Removal Performance of KOH-Modified Biochar from Tropical Biomass on Tetracycline and Cr(VI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biochar
2.3. Adsorption Experiments
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
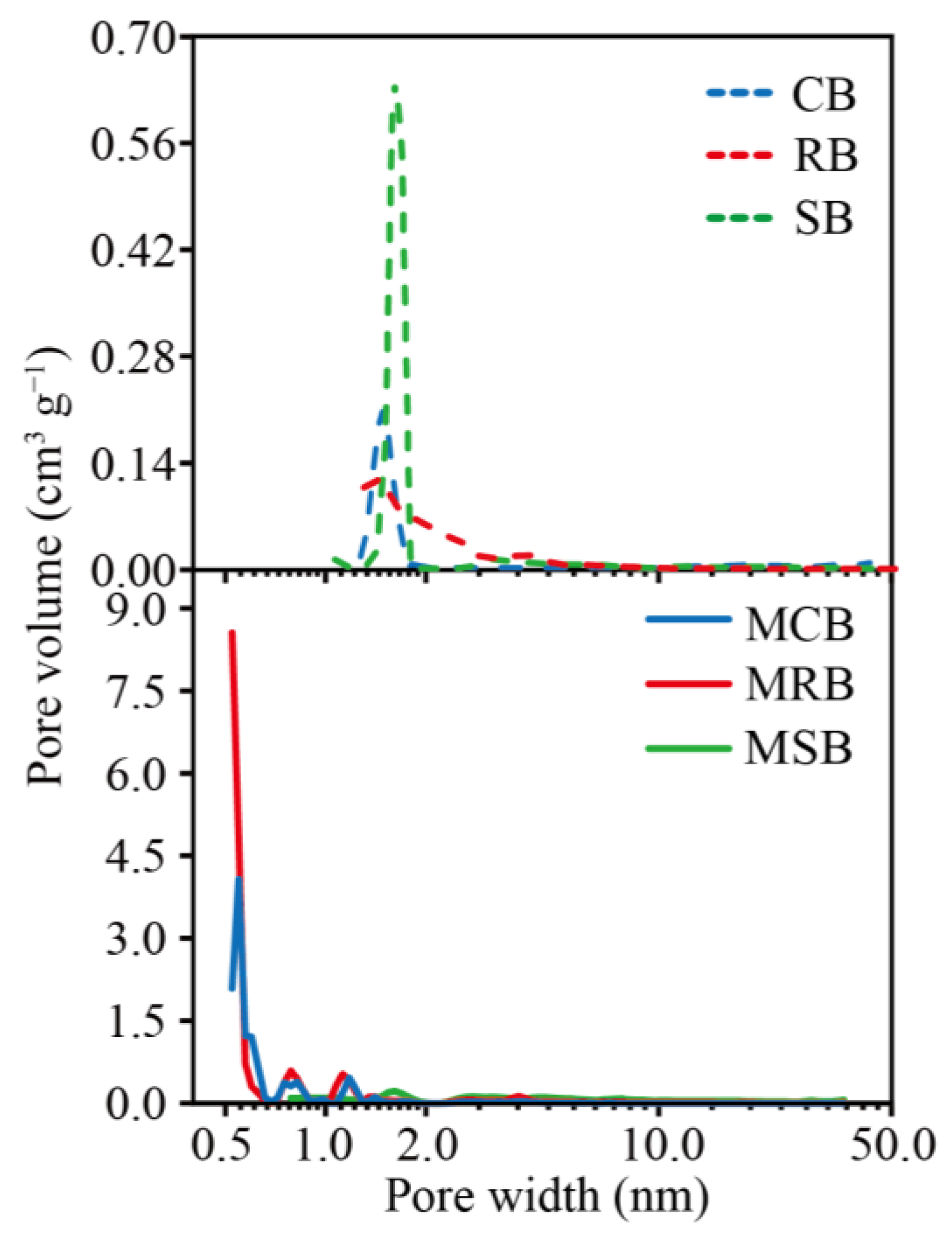
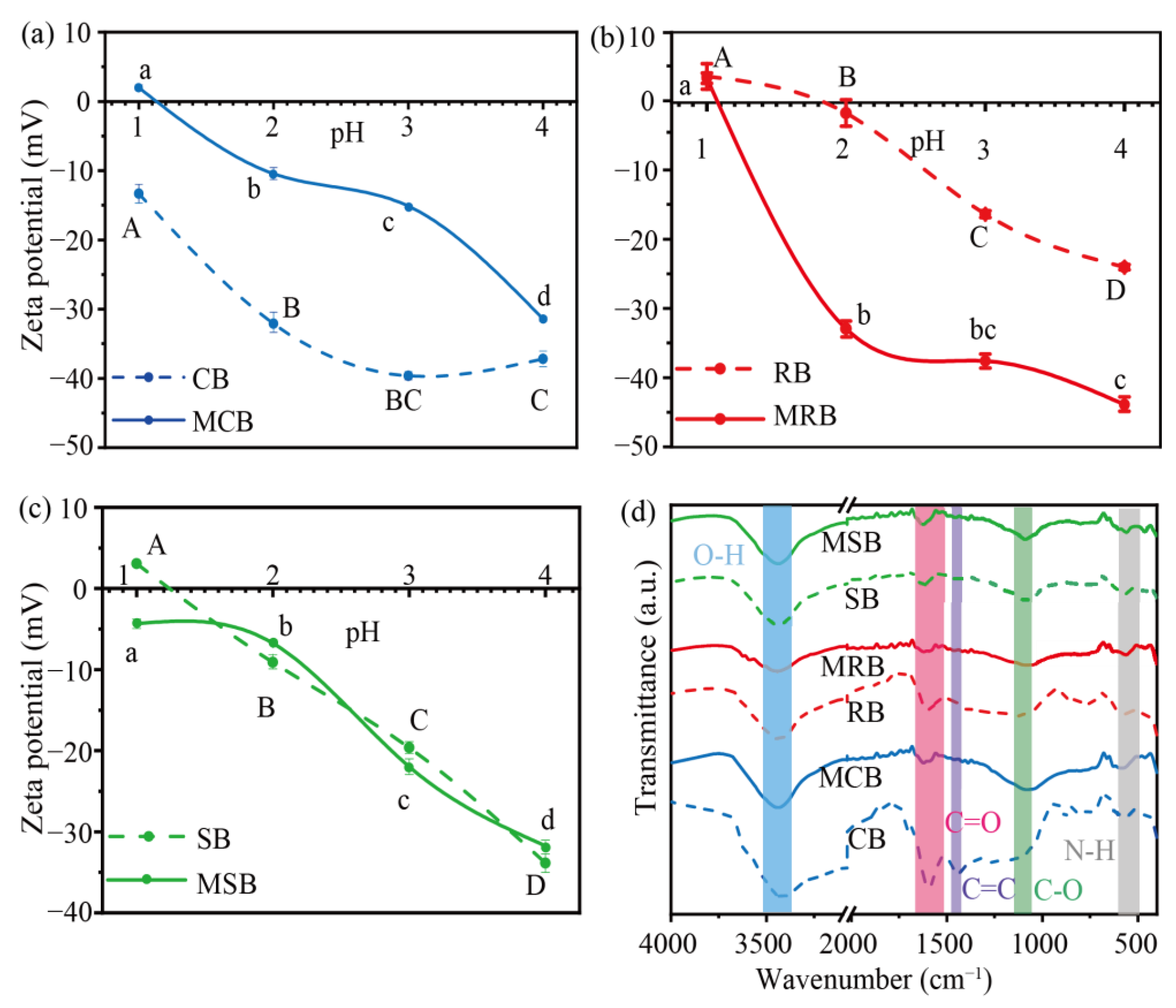
3.1. Characters of Biochar Derived from Cassava Stalk, Rubber Wood and Sugarcane Bagasse
3.2. Environmental pH Affected the Removal of TC
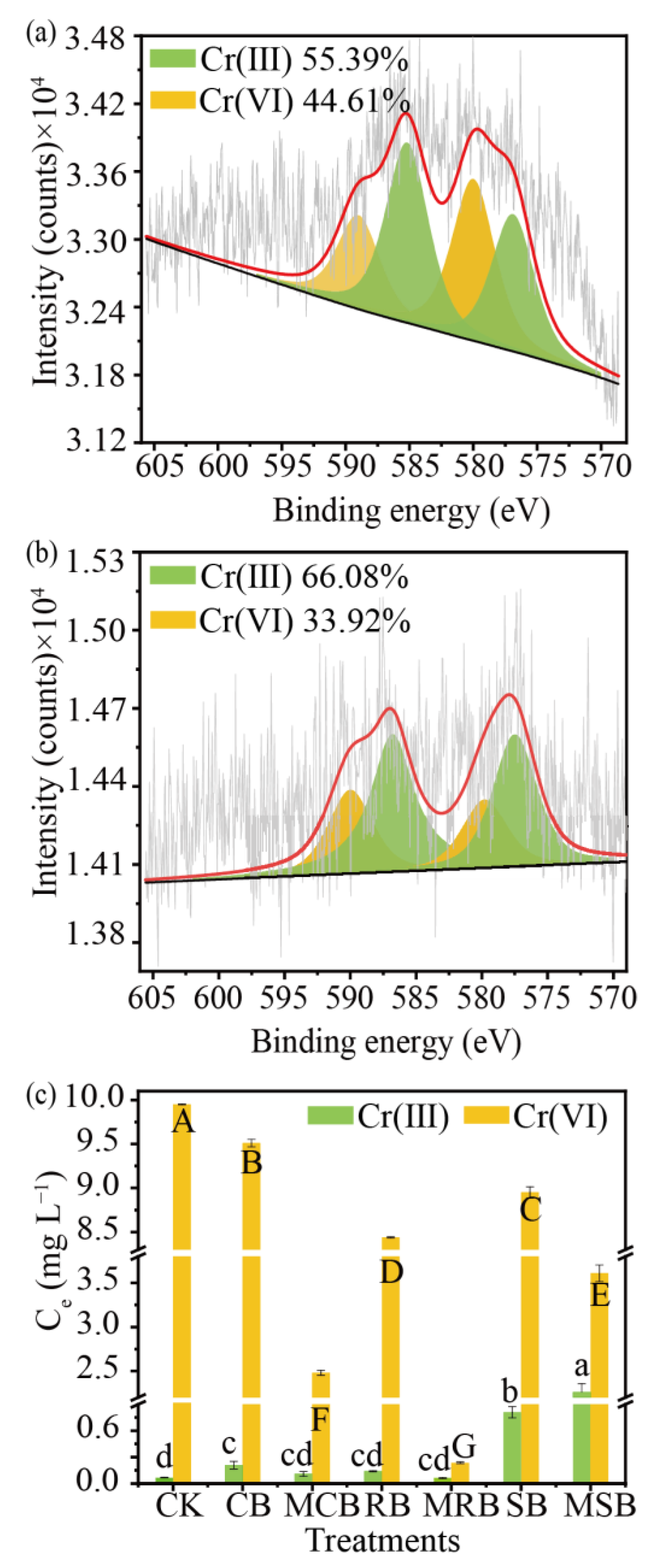
3.3. TC and Cr(VI) Removal Greatly Increased by KOH-Modified Biochar in the Binary System
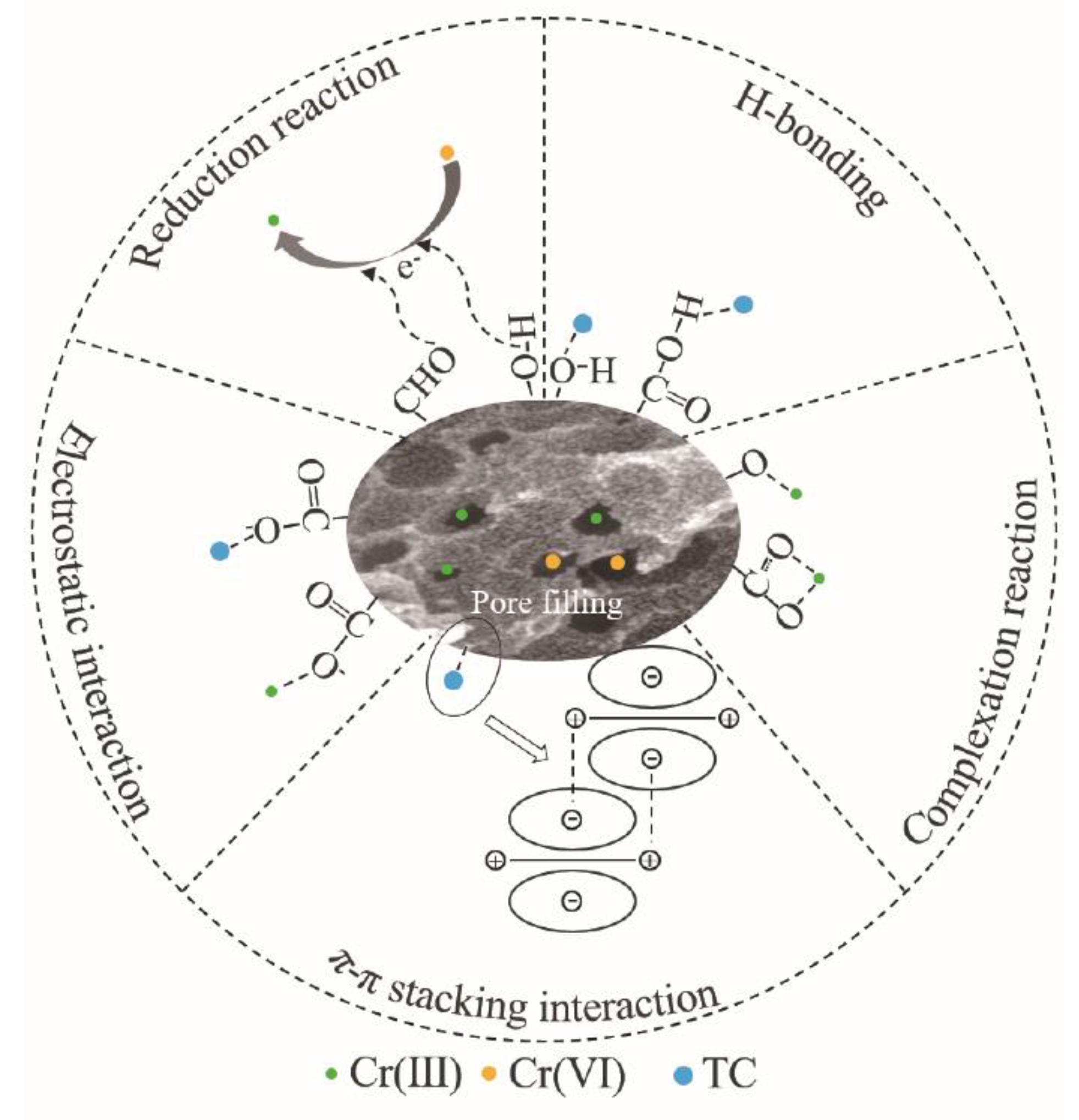
3.4. Mechanism of TC and Cr(VI) Removed by Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, S.; Selvaraj, S. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Bai, C.; Yang, G. A synergistic system of electrocatalytic-anode/α-MnO2/peroxymonosulfate for removing combined pollution of tetracycline and Cr(VI). Chem. Eng. J. 2021, 423, 130284. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Giese, R.F. Investigating the Molecular Interactions of Oxytetracycline in Clay and Organic Matter: Insights on Factors Affecting Its Mobility in Soil. Environ. Sci. Technol. 2004, 38, 4097–4105. [Google Scholar] [CrossRef]
- Gammelgaard, B.; Jensen, K. In vitro Metabolism and Permeation Studies in Rat Jejunum: Organic Chromium Compared to Inorganic Chromium. J. Trace Elem. Med. Biol. 1999, 13, 82–88. [Google Scholar] [CrossRef]
- Altındağ, A.; Yiğit, S. Assessment of heavy metal concentrations in the food web of lake Beyşehir, Turkey. Chemosphere 2005, 60, 552–556. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Hesselberg, T. Trophic transfer of heavy metals along a pollution gradient in a terrestrial agro-industrial food web. Geoderma 2022, 413, 115748. [Google Scholar] [CrossRef]
- Wan, J.; Xue, P. Synergistic effects in simultaneous photocatalytic removal of Cr(VI) and tetracycline hydrochloride by Z-scheme Co3O4/Ag/Bi2WO6 heterojunction. Appl. Surf. Sci. 2019, 483, 677–687. [Google Scholar] [CrossRef]
- Qu, J.; Wang, S. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr(VI) and tetracycline adsorption from water. Bioresour. Technol. 2021, 340, 125692. [Google Scholar] [CrossRef]
- Li, M.f.; Liu, Y.g. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J. Amino-functionalized Ti-metal-organic framework decorated BiOI sphere for simultaneous elimination of Cr(VI) and tetracycline. J. Colloid Interf. Sci. 2022, 607, 933–941. [Google Scholar] [CrossRef]
- Song, Q.; Liang, J. Selective adsorption behavior/mechanism of antibiotic contaminants on novel boron nitride bundles. J. Hazard. Mater. 2019, 364, 654–662. [Google Scholar] [CrossRef]
- Yuan, J.; Passeport, E. Understanding adsorption and biodegradation in granular activated carbon for drinking water treatment: A critical review. Water Res. 2022, 210, 118026. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, T.B. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour. Technol. 2019, 284, 197–203. [Google Scholar] [CrossRef]
- Yan, C.; Jin, J. Metal–organic frameworks (MOFs) for the efficient removal of contaminants from water: Underlying mechanisms, recent advances, challenges, and future prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P. Nitrogen-doped biochar as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J. Removal of chromium(VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J. Hierarchical porous Al2O3@ZnO core-shell microfibres with excellent adsorption affinity for Congo red molecule. Appl. Surf. Sci. 2019, 473, 251–260. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B. Physicochemical and sorptive properties of biochar derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G. Pyrolysis of different biomass pre-impregnated with steel pickling waste liquor to prepare magnetic biochar and their use for the degradation of metronidazole. Bioresour. Technol. 2019, 289, 121613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhai, P. Banana, pineapple, cassava and sugarcane bagasse biochar cannot mitigate ammonia volatilization from latosols in tropical farmland. Sci. Total Environ. 2022, 821, 153427. [Google Scholar] [CrossRef]
- Mu, Y.; He, W. Enhanced adsorption of tetracycline by the modified tea-based biochar with the developed mesoporous and surface alkalinity. Bioresour. Technol. 2021, 342, 126001. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.M. Characterization of Biochar Derived from Rubber Wood Sawdust through Slow Pyrolysis on Surface Porosities and Functional Groups. Procedia Eng. 2013, 68, 365–371. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Li, F.; Feng, D. Effects of Biochars Prepared from Cassava Dregs on Sorption Behavior of Ciprofloxacin. Procedia Environ. Sci. 2016, 31, 795–803. [Google Scholar] [CrossRef]
- An, Q.; Jiang, Y.Q. Unraveling sorption of nickel from aqueous solution by KMnO4 and KOH-modified peanut shell biochar: Implicit mechanism. Chemosphere 2019, 214, 846–854. [Google Scholar] [CrossRef]
- Yuan, S.; Hong, M. Contributions and mechanisms of components in modified biochar to adsorb cadmium in aqueous solution. Sci. Total Environ. 2020, 733, 139320. [Google Scholar] [CrossRef]
- Chen, Z.; Jing, Y. Enhanced removal of aqueous Cd(II) by a biochar derived from salt-sealing pyrolysis coupled with NaOH treatment. Appl. Surf. Sci. 2020, 511, 145619. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J. Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883. [Google Scholar] [CrossRef]
- Guo, D.; Wu, J. Mechanism of efficient magnetic biochar for typical aqueous organic contaminant combined-adsorption removal. Fuel Process. Technol. 2023, 247, 107795. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef]
- Xiao, Y.; Lyu, H. Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms. Chem. Eng. J. 2020, 384, 123311. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J. Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: Synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour. Technol. 2021, 329, 124856. [Google Scholar] [CrossRef]
- Lian, W.; Yang, L. Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II). Bioresour. Technol. 2020, 317, 124011. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Lv, F.; Lu, X. Dozens-fold improvement of biochar redox properties by KOH activation. Chem. Eng. J. 2022, 429, 132203. [Google Scholar]
- Shen, Q.; Wang, Z. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ. Res. 2020, 183, 108195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y. Study on a novel immobilized microbe pellets constructed with Alcaligenes sp. R3 and its ability to remove tetracycline. J. Environ. Chem. Eng. 2023, 11, 109378. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y. Nanosheets-MnxOy anchored biochar for efficient removal of methyl blue and tetracycline from water. Chem. Eng. Res. Des. 2022, 182, 13–24. [Google Scholar] [CrossRef]
- Zhuang, W.R.; Wang, Y. Applications of π-π stacking interactions in the design of drug-delivery systems. J. Control Release 2019, 294, 311–326. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S. Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Yang, C. Chromate(VI)-induced homogeneous oxidation and photolysis of aqueous tetracycline: Kinetics and mechanism. Chem. Eng. J. 2020, 379, 122276. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W. Two-step ball milling-assisted synthesis of N-doped biochar loaded with ferrous sulfide for enhanced adsorptive removal of Cr(VI) and tetracycline from water. Environ. Pollut. 2022, 306, 119398. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, H. A novel nitrogen-doped porous carbon derived from black liquor for efficient removal of Cr(VI) and tetracycline: Comparison with lignin porous carbon. J. Clean. Prod. 2022, 333, 130106. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, Y. Enhancement of Cr(VI) decontamination by irradiated sludge biochar in neutral conditions: Evidence of a possible role of persistent free radicals. Sep. Purif. Technol. 2021, 277, 119414. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G. Preparation of porous biochar based on pharmaceutical sludge activated by NaOH and its application in the adsorption of tetracycline. J. Colloid Interface Sci. 2021, 587, 271–278. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Q. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation. Environ. Pollut. 2020, 265, 115018. [Google Scholar] [CrossRef]
- Bai, L.; Su, X. Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr(VI) adsorption in water. J. Clean. Prod. 2021, 320, 128723. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef]


| Biomass Type | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Cassava stalk | 21.90 | 20.55 | 16.95 |
| Rubber wood | 46.55 | 22.05 | 13.90 |
| Sugarcane bagasse | 42.65 | 20.70 | 28.45 |
| Biochar Type | SBET 1 (m2 g−1) | Smic 2 (m2 g−1) | Smic/SBET (%) | Vtot 3 (cm3 g−1) | Vmic 4 (cm3 g−1) | Vmic/Vtot (%) |
|---|---|---|---|---|---|---|
| CB | 13.70 | 0.99 | 7.23 | 0.018 | 0.000 | – |
| MCB | 1282.32 | 1140.29 | 88.92 | 0.582 | 0.442 | 75.95 |
| RB | 142.06 | 52.90 | 37.24 | 0.101 | 0.018 | 17.82 |
| MRB | 973.46 | 904.37 | 92.90 | 0.420 | 0.348 | 82.86 |
| SB | 138.22 | 107.79 | 77.98 | 0.074 | 0.044 | 59.46 |
| MSB | 156.38 | 43.84 | 28.03 | 0.149 | 0.020 | 13.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yue, Y.; Liu, W.; Liu, Q.; Song, Y.; Ge, C.; Ma, H. Removal Performance of KOH-Modified Biochar from Tropical Biomass on Tetracycline and Cr(VI). Materials 2023, 16, 3994. https://doi.org/10.3390/ma16113994
Wang Q, Yue Y, Liu W, Liu Q, Song Y, Ge C, Ma H. Removal Performance of KOH-Modified Biochar from Tropical Biomass on Tetracycline and Cr(VI). Materials. 2023; 16(11):3994. https://doi.org/10.3390/ma16113994
Chicago/Turabian StyleWang, Qingxiang, Yan Yue, Wenfei Liu, Qing Liu, Yu Song, Chengjun Ge, and Hongfang Ma. 2023. "Removal Performance of KOH-Modified Biochar from Tropical Biomass on Tetracycline and Cr(VI)" Materials 16, no. 11: 3994. https://doi.org/10.3390/ma16113994
APA StyleWang, Q., Yue, Y., Liu, W., Liu, Q., Song, Y., Ge, C., & Ma, H. (2023). Removal Performance of KOH-Modified Biochar from Tropical Biomass on Tetracycline and Cr(VI). Materials, 16(11), 3994. https://doi.org/10.3390/ma16113994





