Abstract
Interest in calcium phosphate cements as materials for the restoration and treatment of bone tissue defects is still high. Despite commercialization and use in the clinic, the calcium phosphate cements have great potential for development. Existing approaches to the production of calcium phosphate cements as drugs are analyzed. A description of the pathogenesis of the main diseases of bone tissue (trauma, osteomyelitis, osteoporosis and tumor) and effective common treatment strategies are presented in the review. An analysis of the modern understanding of the complex action of the cement matrix and the additives and drugs distributed in it in relation to the successful treatment of bone defects is given. The mechanisms of biological action of functional substances determine the effectiveness of use in certain clinical cases. An important direction of using calcium phosphate cements as a carrier of functional substances is the volumetric incorporation of anti-inflammatory, antitumor, antiresorptive and osteogenic functional substances. The main functionalization requirement for carrier materials is prolonged elution. Various release factors related to the matrix, functional substances and elution conditions are considered in the work. It is shown that cements are a complex system. Changing one of the many initial parameters in a wide range changes the final characteristics of the matrix and, accordingly, the kinetics. The main approaches to the effective functionalization of calcium phosphate cements are considered in the review.
1. Introduction
Bone tissue is a part of the human musculoskeletal system, which participates in the transfer of force from one part of the body to another under controlled tension, and protects and fixes internal organs. In addition to performing a mechanical function, bone tissue performs a biological function, as it participates in metabolism [1,2,3,4]. Bone tissue is a reservoir of calcium and phosphate ions in the form of hydroxyapatite, so it plays an important role maintaining the proper calcium levels, along with in other organs [5,6].
Bone tissue’s ability to regenerate effectively, maintain mineralization and repair itself after damage depends on its ability to dynamically remodel. However, the regenerative process is limited by the ability to self-repair: osteogenic insufficiency occurs if the critical size of the defect is exceeded, and the defect is filled with fibrous connective tissue.
There are many different clinical circumstances under which a significant part of bone or a whole bone is lost. Bone defects can be caused by various reasons. They can be associated with various pathogenic conditions and clinical outcomes, including injuries (fractures), infections (osteomyelitis), tumors, osteoporosis, and many other bone diseases [7].
According to statistics, 20 million orthopedic surgeries in the world per year are performed, 70% of which require the use of bone implant material for filling and repairing bone defects [8].
Various osteoplastic materials can be used to fill in bone defects caused by various diseases, or for the purpose of their prevention. They are able to deliver functional substances locally, fill in defects and serve as a material for bone tissue reconstruction.
Calcium phosphate cements are similar in composition to the mineral component of bone tissue. They have a high specific surface area and are used in medicine as osteoplastic materials. Blocks and granules made of pre-hardened cement are a promising type of skeleton for the restoration of bone defects. They have an increased rate of resorption compared to matrices obtained by high-temperature processing. Functional substances can be volumetrically incorporated into them at the stage of mixing the components. The kinetics of the release of functional substances may vary. The release of Ca2+ ions during resorption can affect the differentiation of osteogenic cells and the level of inflammatory cytokines.
The functionalization of calcium phosphate cements is of great clinical interest for the treatment or prevention of various diseases of bone tissue. Prolonged elution is the main requirement for carrier materials of pharmaceutical substances. Another important requirement is the absence of a mutual negative influence of the functional substance and the matrix on each other’s properties. The release of functional substances from calcium phosphate cements depends on many parameters of the matrix, specific interactions between the functional substance and the matrix, as well as environmental factors.
A significant advantage of calcium phosphate cements is the wide range of changes in the properties of matrices that can affect the release of functional substances and the process of bone tissue restoration.
2. Composition and Structure of Bone Tissue
Bone is a highly organized composite material consisting of 50–70% inorganic components (mainly hydroxyapatite), 20–40% organic components, 5–10% water and 3% lipids [9]. That is, the structure of bone tissue is a composite of reinforcing and matrix phases. The reinforcing phase mainly consists of hydroxyapatite crystals. It provides strength and rigidity, while the matrix phase mainly consists of collagen fibrils. They provide flexibility and elasticity to the bone [10]. The organic part consists mainly of type I collagen, 10% non-collagen proteins, lipids, proteoglycan molecules, osteopontin, osteonectin, osteocalcin, sialoproteins, morphogenetic proteins and phosphoproteins. There are more than 200 types of non-collagen proteins; among them, 12 species predominate [10]. Bone matrix proteins also play a vital role in the mechanical strength and adhesive characteristics of tissues. Osteoblasts synthesize the organic substances of the bone matrix. The mineralization of osteoids (non-mineralized organic matrix) occurs due to the appearance of matrix vesicles in the osteoid, secreted by osteoblasts. Vesicles contain Ca2+ ions and phosphatases, and form amorphous calcium phosphate on the surface, followed by the formation of hydroxyapatite crystals from it. Hydroxyapatite is the most important inorganic phase in bone (molecular formula Ca10(PO4)6(OH)2; it contains impurity ions such as CO32−, Cl−, F−, Na+, Mg2+, K+, Zn2+, Fe2+, Cu2+, Sr2+ and Pb2+ [8].
In addition, various other mineral phases, such as amorphous calcium phosphate, monocalcium phosphate, and dicalcium phosphate dehydrate, are present in bones [8]. Carbonate groups, lining up in the structure of hydroxyapatite at 2–8%, form a phase of carbonate-containing non-stoichiometric hydroxyapatite, with the general formula Ca10−x−y/2(HPO4)x(CO3)y(PO4)6−x−y(OH)2−x [11,12].
Bone tissue has a complex multiphase, heterogeneous, anisotropic microstructure [1]. Due to its heterogeneity and anisotropy, the hierarchical structure of natural bone is divided into several different levels, from nano and macro to the level of whole bone [11,13]. In the literature, there is a division into four, seven or nine levels [10,13,14,15]. The unevenness of the bone structure largely depends on the number of levels in the assessment scale and the level used for analysis.
The bone is mainly formed by the external cortical bone and the internal spongy bone at the macroscopic or mesoscopic levels [10]. Compact and spongy macroscopic structures have a smooth structural transition inside the bone and make up 80% and 20% of the total bone mass, respectively [16]. The main function of the cortical bone, as a dense bone tissue, is to stabilize and support the internal porous structure. Spongy trabecular bone provides a favorable environment for tissue metabolism and hematopoiesis of the bone system [17,18].
The mechanical properties of the cortical bone at the macro level are closely related to its microstructure and composition. Moduli of elasticity and strength decrease with increasing porosity or area of osteons. Changes in the mineral content, for example, as a result of aging, and the accumulation of microcracks due to a decrease in remodeling activity are factors affecting the physico-mechanical properties of the cortical bone [19].
Compact and spongy bones have different mechanical properties due to the different contents of inorganic and organic substances [20,21]. A large number of ex vivo studies have reported values of elasticity of the cortical bone layer [22]. The average Young’s modulus along the diaphysis is about 14–20 GPa [19,23], while perpendicular to the diaphysis it is about 11 GPa [24]. The average shear modulus corresponding to the torsion experiment around an axis parallel to the diaphysis is about 4–6 GPa [20,24]. The compressive strength of the cortical bone along the diaphysis is 188–222 MPa, while perpendicular to the diaphysis it is 110–152 MPa, and the bending strength is 119–151 MPa and 42–64 MPa, respectively [8]. The strength of the trabecular bone is 1–12 MPa, with an elastic modulus of 0.022–0.702 GPa. The strengths of vertebrae and tubular bones differ significantly [8].
3. Causes of Bone Tissue Damage and Ways of Treatment
3.1. Injury
Bone injuries caused by trauma can occur in patients of all ages. This can be the result of traffic accidents, falls, and many other reasons. Bone fractures are one of the most common types of injuries.
Injuries caused by trauma can be divided into long bones and spine, or maxillofacial and craniofacial, depending on their localization. The most common places of bone fractures are the femur, shoulder (mainly humerus), hip (femoral neck), wrist (radius/ulna), tibia (distal third), ankle, vertebra, and maxillofacial and craniofacial areas (jaw bone, cranial vault) [25].
The mechanism of bone repair is a multi-stage organized restorative procedure, involving a number of vital progenitor cells along with inflammatory, endothelial and hematopoietic cells [26,27]. Cortical tissue, periosteum, bone marrow and external soft tissues contribute to the healing process. It depends on many parameters present in the damaged tissue, such as growth factors, hormones and nutrients, pH, oxygen saturation, and the performed mechanical stabilization of the fracture [28].
The bone restoration procedure consists of several overlapping phases [8,26,29], which can be combined into three main phases: inflammation, bone formation, and bone modeling (Figure 1).
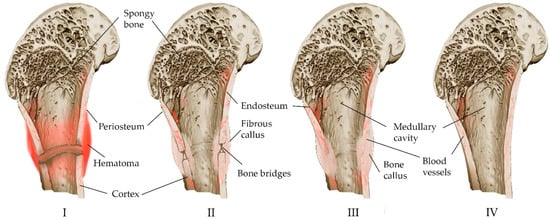
Figure 1.
Schematic representation of the stages of fracture self-healing: (I) The acute stage of the fracture, a hematoma forms around the damaged area of the bone, the bone tissue of the ends of the fragments partially dies (colored dark), local enzymatic activity increases. (II) Development of connective tissue corn. Accompanied by inflammation: cells participating in the inflammatory response appear at the site of the fracture; osteoclasts and osteoblasts are active, non-viable tissue is processed; with the formation of a “cloud” of new tissue at the site of the fracture, structured bone bridges appear inside the corn connecting the fragments. (III) Consolidation: the newly formed tissue acquires the correct bone structure, and trabeculae appear. (IV) Remodeling: the newly formed bone acquires its final structure.
Inflammation begins immediately after a bone fracture and lasts for several days. In the fracture area, blood vessels are damaged, bleeding occurs, hematoma forms caused by osteocyte necrosis due to hypoxia, and thrombosis ensues with the formation of fibrin mesh along the fracture line [8,25,26,30,31,32]. The fibrin mesh isolates the fracture site and serves as a framework for the infiltration of inflammatory cells and macrophages, neutrophils and mast cells that release cytokines and growth factors. Macrophages that have migrated to the site of inflammation remove the temporary fibrin matrix, necrotic cells and bone fragments [25]. As a result, the hematoma and acute inflammatory reactions disappear after a week.
The release of cytokines and growth factors together with pro-inflammatory stimuli leads to a high production of prostaglandins [28]. Newly formed capillaries from the periosteum, and fibroblasts and bone formations of an invasive hematoma appear. Fibroblasts secrete a large number of collagen fibers, and after differentiation, the hematoma forms a fibrous callus, which is characterized by neovascularization, migration of mesenchymal cells, and fibroblast ingrowth [8,31]. Neovasculogenesis in combination with the further production of growth factor and prostaglandins contributes to the differentiation of mesenchymal stem cells towards chondrogenic or osteogenic, and the initial formation of bone tissue [8,33].
The process of the transformation of fibrous callus into bone callus occurs at 2–3 weeks, mainly due to the differentiation of progenitor cells into osteogenic cells (chondrocytes and osteoblasts), secreting matrix osteoids, which gradually replace the fibrous callus due to the deposition of calcium salts (mineralization) [8,32]. It is believed that the relative distance of cells to blood vessels is one of the factors in the differentiation of progenitor cells into osteoblasts, along with the release profiles of cytokines and growth factors. The distance must be kept to a minimum because osteoblasts depend on oxidative metabolism, and require a constant and substantial supply of oxygen and nutrients. Therefore, they accumulate in the immediate vicinity of the newly formed blood vessels. The adapted metabolism of chondrocytes is designed to survive and function in a poorly vascularized environment, so they mature farther from the blood vessels [25].
Chondrocytes proliferate, forming a cartilaginous callus, and then turn into a bone callus via the internal osteogenesis of cartilage [8]. Gradually, the soft callus is replaced by a hard callus at 3–4 months [32], which is visible on radiographs [26].
Bone remodeling, the final stage of healing, lasts for more than several months. In the process of regeneration, the bone regenerates and returns to its original shape [26,28], and the callus is rebuilt according to the needs of biomechanics. Osteoclasts absorb excess callus and recanalize the bone marrow cavity. Insufficient bone callus is replenished due to membrane osteogenesis [8].
Trabecular bone repair occurs without significant external callus formation. After the inflammatory stage, intramembranous ossification predominates in bone formation. This is explained by a significant angiogenic response [32].
The healing of bone fractures is a complex regenerative process. Pathological conditions (the presence of cracks, impaired blood flow, concomitant infection and extensive damage to soft tissues), insufficient mechanical stability and metabolic disorders (diabetes, age-related osteoporosis, genetic factors) are inhibitory factors [33,34]. Up to 10% of people experience delayed healing or bone non-fusion. Biomechanical stability is a critical factor in the healing process of fractures. Internal or external fixation is intended to improve stability and promote healing [35]. The stability of the fracture is of paramount importance to the prevention and treatment of fracture-associated infection [32,36]. Antibiotic-loaded spacers are used in the staged reconstruction of bone non-fusion and bone defects [37].
Poor bone quality in patients with osteoporosis and diabetes can lead to a more complex fracture structure and problems with fixation [7]. The shell of soft tissues can be disrupted due to chronic vascular disease, the prolonged use of steroids, and a decrease in skin turgor. This leads to open injuries, even with low-energy fractures [38].
The use of exogenous bioactive factors at the site of a defect to accelerate bone repair has been under extensive investigation in the field of bone regeneration. Differences in structure determine their different physiological roles and their special role in ensuring the growth of new bones [27,39,40,41]. Vascular endothelial growth factor (VEGF), insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), platelet-derived growth factor (PDGF), and transforming growth factors (TGFs) are the most widely studied in areas of bone regeneration at present. Effective results of regeneration in bone defects depend on the growth factor delivery system.
The concept of using bioactive agents such as tetracycline (antibiotic) and flurbiprofen (non-steroidal anti-inflammatory drug—NSAID) and their chemically modified analogues for early bone formation has recently been proposed in bone regeneration studies [39]. They are effective antiresorptive agents, interfere with intracellular calcium concentration and act as strong inhibitors of osteoclasts. However, there are conflicting data on the effectiveness of the use of NSAIDs. They lead to a delay in healing and a decrease in the content of minerals and matrix of the callus, inhibit remodeling, and cause non-fusion, according to the results of some studies [33,42], and have shown almost no effect on fracture healing in other studies [43]. NSAIDs are useful in everyday clinical practice for pain relief due to their pronounced analgesic activity and anti-inflammatory effects, but conflicting preclinical results, as well as the lack of well-randomized clinical data, suggest that more research is needed [33].
Bisphosphonates and anabolic agents inhibit bone resorption and can be used to accelerate bone tissue repair [44]. Despite the possible stoppage of remodeling and an increase in bone fragility [45], the negative consequences do not outweigh their beneficial effect at the moment; for example, in the prevention of additional fractures in patients with osteoporosis, who are often diagnosed after primary fractures [33].
3.2. Osteomyelitis
Osteomyelitis is an inflammatory bone disease caused by an infectious microorganism, often accompanied by bone destruction. It is most often caused by the local spread of infection after injuries, orthopedic operations or joint replacement. The disease may be limited to a specific area of bone or several areas, such as the bone marrow, cortical, periosteum and surrounding soft tissues [34,46,47]. Different types of osteomyelitis require different medical and surgical therapeutic strategies. The most common osteomyelitis is secondary to the site of infection after injury, surgery, or the installation of an articular prosthesis. Osteomyelitis secondary to vascular insufficiency (diabetic foot) or hematogenic origin is less common.
The main causative agents of bone infections are Gram-positive cocci, including Staphylococcus aureus, coagulase-negative staphylococci (Staphylococcus epidermidis), enterococci (Enterococcus faecalis) and streptococci, and Gram-negative anaerobic bacteria (Escherichia coli, Pseudomonas aeruginosa) [48,49,50,51]. S. aureus has a high level of antibiotic resistance, which has long been recognized. Many other microorganisms, including S. epidermidis [52] and a number of staphylococci, have demonstrated increasing antibiotic resistance in recent years [53]. It has been reported that up to 40% of S. epidermidis strains [54] and 32% of S. aureus strains [55] isolated from orthopedic postoperative and implant-related infections are resistant to gentamicin.
Methicillin-resistant S. aureus (MRSA) is considered particularly virulent due to the production and release of a number of extracellular and cell-associated factors [48,56,57]: bacterial adhesins (microbial surface components that recognize and specifically interact with a single host protein component such as fibrinogen, fibronectin, collagen, and others), toxins, capsular polysaccharides (avoiding host defenses), exotoxins, and various hydrolases (invasion or penetration into tissues by a specific attack on host cells or degradation of extracellular matrix components). S. aureus internalized by cultured osteoblasts can survive inside cells [57,58]. As soon as S. aureus enters the cell, the activity and viability of osteoblasts decrease, and the expression of an apoptosis-inducing ligand associated with tumor necrosis factor is induced [48]. Infected osteoblasts secrete cytokines, chemokines, and growth factors. They are involved in the immune response and promote bone destruction, bacterial adhesion, and biofilm formation [48,59]. A biofilm is a microbial community and consists of cells that adhere to a substrate, an interface, or to each other. They are embedded in an extracellular polymer matrix and exhibit an altered phenotype in terms of growth, gene expression, and protein production [57,60]. Biofilm complicates treatment by acting as an impenetrable barrier that prevents the penetration of antibiotics and immune cells.
The strategy for the treatment of osteomyelitis is based on the use of antibiotics systemically and/or locally, alone or in combination with debridement. Many factors, including patient-specific factors, microorganism type and antibiotic susceptibility, location, spread, implant loosening, and most importantly, the type of infection determined by the time of onset (acute or chronic), affect the overall treatment algorithm [61,62].
In periprosthetic infections, methicillin-resistant S. aureus (MRSA) predominantly causes early infections, whereas methicillin-sensitive S. aureus (MSSA) causes delayed and late infections [49].
Acute purulent inflammation is characteristic of acute osteomyelitis (Figure 2). Various inflammatory factors and leukocytes contribute to tissue necrosis and destruction of bone trabeculae and bone matrix. Vascular channels are compressed and obliterated by the inflammatory process. The ischemia resulting from the process also contributes to bone necrosis. Segments of bone that are deprived of blood supply may separate as sequesters and continue to contain bacteria despite antibiotic treatment [57]. However, antibiotic therapy alone is often sufficient to treat acute osteomyelitis [34]. The high activity of osteoclasts causes bone loss and localized osteoporosis. Meanwhile, the bones converge, in some cases excessively, causing the periosteum to converge and new bone to form [57].
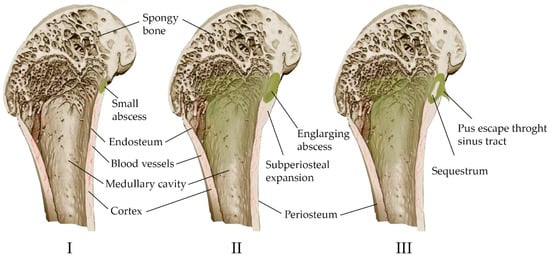
Figure 2.
Scheme of the development of hematogenous osteomyelitis in the bone. (I) Formation of a primary bone abscess (necrosis and purulent fusion of the bone marrow and adjacent bone, limited by the walls of healthy tissue). (II) Subperiosteal abscess (pus through the haversian canals of the bone spreads under the periosteum, exfoliating it from the bone). (III) Fistula formation (pus breaks out through soft tissues); the formation of sequesters is possible—areas of the bone completely devoid of vascular nutrition and lying freely.
Chronic osteomyelitis is still difficult to treat, and has significant morbidity and a high risk of recurrence. It is associated with avascular bone necrosis and sequestration. The infection usually does not begin to regress until the site of persistent contamination has been surgically removed. Antibiotic therapy alone is usually not enough to treat chronic osteomyelitis, although antibiotics relieve many symptoms [34,63]. Rare complications of chronic osteomyelitis include squamous cell carcinoma at the site of tissue drainage and amyloidosis [64].
Systemic antibacterial therapy becomes ineffective in the presence of a vascular area or poorly vascularized scar tissue in osteomyelitis, since antibiotics cannot reach infected tissue [57,61].
In such cases, there is a significant need to develop matrices with antibacterial properties.
Thus, the specific etiology of bone defects caused by inflammation imposes many requirements on the design of medical materials, including the ability to cope with both the inhibition of inflammatory responses and the stimulation of bone tissue regeneration [34,61]. Several principles for topical antibiotic treatment are particularly important: maintaining a concentration above the minimum inhibitory concentration (MIC) of antibiotics that affects bacteria for at least three to six weeks, and adequate tissue penetration so as not to cause local and systemic toxicity. High concentrations of antibiotics (up to 1000-fold increase) are needed to destroy the introduced microorganisms in the case of a formed biofilm.
One of the main advantages of the biodegradable system is the absence of secondary surgical procedures to remove foreign material after the release of antibiotics, such as PMMA cement, has ceased. Additional possibilities for using resorbable systems include changing the release of antibiotics, and the possibility of targeted adjustment of the wound environment by the products of material degradation [65]. Resorption must be complete to leave no substrate for bacterial colonization and to promote the integration of the host tissue. The kinetics of elution of an antibiotic from a material are closely related to its characteristics, provided by composition, surface area, porosity, and other factors (Hanssen, 2005).
The most commonly used antibiotics are gentamicin, rifampicin, vancomycin and tobramycin [62].
It is necessary to take into account the local concentration of antibiotics when they are applied topically, since high concentrations of antibacterial drugs have a cytotoxic effect on cell viability, cause osteogenic differentiation, and reduce the expression levels of genes and the proteins of collagen [66].
3.3. Tumor
The main concept of orthopedic oncology is the prevention of amputation. It is performed in 10–20% of patients with malignant bone tumors [67]. The tumor is characterized by the replacement of healthy bone tissue with tumor tissue, with the inclusion of the medullary canal and the soft tissues surrounding the bone in the pathological process (Figure 3). Many tumors originate in the metaphyseal–diaphyseal regions of long bones and can be segmentally resected while preserving the joint [68]. Approaches have been developed for the treatment of complications associated with malignant neoplasms. These include either anticancer intervention, such as chemotherapy, hormone therapy, radiation therapy, and resection, or bone-supportive care, such as calcium, analgesics, and vitamin D supplements [69].
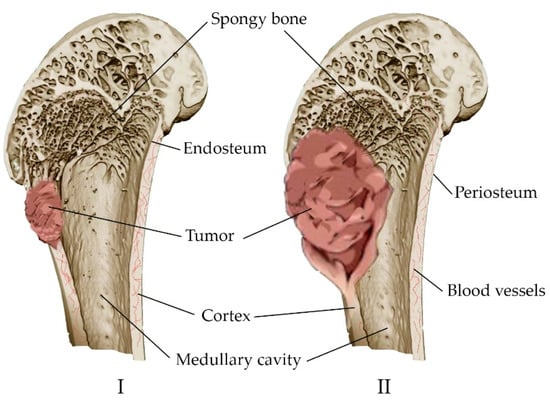
Figure 3.
Development of a bone tumor: (I) Destruction of a bone site by a tumor; (II) replacement of healthy bone tissue with tumor tissue, inclusion of the bone marrow canal and soft tissues in the pathological process.
Metastatic bone lesions are common in patients with other types of cancer (lung, breast, prostate, and colorectal cancer). Most severe bone metastases are also treated with reconstructive surgery. This is followed in some cases by postoperative radiation or chemotherapy. Patients after body reconstruction still show a risk of developing severe complications such as tumor recurrence [70].
Depending on the type of cancer, metastatic lesions can be characterized as osteolytic, osteoblastic or mixed lesions (containing both elements), in which the regulation of the normal process of bone remodeling is disrupted [71]. Osteolytic lesions are characterized by the leaching of the mineral part of the bone, its thinning, and fractures. Osteoblastic metastases, on the contrary, are characterized by compaction of the mineral part of the bone, since the cells of different tumors can both directly destroy bone tissue and stimulate cells that renew it. Osteolysis is also observed in prostate cancer, despite the tendency to osteoblasting in metastases. However, there is a shift in the balance towards the formation of a new matrix and its mineralization. The increase in bone volume is due to the replacement of existing trabecular tissue with abnormally woven bone, which creates a general appearance of sclerosis [72].
Osteolytic metastases can cause severe pain, pathological fractures, life-threatening hypercalcemia due to elevated blood calcium levels, spinal cord compression, and other nerve compression syndromes [73]. Patients with osteoblastic metastases feel pain in bones and pathological fractures due to poor bone quality produced by osteoblasts [71].
Drugs that block bone resorption may reduce bone pain and the risk of pathological fractures in metastatic lesions. One of the effective groups of drugs used in osteolytic bone disease and osteoblastic bone disease associated with prostate cancer metastasis is bisphosphonates [69,71,72,74,75]. Bisphosphonates have the ability to inhibit resorption processes in the bone by reducing the activity of osteoclasts. Bisphosphonates inhibit the release of growth factors, inhibiting bone resorption, and thus block the feedback from tumor cells. This helps to reduce the activity of tumor cell proliferation. Bisphosphonates can induce apoptosis in malignant cells similar to the process observed in osteoclasts, and reduce the adhesion of tumor cells to bone, reducing the risk of new metastatic lesions.
If there is a high probability of a pathological fracture of metastatic bone tissue, prophylactic fixation is recommended [76]. This involves the resection of the tumor and reconstruction of the damaged bone [77]. Plates, intramedullary rods and screws are used as fixators in the reconstruction of a damaged bone. With lesions of more than 50% of the bone diameter or joint lesions, prostheses are installed [78,79]. The purpose of reconstruction is palliative. It reduces pain and restores the function of the affected bone throughout the life of the patient. Among the complications of reconstructive treatment of metastatic lesions of the bone tissue are infection (0–11.7%), aseptic loosening (0–12.5%), mechanical damage (0–14.7%), and tumor recurrence (3.1–14.7%), despite the fact that chemotherapy is prescribed after reconstruction [70,80,81,82,83].
The combination of high doses of methotrexate, cisplatin, ifosfamide, and doxorubicin results in increased survival, but the use of anticancer drugs is limited by serious side effects. Poor bone blood supply, drug resistance, and nonspecific absorption require the use of highly toxic doses of anticancer drugs [74]. Therefore, there is a need to develop locally delivered carrier materials with reduced side effects and/or without effects for the treatment and prevention of growth-related bone cancers.
Surgical resection together with radiation/chemotherapy is a clinically accepted treatment regimen. For biomaterial therapy, surgical intervention is necessary to remove tissue and to provide space for the formation and integration of new bone. Biomaterials are used as bone substitutes after tumor surgery. Adjuvant treatments, such as systemic radiation therapy and chemotherapy, are used to prevent relapse.
The localization of radio/chemotherapy is a more highly effective method of the treatment and prevention of relapse after tissue reconstruction, since simultaneous processes of tumor inhibition and bone regeneration are possible with the mobilization of drugs via bone replacement material [84].
Many surgeons use cement reconstruction techniques to minimize postoperative complications. Bone cement made of polymethylmethacrylate is used as a carrier of chemotherapeutic drugs in an attempt to reduce tumor recurrence, and serves as an addition to bone reconstruction [70].
At the same time, inorganic calcium phosphate cements attract the attention of researchers because their biological properties can counteract the toxic nature of the inclusion of anticancer drugs, and promote osteogenesis. The presence of calcium phosphate cement can help to avoid the spread of tumor cells, and prevent the development of new lesions in the surrounding tissues during resection inside the focus [85]. They can also be used as carriers of antitumor drugs or radioactive substances, and administered to patients to achieve antitumor effects [86,87]. These cement modifications for the delayed release of antitumor drugs, magnetic tumor targeting, or radiological modifications are designed to simplify the treatment process and reduce systemic side effects and pain in patients [88].
3.4. Osteoporosis
An imbalance in bone metabolism between bone resorption mediated by osteoclasts and bone formation mediated by osteoblasts leads to the occurrence of metabolic diseases in bones, including osteoporosis and osteomalacia (mineralization deficiency caused by a a lack of calcium or phosphorus, or insufficient activity of osteoblasts) [89,90]. Osteoporosis is a leading public health problem and one of the most common chronic diseases. It affects more than 200 million people worldwide [91]. A systemic metabolic disorder in the bones leads to a gradual loss of bone mass and damage to microarchitectonics, along with the weakening of bone strength (Figure 4). This leads to low-energy fractures [34,92]. A fracture can occur anywhere in the skeleton, although fractures of the wrist, hip and spine are the most common [89]. Both men and women lose bone mass as they age, with women gradually losing 50% of trabecular and 30% of cortical bone over a lifetime, while men lose two-thirds of this amount [93]. The prevention of osteoporosis can be achieved via a balanced diet containing calcium, phosphorus and vitamin D. These improve bone reabsorption and repair, except in cases of hereditary bone diseases [89,94].
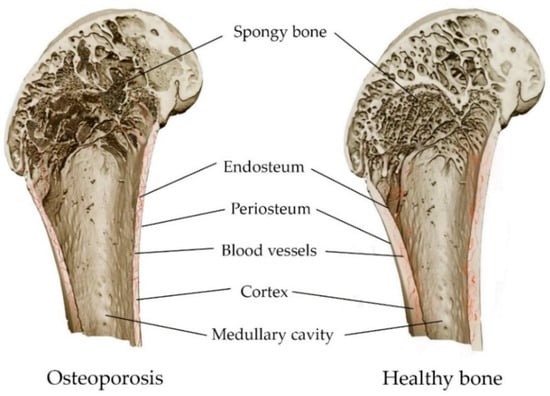
Figure 4.
Comparison of osteoporosis and healthy bone: decrease in bone density, and thinning of bone structures, as a result of osteoporosis.
The pathogenesis of osteoporosis is mainly associated with bone homeostasis, with the balance of bone remodeling between formation and resorption by specific cells, including osteoblasts, osteocytes and osteoclasts. Mesenchymal stem cells of the bone marrow are multipotent cells with the ability to differentiate into lines of osteoblasts, chondrocytes and adipocytes [95]. Osteoporosis is an increase in the adipose tissue of the bone marrow due to a shift in differentiation into adipocytes rather than osteoblasts. In addition, activation of the main signaling pathways of bone metabolism can both promote the differentiation of pre-osteoclasts into osteoclasts, and prevent it by suppressing the RANKL membrane protein [34].
A common and effective strategy for the treatment of osteoporosis is antiresorptive therapy, which targets osteoclasts and reduces the rate of bone resorption [34,74,92,96]. The therapeutic efficacy of bisphosphonates and monoclonal antibodies has been confirmed by the successful use of pharmaceutical preparations alendronate, risedronate, zoledronate, raloxifene ibandronate, teriparatide, abaloparatide and denosumab [74,89,92,97]. Bisphosphonates can minimize the chances of vertebral fractures by 50–60% and hip fractures by 50% [98]. Anabolic agents (romosozumab, teriparatide, and abaloparatide) are currently approved for the treatment of osteoporosis because the drugs may promote bone regeneration and reduce bone fractures [89,97].
In addition, osteoblasts are able to respond to various modalities of the extracellular signal, including the concentration of extracellular free ionized calcium Ca2+, regardless of systemic factors [99].
Local prolonged drug delivery systems in effective therapeutic doses to the site of bone disease can contribute to the effective treatment of various metabolic diseases of the bone tissue, with less adverse effects [89,100,101]. Minimal invasiveness can be considered as a concept to reduce the risk and number of complications [34].
Thus, calcium phosphate cements can be used as carriers of antitumor, antibacterial, radioactive, anabolic, antiresorptive, anti-inflammatory and osteoinductive drugs, providing the output of functional substances at the implantation site (Figure 5).
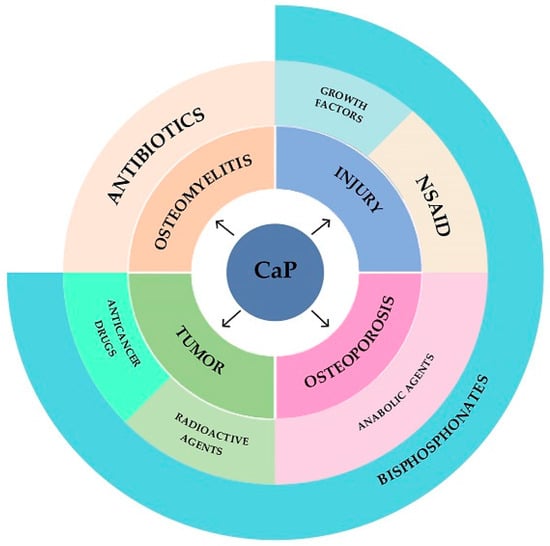
Figure 5.
Diagram of applicability of calcium phosphate cements.
3.5. Calcium Phosphate Cements for Bone Treatment
Bone tissue is able to spontaneously heal fractures or defects, but regeneration is limited to small areas of the defect. The critical bone tissue defect size is commonly taken to mean the smallest bone defect in a particular bone of a particular living organism that does not spontaneously heal up, or shows less than 10% bone regeneration over its life [102]. Bone grafts are needed to facilitate the repair process if the size of the defect is too large for the bone’s natural ability to heal. Calcium phosphate cements have a similar bone mineral chemistry, and can adapt perfectly to the shape of the defect, making them a convenient option for use as a synthetic bone graft.
Bone is the main storage site for calcium and other ions in the body. Bone fractures, especially during surgical treatment, are associated with changes in microelement homeostasis [103]. Calcium introduction is effective in reducing the risk of postoperative hypocalcemia [104,105,106]. Together with other ions such as magnesium, they have a positive effect on the healing of bone fractures [107]. Ca2+ is an important homing signal; it brings together various cell types required to initiate bone remodeling. A high concentration of Ca2+ can induce osteoblast proliferation and chemotaxis by binding to an extracellular calcium-sensitive G-protein-coupled receptor [108,109].
Cytokines C-reactive protein (CRP), interleukins IL-1β, IL-6 and tumor necrosis factor alpha TNF-α play a pro-inflammatory role, and are important mediators of the immune response and inflammatory response. The disordered expression of IL-1β, IL-6 and TNF-α is considered an effective biomarker of inflammation associated with the development and process of fractures [110]. An increase in the content of Ca and Mg, and a decrease in inflammatory cytokines, are observed after short-term treatment with trace elements. Their addition can be an effective way to combat inflammation, as it contributes to the restoration of bone fractures [105]. The disordered expression of IL-1β, IL-6 and TNF-α is considered an effective biomarker of inflammation associated with the development and process of fractures [110]. There is an increase in the content of Ca and Mg, and a decrease in inflammatory cytokines, after short-term treatment with trace elements. The addition of trace elements can be an effective way to fighting inflammation, and contribute to the reconstruction of bone fractures [105].
Calcium phosphate cements can be used to strengthen vertebral bodies affected by osteoporosis [111] in cranio-maxillofacial surgery [112,113], as well as for treating fractures and revisions, mainly to fill defects resulting from the surgical removal of cysts and tumors, trauma and osteolytic defects, or in the surgical treatment of infections [114].
Resorbable calcium phosphate cements prepared by low-temperature technology containing drugs or other biologically active substances and cells can potentially act as multifunctional carriers and drug delivery systems [115,116]. Figure 6 shows the process of bone defect replacement during the implantation of calcium phosphate material in the form of a block, granules or cement paste.
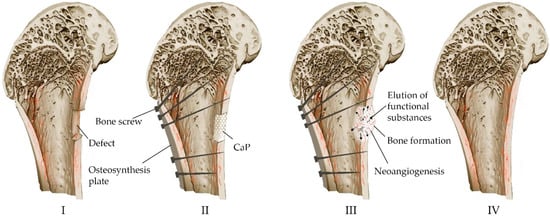
Figure 6.
Scheme of replacement of bone defects with the help of calcium phosphate material: (I) Formation of a defect along the boundaries of viable tissues (resection of compromised areas as a result of high-energy trauma, inflamed areas in osteomyelitis, tumor lesions, etc.). (II) Installation of metal structures for mechanical strength for the period of healing and implantation of osteoplastic material to close the defect. (III) Reconstruction of osteoplastic material in the defect, resorption, biodegradation and formation of bone. (IV) Bone remodeling.
Currently, calcium phosphate materials are used in the field of traumatology and orthopedics in the form of powders, granules, blocks, cements and coatings on metal implants [117,118,119,120,121,122]. Due to the high strength of ionic bonds, calcium phosphate materials are brittle and cannot carry heavy loads. They are used as osteoplastic materials in small defects or operated with the additional use of structures on which the load is shifted.
Calcium phosphate cements are osteoconductive and bioactive, and they integrate into bone tissues without forming a connective tissue capsule [123]. The cements are resorbable, moldable and easy to handle. They can be injected into bone cavities under conditions of limited surgical access, and completely fill cracks (defects) in situ in the operating room, thus minimizing surgical intervention. They provide good fixation and close contact between the bone and the material [124]. Cements harden, acquiring their own mechanical strength [121].
4. Concepts for the Production of Calcium Phosphate Cements
Calcium phosphate blocks and granules obtained as a result of the hardening of calcium phosphate cement are a promising type of scaffold for restoring bone defects due to their similarity in composition with the mineral component of bone tissue, high specific surface area, and increased resorption rate compared to matrices obtained by high-temperature processing. In addition, the volumetric incorporation of functional substances at the stage of mixing the components can be used to change the release kinetics, in contrast to the surface impregnation of high-temperature calcium phosphate materials [117,125,126,127,128]. Matrixes can be formed by casting cement paste into molds, by interacting pre-pressed initial components in an aqueous medium via setting and hardening, and by 3D printing; they can serve as a substrate in tissue engineering and are an excellent platform for incorporating functional substances [117,129,130,131].
Cements are usually obtained from powders of one or more calcium phosphates and an aqueous solution. When mixing powders of calcium phosphates and an aqueous solution, a paste is obtained. It hardens within minutes. Another form of supply of calcium phosphate cement is pre-mixed cement components in the form of a cement paste. There are three main approaches used to prepare premixed cements: (1) one-component cement in the form of calcium phosphate paste mixed with a non-aqueous liquid (hardening when mixed with biological fluids); (2) two- or more-component cement in the form of pastes (hardening when mixed); (3) one-component cement in the form of a paste of calcium phosphate in an aqueous liquid, followed by freezing (hardening during thawing). This form of delivery is presented in the commercial products of VitalOs from CalciphOs/Produits Dentaires SA and VELOX from InnoTERE GmbH [132].
The constant solidification volume of cements and low heat release (minor exothermicity at low heat release rate) [88,125,133] are properties that that enable their use.
An increase in mechanical strength is facilitated by the isolation of particles of non-equiaxed morphology (lamellar, needle-shaped), which provide mechanical engagement. The cements fuse well with the bone, gradually dissolving and being replaced by new bone tissue.
Powders and blocks of cements can be sterilized by γ-irradiation without the loss of biocompatibility and bioactivity [134]. Steam sterilization, ethanol sterilization, and ethylene oxide sterilization with complete degassing after sterilization are also mentioned [128,131,135]. Gamma radiation for powder components and filtration for liquid components are used in the sterilization of cement formulations [136].
Calcium phosphate cements can be divided into two categories according to the final product, despite the large number of possible preparation methods: (1) based on hydroxyapatite; (2) based on dicalcium phosphate dihydrate CaHPO4·2H2O (DCPD) (or brushite) [121,125,137,138].
A significant part of the commercial cement materials used in medicine for the treatment of bone tissue defects contain minerals belonging to the CaO–P2O5–H2O system [139]. The production of calcium phosphate cements is usually realized according to two scenarios, with two chemical processes: acid–base interaction and hydrolysis.
As a result of the acid–base reaction, cements based on hydroxyapatite Ca10(PO4)6(OH)2 (HA) (Ca/P = 1.67)/calcium-deficient hydroxyapatite Ca10−x(HPO4)y(PO4)1−y)6(OH)2 (CDHA) (Ca/P = 1.5) and brushite CaHPO4·2H2O (DCPD) (Ca/P = 1)/monetite (dicalcium phosphate anhydrite) CaHPO4 (DCPA) (Ca/P = 1) are obtained by reacting tetracalcium phosphate Ca4(PO4)2O (TTCP) (Ca/P = 2) or β-tricalcium phosphate β-Ca3(PO4)2 (β-TCP) (Ca/P = 1.5) with DCPD (Ca/P = 1)/DCPA or monocalcium phosphate monohydrate Ca(H2PO4)2·H2O (MCPM) (Ca/P = 0.5), without the formation of acidic or basic co-products. Monetite is formed under conditions of water deficiency and low pH [120,140].
Only one precursor is involved in the hydrolysis reaction. When mixed with the liquid phase, it becomes hydrated. The reaction proceeds with the dissolution of PO43− and Ca2+ ions. On the surface of the α-tricalcium phosphate α-Ca3(PO4)2 (α-TCP) or amorphous calcium phosphate Ca3(PO4)2 (ACP), particles of precipitated crystals of calcium-deficient hydroxyapatite form during the hydrolysis process.
Examples of possible combinations of the main initial components used in the production of calcium phosphate cements as commercial products are shown in Table 1.

Table 1.
The main components of commercial compositions of calcium phosphate cements.
HA and brushite cements differ significantly in setting time, mechanical strength, resorption rate in the body, and pH values. In this regard, approaches to improving the properties of cements differ.
HA cements are long-hardening; the setting time is no earlier than 30 min. A decrease in cohesion upon contact with blood and partial mixing with it may occur during prolonged hardening, and this will lead to a loss of quality or migration from the implantation site [141,142,143].
Miyamoto and colleagues stated that the cement sets consistently despite partial decomposition of the cement paste on contact with blood during setting. However, fast-setting cements may be less sensitive to contact with blood [144].
Particle size and the degree of crystallinity strongly affect the degree of reactivity of cements and, as a result, the rate and integral completeness of the reaction [145,146]. ACP is the most reactive because it has the least stable crystalline phase. It is followed by α-TCP and finally β-TCP [147]. A decrease in the particle size leads to an increase in the surface area, and an increase in the reactivity and the reaction rate [117,148,149].
The introduction of a seed of crystallization increases the rate of hydration and hardening [149,150,151]. The seed plays the role of a substrate that can be used for heterogeneous nucleation. The nucleation barrier does not exist if the substrate is identical to the nascent crystal.
HA cements are relatively insoluble in aqueous solutions at neutral pH. Solubility increases at acidic pH values, as their own pH values correspond to physiological values.
Brushite cements are fast-setting, with setting times less than 1 min, as well as being soluble, highly bioresorbable and biocompatible, and relatively acidic (pH around 4).
The rate of resorption of cements in the body can be regulated by various technological parameters: L/P ratio, porosity, phase composition (ion substitutions, introduction of additional components of cement powder), crystallinity, as well as the presence and quantity of additives.
The introduction of additives into the system or due to ionic substitutions can increase the setting time of cement. For example, in the production of brushite cement, the setting time and compressive strength were increased by replacing calcium in TCP with magnesium by up to 10%. Moreover, the initial setting time increased to 33 min with magnesium content [152]. Changing the particle size of the initial components affects the setting time and strength of the cement.
In the literature, the compressive strength of HA cements is 20–83 MPa, while that of brushite cements −1–24 MPa, and tensile strength of brushite cements −0.7–4.5 MPa, and that of HA cements is up to 15 MPa. Such data were obtained by measuring the strength under different conditions. Cement samples dried in air or at a slight increase in temperature have the maximum strength values.
Among the ways to increase the strength of cements are the following: reducing the liquid/powder ratio (L/P or LPR) when receiving cement paste, introducing additives, changing the particle size [153,154], and introducing fillers (fibers, granules) inert with respect to the cement stone. As fillers, granules or fibers of the inorganic compounds β-TCP, HA, gypsum, bioglass, carbon, silica, wollastonite and zirconium dioxide can be used. They are biocompatible, non-resorbable or poorly resorbable compounds [155,156,157], with the exception of resorbable β-TCP and some bioglasses.
Fibers and granules made from resorbable polymers such as polylactic acid (PLA) [158], polylactide-co-glycolide (PLGA) [159], polyvinyl alcohol (PVA) [160], gelatin or chitosan [161,162] increase initial strength and create porosity over time. In this regard, the characteristics of the cement stone change; this affects the behavior of the material in vivo. Such fillers can be attributed to additives as modifiers of cements.
Calcium phosphate cements have a porosity of 30–60% by volume, typically depending on the composition of the cement. The porosity is mostly open. Pore sizes up to 1 micron do not provide bone tissue ingrowth, and resorption occurs from the surface of the material. The porosity of calcium phosphate cements is due to excess mixing water. As the amount of water decreases, the porosity decreases, and hence the mechanical properties improve. This leads to a decrease in the resorption rate and the deterioration of rheological properties [125].
The absence of macroporosity is attributed to the disadvantages of calcium phosphate cements, in particular HA, while the presence of microporosity is an advantage. This is due to the fact that microporosity creates a surface microrelief for cell retention, and improves osteogenesis [162,163,164,165,166]. In addition, microporosity is a positive factor in calcium phosphate cements–functional substance delivery systems. However, microporosity reduction due to prepressing is used to obtain cement scaffolds with increased strength [167], and in combination with removable fillers to form macroporosity, it reduces strength to a lesser extent [126,168]. Another approach to reducing porosity is to reduce the particle size of the original components [169].
Ionic substitutions can also affect porosity. For example, when using silicon beta-tricalcium phosphate (Si-β-TCP) in the preparation of brushite cement, the pore size decreases in direct proportion to the amount of Si from micro- to nano-size, and the surface area increases [170].
Macroporosity provides blood with access to contact surfaces, and allows angiogenesis [162,171]. The absence of macroporosity is solved by introducing soluble additives and removing them before or after implantation (in vivo).
4.1. Osteogenic Ionic Substitutions in Calcium Phosphate Cement
The calcium of hydroxyapatite and brushite can be replaced by the following cations: magnesium, radium, strontium, barium, sodium, zinc, etc. The phosphate ions of hydroxyapatite can be replaced by carbonate or silicate ions. Currently, more than 30 osteotropic microelements (copper, strontium, zinc, barium, aluminum, silicon, fluorine) are known, the lack or excess of which can cause various rickets, leading to skeletal growth arrest and other consequences. Substitutions with magnesium ions, strontium and carbonate ions are already used in the preparation of commercial products (see Table 1). For example, calcium carbonate is used to produce carbonate hydroxyapatite as the target phase in commercial products Calcibon® and Norian. Calcium carbonate participates in the hydrolysis process together with α-TCP, facilitating the incorporation of CO32− ions into the crystal lattice [172]. A negative charge deficiency is formed when PO43− is replaced by CO32− or HPO42−; it is compensated by calcium deficiency and the replacement of Ca2+ ions by Na+.
It is known that the presence of the bioactive Mg2+ ion in the structure of calcium phosphates plays a significant role in the biological process and stimulates bone formation [173,174,175,176,177]. Magnesium is an obligate cofactor of alkaline phosphatase and many enzymatic reactions, inhibits the formation of osteoclasts, and participates in the synthesis of bone collagen, cell proliferation and differentiation, and the interaction of the cell with the matrix, as well as the normal functioning of organs [178,179,180,181,182]. Mg2+ ions in HA cement can reduce the setting time, and increase it in brushite cement. This is a beneficial effect for both cements [152,177].
One of the important features of the substitution of calcium ions Ca2+ for magnesium ions Mg2+ in brushite is the stabilization of the phase composition [180,183,184,185]. Magnesium ions suppress the formation of less soluble calcium phosphates (hydroxypatite, tricalcium phosphate, octacalcium phosphate) in vivo in brushite materials [185,186,187,188,189]. Magnesium acts as a strong inhibitor of the crystal growth of less soluble calcium phosphates. Magnesium–calcium phosphate cement does not recrystallize with time, in contrast to brushite, and retains a high resorption rate [181,190].
Strontium is very close to calcium in nature, and therefore they are associated in the metabolic processes of bone tissue. Strontium accumulates mainly in the newly formed bone and in the areas of ossification, being part of the trabeculae. Ca2+ and Sr2+ ions can be replaced by Sr2+ ions in the cement composition. Sr2+ has been proven to be effective in stimulating osteogenesis, and in the treatment of osteoporotic bone fractures. It acts as an inhibitor of bone resorption and as a stimulator of bone formation [177,191].
The replacement of calcium ions with zinc ions significantly contributes to the formation of new bone without an inflammatory reaction. With the introduction of zinc into the composition of the cement, the proliferation of primary human mesenchymal stem cells is significantly stimulated, and the activity of alkaline phosphatase increases [192,193,194,195].
The co-introduction of Si and Zn ions increases the rate of resorption of calcium phosphate cement significantly, as well as angiogenesis and osteogenesis, due to the synergistic effects of Si and Zn on biostimulation and immunoregulation [196].
There are two approaches to ionic substitution: introducing additional compounds into the composition of the powder part of cement [195,196], or by doping one of the original components [120,170,194,197].
In addition to alkaline earth metals, ions of other bioactive metals such as Cu2+, Co2+, Cr2+ and Ga3+ at low doses can also accelerate bone tissue repair [88]. Cu2+-doped cement has antibacterial properties and stimulates angiogenesis, bone marrow mesenchymal stem cell (BMSC) differentiation and bone mineralization. Co2+ shows conflicting results. Cr3+ has a positive effect on bone formation, and supports the proliferation of osteogenesis precursor cells and osteoclast resorption. Ga3+ has a positive effect on the synthesis of mature organized collagen and an inhibitory effect on osteoclasts [88]. The inhibitory effect of Ag-doped calcium phosphate cements on pathogenic Escherichia coli has been proven [197].
Ionic substitutions are used to improve the properties of cements for the treatment of bone cancer. These ions include holmium (Ho) and samarium (Sm) ions, and a collection of manganese (Mn), lanthanum (La), strontium (Sr), cobalt (Co), and iron (Fe) ions, including iron oxide and magnetite [198].
The medical impact of any micronutrient needs to be assessed in order to establish toxic thresholds. For example, the use of high doses of strontium causes defects in bone mineralization [199], while high doses of zinc cause a slowdown in bone repair [200] and Cu2+ cytotoxicity [88].
4.2. Influence of Modifier Additives on the Properties of Cements
Inorganic and polymer additives greatly affect the properties of cements. They are necessary to obtain medical devices suitable for use in the field of traumatology and orthopedics (Table 2).

Table 2.
Additives and their effect on cement.
4.3. Porosity and Features of the Pores
Slowly resorbed HA cements require the formation of macroporosity. It increases the surface area and the rate of resorption. The pores provide fluid flow (perfusion in the case of interconnected porosity), as well as the migration and proliferation of osteoblasts in cements, and vascularization. In addition, in the presence of pores, the stability of the tissue–implant interface improves. This is due to a larger surface area for cell proliferation and the regeneration of new tissue [121].
Depending on the size of the pores in calcium phosphate cement, they are classified into micropores (pore diameter <1 microns), mesopores (pore diameter 1–100 microns) and macropores (pore diameter >100 microns) [121]. The size of osteoblasts is about 10–50 microns [212]. However, osteoblasts prefer larger pores (100–200 microns) for the regeneration of mineralized bone after implantation. Macrophages can enter these pores, destroy bacteria and cause the infiltration of other cells involved in colonization, migration and vascularization in vivo [213]. As a rule, a pore size of ≥300 microns is required for the formation of new bone and vascularization. The minimum allowable size is ≈100 microns [121,214,215]. The pore size <100 microns prevents angiogenesis [216]. According to the results of many studies, bone tissue is formed in such pores [121,215,217]. Small pores favor hypoxic conditions and induce osteochondral formation before osteogenesis. Large pores with good vascularization can lead to direct osteogenesis (without prior cartilage formation) [215].
Pores larger than 300 microns are well supplied with oxygen and nutrients. This promotes vascularization in new bone tissue and, accordingly, osteogenesis. [215,216,218,219,220].
Another important parameter of the porosity of calcium phosphate cements is the interconnectedness of the pores. Porosity can be open and closed. Open porosity has an advantage. The interconnected open microporous system ensures good impregnation of the material with biological fluids and oxygen diffusion, and creates a surface roughness. Roughness plays an important role in the adsorption and retention of osteogenic cells on the implant surface. Macropores facilitate cell infiltration and migration into the scaffold, as well as angiogenesis and osseointegration [221,222,223,224]. Macroporosity allows cells to migrate and proliferate into the matrix, provides easy access for cells (inflammatory, stem) and soluble proteins, including signaling molecules and osteogenic growth factors, and enhances active (cell-mediated) and passive (solubility) cement resorption. [224].
Interconnected macroporosity is a necessary but not sufficient requirement. The shape and architecture of pores are of great importance to the behavior of cement scaffolds in vivo. The spherical concave surface of the pores of various types of materials has a positive effect on in vivo behavior [224,225,226]. Triangular, rectangular and elliptical pores support angiogenesis and faster cell migration due to their greater curvature [218].
The closed spaces of spherical macropores act as niches for the differentiation of mesenchymal stem cells into osteoblasts due to the presence of calcium and phosphate near the scaffold, and osteoinductive growth factors. Such a microenvironment does not occur in an open structure where ions and proteins diffuse easily [224].
Thus, scaffolds based on calcium-deficient hydroxyapatite with spherical, concave macropores form due to the release of CO2 in the process of cement stone formation, and these cause significantly more intense ectopic bone formation during intramuscular implantation than 3D-printed scaffolds with convex, prismatic macropores. Ectopic osteogenesis in 3D-printed scaffolds was present only at the corners where a concave surface formed. The rate of local growth of tissue in concave spaces is proportional to the curvature of the concavity. Moreover, no difference in angiogenesis was observed with different pore shapes [224].
4.4. Resorbability
The main difference between the end products of hydroxyapatite and brushite calcium phosphate cements is their solubility and resorption rate. Brushite cements are more soluble than HA. Therefore, they are more rapidly resorbed in vivo [125,227]. Ideally, the rate of biodegradation of calcium phosphate cements should be nearly the same as the rate of new bone formation. This will ensure the gradual restoration of the mechanical properties of the new bone tissue. Although HA cement resorbs faster than high-temperature HA, resorption can take several years to decades [123].
The resorption of both types of cements proceeds from the periphery to the center (creeping substitution) at the bone–cement boundary [123]. Osteoclast-like cells are present on the interface. They resorb cement stone. At the sites of resorption, new bone tissue is formed, and the integrity between the bone bed and cement is preserved (osteotransductive property of cement) [228].
In vivo resorption is carried out in two different ways: (i) passive resorption by dissolving cement stone in extracellular fluid and (ii) active resorption due to cell activity [121].
The rate of passive resorption by extracellular fluid depends on the properties of cements—L/P ratio, porosity, surface area, phase composition, Ca/P ratio and crystallinity—as well as the microenvironment properties—pH and perfusion by body fluids [229,230].
The active resorption of calcium phosphate cements is mediated by giant cells and osteoclasts. Macrophages absorb fragmented cement particles [231,232]. Macrophages are among the first cells to enter the fracture site. They contribute to the initial inflammation and rehabilitation of the injury site, as it was thought for a long time. [2]. The main role of macrophages is to regulate bone regeneration during normal homeostasis and during fracture healing. In addition to macrophages, osteoclasts play complex roles in bone growth and regeneration [233,234]. The mature osteoclast is tightly attached to the mineral surface. It lowers the pH locally near the biomaterial and dissolves the inorganic calcium phosphate underneath [235].
The rate of resorption of calcium phosphate cements affects cell proliferation. This is due to the interaction of the released calcium with the extracellular calcium-sensitive receptor associated with the G-protein [108,109]. In this regard, cellular proliferation is higher in carbonate-substituted HA cements [126] than in HA cements.
Brushite cements show a higher rate of resorption than HA cements, but there is a possibility of the recrystallization of brushite into low-soluble phases [187,236]. The resorption rate of brushite cement should be increased or decreased depending on the purposes of its use (bone restoration, drug delivery system). To prevent the recrystallization of brushite into low-resorbed phases (for example, hydroxyapatite), a highly resorbed phase of newberyite is introduced into the composition [76,120]. Slowing down the rate of resorption can be achieved by inhibiting osteoclast-mediated resorption, for example, by including simvastatin to stimulate bone formation and inhibit cement resorption [232].
A chemical approach to improve cell adhesion and osteogenic differentiation aims to create functional groups on the surface of matrices, such as −COOH and −NH2. Through functional groups, the surface binds to proteins using hydrogen bonds [237].
An increase in the resorption rate of HA cement is possible due to the presence of macroporosity. The active and passive resorption of calcium phosphate cements is enhanced in the presence of macroporosity. It allows cells to migrate and proliferate into the matrix, and increases the surface area [116]. The introduction of particles of poly(D,L-lactic-co-glycolic acid) (PLGA) increases the rate of dissolution of the cement matrix due to the formation of marcoporosity and the presence of acidic monomers (lactic and glycolic acids). Acidic monomers accelerate matrix degradation [208].
The structure of macropores affects not only bone formation, but also the resorption of the material. Matrices with concave macropores have a higher cell-mediated resorption compared to matrices with convex prismatic pores. The interstitial microenvironment in concave pores influences osteoclastic activity [224].
Preclinical studies of calcium phosphate cements unambiguously confirm their biocompatibility, bioactivity and resorbability. However, the resorption rate depends on a large number of the technological parameters of the preparation of cements (precursor synthesis methods—firing temperature, particle size, crystallinity; cement paste and cement stone parameters—he presence and amount of additives, strength, porosity, cohesion, phase and chemical composition, phase distribution, pH) and the different osteogenesis processes in different animals, as well as the place of implantation. The bone metabolism of sheep, pigs (1.2–1.5 mm/day), dogs (1.5–2.0 mm/day) and goats more closely mimics human bone physiology in terms of bone metabolism (1.0–1.5 mm/day), long bone size, and mechanical loading conditions, but there are many other differences. Bone metabolism and regenerative capacity are faster in rodents and rabbits [138].
The difficulty in predicting the clinical outcome of calcium phosphate cement implantation lies in the individual characteristics of the patients, such as age, gender, metabolism and comorbidities (e.g., osteoporosis or inflammatory diseases). However, non-clinical evaluation is important for the study of material behavior, including comparative evaluations with existing commercial medical devices.
5. Calcium Phosphate Cements as Carriers of Functional Substances
The incorporation of active molecules into calcium phosphate cement can be achieved by dissolving it in the liquid phase, by mixing with the powder phase, or by simultaneously mixing with the powder and liquid phases [238], including with granules of resorbable fillers. The surface adsorption of drugs to the cement surface by incubating the scaffold in a drug solution is another possible approach. Surface impregnation for cements is rarely used. The kinetic release of drugs depends on the functionalization, microstructure and resorbability of the CPC matrix [116,129]. The gradual release of drugs is ensured by a homogeneous distribution in the cement stone volume, which can be effective in the treatment of various bone diseases such as tumors, osteoporosis or osteomyelitis [115]. Cells, injectable calcium phosphate cements, and functional agents, used alone or in combination, can promote tissue regeneration in a minimally invasive manner to restore function, reduce risk, reduce complications, and reduce treatment costs [34].
The functionalization of calcium phosphate cements is of great clinical interest for the treatment or prevention of various bone diseases. The main requirement of this for all carrier materials is prolonged elution. The release of functional substances depends on many factors:
- -
- Resorption rate (depends on crystallinity, porosity, phase composition, presence of additives, surface roughness, L/P ratio, molding method, curing conditions, geometric shape and matrix size);
- -
- Size and size distribution of pores (depends on the phase composition, presence and concentration of additives and their nature, L/P ratio, molding method, hardening conditions);
- -
- pH of the cement stone (depends on the phase composition, solubility);
- -
- Solubility of a functional substance (depending on the type, chemical nature);
- -
- The possibility of interaction between the functional substance and the matrix (depends on the chemical formula);
- -
- The size of the molecule of the functional substance;
- -
- Quantity and uniformity of distribution of the immobilized functional substance;
- -
- Method of immobilization of the functional substance;
- -
- Type of supply of calcium phosphate cement (paste or cement stone).
The release of functional substances also depends on environmental factors. There are specific interactions between the ions formed during the dissolution of the drug and the ions of saline buffer solutions. This may play an important role in determining release mechanisms and in shaping the release profile [239]. Saline buffer solutions such as PBS or SBF promote the precipitation of HA and its deposition on the surface of the cement. It acts as a barrier to the diffusion of drugs from the volume, thereby reducing the release rate [137].
Environmental conditions are different in experiments in vivo and in vitro. This affects the release. The amount of vancomycin released in vivo has been reported to be half that of vancomycin released in vitro [240]. It is necessary to take into account that high concentrations of the antibiotic can adversely affect osteogenesis. It was reported that high concentrations of gentamicin sulfate inhibited the production of alkaline phosphatase by cells [241].
The kinetics of release of functional substances from calcium phosphate cements are controlled by diffusion, since matrices are resorbed more slowly compared to the release of functional substances. The mechanism of material resorption can be connected to the diffusion mechanism in the case of a more highly resorbable brushite cement [242,243].
Mathematical models taking into account various internal and external parameters are used to determine the kinetics of drug release from delivery systems (Table 3) [244].

Table 3.
Mathematical models for the determination of the kinetics of drug release.
The release curves of functional substances from calcium phosphate matrices most often show bimodal release. In this case, a typical initial release occurs within the first 24 h, followed by a sustained slow release [152]. This corresponds to the Higuchi model. It describes the release of functional substances from matrix systems as a diffusion process, based on Fick’s law and depending on the square root of time. The burst of release in the initial period of time reflects the slight diffusion of functional substances from the surface layer. After this period of time, diffusion from the inner surface of the matrix is somewhat difficult; this slows down the release of functional substances and thereby prolongs the release.
Multi-stage release profiles potentially offer much greater advantages over monotonic drug elution kinetics. The rapid initial release is able to effectively stop the pathological process, while its longer second release phase will gradually support the healing process [245].
The rate of release of functional substances depends on the morphology of the cement stone particles, as it leads to a different degree of adsorption of the substance on the inner surface. Desorption of functional substances from the surface of calcium phosphates with needle morphology of crystals is higher compared to desorption with lamellar morphology [246].
The surface charge of molecules of functional substances increases adsorption with the surface of calcium phosphate cement due to electrostatic attraction. Positively charged functional substances bind to the surface of calcium phosphate, since there are many negatively charged phosphate and hydroxide ions on the surface. Negatively charged functional substances bind to the surface of calcium phosphate with a large amount of calcium ions [247], such as cefaclor and ciprofloxacin, which have carboxyl groups [248]. The functional groups of BMP-2 (hydroxyl, amine and carboxyl) have a high affinity for calcium phosphates.
However, the presence of cephalexin has been reported to inhibit the growth of hydroxyapatite crystals. This is due to the ability of carboxylic acid molecules to be adsorbed on the surfaces of the initial components and nascent crystals, and to inhibit the growth of the target phase [249].
The values of the surface zeta potential are negative for calcium phosphates, but they differ depending on the arrangement of atoms on two types of crystal planes along the (a) axis and along the (c) axis [247]. Plane (a) is rich in positively charged calcium ions, and plane (c) is rich in negatively charged phosphate and hydroxide ions [250]. The different habitus of calcium phosphate crystals determines the different surface zeta potential due to the different arrangement of atoms. Negatively charged functional substances are easily adsorbed on needle-shaped crystals with developed (a) planes. Positively charged functional substances are adsorbed on lamellar crystals with developed (c) planes [246]. Neutral functional substances are adsorbed to a lesser extent and more easily desorbed from the surface of calcium phosphates, for example, metronidazole [248] or di(ethylenediamineplatinum)medronate [246]. Irregular crystals have intermediate zeta potentials and differently oriented planes [247].
Low crystallinity and high specific surface area allow the mobilization of more functional substances [251].
An increase in open porosity leads to an increase in surface area and the faster elution of functional substances from the cement matrix, since the drug solution is released through the phenomenon of capillary flow [252]. The volumetric flow rate of the eluted substance is proportional to the radius of the capillary. The influence of the pore size is more intense when it is similar to the size of the molecules of functional substances. Molecules of functional substances can freely diffuse from much larger pores. Release is controlled by diffusion to a greater extent than by structural factors [253]. This aspect is extremely important in the incorporation of giant molecules of some protein growth factors, such as the bone morphogenetic protein (BMP).
The release rate of functional substances from injection formulations is higher due to the setting period and the initial hardening time until the structure of the cement stone is formed. The kinetics depend on setting time, cohesion, microenvironment and hardening conditions [254,255]. Thus, drug release rates from brushite cement after 3 min and 1 h of curing showed explosive release during the first 8 h and slower release over 4 days. At the same time, the initial substances were found in the composition after 3 min. Most of the reagents turned into brushite after 1 h. The morphology of brushite crystals changed slightly from 1 to 15 h, which is confirmed by the values of the total porosity and tortuosity coefficient [255].
The functional substance should not impair the physical properties of the cements, and the cement should not change the active principle of the functional substance in delivery systems [238]. Brushite cements have a high ion concentration and an acid setting reaction—properties that can reduce or inhibit the effects of certain drugs [238].
In particular, the antibiotic groups of tetracyclines tend to chelate Ca2+ ions. This affects the primary formation of the nuclei of crystallization in brushite cements, prevents the deposition of minerals, and inhibits mineralization. Consequently, the incorporation of tetracyclines increases the setting time [256,257].
On the other hand, functional substances loaded in the form of salts into cement stone can affect the structure of the cement stone. For example, the addition of lidocaine hydrochloride increases the size of needle and plate CDHA crystals in TCP-based cement stone [239]. Increasing the size of the crystals ensures a good interaction between the preparation and the surface of the cement crystals. Large needles and plates provide greater adsorption, greater chemical binding and greater dissolution of lidocaine hydrochloride from the surface of cement crystals. This increases the release rate without changing the phase composition of the cement [239,258].
The excipients in the composition of pharmaceuticals ensure their shape, consistency, strength and degradation properties, and can affect the properties of cement stone. For example, sodium stearate inhibits the hydration of α-TCP and slows down the setting reaction. This contributes to an increase in the release rate of the functional substance at the first stage [137].
One of the options for changing the kinetics of the output of a functional agent is its encapsulation in a biodegradable polymer. As a result of encapsulation, the kinetics of elution are limited by diffusion from the polymer. Reduced initial explosive elution of the drug and more prolonged elution at a later date were observed when the substance was incorporated into PGLA microspheres, compared with direct incorporation [259].
The kinetics of elution of functional substances can be determined using the following methods: ultraviolet–visible spectroscopy (UV-Vis), high-performance liquid chromatography (HPLC) and fluorescent polarization immunoassay (FPIA). The electrochemical impedance spectroscopy (EIS) of Pasqual Group positions is a method for determining the amount of a drug without aliquot selection, as required by traditional methods [239].
A brief review of the scientific literature over the past 5 years on calcium phosphate cements as carriers of functional substances for the treatment of bone tissue is presented in Table 4.

Table 4.
Calcium phosphate cements as carriers of functional substances for the treatment of bone tissue.
6. Conclusions
Bone tissue repair is often limited by large defects and associated complications (osteoporosis, infection, and metastasis). Effective treatment strategies are aimed at the resection of the affected area of bone tissue, followed by treatment. Due to the limited availability of the affected bone for systemic therapy and for the prevention of postoperative complications, a local therapy method is used with the delivery of functional substances during surgery, or using minimally invasive procedures. Local prolonged drug delivery systems in effective therapeutic doses to the site of bone disease can contribute to the effective treatment of various bone diseases with the least side effects.
Calcium phosphate cements have a chemical composition similar to the inorganic component of bone tissue. They are biocompatible, resorbable, injectable and self-hardening. They can take the form of a bone defect and fit tightly to the bone bed. The Ca2+ ion is an important homing signal; it brings together various cell types necessary to initiate bone remodeling. Ca2+ ions affect protein growth factors, and additionally stimulate the expression of osteogenic marker genes.
At present, the most common approach to using calcium phosphate cements as scaffolds is the bulk incorporation of anti-inflammatory, antitumor, antiresorptive, and osteogenic functional substances.
The functionalization of calcium phosphate cements for a specific task requires an understanding of cement systems. Cements are complex systems. Changing one of the many parameters of the system changes the final characteristics of the matrix in a wide range, including the kinetics of elution of functional substances.
In this paper, the diseases of bone tissue are described, in the treatment of which calcium phosphate cements can be used as carriers of functional substances. Knowledge of the pathogenesis of diseases and the methods of treatment used—in particular, drug therapy—can help develop the concepts of directed functionalization for various clinical cases. We tried to isolate and describe in detail the factors that affect the release of functional substances from calcium phosphate cements, as related to the parameters of the cement system and the nature and affinity of functional substances for restoring bone tissue with the matrix.
Over the past two years, interesting reviews have been published by various scientific groups, which indicate an undying interest in calcium phosphate cements. A more detailed description of the properties of injectable calcium phosphate cements and the possibilities of their regulation can be found in the Vezenkova review, where cements are considered from the point of view of obtaining an osteoinductive product [279]. The mechanism of decomposition, recrystallization, resorption assessment and clinical characteristics of biodegradable cement are widely described in the Liu review [280]. A detailed review of composite materials based on cements with bioactive glass was carried out by the Demir-Oğuz scientific group [281].
The functionalization of calcium phosphate cement is one of the most important directions for solving the problems of the treatment and restoration of bone tissue.
The use of calcium phosphate cements as carriers of anticancer drugs and bisphosphonates is addressed in the reviews of Zogakis et al. [282] and Ntep et al. [283]. The possibility of using SaR-bisphosphonate and SaR-doxycycline cements in the non-surgical treatment of osteolytic tumors of the jaws is considered in the review by Ntep et al. Factors for achieving the sustained release of anti-bone cancer drugs are discussed in a review by Pylostomou et al. The influences of drugs, nanoparticles and ion substitutions on the properties of calcium phosphate cements have been analyzed [198]. General issues of functionalization are very thoroughly discussed in the Fosca review article [137].
Based on an understanding of the importance of calcium phosphate cements in the process of bone tissue restoration, and the possibility of functionalization by incorporating various drugs and growth factors directly into the volume of the cement matrix, or via encapsulation in polymer carriers, we assume that future research will aim at solving the problems of interaction between the carrier and functional substances, stabilizing (controlling) the release of functional substances from the matrix, and creating ideal frameworks (structure, geometry, resorption rate) aimed at faster restoration of the integrity of bone tissue.
Author Contributions
Conceptualization, Y.L.; investigation, Y.L., T.S., D.S. and O.T.; writing—original draft preparation, Y.L., T.S. and O.T.; writing—review and editing, Y.L. and T.S. visualization, Y.L. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out within the framework of the state task “Development of technology for obtaining osteoplastic polymer and calcium phosphate materials with a controlled rate of release of antibiotics and targeted pharmaceutical substances for the surgical treatment of purulent processes of bone tissue and prevention of the formation of bacterial biofilms on implantable metal structures”, and was funded by the Ministry of Health of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to give thanks to Alina Nesterenko for the illustrations in the review.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Doblaré, M.; Garcıa, J.M.; Gómez, M.J. Modelling bone tissue fracture and healing: A review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hassan, M.G.; Scheller, E.L. Neural regulation of bone marrow adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101522. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Y.; Yu, X. The unique metabolic characteristics of bone marrow adipose tissue. Front. Endocrinol. 2019, 10, 69. [Google Scholar] [CrossRef]
- Jouret, F.; Wu, J.; Hull, M.; Rajendran, V.; Mayr, B.; Schöfl, C.; Geibel, J.; Caplan, M.J. Activation of the Ca2+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. J. Cell Sci. 2013, 126, 5132–5142. [Google Scholar] [CrossRef]
- Kitay, A.M.; Geibel, J.P. Stomach and bone. In Understanding the Gut-Bone Signaling Axis: Mechanisms and Therapeutic Implications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–131. [Google Scholar] [CrossRef]
- Rammelt, S. Management of ankle fractures in the elderly. EFORT Open Rev. 2016, 1, 239–246. [Google Scholar] [CrossRef]
- Qiu, Z.-Y.; Cui, Y.; Wang, X.-M. Natural Bone Tissue and Its Biomimetic. In Mineralized Collagen Bone Graft Substitutes; Woodhead Publishing: Sawston, UK, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 2, 97–108. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. The structure and mechanics of bone. J. Mater. Sci. 2012, 47, 41–54. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Ur Rehman, F.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, M.; Buchwald, T.; Szybowicz, M.; Błaszczak, Z.; Piotrowski, A.; Ciesielczyk, B. Determination of composition and structure of spongy bone tissue in human head of femur by Raman spectral mapping. J. Mater. Sci. Mater. Med. 2011, 22, 1653–1661. [Google Scholar] [CrossRef]
- Cross, L.M.; Thakur, A.; Jalili, N.A.; Detamore, M.; Gaharwar, A.K. Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater. 2016, 42, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Augat, P.; Schorlemmer, S. The role of cortical bone and its microstructure in bone strength. Age Ageing 2006, 35, ii27–ii31. [Google Scholar] [CrossRef]
- Grimal, Q.; Laugier, P. Quantitative ultrasound assessment of cortical bone properties beyond bone mineral density. IRBM 2019, 40, 16–24. [Google Scholar] [CrossRef]
- Boughton, O.R.; Ma, S.; Cai, X.; Yan, L.; Peralta, L.; Laugier, P.; Marrow, J.; Giuliani, F.; Hansen, U.; Abel, R.L.; et al. Computed tomography porosity and spherical indentation for determining cortical bone millimetre-scale mechanical properties. Sci. Rep. 2019, 9, 7416. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Schneider, J.; Varga, P.; Laugier, P.; Raum, K.; Grimal, Q. Elasticity-density and viscoelasticity-density relationships at the tibia mid-diaphysis assessed from resonant ultrasound spectroscopy measurements. Biomech. Model. Mechanobiol. 2016, 15, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2014, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 19, pp. 459–466. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Khan, N.K. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. J. Inflamm. Res. 2005, 54, 358–366. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.-A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Blokhuis, T.J.; Pape, H.C.; Giannoudis, P.V. Pharmacological agents and impairment of fracture healing: What is the evidence? Injury 2008, 39, 384–394. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef]
- Perren, S.M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar] [PubMed]
- Court-Brown, C.M.; Biant, L.C.; Clement, N.D.; Bugler, K.E.; Duckworth, A.D.; McQueen, M.M. Open fractures in the elderly. The importance of skin ageing. Injury 2015, 46, 189–194. [Google Scholar] [CrossRef]
- Toosi, S.; Behravan, J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors 2019, 46, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological bone remodeling: Systemic regulation and growth factor involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Yun, Y.R.; Jang, J.H.; Jeon, E.; Kang, W.; Lee, S.; Won, J.E.; Kim, H.W.; Wall, I. Administration of growth factors for bone regeneration. Regen. Med. 2012, 7, 369–385. [Google Scholar] [CrossRef]
- Goodman, S.; Ma, T.; Trindade, M.; Ikenoue, T.; Matsuura, I.; Wong, N.; Fox, N.; Genovese, M.; Regula, D.; Smith, R.L. COX-2 selective NSAID decreases bone ingrowth in vivo. J. Orthop. Res. 2002, 20, 1164–1169. [Google Scholar] [CrossRef]
- Reikeraas, O.; Engebretsen, L. Effects of ketoralac tromethamine and indomethacin on primary and secondary bone healing. Arch. Orthop. Traum. Surg. 1998, 118, 50–52. [Google Scholar] [CrossRef]
- Amanat, N.; Brown, R.; Bilston, L.E.; Little, D.G. A single systemic dose of pamidronate improves bone mineral content and accelerates restoration of strength in a rat model of fracture repair. J. Orthop. Res. 2005, 235, 1029–1034. [Google Scholar] [CrossRef]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wu, Y.; Lin, H.; Liu, R. Advances in the antimicrobial treatment of osteomyelitis. Compos. B Eng. 2022, 249, 110428. [Google Scholar] [CrossRef]
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018, 31, e00084-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Gao, S.; Zhang, Y.W.; Zhou, R.B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kernéis, S.; Plainvert, C.; Barnier, J.P.; Tazi, A.; Dmytruk, N.; Gislain, B.; Loubinoux, J.; Sayed, F.E.; Cattoir, V.; Desplaces, N.; et al. Clinical and microbiological features associated with group B Streptococcus bone and joint infections, France 2004–2014. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and hydroxyapatite based biomaterials to circumvent periprosthetic joint infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef]
- Cherifi, S.; Byl, B.; Deplano, A.; Nonhoff, C.; Denis, O.; Hallin, M. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J. Clin. Microbiol. 2013, 51, 1541–1547. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant—Associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Von Eiff, C.; Pirini, V.; Ravaioli, S.; Becker, K.; Arciola, C.R. Cluster analysis of ribotyping profiles of Staphylococcus epidermidis isolates recovered from foreign body—Associated orthopedic infections. J. Biomed. Mater. Res. A 2009, 88, 664–672. [Google Scholar] [CrossRef]
- Campoccia, D.; Baldassarri, L.; Pirini, V.; Ravaioli, S.; Montanaro, L.; Arciola, C.R. Molecular epidemiology of Staphylococcus aureus from implant orthopaedic infections: Ribotypes, agr polymorphism, leukocidal toxins and antibiotic resistance. Biomaterials 2008, 29, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Sarkissian, E.J.; Gans, I.; Gunderson, M.A.; Myers, S.H.; Spiegel, D.A.; Flynn, J.M. Community-acquired Methicillin-resistant Staphylococcus aureus Musculoskeletal Infections. J. Pediatr. Orthop. 2016, 36, 323–327. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.C.; Ramp, W.K.; Nicholson, N.C.; Williams, A.S.; Nousiainen, M.T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 1995, 19, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Ene, R.; Nica, M.; Ene, D.; Cursaru, A.; Cirstoiu, C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Rev. 2021, 6, 297. [Google Scholar] [CrossRef]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Calhoun, J.H.; Manring, M.M. Adult Osteomyelitis. Infect. Dis. Clin. N. Am. 2005, 19, 765–786. [Google Scholar] [CrossRef]
- Gruber, H.E. Bone and the immune system. Proc. Soc. Exp. Biol. Med. 1991, 197, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 437, 91–96. [Google Scholar] [CrossRef]
- Wiesli, M.G.; Kaiser, J.P.; Gautier, E.; Wick, P.; Maniura-Weber, K.; Rottmar, M.; Wahl, P. Influence of ceftriaxone on human bone cell viability and in vitro mineralization potential is concentration-and time-dependent. Bone Jt. Res. 2021, 10, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Ossendorf, C.; Leerapun, T.; Sim, F.H. Intercalary segmental reconstruction after bone tumor resection. Eur. J. Surg. Oncol. 2008, 34, 1271–1276. [Google Scholar] [CrossRef]
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Alkhooly, A.Z.A.; Abd-Elfattah, A.S.; Elsaid, A.N.S.; Ahmed, A.R.; Tsuchiya, H. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J. Orthop. Surg. 2019, 27, 2309499019832970. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, I.; Kousidou, O.C.; Karamanos, N.K. In vitro and in vivo antiresorptive effects of bisphosphonates in metastatic bone disease. In Vivo 2005, 19, 311–318. [Google Scholar]
- Phull, S.S.; Yazdi, A.R.; Ghert, M.; Towler, M.R. Bone cement as a local chemotherapeutic drug delivery carrier in orthopedic oncology: A review. J. Bone Oncol. 2021, 26, 100345. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Oades, G.M.; Coxon, J.; Colston, K.W. The potential role of bisphosphonates in prostate cancer. Prostate Cancer Prostatic Dis. 2002, 5, 264–272. [Google Scholar] [CrossRef]
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. CA Cancer J. Clin. 2018, 68, 377–386. [Google Scholar] [CrossRef]
- Mbese, Z.; Aderibigbe, B.A. Bisphosphonate-Based conjugates and derivatives as potential therapeutic agents in osteoporosis, bone cancer and metastatic bone cancer. Int. J. Mol. Sci. 2021, 22, 6869. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Rosen, L.S.; Howell, A.; Porter, L.; Coleman, R.E.; Morley, W.; Dreicer, R.; Kuross, S.A.; Lipton, A.; Seaman, J.J. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: A double-blind, randomized dose–response study. Cancer 2001, 91, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Mirels, H. Metastatic Disease in Long Bones a Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 1989, 249, 256–264. [Google Scholar] [CrossRef]
- Chen, T.H.; Chen, W.M.; Huang, C.K. Reconstruction after intercalary resection of malignant bone tumours: Comparison between segmental allograft and extracorporeally-irradiated autograft. J. Bone Jt. Surg. Br. Vol. 2005, 87, 704–709. [Google Scholar] [CrossRef]
- Harvey, N.; Ahlmann, E.R.; Allison, D.C.; Wang, L.; Menendez, L.R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin. Orthop. Relat. Res. 2012, 470, 684–691. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, X.C.; Xu, M.; Zheng, K.; Hu, Y.C.; Wang, F.; Lun, D.X. Intercalary prosthetic reconstruction for pathologic diaphyseal humeral fractures due to metastatic tumors: Outcomes and improvements. J. Shoulder Elb. Surg. 2018, 27, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Henrichs, M.P.; Krebs, J.; Gosheger, G.; Streitbuerger, A.; Nottrott, M.; Sauer, T.; Hoell, S.; Singh, G.; Hardes, J. Modular tumor endoprostheses in surgical palliation of long-bone metastases: A reduction in tumor burden and a durable reconstruction. World J. Surg. Oncol. 2014, 12, 330. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Schwartz, A.J.; Oakes, D.A.; Kabo, J.M.; Eckardt, J.J. How long do endoprosthetic reconstructions for proximal femoral tumors last? Clin. Orthop. Relat. Res. 2010, 468, 2867–2874. [Google Scholar] [CrossRef]
- Bickels, J.; Wittig, J.C.; Kollender, Y.; Henshaw, R.M.; Kellar-Graney, K.L.; Meller, I.; Malawer, M.M. Distal femur resection with endoprosthetic reconstruction: A long-term followup study. Clin. Orthop. Relat. Res. 2002, 400, 225–235. [Google Scholar] [CrossRef]
- Wafa, H.; Reddy, K.; Grimer, R.; Abudu, A.; Jeys, L.; Carter, S.; Tillman, R. Does total humeral endoprosthetic replacement provide reliable reconstruction with preservation of a useful extremity? Clin. Orthop. Relat. Res. 2015, 473, 917–925. [Google Scholar] [CrossRef]
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanzawa, Y.; Tsuchiya, H.; Shirai, T.; Nishida, H.; Hayashi, K.; Takeuchi, A.; Tomita, K.; Kawahara, M. Potentiation of the antitumor effect of calcium phosphate cement containing anticancer drug and caffeine on rat osteosarcoma. J. Orthop. Sci. 2011, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Donanzam, B.A.; Campos, T.P.R.; Dalmázio, I.; Valente, E.S. Synthesis and Characterization of Calcium Phosphate Loaded with Ho-166 and Sm-153: A Novel Biomaterial for Treatment of Spine Metastases. J. Mater. Sci. Mater. Med. 2013, 24, 2873–2880. [Google Scholar] [CrossRef]
- Dolci, L.S.; Panzavolta, S.; Torricelli, P.; Albertini, B.; Sicuro, L.; Fini, M.; Bigi, A.; Passerini, N. Modulation of Alendronate release from a calcium phosphate bone cement: An in vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019, 554, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Jia, S.J.; Hao, D.J. Advances in the modification of injectable calcium-phosphate-based bone cements for clinical application. Chin. Med. J. 2020, 133, 2610–2612. [Google Scholar] [CrossRef]
- Natesan, V.; Kim, S.J. Metabolic bone diseases and new drug developments. Biomol. Ther. 2022, 30, 309. [Google Scholar] [CrossRef]
- Anderson, H.C.; Harmey, D.; Camacho, N.P.; Garimella, R.; Sipe, J.B.; Tague, S.; Bi, X.; Johnson, K.; Terkeltaub, R.; Millán, J.L. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am. J. Pathol. 2005, 166, 1711–1720. [Google Scholar] [CrossRef]
- Albahri, A.S.; Alwan, J.K.; Taha, Z.K.; Ismail, S.F.; Hamid, R.A.; Zaidan, A.A.; Albahri, O.S.; Zaidan, B.B.; Alamoodi, A.H.; Alsalem, M.A. IoT-based telemedicine for disease prevention and health promotion: State-of-the-art. J. Netw. Comput. Appl. 2021, 173, 102873. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P. Therapy of endocrine disease: Denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur. J. Endocrinol. 2018, 179, R31–R45. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The pathophysiology and treatment of osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- Chinoy, A.; Mughal, M.Z.; Padidela, R. Metabolic bone disease of prematurity: Causes, recognition, prevention, treatment and long-term consequences. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F560–F566. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H. Bisphosphonates in osteoporosis. Eur. Spine J. 2003, 12, S142–S146. [Google Scholar] [CrossRef] [PubMed]
- Fabre, S.; Funck-Brentano, T.; Cohen-Solal, M. Anti-sclerostin antibodies in osteoporosis and other bone diseases. J. Clin. Med. 2020, 9, 3439. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.D.; Gonzalez, B.; Miskevics, S.; Ray, C.; Etingen, B.; Guihan, M.; Craven, B.C.; George, V.; Weaver, F.M. Association of bisphosphonate therapy with incident of lower extremity fractures in persons with spinal cord injuries or disorders. Arch. Phys. Med. Rehabil. 2020, 101, 633–641. [Google Scholar] [CrossRef]
- Dvorak, M.M.; Riccardi, D. Ca2+ as an extracellular signal in bone. Cell Calcium 2004, 35, 249–255. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef]
- Nandi, S.K.; Bandyopadhyay, S.; Das, P.; Samanta, I.; Mukherjee, P.; Roy, S.; Kundu, B. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol. Adv. 2016, 34, 1305–1317. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Galusha, A.L.; Howard, L.J.; Kruger, P.C.; Marks, T.; Parsons, P.J. Bone mineral composition among long-term parenteral nutrition patients: Postmortem assessment of calcium, phosphorus, magnesium, and select trace elements. J. Parenter. Enteral. Nutr. 2020, 45, 175–182. [Google Scholar] [CrossRef]
- Xing, T.; Hu, Y.; Wang, B.; Zhu, J. Role of oral calcium supplementation alone or with vitamin D in preventing post-thyroidectomy hypocalcaemia: A meta-analysis. Medicine 2019, 98, e14455. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, L.; Jin, X.; Chen, D.; Jin, X.; Xu, G. Effect of calcium and magnesium on inflammatory cytokines in accidentally multiple fracture adults: A short-term follow-up. Medicine 2022, 101, e28538. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef]
- Notomi, T.; Kuno, M.; Hiyama, A.; Nozaki, T.; Ohura, K.; Ezura, Y.; Noda, M. Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J. Biol. Chem. 2017, 292, 20998–21010. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Faber, P.; Ekema, G.; Jackson, P.D.; Ting, A.; Wang, N.; Fontilla-Poole, M.; Mays, R.W.; Brunden, K.R.; Harrington, J.J.; et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 40201–40209. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Lin, Y. The efficacy of parathyroid hormone analogues in combination with bisphosphonates for the treatment of osteoporosis: A meta-analysis of randomized controlled trials. Medicine 2015, 94, e1156. [Google Scholar] [CrossRef]
- Che, J.; Yang, J.; Zhao, B.; Zhang, G.; Wang, L.; Peng, S.; Shang, P. The effect of abnormal iron metabolism on osteoporosis. Biol. Trace Elem. Res. 2020, 195, 353–365. [Google Scholar] [CrossRef]
- Krenzlin, H.; Foelger, A.; Mailänder, V.; Blase, C.; Brockmann, M.; Düber, C.; Ringel, C.; Keric, N. Novel biodegradable composite of calcium phosphate cement and the collagen I mimetic P-15 for pedicle screw augmentation in osteoporotic bone. Biomedicines 2021, 9, 1392. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, S.; Zou, R.; Gao, X.; Ruan, J.; Weir, M.D.; Reynolds, M.A.; Qin, W.; Chang, X.; Fu, H.; et al. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent. Mater. 2019, 35, 1031–1041. [Google Scholar] [CrossRef]
- Van Oirschot, B.; Mikos, A.G.; Liu, Q.; van den Beucken, J.J.; Jansen, J.A. Fast Degradable Calcium Phosphate Cement for Maxillofacial Bone Regeneration. Tissue Eng. Part A 2023, 29, 161–171. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; DGresser, J.; Wise, D.L.; Trantolo, D.J. Bioresorbable bone graft substitutes of different osteoconductivities: A histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials 2000, 21, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics 2022, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive calcium phosphate-based composites for bone regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Lukina, Y.; Panov, Y.; Panova, L.; Senyagin, A.; Bionyshev-Abramov, L.; Serejnikova, N.; Kireynov, A.; Sivkov, S.; Gavryushenko, N.; Smolentsev, D.; et al. Chemically Bound Resorbable Ceramics as an Antibiotic Delivery System in the Treatment of Purulent–Septic Inflammation of Bone Tissue. Ceramics 2022, 5, 26. [Google Scholar] [CrossRef]
- Skriabin, A.S.; Shakurov, A.V.; Vesnin, V.R.; Lukina, Y.S.; Tsygankov, P.A.; Bionyshev-Abramov, L.L.; Serejnikova, N.B.; Vorob’ev, E.V. Titanium Membranes with Hydroxyapatite/Titania Bioactive Ceramic Coatings: Characterization and In Vivo Biocompatibility Testing. ACS Omega 2022, 7, 47880–47891. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Lukina, Y.; Kotov, S.; Bionyshev-Abramov, L.; Serejnikova, N.; Chelmodeev, R.; Fadeev, R.; Toshev, O.; Tavtorkin, A.; Ryndyk, M.; Smolentsev, D.; et al. Low-Temperature Magnesium Calcium Phosphate Ceramics with Adjustable Resorption Rate. Ceramics 2023, 6, 11. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Safronova, T.V.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.S.; Davydova, G.A.; Shatalova, T.B.; Larionov, D.S.; Rau, J.V. Biocompatibility of biphasic α, β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; de Bruijn, J.; de Groot, K.; Zhang, X. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000, 21, 1283–1290. [Google Scholar] [CrossRef]
- Hurle, K.; Oliveira, J.M.; Reis, R.L.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped brushite cements for bone regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur. Spine J. 2001, 10 (Suppl. S2), S114–S121. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Su, J.; Wei, J.; Kong, H.; Liu, C. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 2009, 5, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Maazouz, Y.; Diez-Escudero, A.; Rappe, K.; Espanol, M.; Montufar, E.B.; Öhman-Mägi, C.; Persson, C.; Fontecha, P.; Manzanares, M.-C.; et al. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: Effect of pore architecture. Acta Biomater. 2018, 79, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, H.; Maruta, M.; Sato, T.; Kajimoto, N.; Fujii, E.; Matsuura, T.; Tsuru, K. Fabrication of bioresorbable hydroxyapatite bone grafts through the setting reaction of calcium phosphate cement. Dent. Mater. J. 2022, 41, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D printed calcium phosphate cement (CPC) scaffolds for anti-cancer drug delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; Bentley, K.L.D.M.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef]
- Bagnol, R.; Sprecher, C.; Peroglio, M.; Chevalier, J.; Mahou, R.; Büchler, P.; Richards, G.; Eglin, D. Coaxial micro-extrusion of a calcium phosphate ink with aqueous solvents improves printing stability, structure fidelity and mechanical properties. Acta Biomater. 2021, 125, 322–332. [Google Scholar] [CrossRef]
- Irbe, Z.; Loca, D. Soluble phosphate salts as setting aids for premixed calcium phosphate bone cement pastes. Ceram. Int. 2021, 47, 24012–24019. [Google Scholar] [CrossRef]
- Criado, A.; Yokhana, S.; Rahman, T.; McCarty, S.; Andrecovich, C.; Ren, W.; Yassir, W.K. Biomechanical strength comparison of pedicle screw augmentation using poly-dicalcium phosphate dihydrate (P-DCPD) and polymethylmethacrylate (PMMA) cements. Spine Deform. 2020, 8, 165–170. [Google Scholar] [CrossRef]
- Kucko, N.W.; Li, W.; García Martinez, M.A.; Rehman, I.U.; Ulset, A.S.T.; Christensen, B.E.; Leewenburgh, S.C.G.; Herber, R.P. Sterilization effects on the handling and degradation properties of calcium phosphate cements containing poly (D, L–lactic–co–glycolic acid) porogens and carboxymethyl cellulose. J. Biomed. Mater. Res. 2019, 107, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, E.; Czechowska, J.P.; Krok-Borkowicz, M.; Allinson, S.L.; Stępień, K.; Smith, A.; Douglas, T.E.L.; Zima, A. Biosurfactants as foaming agents in calcium phosphate bone cements. Biomater. Adv. 2023, 145, 213273. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda Schickert, S.; Jansen, J.A.; Bronkhorst, E.M.; van den Beucken, J.J.; Leeuwenburgh, S.C. C. Stabilizing dental implants with a fiber-reinforced calcium phosphate cement: An in vitro and in vivo study. Acta Biomater. 2020, 110, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V. Inorganic Materials for Regenerative Medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- Aberg, J.; Brisby, H.; Henriksson, H.; Lindahl, A.; Thomsen, P.; Engqvist, H. Premixed acidic calcium phosphate cement: Characterization of strength and microstructure. J. Biomed. Mater. Res. 2010, 93, 436–441. [Google Scholar] [CrossRef]
- Bohner, M.; Doebelin, N.; Baroud, G. Theoretical and experimental approach to test the cohesion of calcium phosphate pastes. Eur. Cell. Mater. 2006, 12, 26–35. [Google Scholar] [CrossRef]
- Martin, R.I.; Brown, P.W. Formation of hydroxyapatite in serum. J. Mater. Sci. Mater. Med. 1994, 5, 96–102. [Google Scholar] [CrossRef]
- Eanes, E.; Hailer, A. Anionic Effects on the Size and Shape of Apatite Crystals Grown from Physiological Solutions. Calcif. Tissue Int. 2000, 66, 449–455. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ishikawa, R.; Fukao, H.; Sawada, M.; Nagayama, M.; Kon, M.; Asaoka, K. In vivo setting behavior of fast-setting calcium phosphate cement. Biomaterials 1995, 16, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Vargas, F.; Ruiz, J.; López, M.E. Solid-state synthesis of alpha tricalcium phosphate for cements used in biomedical applications. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 193–200. [Google Scholar] [CrossRef]
- Hurle, K.; Neubauer, J.; Bohner, M.; Doebelin, N.; Goetz-Neunhoeffer, F. Effect of amorphous phases during the hydraulic conversion of α-TCP into calcium-deficient hydroxyapatite. Acta Biomater. 2014, 10, 3931–3941. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Grass, R.N.; Bohner, M.; Stark, W.J. Effect of particle size, crystal phase and crystallinity on the reactivity of tricalcium phosphate cements for bone reconstruction. J. Mater. Chem. 2007, 17, 4072–4078. [Google Scholar] [CrossRef]
- Bohner, M.; Brunner, T.J.; Stark, W.J. Controlling the reactivity of calcium phosphate cements. J. Mater. Chem. 2008, 18, 5669–5675. [Google Scholar] [CrossRef]
- Liu, C.; Shao, H.; Chen, W.; Zheng, H. Effect of granularity of raw materials on the hydration and hardening process of calcium phosphate cement. Biomaterials 2003, 24, 4103–4113. [Google Scholar] [CrossRef]
- Bermudez, O.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Development of an octocalcium phosphate cement. Mater. Sci. Mater. Med. 1994, 5, 144–146. [Google Scholar] [CrossRef]
- Brown, P.W.; Hocker, N.; Hoyle, S. Variations in Solution Chemistry During the Low-Temperature Formation of Hydroxyapatite. J. Am. Ceram. Soc. 1991, 74, 1848–1854. [Google Scholar] [CrossRef]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Hofmann, M.P.; Mohammed, A.R.; Perrie, Y.; Gbureck, U.; Barralet, J.E. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009, 5, 43–49. [Google Scholar] [CrossRef]
- Mirtchi, A.; Lemaitre, J.; Munting, E. Calcium phosphate cements: Action of setting regulators in the properties of the β-tricalcium phosphate—Monocalcium phosphate cements. Biomaterials 1989, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Eichmiller, F.C.; Giuseppetty, A.A. Reinforcement of a self-setting calcium phosphate cement with different fibers. J. Biomed. Mater. Res. 2000, 52, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Nezafati, N.; Moztarzadeh, F.; Hesaraki, S.; Mozafari, M. Synergistically reinforcement of a self-setting calcium phosphate cement with bioactive glass fibers. Ceram. Int. 2011, 37, 927–934. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite–carbon nanotube composites for biomedical applications: A review International. J. Appl. Ceram. Technol. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Zuo, Y.; Yang, F.; Wolke, J.G.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247. [Google Scholar] [CrossRef]
- Maenz, S.; Kunisch, E.; Mühlstädt, M.; Böhm, A.; Kopsch, V.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Enhanced mechanical properties of a novel, injectable, fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2014, 39, 328–338. [Google Scholar] [CrossRef]
- Kucko, N.W.; de Lacerda Schickert, S.; Sobral Marques, T.; Herber, R.P.; Van den Beuken, J.J.; Zuo, Y.; Leeuwenburgh, S.C. Tough and osteocompatible calcium phosphate cements reinforced with poly (vinyl alcohol) fibers. ACS Biomater. Sci. Eng. 2019, 5, 2491–2505. [Google Scholar] [CrossRef]
- Pan, Z.; Jiang, P.; Fan, Q.; Ma, B.; Cai, H. Mechanical and biocompatible influences of chitosan fiber and gelatin on calcium phosphate cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 246–252. [Google Scholar] [CrossRef]
- Yin, Y.; Ye, F.; Yao, K.; Cui, J.; Song, X. Gelatin manipulation of latent macropores formulation in brushite cement. J. Mater. Sci. Mater. Med. 2003, 14, 255–261. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; Stumpel, F.; Jansen, J.A.; van den Beucken, J.J. Early-stage macroporosity enhancement in calcium phosphate cements by inclusion of poly (N-vinylpyrrolidone) particles as a porogen. Mater. Today Commun. 2020, 23, 100901. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, F.; Chen, X.; Xiao, Y.; Zhang, X. Design of macropore structure and micro-nano topography to promote the early neovascularization and osteoinductivity of biphasic calcium phosphate bioceramics. Mater. Des. 2022, 216, 110581. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.C.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. The Size of Surface Microstructures as an Osteogenic Factor in Calcium Phosphate—Ceramics. Acta Biomater. 2014, 10, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.; Coathup, M.J.; Nesbitt, A.; Ho, C.Y.; Hing, K.A.; Buckland, T.; Campion, C.; Blunn, G.W. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 2012, 8, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.E.; Grover, L.M.; Gbureck, U. Ionic modification of calcium phosphate cement viscosity. Part II: Hypodermic injection and strength improvement of brushite cement. Biomaterials 2004, 25, 2197–2203. [Google Scholar] [CrossRef]
- Lukina, Y.S.; Panova, L.V.; Panov, Y.M.; Krut’ko, D.P.; Gavryushenko, N.S.; Lemenovskii, D.A. Application of SC-CO2 in the Technology of the Preparation of Calcium Phosphate Matrices for the Treatment of Septic Purulent Inflammations of Bone Tissues. Russ. J. Phys. Chem. B 2022, 16, 1221–1230. [Google Scholar] [CrossRef]
- Espanol, M.; Perez, R.A.; Montufar, E.B.; Marichal, C.; Sacco, A.; Ginebra, M.P. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009, 5, 2752–2762. [Google Scholar] [CrossRef]
- Lucas-Aparicio, J.; Manchón, Á.; Rueda, C.; Pintado, C.; Torres, J.; Alkhraisat, M.H.; López-Cabarcos, E. Silicon-calcium phosphate ceramics and silicon-calcium phosphate cements: Substrates to customize the release of antibiotics according to the idiosyncrasies of the patient. Mater. Sci. Eng. C 2020, 106, 110173. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L. Formation of macropores in calcium phosphate cement implants. Mater. Sci. Mater. Med. 2001, 12, 135–139. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Fernández, E.; Driessens, F.C.M.; Planell, J.A. Modeling of the Hydrolysis of α-Tricalcium Phosphate. J. Am. Ceram. Soc. 2004, 82, 2808–2812. [Google Scholar] [CrossRef]
- Kim, J.-A.; Lim, J.; Naren, R.; Yun, H.; Park, E.K. Effect of the biodegradation rate controlled by pore structures in magnesium phosphate ceramic scaffolds on bone tissue regeneration in vivo. Acta Biomater. 2016, 44, 155–167. [Google Scholar] [CrossRef]
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Sayahi, M.; Santos, J.; El-Feki, H.; Charvillat, C.; Bosc, F.; Karacan, I.; Milthorpe, B.; Drouet, C. Brushite (Ca,M)HPO4, 2H2O doping with bioactive ions (M = Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): A new path to functional biomaterials? Mater. Today Chem. 2020, 16, 100230. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and in vitro evaluations of injectable calcium phosphate cement doped with magnesium and strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kumta, P.N. Chemical synthesis and stabilization of magnesium substituted brushite. Mater. Sci. Eng. C 2010, 30, 934–943. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A. High thermally stable Mg-substituted tricalcium phosphate via precipitation. Ceram. Int. 2011, 37, 127–137. [Google Scholar] [CrossRef]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements for enhanced bone tissue regeneration. Mater. Sci. Eng. C 2014, 43, 403–410. [Google Scholar] [CrossRef]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.E.; Grover, L.M.; Kübler, A.C.; Gbureck, U. Phase composition, mechanical performance and in vitro biocompatibility of hydraulic setting calcium magnesium phosphate cement. Acta Biomater. 2010, 6, 1529–1535. [Google Scholar] [CrossRef]
- Wu, L.; Luthringer, B.J.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef]
- Pina, S.; Ferreira, J.M.F. Injectability of brushite-forming Mg-substituted and Sr-substituted α-TCP bone cements. J. Mater. Sci. Mater. Med. 2010, 21, 431–438. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Cabrejos-Azama, J.; Rodríguez, C.R.; Jerez, L.B.; Cabarcos, E.L. Magnesium substitution in brushite cements. Mater. Sci. Eng. C 2013, 33, 475–481. [Google Scholar] [CrossRef] [PubMed]
- TenHuisen, K.S.; Brown, P.W. Effects of magnesium on the formation of calcium-deficient hydroxyapatite from CaHPO4.2H2O and Ca4(PO4)2O. J. Biomed. Mater. Res. 1997, 36, 306–314. [Google Scholar] [CrossRef]
- Rousseau, S.; Lemaitre, J.; Bohner, M.; Frei, C. Long-Term aging of brushite cements in physiological conditions: An in vitro study. Eur. Cells Mater. 2002, 5, 83. [Google Scholar]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Zhang, Y.L.; Grover, L.; Merle, G.E.; Tamimi, F.; Barralet, J. In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater. 2015, 26, 338–346. [Google Scholar] [CrossRef]
- Sheikh, Z.; Geffers, M.; Christel, T.; Barralet, J.E.; Gbureck, U. Chelate setting of alkali ion substituted calcium phosphates. Ceram. Int. 2015, 41, 10010–10017. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Li, X.; Sogo, Y.; Ito, A.; Mutsuzaki, H.; Ochiai, N.; Kobayashi, T.; Nakamura, S.; Yamashita, K.; LeGeros, R.Z. The optimum zinc content in set calcium phosphate cement for promoting bone formation in vivo. Mater. Sci. Eng. C 2009, 29, 969–975. [Google Scholar] [CrossRef]
- Horiuchi, S.; Hiasa, M.; Yasue, A.; Sekine, K.; Hamada, K.; Asaoka, K.; Tanaka, E. Fabrications of zinc-releasing biocement combining zinc calcium phosphate to calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2014, 29, 151–160. [Google Scholar] [CrossRef]
- Cama, G.; Nkhwa, S.; Gharibi, B.; Lagazzo, A.; Cabella, R.; Carbone, C.; Dubruel, O.; Haugen, H.; Di Silvio, L.; Deb, S. The role of new zinc incorporated monetite cements on osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2017, 78, 485–494. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, J.; Zhu, Y.; Chen, L.; Ye, J. Zinc doping induced differences in the surface composition, surface morphology and Fosteogenesis performance of the calcium phosphate cement hydration products. Mater. Sci. Eng. C 2019, 105, 110065. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wang, J.; Yuan, X.; Tang, C.; Wang, X.; He, F.; Ye, J. Zinc-doped calcium silicate additive accelerates early angiogenesis and bone regeneration of calcium phosphate cement by double bioactive ions stimulation and immunoregulation. Biomater. Adv. 2022, 141, 213120. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-Doped Calcium Phosphate Bone Cements with Antibacterial Properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Pylostomou, A.; Demir-Oguz, O.; Loca, D. Calcium phosphate bone cements as local drug delivery systems for bone cancer treatment. Biomater. Adv. 2023, 148, 213367. [Google Scholar] [CrossRef]
- Cohen-Solal, M. Strontium overload and toxicity: Impact on renal osteodystrophy. Nephrol. Dial. Transplant. 2002, 17, 30–34. [Google Scholar] [CrossRef]
- Paul, K.; Lee, B.Y.; Abueva, C.; Kim, B.; Choi, H.J.; Bae, S.H.; Lee, B.T. In vivo evaluation of injectable calcium phosphate cement composed of Zn- and Si-incorporated β-tricalcium phosphate and monocalcium phosphate monohydrate for a critical sized defect of the rabbit femoral condyle. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 260–271. [Google Scholar] [CrossRef]
- Mariño, F.T.; Torres, J.; Hamdan, M.; Rodríguez, C.R.; Cabarcos, E.L. Advantages of using glycolic acid as a retardant in a brushite forming cement. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 571. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, N.; Van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaitre, J.; Leroy, G. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 1999, 25, 81S–84S. [Google Scholar] [CrossRef]
- Leroux, L.; Hatim, Z.; Freche, M.; Lacout, J.L. Effect of various adjuvants (lactic asid, glycerol, and chitosan) on the injectability of a calcium phosphate cement. Bone 1999, 25, 31S–34S. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Chen, Z.; Zhang, M.; Liu, C.; Su, K.; Wang, J.; Li, L.; Wang, R.; Li, B.; et al. Hyaluronic acid facilitates bone repair effects of calcium phosphate cement by accelerating osteogenic expression. Bioact. Mater. 2021, 6, 3801–3811. [Google Scholar] [CrossRef] [PubMed]
- Morilla, C.; Perdomo, E.; Hernández, A.K.; Regalado, R.; Almirall, A.; Fuentes, G.; Mora, Y.C.; Schomann, T.; Chan, A.; Cruz, L.J. Effect of the Addition of Alginate and/or Tetracycline on Brushite Cement Properties. Molecules 2021, 26, 3272. [Google Scholar] [CrossRef] [PubMed]
- Grosfeld, E.-C.; Hoekstra, J.W.M.; Herber, R.-P.; Ulrich, D.J.O.; Jansen, J.A.; van den Beucken, J.J.J.P. Long-term biological performance of injectable and degradable calcium phosphate cement. Biomed. Mater. 2016, 12, 015009. [Google Scholar] [CrossRef] [PubMed]
- Timimi, F.; Kumarasami, B.; Doillon, C.; Gbureck, U.; Le Nihouannen, D.; Cabarcos, E.L.; Barralet, J.E. Brushite-collagen composites for bone regeneration. Acta Biomater. 2008, 4, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Lanao, R.F.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011, 7, 3459–3468. [Google Scholar] [CrossRef]
- Del Real, R.; Wolke, J.G.; Vallet-Regí, M.; Jansen, J. A new method to produce macropores in calcium phosphate cements. Biomaterials 2002, 23, 3673–3680. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Suwa, Y.; Ohgushi, H.; Tamai, S.; Ichijima, K. Self-setting hydroxyapatite cement as a carried for bone-forming cells. Biomed. Mater. Eng. 1996, 6, 345–351. [Google Scholar] [CrossRef]
- Khairoun, I.; Driessens FC, M.; Boltong, M.G.; Planell, J.A.; Wenz, R. Addition of cohesion promotors to calcium phosphate cements. Biomaterials 1999, 20, 393–398. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kamioka, H.; Honjo, T.; Tezuka, K.I.; Takano-Yamamoto, T. Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 2005, 36, 877–883. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Itala, A.I.; Ylanen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 micron is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Van de Belt, H.; Neut, D.; Uges, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.J.J.; Amerongen, A.V.N.; Wuisman, P.I.J.M. In vitro gentamicin release from commercially available calcium-phosphate bone substitutes influence of carrier type on duration of the release profile. BMC Musculoskelet. Disord. 2006, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Uchida, K.; Sugo, K.; Nakasu, M.; Nakajima, T.; Takata, K.; Takaso, M.; Urabe, K. Long-term antibacterial activity of vancomycin from calcium phosphate cement in vivo. Bio-Med. Mater. Eng. 2022, 33, 41–50. [Google Scholar] [CrossRef]
- Barba, A.; Diez-Escudero, A.; Maazouz, Y.; Rappe, K.; Espanol, M.; Montufar, E.B.; Bonany, M.; Sadowska, J.M.; Guillem-Marti, J.; Öhman-Mägi, C.; et al. Osteoinduction by Foamed and 3D-Printed Calcium Phosphate Scaffolds: Effect of Nanostructure and Pore Architecture. ACS Appl. Mater. Interfaces 2017, 9, 41722–41736. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Shao, H.; Liu, H.; Xu, J.; Cong, M.; Zhao, K.; Lin, T. 3D gel-printing of hierarchically porous BCP scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2023, 43, 2646–2653. [Google Scholar] [CrossRef]
- Hympanova, M.; Oliver-Urrutia, C.; Vojta, M.; Macháček, M.; Krupka, P.; Kukla, R.; Celko, L.; Montufar, E.B.; Marek, J. Assessment of Streptococcus mutans biofilm formation on calcium phosphate ceramics: The role of crystalline composition and microstructure. Biomater. Adv. 2022, 135, 212750. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Ooms, E.M.; Wolke JG, C.; van der Waerden JP, C.M.; Jansen, J.A. Trabecular bone response to injectable calcium phosphate (Ca-P) cement. J. Biomed. Mater. Res. 2002, 61, 9–18. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. Available online: https://journals.lww.com/clinorthop/Fulltext/2002/02000/Properties_of_Osteoconductive_Biomaterials_.9.aspx (accessed on 20 April 2023). [CrossRef]
- Grossardt, C.; Ewald, A.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Passive and active in vitro resorption of calcium and magnesium phosphate cements by osteclastic cells. Tissue Eng. Part A 2010, 16, 3687–3695. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Rasmusson, A.; Melhus, H.; Engqvist, H.; Karlsson Ott, M. Simvastatin-doped pre-mixed calcium phosphate cement inhibits osteoclast differentiation and resorption. J. Mater. Sci. Mater. Med. 2016, 27, 83. [Google Scholar] [CrossRef]
- Davison, N.L.; Gamblin, A.-L.; Layrolle, P.; Yuan, H.; de Bruijn, J.D.; Barrère-de Groot, F. Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials 2014, 35, 5088–5097. [Google Scholar] [CrossRef]
- Odgren, P.R.; Witwicka, H.; Reyes-Gutierrez, P. The cast of clasts: Catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect. Tissue Res. 2016, 57, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Zhang, Y.L.; Tamimi, F.; Barralet, J. Effect of processing conditions of dicalcium phosphate cements on graft resorption and bone formation. Acta Biomater. 2017, 53, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Pasqual, J.A.R.; Pereira, B.L.R.; Colpo, J.C.; Egea, J.R.J.; Santos, L.A.L.; Sousa, V.C. Monitoring of the interaction of calcium phosphate cement and lidocaine hydrochloride by electrochemical impedance spectroscopy during the drug release process. J. Appl. Electrochem. 2021, 51, 463–471. [Google Scholar] [CrossRef]
- Uchida, K.; Sugo, K.; Nakajima, T.; Nakawaki, M.; Takano, S.; Nagura, N.; Takaso, M.; Urabe, K. In Vivo Release of Vancomycin from Calcium Phosphate Cement. BioMed Res. Int. 2018, 2018, 4560647. [Google Scholar] [CrossRef]
- Liu, S.-M.; Chen, W.-C.; Ko, C.-L.; Chang, H.-T.; Chen, Y.-S.; Haung, S.-M.; Chang, K.-C.; Chen, J.-C. In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold. Pharmaceuticals 2021, 14, 1000. [Google Scholar] [CrossRef]
- Haghbin-Nazarpak, M.; Moztarzadeh, F.; Solati-Hashjin, M.; Mirhabibi, A.R.; Tahriri, M. Preparation, characterization and gentamicin sulfate release investigation of biphasic injectable calcium phosphate bone cement. Ceram. Silikáty 2010, 54, 334–340. [Google Scholar]
- Khashaba, R.M.; Lockwood, P.E.; Lewis, J.B.; Messer, R.L.; Chutkan, N.B.; Borke, J.L. Cytotoxicity, calcium release, and pH changes generated by novel calcium phosphate cement formulations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 297–303. [Google Scholar] [CrossRef]
- Ramteke, K.H.; Dighe, P.A.; Kharat, A.R.; Patil, S.V. Mathematical models of drug dissolution: A review. Sch. Acad. J. Pharm. 2014, 3, 388–396. [Google Scholar]
- Lu, Z.; Tsai, M.; Lu, D.; Wang, J.; Wientjes, M.G.; Au, J.L.S. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J. Pharmacol. Exp. Ther. 2008, 327, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, B.; Mann, S.; Roveri, N. Biomimetic Hydroxyapatite–Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 2007, 17, 2180–2188. [Google Scholar] [CrossRef]
- Zhuang, Z.; Aizawa, M. Protein adsorption on single-crystal hydroxyapatite particles with preferred orientation to a(b)- and c-axes. J. Mater. Sci. Mater. Med. 2013, 24, 1211–1216. [Google Scholar] [CrossRef]
- Akashi, A.; Matsuya, Y.; Unemori, M.; Akamine, A. Release profile of antimicrobial agents from α-tricalcium phosphate cement. Biomaterials 2001, 22, 2713–2717. [Google Scholar] [CrossRef]
- Hesaraki, S.; Zamanian, A.; Moztarzadeh, F. The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: Acetic acid versus citric acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 208–216. [Google Scholar] [CrossRef]
- Peroos, S.; Du, Z.; de Leeuw, N.H. A computer modelling study of the uptake, structure and distribution of carbonate defects in hydroxy-apatite. Biomaterials 2006, 27, 2150–2161. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef]
- Stähli, C.; Bohner, M.; Bashoor-Zadeh, M.; Doebelin, N.; Baroud, G. Aqueous impregnation of porous β-tricalcium phosphate scaffolds. Acta Biomater. 2010, 6, 2760–2772. [Google Scholar] [CrossRef]
- Uskokovíc, V. Entering the Era of Nanoscience: Time to Be So Small. J. Biomed. Nanotechnol. 2013, 9, 1441–1470. [Google Scholar] [CrossRef]
- Su, W.-Y.; Chen, Y.-C.; Lin, F.-H. A New Type of Biphasic Calcium Phosphate Cement as a Gentamicin Carrier for Osteomyelitis. Evid. Based Complement. Altern. Med. 2013, 2013, 801374. [Google Scholar] [CrossRef] [PubMed]
- Mestres, G.; Kugiejko, K.; Pastorino, D.; Unosson, J.; Öhman, C.; Ott, M.K.; Ginebra M-PPersson, C. Changes in the drug release pattern of fresh and set simvastatin-loaded brushite cement. Mater. Sci. Eng. C 2016, 58, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ratier, A.; Freche, M.; Lacout, J.L.; Rodriguez, F. Behaviour of an injectable calcium phosphate cement with added tetracycline. Int. J. Pharm. 2004, 274, 261–268. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparicio, J.; Mariño, F.T.; García-Denche, J.T.; Blanco Jerez, L.; Gbureck, U.; Cabarcos, E.L. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010, 6, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- Schnieders, J.; Gbureck, U.; Vorndran, E.; Schossig, M.; Kissel, T. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J. Biomed. Mater. Res. Part A 2011, 99, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Huang, M.; Liu, J.; Wang, P.; Schneider, A.; Ren, K.; Oates, T.W.; Weir, M.D.; Xu, H.H.K.; Zhao, L. Antibacterial calcium phosphate cement with human periodontal ligament stem cell-microbeads to enhance bone regeneration and combat infection. J. Tissue Eng. Regen. Med. 2021, 15, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.K.; Sosa, B.; Osagie, L.; Turajane, K.; Bostrom, M.P.; Yang, X. Vancomycin-laden calcium phosphate-calcium sulfate composite allows bone formation in a rat infection model. PLoS ONE 2019, 14, e0222034. [Google Scholar] [CrossRef]
- Hu, M.-H.; Chu, P.-Y.; Huang, S.-M.; Shih, B.-S.; Ko, C.-L.; Hu, J.-J.; Chen, W.-C. Injectability, Processability, Drug Loading, and Antibacterial Activity of Gentamicin-Impregnated Mesoporous Bioactive Glass Composite Calcium Phosphate Bone Cement In Vitro. Biomimetics 2022, 7, 121. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Dolci, L.S.; Albertini, B.; Passerini, N.; Torricelli, P.; Parrilli, A.; Fini, M.; Bonvicini, F.; Gentilomi, G.A.; Panzavolta, S.; et al. A radiopaque calcium phosphate bone cement with long-lasting antibacterial effect: From paste to injectable formulation. Ceram. Int. 2020, 46, 10048–10057. [Google Scholar] [CrossRef]
- Lang, Z.G.; Zhang, X.; Guo, Q.; Liang, Y.X.; Yuan, F. Clinical observations of vancomycin-loaded calcium phosphate cement in the 1-stage treatment of chronic osteomyelitis: A randomized trial. Ann. Palliat. Med. 2021, 10, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.; Varma, P.K.; Arumugam, T.; Vijayan, A.M.; Vasudevan, A.K.; Rangasamy, J. Antibacterial bone adhesive cement for preventing sternal infections after cardiac surgery. Ceram. Int. 2023, 49, 16110–16122. [Google Scholar] [CrossRef]
- Li, K.; Guo, A.; Ran, Q.; Tian, H.; Du, X.; Chen, S.; Wen, Y.; Tang, Y.; Jiang, D. A novel biocomposite scaffold with antibacterial potential and the ability to promote bone repair. J. Biomater. Appl. 2021, 36, 474–480. [Google Scholar] [CrossRef]
- Ribeiro, N.; Reis, M.; Figueiredo, L.; Pimenta, A.; Santos, L.F.; Branco, A.C.; Alves de Matos, A.P.; Salema-Oom, M.; Almeida, A.; Pereira, M.F.C.; et al. Improvement of a commercial calcium phosphate bone cement by means of drug delivery and increased injectability. Ceram. Int. 2022, 48, 33361–33372. [Google Scholar] [CrossRef]
- Mestres, G.; Fernandez-Yague, M.A.; Pastorino, D.; Montufar, E.B.; Canal, C.; Manzanares-Céspedes, M.C.; Ginebra, M.P. In vivo efficiency of antimicrobial inorganic bone grafts in osteomyelitis treatments. Mater. Sci. Eng. C. 2019, 97, 84–95. [Google Scholar] [CrossRef]
- Son, Y.J.; Lee, I.C.; Jo, H.H.; Chung, T.J.; Oh, K.S. Setting behavior and drug release from brushite bone cement prepared with granulated hydroxyapatite and β-tricalcium phosphate. J. Korean Ceram. Soc. 2019, 56, 56–64. [Google Scholar] [CrossRef]
- Ruskin, E.I.; Coomar, P.P.; Sikder, P.; Bhaduri, S.B. Magnetic Calcium Phosphate Cement for Hyperthermia Treatment of Bone Tumors. Materials 2020, 13, 3501. [Google Scholar] [CrossRef]
- Martinez, T.; Sarda, S.; Dupret-Bories, A.; Charvillat, C.; Projetti, F.; Drouet, C. Toward a doxorubicin-loaded bioinspired bone cement for the localized treatment of osteosarcoma. Future Oncol. 2021, 17, 3511–3528. [Google Scholar] [CrossRef]
- Goldin, A.N.; Healey, R.M. The Effectiveness and Mechanical Properties of Chemotherapy-Impregnated Cement in Ewing Sarcoma. J. Orthop. Res. 2023, in press. [Google Scholar] [CrossRef]
- Demir-Oğuz, Ö.; Ege, D. Effect of zoledronic acid and graphene oxide on the physical and in vitro properties of injectable bone substitutes. Mater. Sci. Eng. C. 2021, 120, 111758. [Google Scholar] [CrossRef]
- Kamal, N.H.; Heikal, L.A.; Ali, M.M.; Aly, R.G.; Abdallah, O.Y. Development and evaluation of local regenerative biomimetic bone-extracellular matrix scaffold loaded with nano-formulated quercetin for orthopedic fractures. Biomater. Adv. 2023, 145, 213249. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Liang, W.; Li, L.; Cui, X.; Wei, X.; Pan, H.; Li, B. Novel calcitonin gene-related peptide/chitosan-strontium-calcium phosphate cement: Enhanced proliferation of human umbilical vein endothelial cells in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107B, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gunnella, F.; Kunisch, E.; Horbert, V.; Maenz, S.; Bossert, J.; Jandt, K.D.; Plöger, F.; Kinne, R.W. In Vitro Release of Bioactive Bone Morphogenetic Proteins (GDF5, BB-1, and BMP-2) from a PLGA Fiber-Reinforced, Brushite-Forming Calcium Phosphate Cement. Pharmaceutics 2019, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Huang, Y.; Gu, F. The osteogenesis effect of rhBMP2-loaded calcium phosphate cements in repairing dental extraction sockets. Am. J. Transl. Res. 2022, 14, 7172–7177. [Google Scholar] [PubMed]
- Kim, S.H.; Hwang, C.; Park, S.H. Cell-laden Gelatin Fiber Contained Calcium Phosphate Biomaterials as a Stem Cell Delivery Vehicle for Bone Repair. J. Biomed. Eng. Res. 2022, 43, 61–70. [Google Scholar] [CrossRef]
- Vezenkova, A.; Locs, J. Sudoku of porous, injectable calcium phosphate cements—Path to osteoinductivity. Bioact. Mater. 2022, 17, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cui, C.; Chen, W.; Shi, J.; Li, B.; Chen, S. Biodegradable Cements for Bone Regeneration. J. Funct. Biomater. 2023, 14, 134. [Google Scholar] [CrossRef]
- Demir-Oğuz, Ö.; Boccaccini, A.R.; Loca, D. Injectable bone cements: What benefits the combination of calcium phosphates and bioactive glasses could bring? Bioact. Mater. 2022, 19, 217–236. [Google Scholar] [CrossRef]
- Zogakis, P.N.; Teles, A.R.; Zafeiris, Ε.P.; Zafeiris, C.P. Bisphosphonate-loaded bone cement: Background, clinical indications and future perspectives. J. Musculoskelet. Neuronal. Interact. 2022, 22, 587–595. [Google Scholar]
- Ntep DB, N.; Messanga, C.B.; Ndjoh, J.J.; Fokam, S.T.; Fokunang, C. Suppression of osteoclastogenesis signalling pathways and attenuation of ameloblastic osteolysis induced by local administration of CaP-bisphosphonate and CaP-doxycycline cements: Review of the literature and therapeutic hypothesis. Adv. Oral Maxillofac. Surg. 2022, 5, 100241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).