Coupled Effects of Temperature and Humidity on Fracture Toughness of Al–Mg–Si–Mn Alloy
Abstract
1. Introduction
2. Experimental Methods
2.1. Material
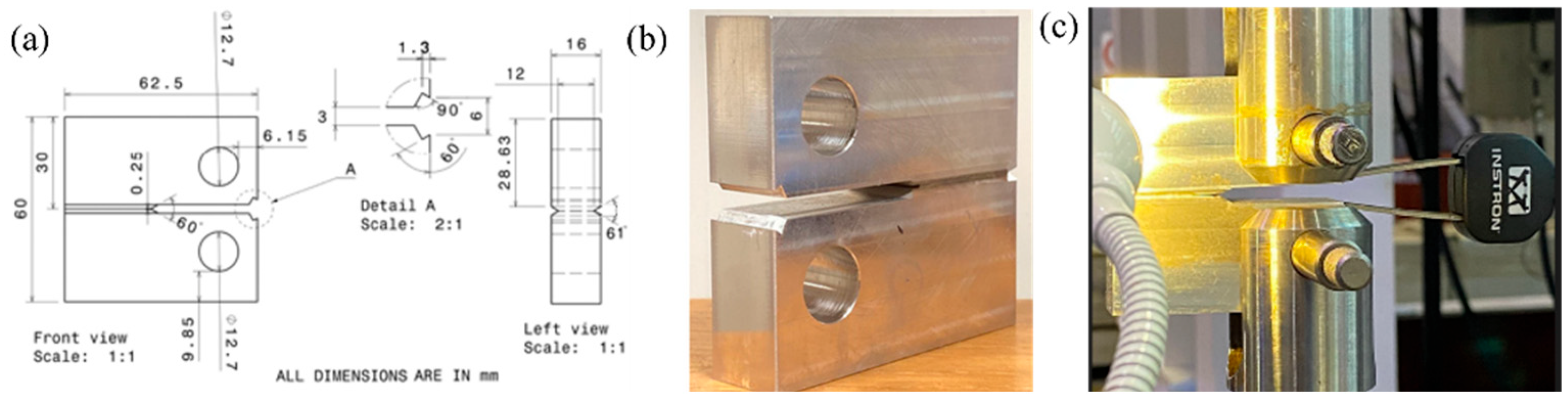
2.2. Experimentation
3. Results and Discussion
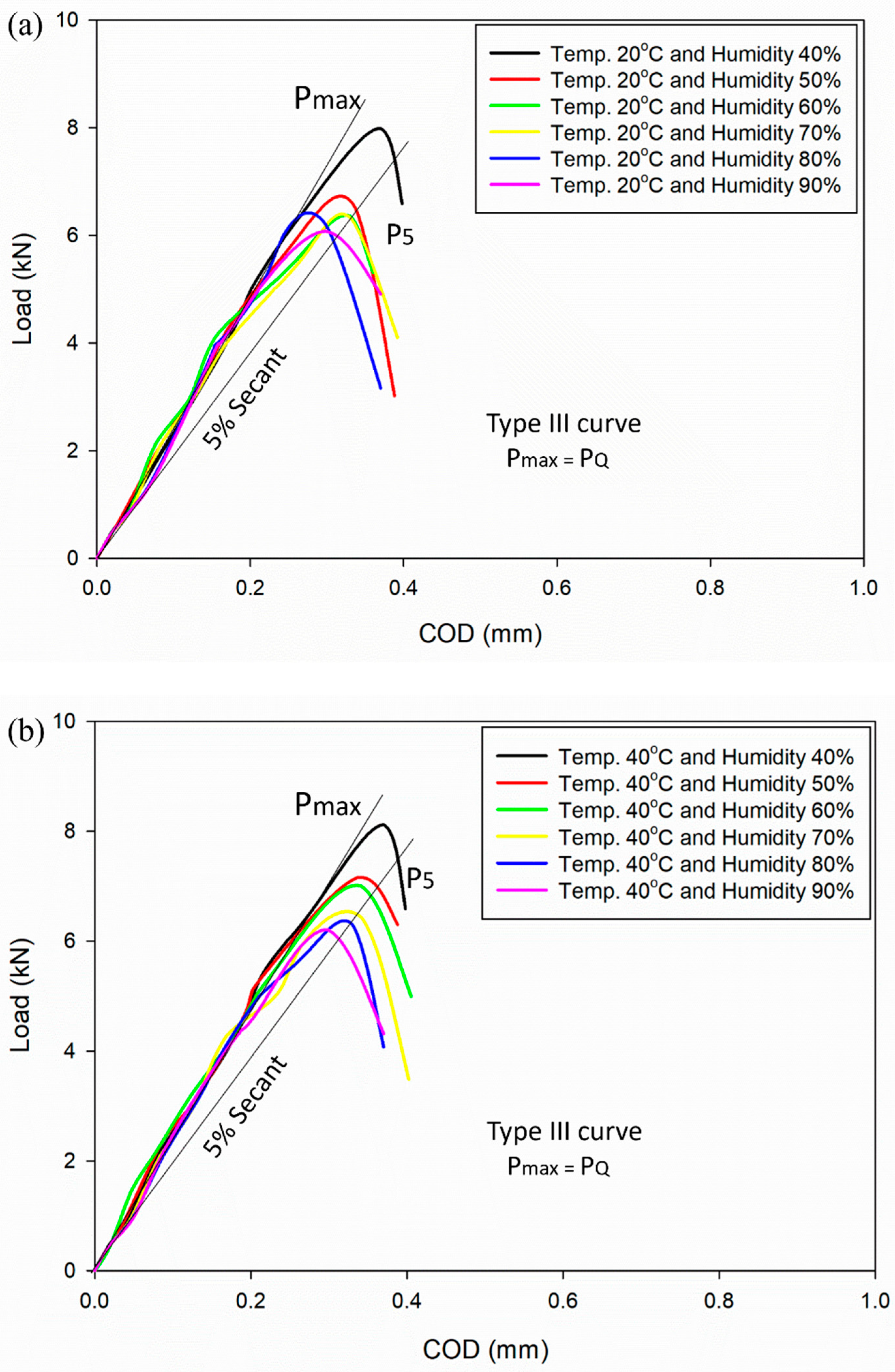
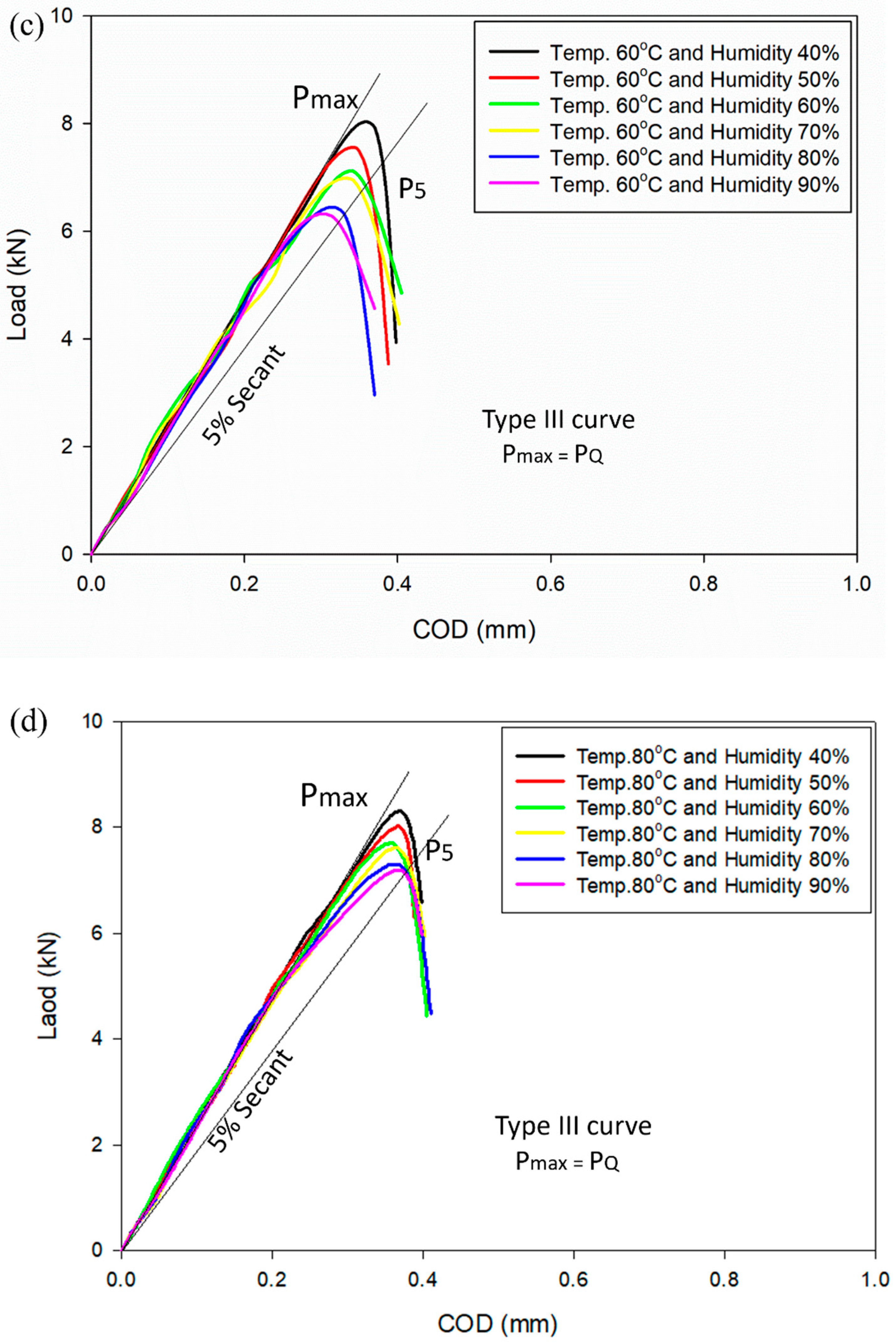
3.1. Subsection Load vs. Crack Opening Displacement
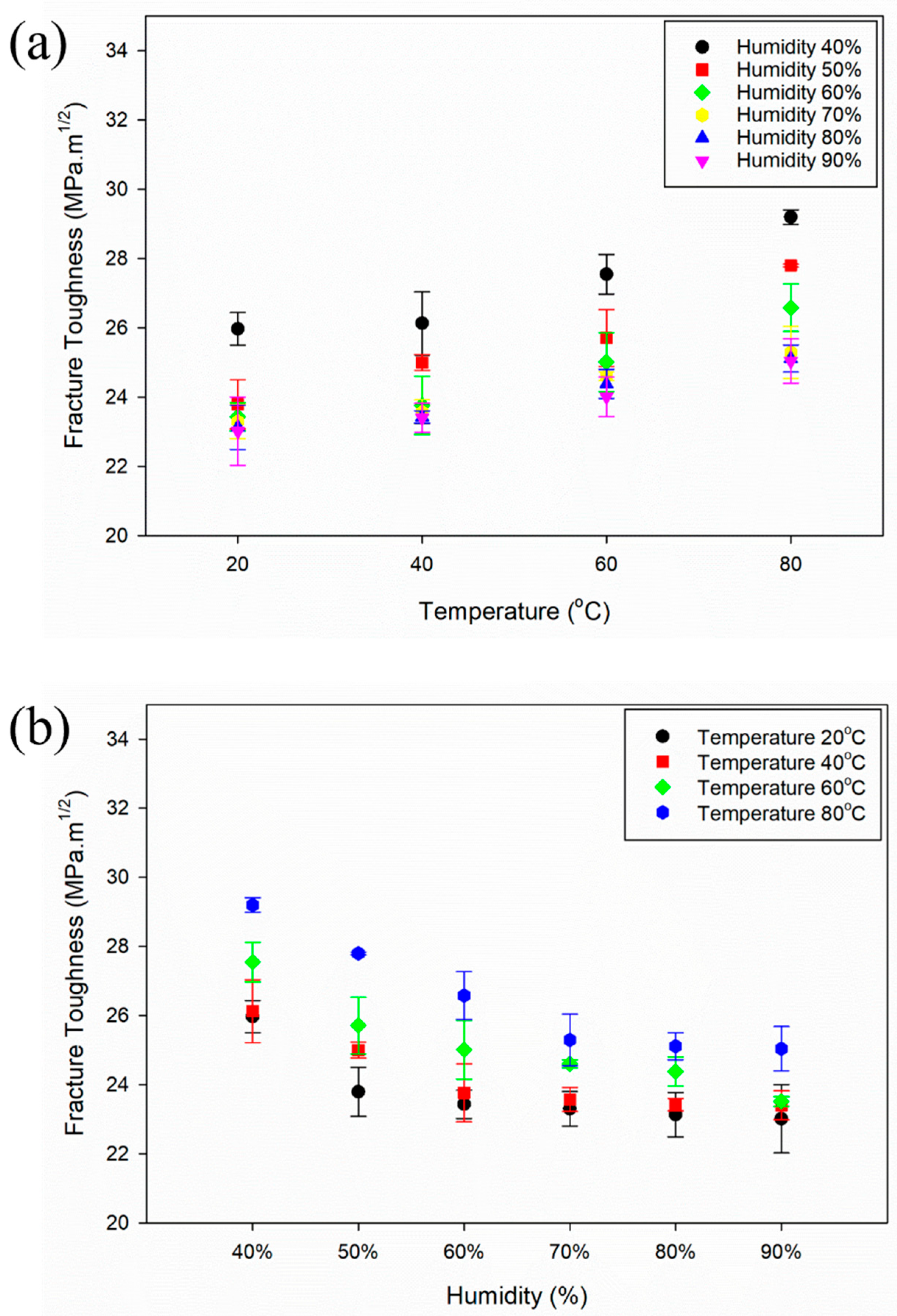
3.2. Fracture Toughness
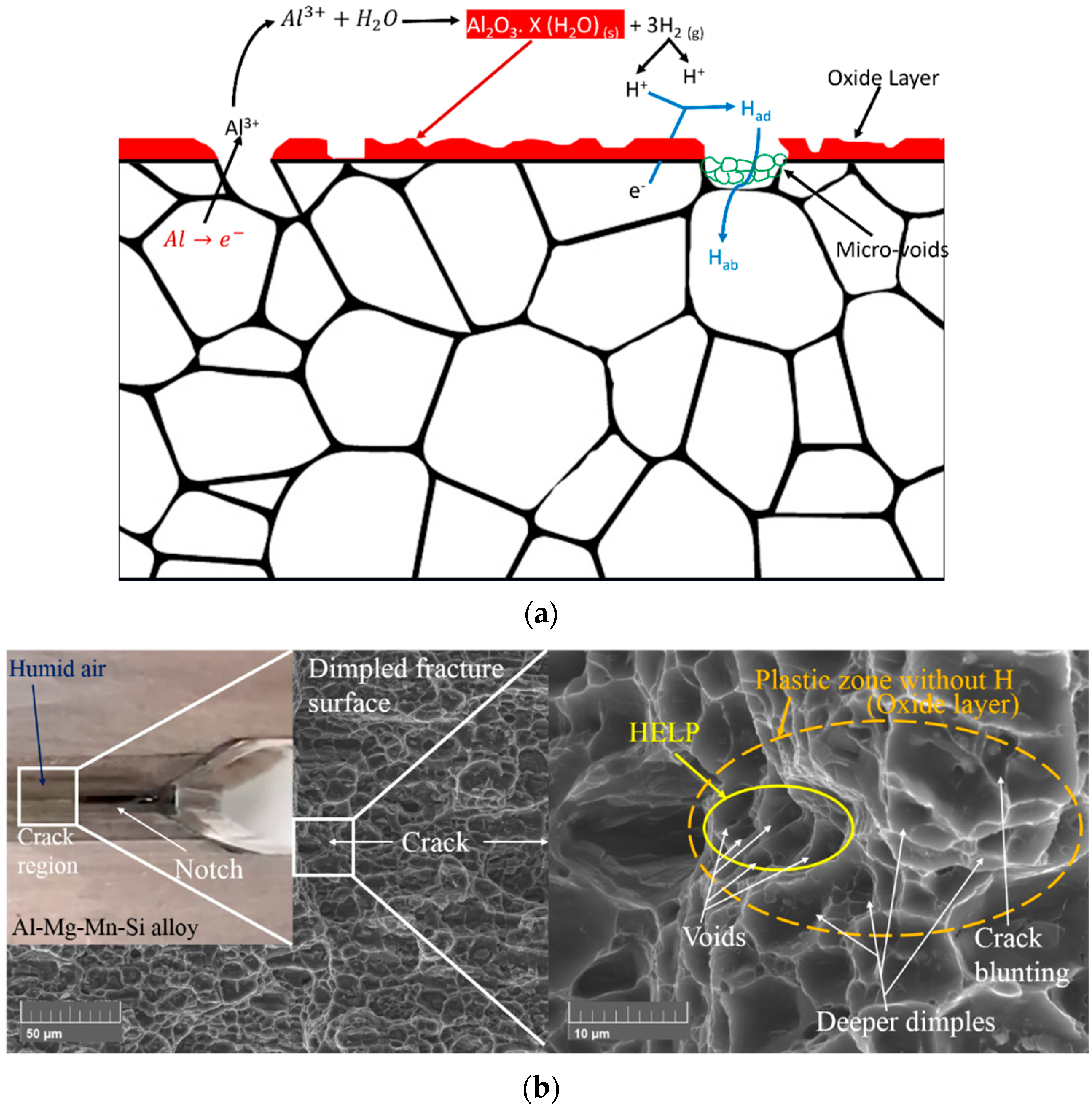
3.3. Fracture Surface Morphology
4. Validation of Results
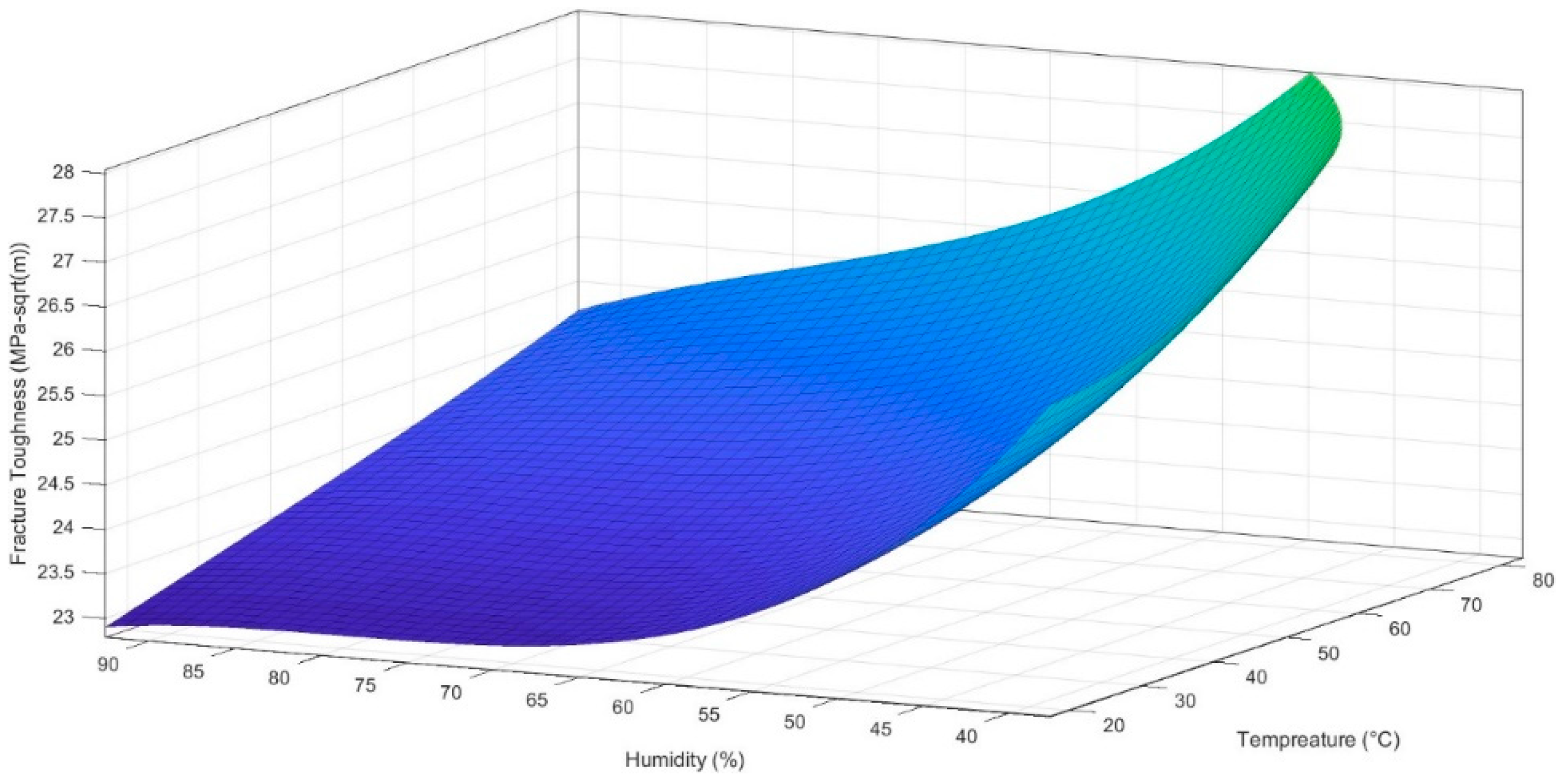
4.1. Empirical Modelling
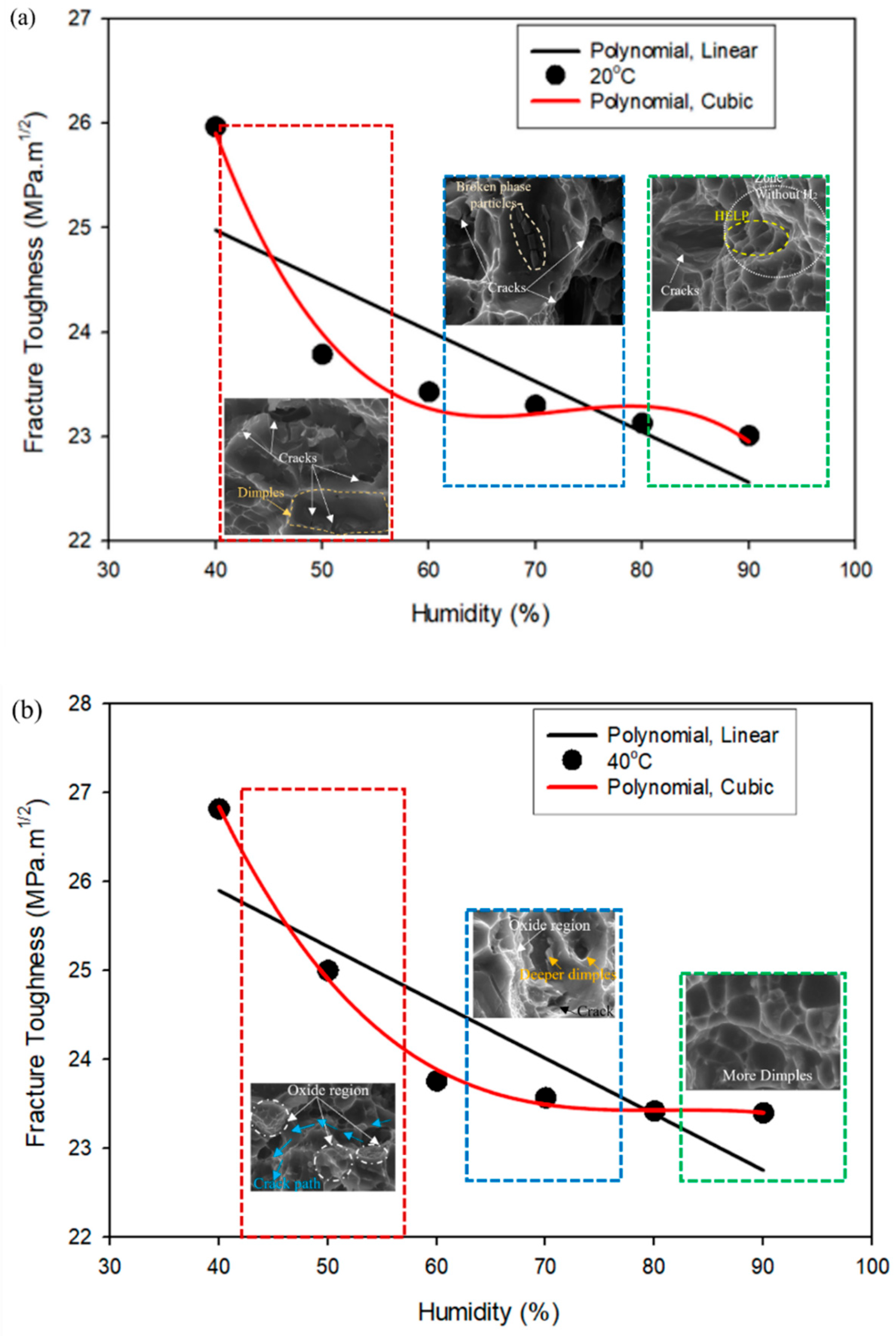
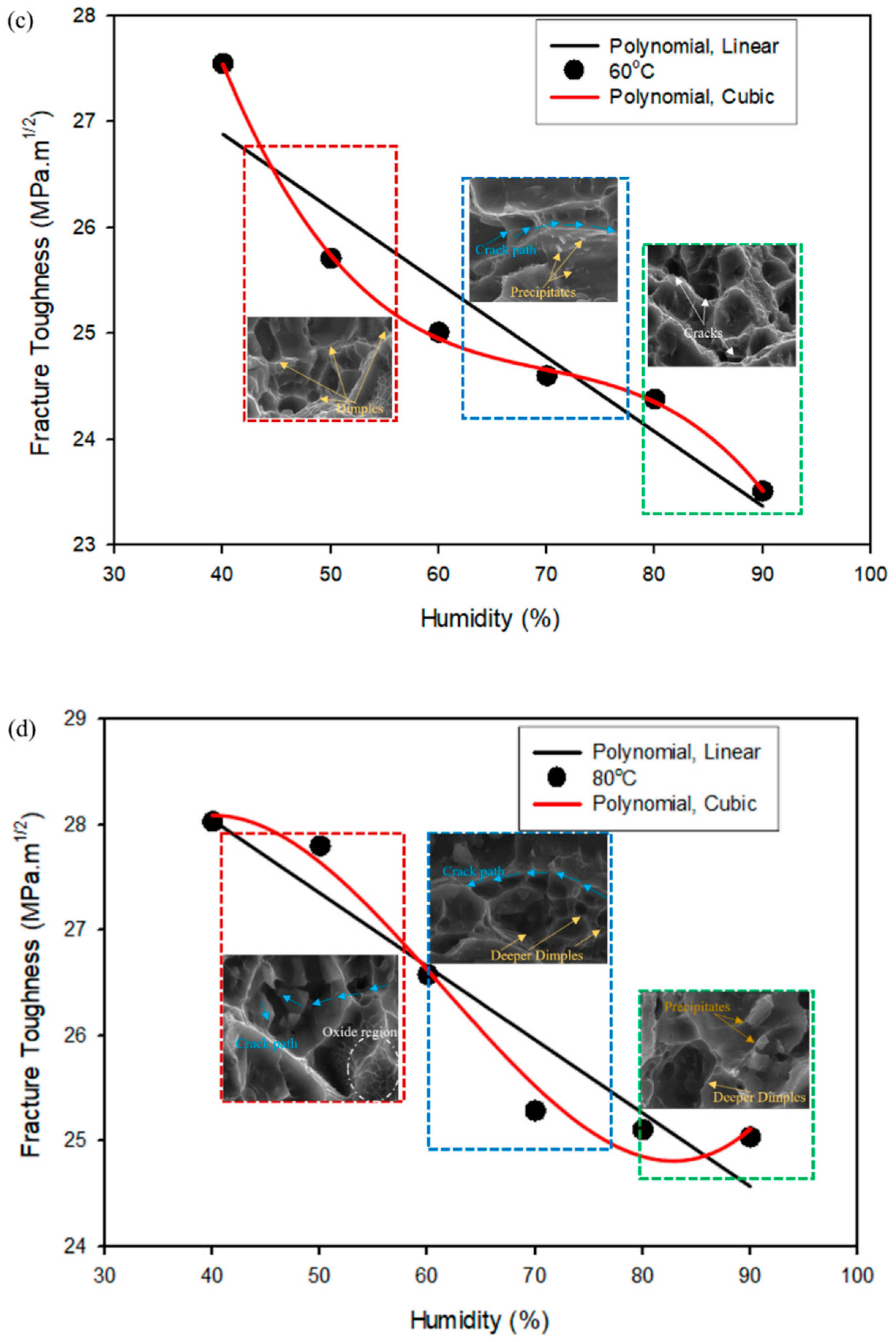
4.2. Mapping of Curve-Fitting Graphs with Micrographs
4.3. Comparison of Experimental and Curve-Fitting Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Zhang, X.; Liu, Y.; Li, X.; Chen, J.; Zhang, X.; Gao, L. Environmental Failure Behavior Analysis of 7085 High Strength Aluminum Alloy under High Temperature and High Humidity. Metals 2022, 12, 968. [Google Scholar] [CrossRef]
- Liang, M.; Melchers, R.; Chaves, I. Corrosion and Pitting of 6060 Series Aluminium after 2 Years Exposure in Seawater Splash, Tidal and Immersion Zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Yang, X.K.; Zhang, L.W.; Zhang, S.Y.; Liu, M.; Zhou, K.M. Properties Degradation and Atmospheric Corrosion Mechanism of 6061 Aluminum Alloy in Industrial and Marine Atmosphere Environments. Mater. Corros. 2017, 68, 529–535. [Google Scholar] [CrossRef]
- Peng, C.; Cao, G.; Gu, T.; Wang, C.; Wang, Z.; Sun, C. The Corrosion Behavior of the 6061 Al Alloy in Simulated Nansha Marine Atmosphere. J. Mater. Res. Technol. 2022, 19, 709–721. [Google Scholar] [CrossRef]
- Algahtani, A.; Mahmoud, E.R.I.; Khan, S.Z.; Tirth, V. Experimental Studies on Corrosion Behavior of Ceramic Surface Coating Using Different Deposition Techniques on 6082-T6 Aluminum Alloy. Processes 2018, 6, 240. [Google Scholar] [CrossRef]
- Alqahtani, I.M.; Starr, A.; Khan, M. Experimental and Theoretical Aspects of Crack Assisted Failures of Metallic Alloys in Corrosive Environments–A Review. Mater. Today Proc. 2022, 66, 2530–2535. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and Corrosion Behavior of Al/SiC Metal Matrix Composites. Ain. Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, C.; Yang, L.; Bai, D.; Olugbade, E.O.; Huang, G. zhe Corrosion Behaviour and Mechanism of 6082 Aluminium Alloy in NaCl and Na2SO4 Etchants. Mater. Corros. 2020, 71, 392–400. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Bjerrum, N. Chemistry, Electrochemistry, And Electrochemical Applications|Aluminum. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 695–708. [Google Scholar]
- Cicolin, D.; Trueba, M.; Trasatti, S. Effect of Chloride Concentration, PH and Dissolved Oxygen, on the Repassivation of 6082-T6 Al Alloy. Electrochim. Acta 2014, 124, 27–35. [Google Scholar] [CrossRef]
- Cao, M.; Liu, L.; Fan, L.; Yu, Z.; Li, Y.; Oguzie, E.E.; Wang, F. Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments. Materials 2018, 11, 235. [Google Scholar] [CrossRef]
- Vargel, C. Chapter D.1-Freshwater. In Corrosion of Aluminium; Vargel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 299–327. ISBN 9780080444956. [Google Scholar]
- Ahmad, Z. Chapter 10-Atmospheric Corrosion. In Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann: Oxford, UK, 2006; pp. 550–575. ISBN 9780750659246. [Google Scholar]
- Zheng, Y.-Y.; Luo, B.H.; He, C.; Gao, Y.; Bai, Z.-H. Corrosion Evolution and Behaviour of Al–2.1Mg–1.6Si Alloy in Chloride Media. Rare Met. 2021, 40, 908–919. [Google Scholar] [CrossRef]
- Otieno, M.T.M. Marine Exposure Environments and Marine Exposure Sites. In Marine Concrete Structures; Alexander, M.G., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 171–196. [Google Scholar]
- Amura, M.; Aiello, L.; Colavita, M.; De Paolis, F.; Bernabei, M. Failure of a Helicopter Main Rotor Blade. Procedia Mater. Sci. 2014, 3, 726–731. [Google Scholar] [CrossRef]
- Romeyn, A. Main Rotor Blade Failure Analysis Report. 2005. Available online: https://www.atsb.gov.au/publications/investigation_reports/2016/aair/ao-2016-174 (accessed on 11 May 2023).
- Sunrise Helicopters Inc. In-Flight Separation of Main Rotor Blade and Collision with Terrain; Transport Safety Board Canada: Kapusakasing, ON, Canada, 2011. [Google Scholar]
- Francese, A.; Khan, M.; He, F. Role of Dynamic Response in Inclined Transverse Crack Inspection for 3D-Printed Polymeric Beam with Metal Stiffener. Materials 2023, 16, 3095. [Google Scholar] [CrossRef]
- Moreira, P.M.G.P.; Jesus, A.M.P.; Ribeiro, A.S.; Castro, P.M.S.T. Fatigue Crack Growth in Friction Stir Welds of 6082-T6 and 6061-T6 Aluminium Alloys: A Comparison. Theor. Appl. Fract. Mech. 2008, 50, 81–91. [Google Scholar] [CrossRef]
- Moreira, P.M.G.P.; Santos, T.; Tavares, S.M.O.; Richter-Trummer, V.; Vilaça, P.; Castro, P.M.S.T. Mechanical and Metallurgical Characterization of Friction Stir Welding Joints of AA6061-T6 with AA6082-T6. Mater. Des. 2009, 30, 180–187. [Google Scholar] [CrossRef]
- Abdellah, M.Y. Essential Work of Fracture Assessment for Thin Aluminium Strips Using Finite Element Analysis. Eng. Fract. Mech. 2017, 179, 190–202. [Google Scholar] [CrossRef]
- Gilioli, A.; Manes, A.; Giglio, M. Numerical Simulation of a Fracture Toughness Test of an Al6061-T6 Aluminium Alloy Using a Ductile Criterion. Mech. Res. Commun. 2014, 58, 2–9. [Google Scholar] [CrossRef]
- ASTME399-22; Standard Test Method for Linear-Elastic Plane Strain Fracture Toughness KIc of Metallic Materials. ASTM International: West Conchhocken, PA, USA, 2022.
- Dietzel, W.; Srinivasan, P.B.; Atrens, A. 3-Testing and Evaluation Methods for Stress Corrosion Cracking (SCC) in Metals. In Metals and Surface Engineering, Stress Corrosion Cracking; Raja, V.S., Shoji, T., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 133–166. [Google Scholar]
- Zhu, X.K.; Joyce, J.A. Review of Fracture Toughness (G, K, J, CTOD, CTOA) Testing and Standardization. Eng. Fract. Mech. 2012, 85, 1–46. [Google Scholar] [CrossRef]
- Ogawa, T.; Hasunuma, S.; Sirawachi, T.; Fukada, N. Effect of Chemical Composition and Relative Humidity on the Humid Gas Stress Corrosion Cracking of Aluminum Alloys. J. High Press. Inst. Jpn. 2019, 57, 24–33. [Google Scholar] [CrossRef]
- Safyari, M.; Hojo, T.; Moshtaghi, M. Effect of Environmental Relative Humidity on Hydrogen-Induced Mechanical Degradation in an Al–Zn–Mg–Cu Alloy. Vacuum 2021, 192, 110489. [Google Scholar] [CrossRef]
- Schwarzenböck, E.; Wiehler, L.; Heidenblut, T.; Hack, T.; Engel, C.; Maier, H.J. Crack Initiation of an Industrial 7XXX Aluminum Alloy in Humid Air Analyzed via Slow Strain Rate Testing and Constant Displacement Testing. Mater. Sci. Eng. A 2021, 804, 140776. [Google Scholar] [CrossRef]
- Lynch, S. Discussion of Some Recent Literature on Hydrogen-Embrittlement Mechanisms: Addressing Common Misunderstandings. Corros. Rev. 2019, 37, 377–395. [Google Scholar] [CrossRef]
- Sergeev, N.N.; Kutepov, S.N.; Kolmakov, A.G.; Gvozdev, A.E. Mechanism of the Hydrogen Cracking of Metals and Alloys, Part II (Review). Inorg. Mater. Appl. Res. 2019, 10, 32–41. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Zhang, D.; Li, K.; Gao, L.; Lin, T. Electrochemical and DFT Studies of Quinoline Derivatives on Corrosion Inhibition of AA5052 Aluminium Alloy in NaCl Solution. Appl. Surf. Sci. 2015, 357, 2176–2183. [Google Scholar] [CrossRef]
- Pu, J.; Zhang, Y.; Zhang, X.; Yuan, X.; Ren, P.; Jin, Z. Mapping the Fretting Corrosion Behaviors of 6082 Aluminum Alloy in 3.5% NaCl Solution. Wear 2021, 482–483, 203975. [Google Scholar] [CrossRef]
- Gholami, M.D.; Hashemi, R.; Sedighi, M. The Effect of Temperature on the Mechanical Properties and Forming Limit Diagram of Aluminum Strips Fabricated by Accumulative Roll Bonding Process. J. Mater. Res. Technol. 2020, 9, 1831–1846. [Google Scholar] [CrossRef]
- Mouritz, A.P. 19-Fracture Toughness Properties of Aerospace Materials. In Introduction to Aerospace Materials; Mouritz, A.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 454–468. ISBN 9781855739468. [Google Scholar]
- L’haridon-Quaireau, S.; Laot, M.; Colas, K.; Kapusta, B.; Delpech, S.; Gosset, D. Effects of Temperature and PH on Uniform and Pitting Corrosion of Aluminium Alloy 6061-T6 and Characterisation of the Hydroxide Layers. J. Alloys Compd. 2020, 833, 155146. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.-H.; Ma, E.; Han, W.-Z. Relative Mobility of Screw versus Edge Dislocations Controls the Ductile-to-Brittle Transition in Metals. Proc. Natl. Acad. Sci. USA 2021, 118, e2110596118. [Google Scholar] [CrossRef]
- Abdellah, M.Y.; Fadhl, B.M.; Abu El-Ainin, H.M.; Hassan, M.K.; Backar, A.H.; Mohamed, A.F. Experimental Evaluation of Mechanical and Tribological Properties of Segregated Al-Mg-Si Alloy Filled with Alumina and Silicon Carbide through Different Types of Casting Molds. Metals 2023, 13, 316. [Google Scholar] [CrossRef]
- Gamal, A.; EL-Mahdy, K.B.K. AC Impedance Study on the Atmospheric Corrosion of Aluminum under Periodic Wet–Dry Conditions. Electrochim. Acta 2004, 49, 1937–1948. [Google Scholar] [CrossRef]
- Piippo, T.J.; Laitinen, P.S. Corrosion Behaviour of Zinc and Aluminium in Simulated Nuclear Accident Environments; STUK-YTO-TR-123; Finnish Centre for Radiation and Nuclear Safety: Helsinki, Finland, 1997; ISBN 951-712-177-6. [Google Scholar]
- Dai, X.; Wang, H.; Ju, L.-K.; Cheng, G.; Cong, H.; Newby, B.-M.Z. Corrosion of Aluminum Alloy 2024 Caused by Aspergillus Niger. Int. Biodeterior. Biodegrad. 2016, 115, 1–10. [Google Scholar] [CrossRef]
- Young, G.A.; Scully, J.R. The Effects of Test Temperature, Temper, and Alloyed Copper on the Hydrogen-Controlled Crack Growth Rate of an Al-Zn-Mg-(Cu) Alloy. Met. Mater. Trans. A 2002, 33, 1167–1181. [Google Scholar] [CrossRef]
- Abdellah, M.Y. Abdellah Ductile Fracture and S–N Curve Simulation of a 7075-T6 Aluminum Alloy under Static and Constant Low-Cycle Fatigue. J Fail. Anal. Preven. 2021, 21, 1476–1488. [Google Scholar] [CrossRef]
- He, F.; Khan, M.; Aldosari, S. Interdependencies between Dynamic Response and Crack Growth in a 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Cantilever Beam under Thermo-Mechanical Loads. Polymers 2022, 14, 982. [Google Scholar] [CrossRef]
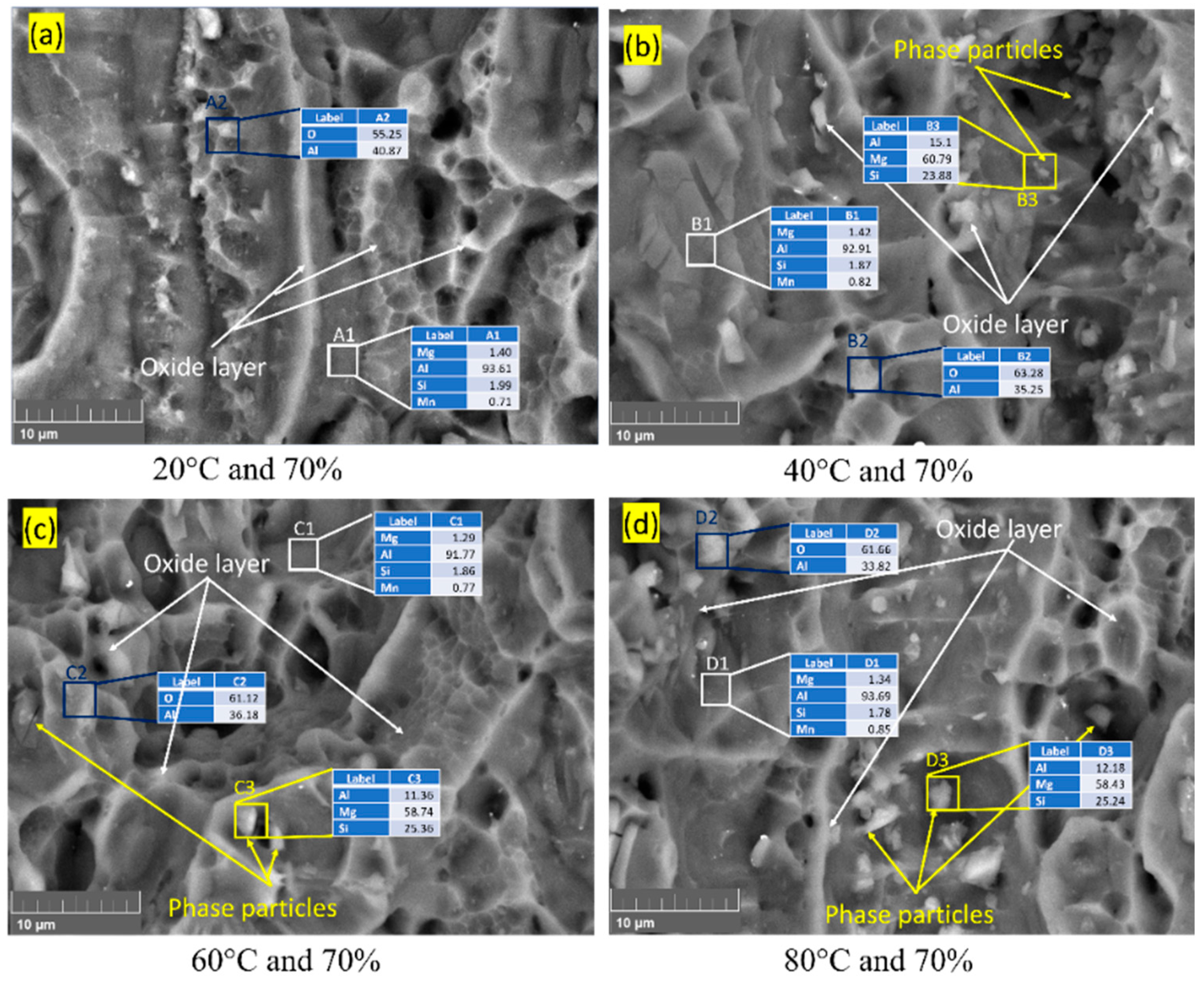
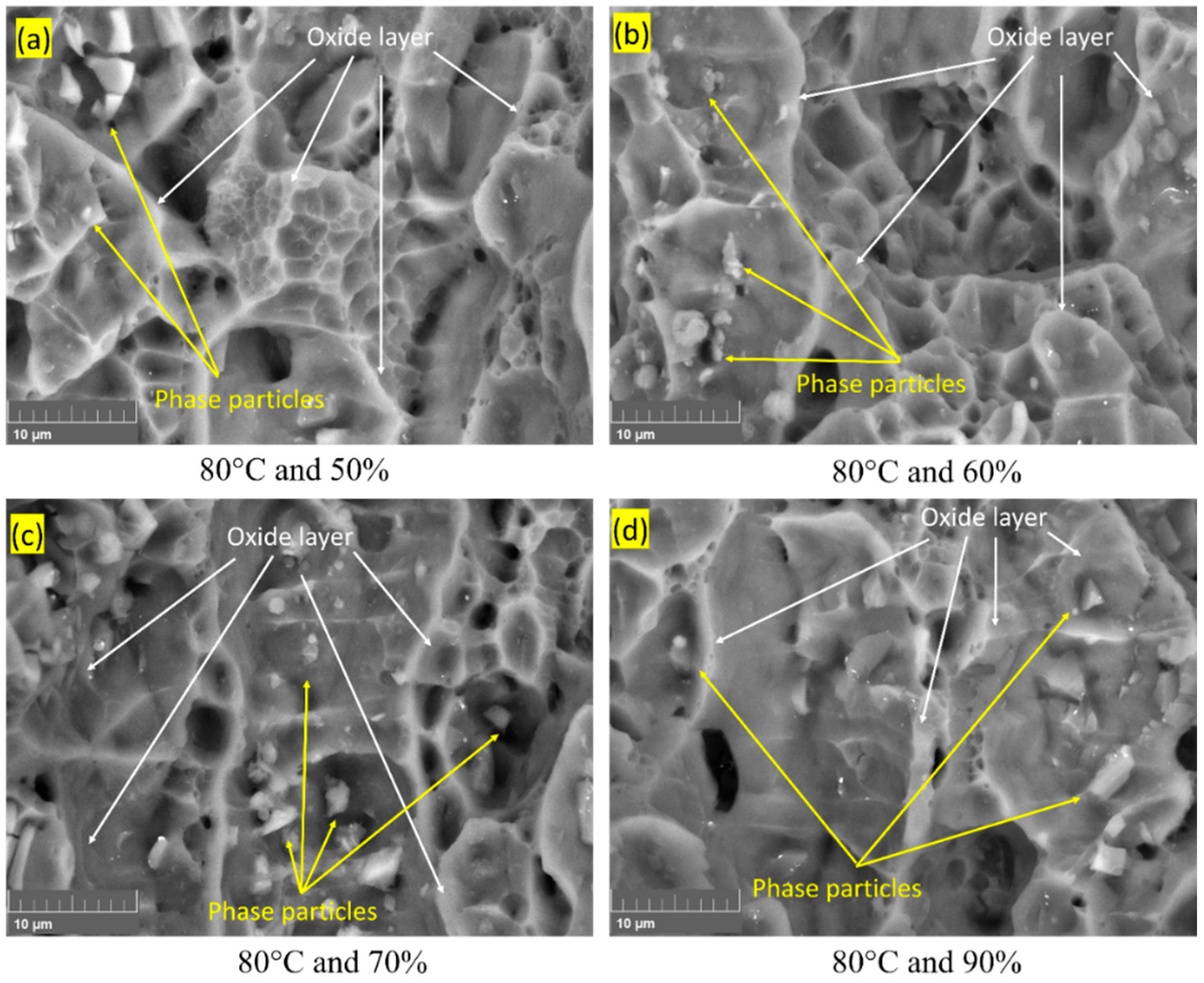
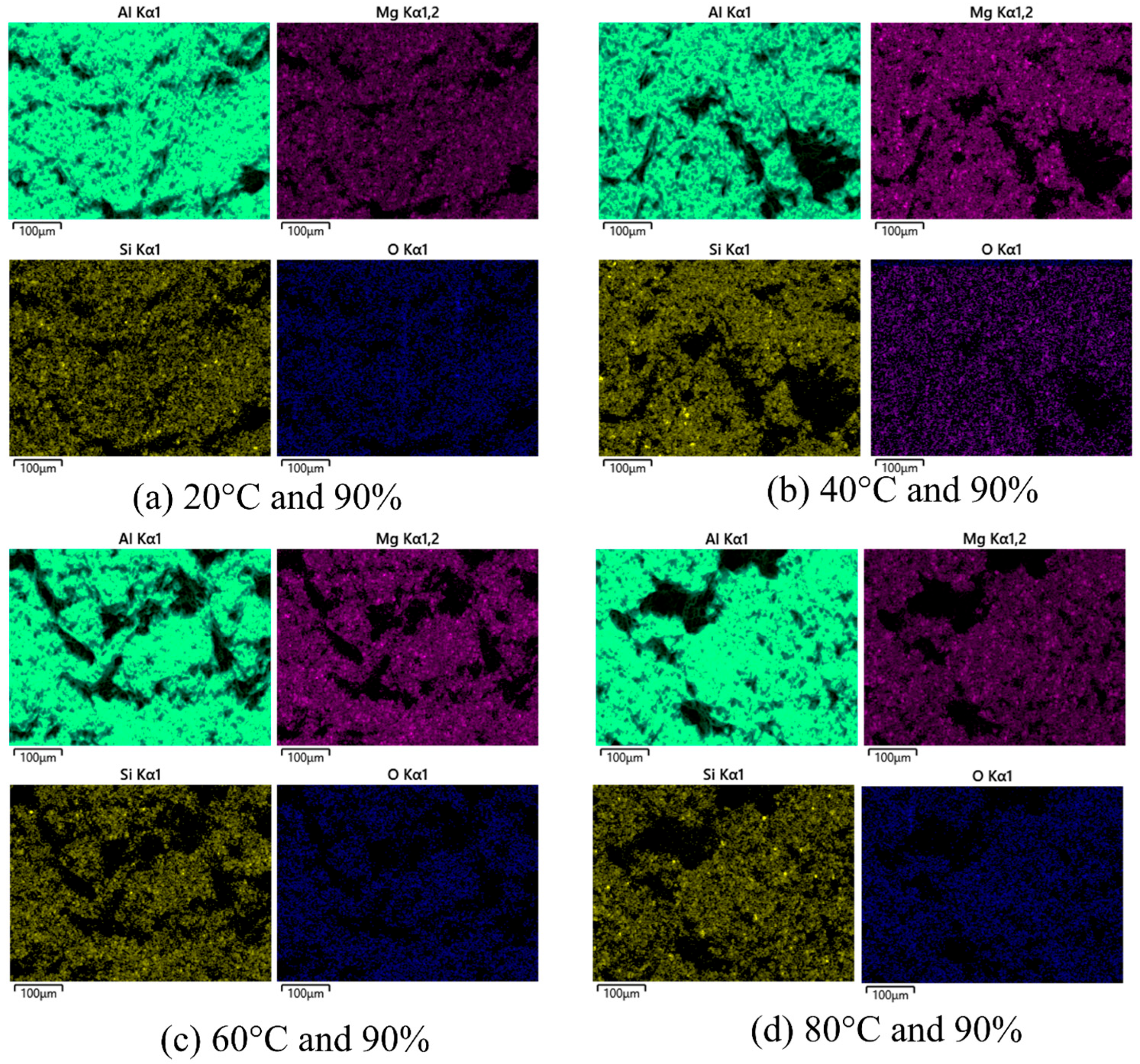
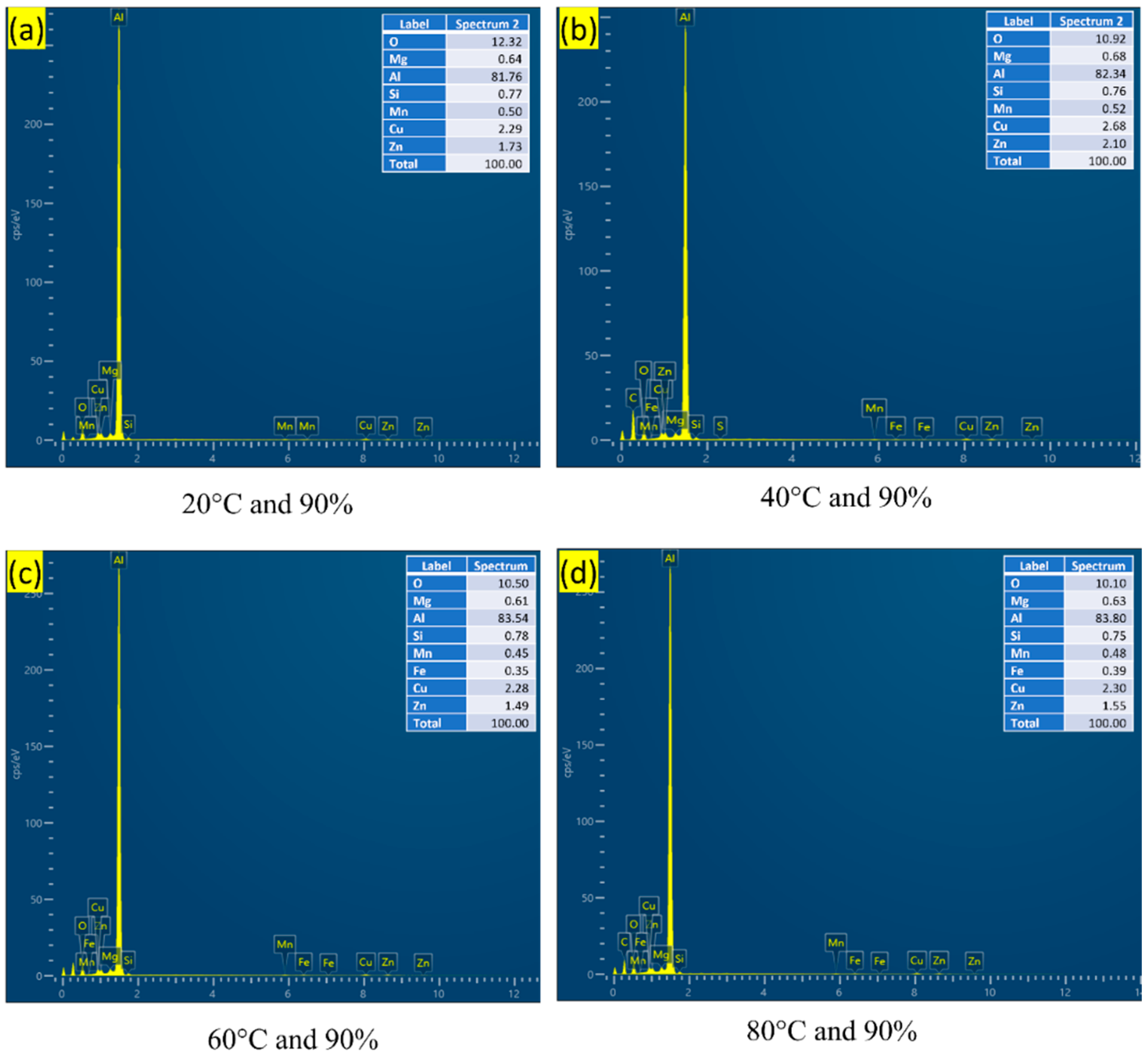
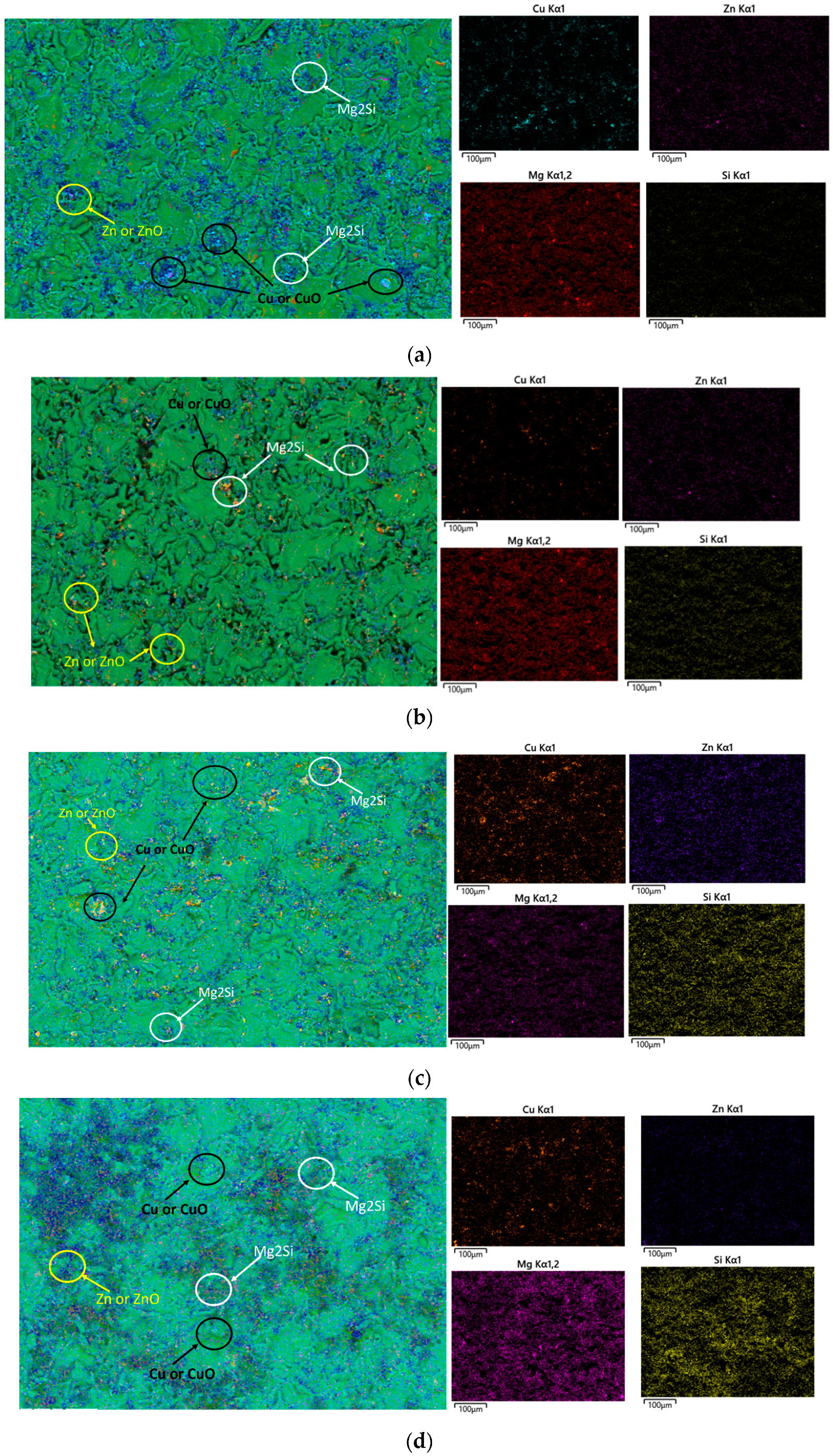


| Element | Cu | Cr | Mn | Mg | Zn | Ti | Si | Fe | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | 0.1 | 0.08 | 0.9 | 1.2 | 0.2 | 0.2 | 1.1 | 0.5 | Balance |
| Sl. No. | Temperature | Humidity | Experimental KIc (MPa.m1/2) | Empirical Model KIc (MPa.m1/2) (Equation (5)) | % Error |
|---|---|---|---|---|---|
| 1 | 20 | 50 | 23.79 | 24.16 | 1.53 |
| 2 | 20 | 60 | 23.43 | 23.37 | −0.26 |
| 3 | 20 | 70 | 23.30 | 23.10 | −0.86 |
| 4 | 20 | 80 | 23.13 | 23.07 | −0.26 |
| 5 | 20 | 90 | 23.01 | 23.01 | −0.01 |
| 6 | 40 | 50 | 25.00 | 24.71 | −1.14 |
| 7 | 40 | 60 | 23.76 | 23.89 | 0.53 |
| 8 | 40 | 70 | 23.57 | 23.60 | 0.12 |
| 9 | 40 | 80 | 23.42 | 23.56 | 0.59 |
| 10 | 40 | 90 | 23.40 | 23.50 | 0.44 |
| 11 | 60 | 50 | 25.71 | 25.87 | 0.61 |
| 12 | 60 | 60 | 25.00 | 24.87 | −0.49 |
| 13 | 60 | 70 | 24.60 | 24.43 | −0.73 |
| 14 | 60 | 80 | 24.38 | 24.25 | −0.54 |
| 15 | 60 | 90 | 24.01 | 24.05 | 0.18 |
| 16 | 80 | 50 | 27.80 | 27.67 | −0.46 |
| 17 | 80 | 60 | 26.58 | 26.37 | −0.80 |
| 18 | 80 | 70 | 25.29 | 25.62 | 1.29 |
| 19 | 80 | 80 | 25.11 | 25.16 | 0.20 |
| 20 | 80 | 90 | 25.04 | 24.69 | −1.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, I.; Starr, A.; Khan, M. Coupled Effects of Temperature and Humidity on Fracture Toughness of Al–Mg–Si–Mn Alloy. Materials 2023, 16, 4066. https://doi.org/10.3390/ma16114066
Alqahtani I, Starr A, Khan M. Coupled Effects of Temperature and Humidity on Fracture Toughness of Al–Mg–Si–Mn Alloy. Materials. 2023; 16(11):4066. https://doi.org/10.3390/ma16114066
Chicago/Turabian StyleAlqahtani, Ibrahim, Andrew Starr, and Muhammad Khan. 2023. "Coupled Effects of Temperature and Humidity on Fracture Toughness of Al–Mg–Si–Mn Alloy" Materials 16, no. 11: 4066. https://doi.org/10.3390/ma16114066
APA StyleAlqahtani, I., Starr, A., & Khan, M. (2023). Coupled Effects of Temperature and Humidity on Fracture Toughness of Al–Mg–Si–Mn Alloy. Materials, 16(11), 4066. https://doi.org/10.3390/ma16114066






