminum Foil under Cold Forming and Annealing. Materials. 2023; 16(11):4128. https://doi.org/10.3390/ma16114128
Chicago/Turabian StyleWang, Yunlei, Taibin Wu, Luchang Che, and Guangjie Huang. 2023. "Microstructure and Recrystallization Behavior of Heating Rate-Controlled Electrolytic Capacitor Aluminum Foil under Cold Forming and Annealing" Materials 16, no. 11: 4128. https://doi.org/10.3390/ma16114128
APA StyleWang, Y., Wu, T., Che, L., & Huang, G. (2023). Microstructure and Recrystallization Behavior of Heating Rate-Controlled Electrolytic Capacitor Aluminum Foil under Cold Forming and Annealing. Materials, 16(11), 4128. https://doi.org/10.3390/ma16114128






