Yttrium and Niobium Elements Co-Doping and the Formation of Double Perovskite Structure Ba2YNbO6 in BCZT
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation
2.2. Characterization
3. Result and Discussion
3.1. XPS (Defect Chemistry Analysis)
3.2. Phase and Structure
3.3. Microscopic Structure
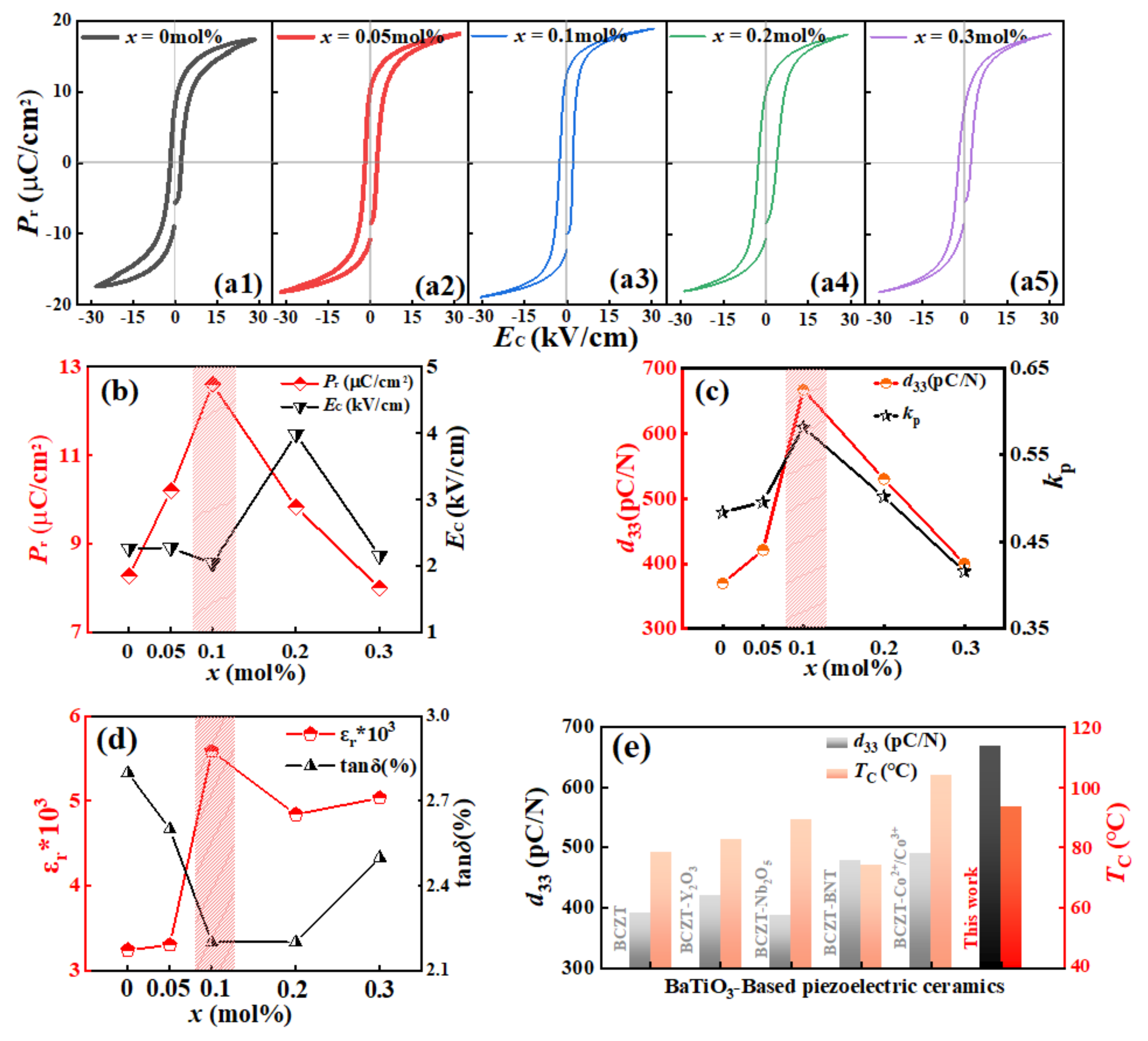
3.4. Electrical Properties
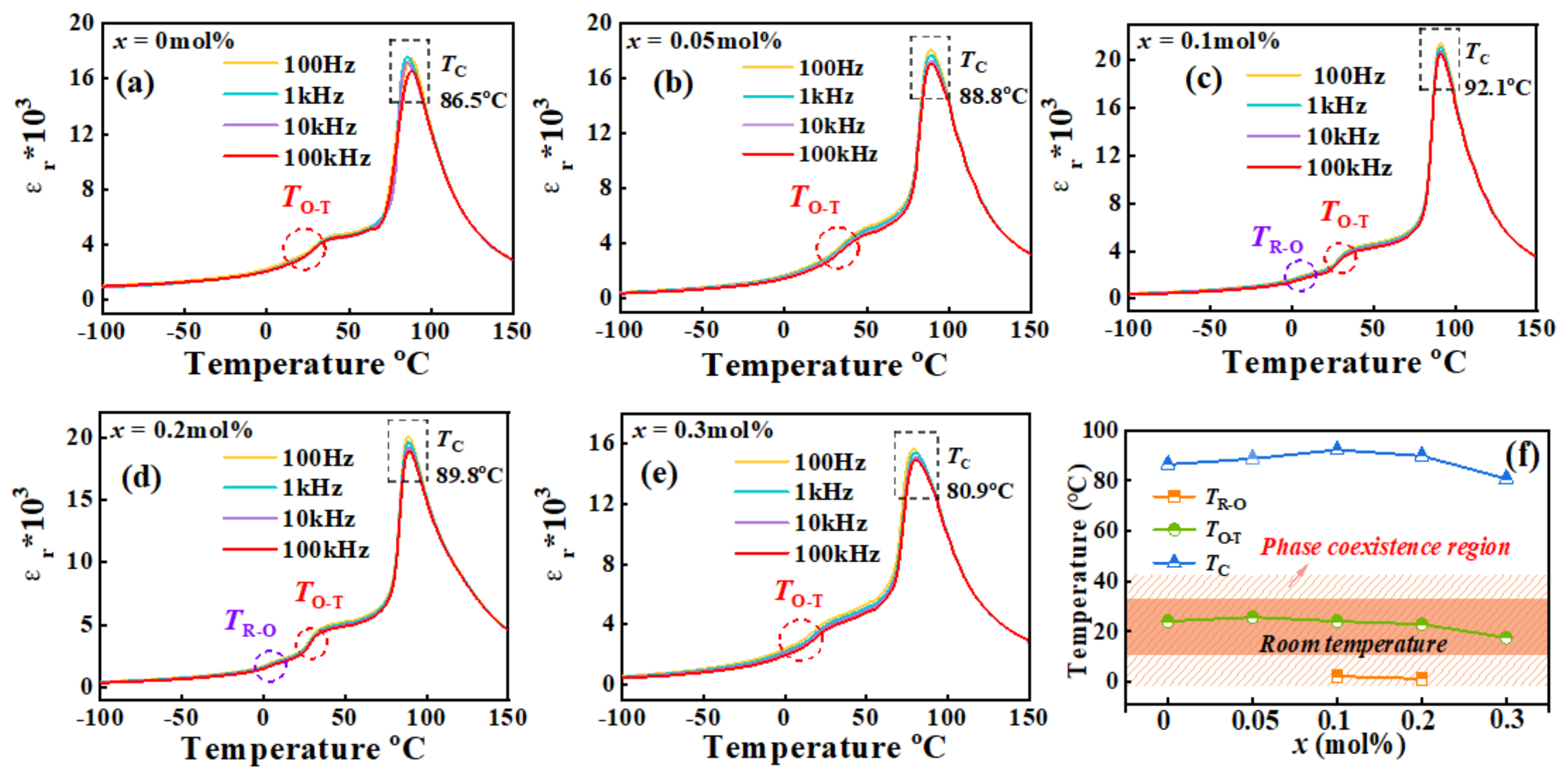
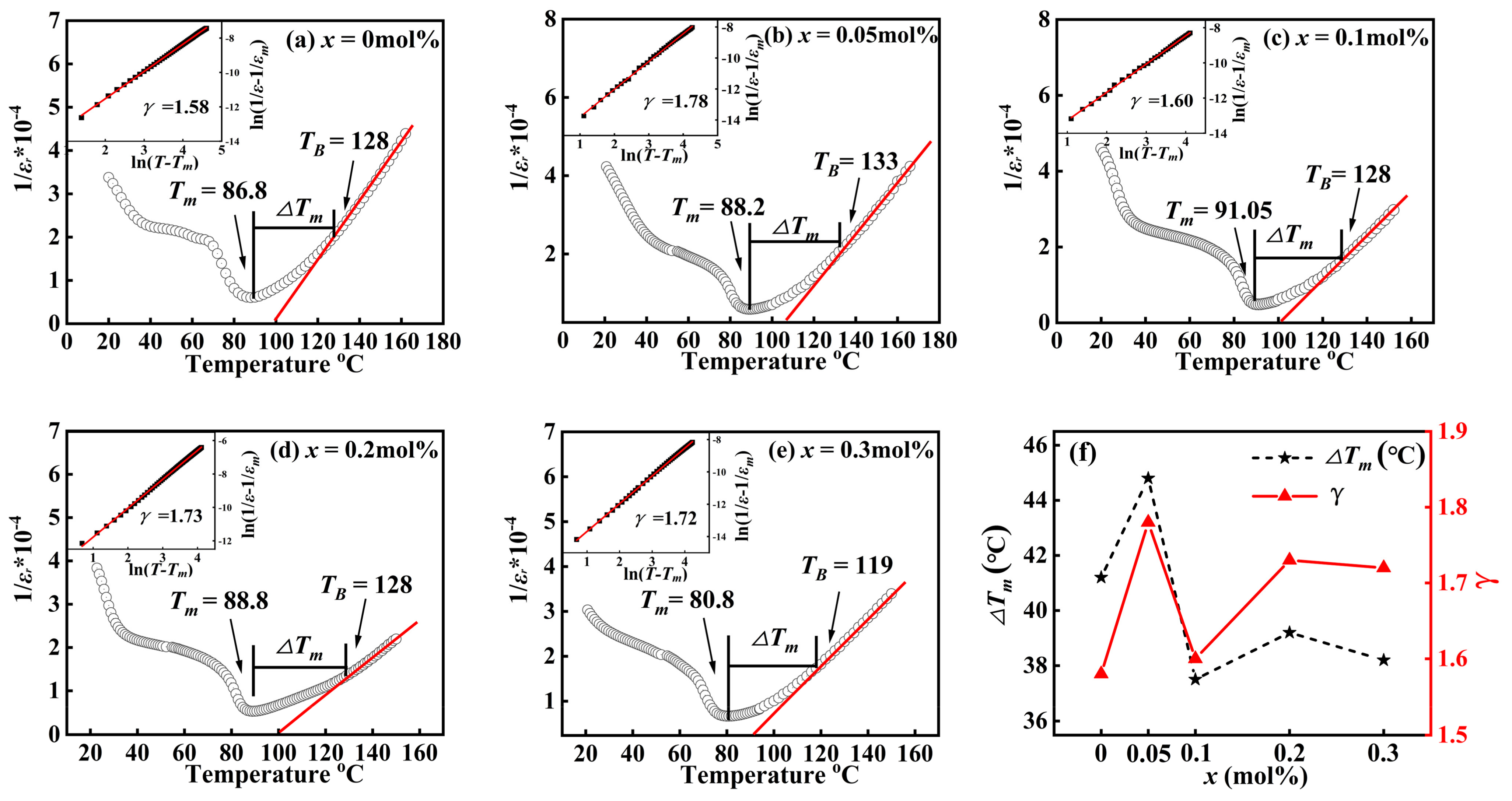
3.5. Dielectric Relaxation Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cross, E. Materials science: Lead-free at last. Nature 2004, 432, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wu, J.; Xiao, D.; Zhu, J. Recent development in lead-free perovskite piezoelectric bulk materials. Prog. Mater. Sci. 2018, 98, 552–624. [Google Scholar] [CrossRef]
- Liu, W.F.; Ren, X.B. Large piezoelectric effect in Pb-free ceramics. Phys. Rep. 2009, 103, 257602. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Wang, C.; Luo, S.; Zheng, D. Effects of BNT precursor prepared by molten salt method on BCZT-based lead-free piezoelectric ceramics. J. Mater. Sci. 2022, 3, 5456–5467. [Google Scholar] [CrossRef]
- Wang, X.; Huan, Y.; Ji, S.; Zhu, Y.; Wei, T.; Cheng, Z. Ultra-high piezoelectric performance by rational tuning of heterovalention doping in lead-free piezoelectric ceramics. Nano Energy 2022, 101, 107580. [Google Scholar] [CrossRef]
- Jaiban, P.; Theethuan, T.; Khumtrong, S.; Lokakaew, S.; Watcharapasorn, A. The effects of donor (Nb5+) and acceptor (Cu2+, Zn2+, Mn2+, Mg2+) doping at B-site on crystal structure, microstructure, and electrical properties of (Ba0.85Ca0.15)Zr0.1Ti0.9O3 ceramics. J. Alloys Compd. 2022, 899, 162909. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Cai, W.; Zhou, C.; Gao, R.; Chen, G.; Deng, X.; Wang, Z.; Fu, C. Enhanced ferroelectric and piezoelectric responses of (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 ceramics by Tm3+ amphoteric substitution. Mater. Chem. Phys. 2020, 25, 123242. [Google Scholar] [CrossRef]
- Mittal, S.; Laishram, R.; Singh, K.C. Multi-phase coexistence in yttrium-substituted Ba0.9Ca0.1Zr0.07Ti0.93O3lead-free piezoceramics. Ceram. Int. 2019, 45, 1237–1245. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Bai, W.; Xu, Z.; Chu, R.; Zhai, J. Enhancement of the temperature stabilities in yttrium doped (Ba0.99Ca0.01)(Ti0.98Zr0.02)O3 ceramics. J. Alloys Compd. 2012, 531, 46–49. [Google Scholar] [CrossRef]
- He, L.; Ji, Y.; Ren, S.; Zhao, L.; Luo, H.; Liu, C.; Hao, Y.; Zhang, L.; Zhang, L.; Ren, X. Large piezoelectric coefficient with enhanced thermal stability in Nb5+-doped Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics. Ceram. Int. 2020, 46, 3236–3241. [Google Scholar] [CrossRef]
- Parjansri, P.; Intatha, U.; Eitssayeam, S. Dielectric, ferroelectric and piezoelectric properties of Nb5+ doped BCZT ceramics. Mater. Res. Bull. 2015, 65, 61–67. [Google Scholar] [CrossRef]
- Zulhadjri, T.P.; Wendari, M.; Ikhram, Y.E.; Putri, U. Septiani, Imelda, Enhanced dielectric and ferroelectric responses in La3+/Ti4+ co-substituted SrBi2Ta2O9 Aurivillius phase. Ceram. Int. 2022, 48, 10328–10332. [Google Scholar] [CrossRef]
- Xia, L.; Hu, T.; Liu, H.; Xie, J.; Asif, S.U.; Xiong, F.; Hu, W. B-site Y3+ assisted charge compensation strategy to synthesize Eu3+ doped Ruddlesden-Popper Ca2SnO4 perovskite and photoluminescence properties. J. Alloys Compd. 2020, 845, 156131. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Verma, R.; Chauhan, A.; Batoo, K.M.; Kumar, R.; Hadi, M.; Raslan, E.H. Structural, morphological, and optical properties of strontium doped lead-free BCZT ceramics. Ceram. Ceram. Int. 2021, 47, 15442–15457. [Google Scholar] [CrossRef]
- Wegmann, M.; Watson, L.; Hendry, A. XPS Analysis of Submicrometer Barium Titanate Powder. J. Am. Ceram. Soc. 2004, 87, 371–377. [Google Scholar] [CrossRef]
- Miao, Z.; Chen, L.; Zhou, F.; Wang, Q. Modulation of Resistive Switching Characteristics for Individual BaTiO3 Microber by Surface Oxygen Vacancies. J. Phys. D. Appl. Phys. 2017, 51, 025107. [Google Scholar] [CrossRef]
- Clabel, H.J.L.; Awan, I.T.; Calderón, G.L.; Pereira-da-Silva, M.A.; Romano, R.A.; Rivera, V.A.G.; Ferreira, S.O.; Marega, E., Jr. Understanding the electronic properties of BaTiO3 and Er doped BaTiO3 films through confocal scanning microscopy and XPS: Role of oxygen vacancy. Phys. Chem. Chem. Phys. 2020, 22, 15022–15034. [Google Scholar] [CrossRef]
- Cai, E.; Liu, Q. A correlative study between the microstructure and electrical properties of lead-free piezoceramics (1-x)BHT-xYLG driven by the synergistic action of multiple factors. J. Eur. Ceram. Soc. 2022, 42, 4539–4555. [Google Scholar] [CrossRef]
- Howard, C.J.; Barnes, P.W.; Kennedy, B.J.; Woodward, P.M. Structures of the ordered double perovskites Sr2YTaO6 and Sr2YNbO6. Acta Crystallogr. B. 2005, 61, 258–262. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Zhang, B. Dielectric and piezoelectric properties of (Ba0.95Ca0.05)(Ti0.88Zr0.12)O3 ceramics sintered in a protective atmosphere. J. Eur. Ceram. Soc. 2009, 29, 3235–3242. [Google Scholar] [CrossRef]
- Clabel, H.J.L.; Awan, I.T.; Rivera, V.A.G.; Nogueira, I.C.; Pereira-da-Silva, M.A.; Siu Lia, M.; Ferreira, S.O.; Marega, E., Jr. Growth process and grain boundary defects in Er doped BaTiO3 processed by EB-PVD: A study by XRD, FTIR, SEM and AFM. Appl. Surf. Sci. 2019, 493, 982–993. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Q.; Gao, J.; Liu, W.; Ding, L.; Yin, S. Structures and luminescence properties of Yb3+ in the double perovskites Ba2YB’ O6 (B’ =Ta5+, Nb5+). Chin. Phys. B. 2011, 20, 016101. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, M.; He, Y.; Zhu, M.; Hou, Y. Origin of superior dielectric and piezoelectric properties in 0.4Ba(Zr0.2Ti0.8)O3-0.6(Ba0.7Ca0.3)TiO3 at intermediate grain sizes. J. Eur. Ceram. Soc. 2020, 40, 3936–3945. [Google Scholar] [CrossRef]
- Zheng, W.; Lin, J.; Liu, X.; Yang, W.; Li, Y. Enhanced ferroelectric and piezoelectric performance of (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free ceramics upon Ce and Sb co-doping. RSC Adv. 2021, 11, 2616–2623. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, L.; Dou, W.; Xu, Q.; Wei, Z.; Qin, Y. Electrospinning lead-free 0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3 nanowires and their application in energy harvesting. J. Mater. Chem. A 2013, 1, 7332–7338. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Chen, W. Li-modified Ba0.99Ca0.01Zr0.02Ti0.98O3 lead-free ceramics with highly improved piezoelectricity. J. Alloys Compd. 2017, 694, 745–751. [Google Scholar] [CrossRef]
- Shi, S.; Hashimoto, H.; Sekino, T. Enhancing piezoelectric properties of Ba0.88Ca0.12Zr0.12Ti0.88O3 lead-free ceramics by doping Co ions. Ceram. Int. 2021, 47, 3272–3278. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Qiu, J.; Zhao, T.; Ding, J.; Jia, X.; Zhu, W.; Xu, J. Y2O3 doped Ba0.9Ca0.1Ti0.9Sn0.1O3 ceramics with improved piezoelectric properties. J. Eur. Ceram. Soc. 2018, 38, 1349–1355. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Li, Q.; Gao, R.; Chen, G.; Deng, X.; Wang, Z.; Cao, X.; Fu, C. Enhanced piezoelectric response of (Ba,Ca)(Ti, Zr)O3 ceramics by super large grain size and construction of phase boundary. J. Alloys Compd. 2019, 794, 542–552. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, N.; Wu, J.; Zhang, X. The role of adding Bi0.5A0.5ZrO3 in affecting orthorhombic-tetragonal phase transition temperature and electrical properties in potassium sodium niobate ceramics. Acta Mater. 2020, 197, 224–234. [Google Scholar] [CrossRef]
- Jiang, L.; Tan, Z.; Xie, L.; Li, Y.; Xing, J.; Wu, J.; Chen, Q.; Xiao, D.; Zhu, J. Study of the relationships among the crystal structure, phase transition behavior and macroscopic properties of modified (K,Na)NbO3-based lead-free piezoceramics. J. Eur. Ceram. Soc. 2018, 38, 2335–2343. [Google Scholar] [CrossRef]
- Hwang, H.J.; Nagai, T.; Ohji, T.; Sando, M.; Toriyama, M.; Niihara, K. Curie Temperature Anomaly in Lead Zirconate Titanate/Silver Composites. J. Am. Ceram. Soc. 1998, 81, 709–712. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, M.; Gao, X.; Zhu, M.; Hou, Y. High-performance lead-free ferroelectric BZT-BCT and its application in energy fields. J. Mater. Chem. C. 2020, 8, 13530–13556. [Google Scholar] [CrossRef]
- Cai, E.; Liu, Q.; Zeng, F.; Wang, Y.; Xue, A. A comparative study of lead-free (Ba0.85Ca0.15)(Ti0.9Zr0.08Sn0.02)O3 ceramics prepared by conventional sintering and microwave sintering techniques. Ceram. Int. 2018, 44, 788–798. [Google Scholar] [CrossRef]
- Jaiban, P.; Wannasut, P.; Kantha, P.; Promsawat, M.; Watcharapasorn, A. Effects of Mg and La co-doping on dielectric, ferroelectric, and piezoelectric properties of barium calcium zirconate titanate ceramics. Chiang Mai J. Sci. 2020, 47, 633–641. [Google Scholar]
- Zhao, C.; Hui, W.; Jie, X.; Wu, J. Composition-driven phase boundary and electrical properties in (Ba0.94Ca0.06)(Ti1-x,Mx)O3 (M = Sn, Hf, Zr) lead-free ceramics. Dalton Trans. 2016, 45, 6466–6480. [Google Scholar] [CrossRef]

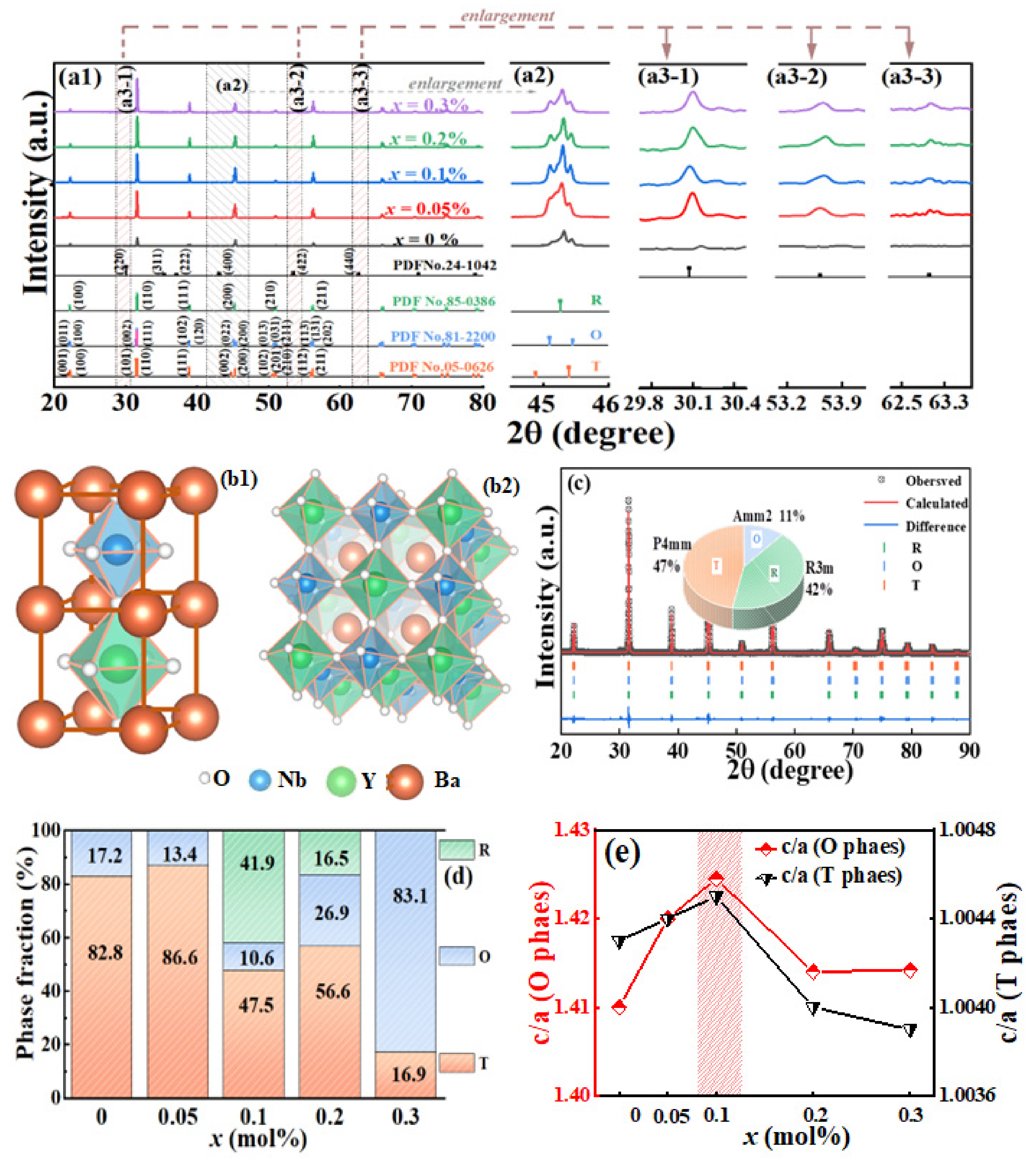

| x (%) | Phase | a (Å) | b(Å) | c (Å) | c/a | α = β = γ (°) | V (Å3) | Fraction (%) | Rp (%) | Rwp (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | O | 4.0295 | 5.6824 | 5.6872 | 1.4113 | 90.00 | 130.223 | 17.1 | 7.66 | 9.59 |
| T | 4.0000 | 4.0000 | 4.0170 | 1.0043 | 90.00 | 64.272 | 82.8 | |||
| 0.05 | O | 4.0259 | 5.7085 | 5.7169 | 1.4200 | 90.00 | 131.38 | 13.4 | 8.37 | 10.88 |
| T | 4.0007 | 4.0007 | 4.0183 | 1.0044 | 90.00 | 64.315 | 86.6 | |||
| 0.1 | R | 4.0014 | 4.0014 | 4.0014 | 1.0000 | 89.91 | 64.069 | 41.9 | ||
| O | 4.0537 | 5.7570 | 5.7746 | 1.4245 | 90.00 | 134.761 | 10.6 | 8.41 | 11.20 | |
| T | 4.0012 | 4.0012 | 4.0192 | 1.0045 | 90.00 | 64.346 | 47.5 | |||
| R | 4.0096 | 4.0096 | 4.0096 | 1.0000 | 89.99 | 64.460 | 16.5 | 8.38 | 10.92 | |
| 0.2 | O | 4.0127 | 5.6702 | 5.6741 | 1.4140 | 90.00 | 129.10 | 26.9 | ||
| T | 4.0015 | 4.0015 | 4.0176 | 1.0040 | 90.00 | 64.330 | 56.6 | |||
| 0.3 | O | 4.0060 | 5.6637 | 5.6669 | 1.4142 | 90.00 | 128.57 | 83.1 | 7.52 | 10.53 |
| T | 4.0017 | 4.0017 | 4.0175 | 1.0039 | 90.00 | 64.335 | 16.9 |
| a (Å) | b (Å) | c (Å) | α (◦) | β (◦) | γ (◦) |
|---|---|---|---|---|---|
| 8.43159 | 8.43159 | 8.43159 | 90 | 90 | 90 |
| x (mol%) | Tcw (°C) | TB (°C) | Tm (°C) | ∆Tm (°C) | εm (100 Hz) | γ |
|---|---|---|---|---|---|---|
| 0 | 100.1 | 128 | 86.8 | 41.2 | 16,301 | 1.58 |
| 0.05 | 106.5 | 133 | 88.2 | 44.8 | 17,062 | 1.78 |
| 0.1 | 101.2 | 128 | 91.05 | 36.95 | 20,545 | 1.60 |
| 0.2 | 99.8 | 128 | 88.8 | 39.2 | 18,905 | 1.73 |
| 0.3 | 93.5 | 119 | 80.8 | 38.2 | 14,920 | 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, R.; Zheng, D.; Wu, Q.; Wang, Y.; Liu, C. Yttrium and Niobium Elements Co-Doping and the Formation of Double Perovskite Structure Ba2YNbO6 in BCZT. Materials 2023, 16, 4044. https://doi.org/10.3390/ma16114044
Mao R, Zheng D, Wu Q, Wang Y, Liu C. Yttrium and Niobium Elements Co-Doping and the Formation of Double Perovskite Structure Ba2YNbO6 in BCZT. Materials. 2023; 16(11):4044. https://doi.org/10.3390/ma16114044
Chicago/Turabian StyleMao, Runyu, Deyi Zheng, Qiyun Wu, Yuying Wang, and Chang Liu. 2023. "Yttrium and Niobium Elements Co-Doping and the Formation of Double Perovskite Structure Ba2YNbO6 in BCZT" Materials 16, no. 11: 4044. https://doi.org/10.3390/ma16114044
APA StyleMao, R., Zheng, D., Wu, Q., Wang, Y., & Liu, C. (2023). Yttrium and Niobium Elements Co-Doping and the Formation of Double Perovskite Structure Ba2YNbO6 in BCZT. Materials, 16(11), 4044. https://doi.org/10.3390/ma16114044





