Microstructural Understanding of Flow Accelerated Corrosion of SA106B Carbon Steel in High-Temperature Water with Different Flow Velocities
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. FAC Tests
2.3. Analyses
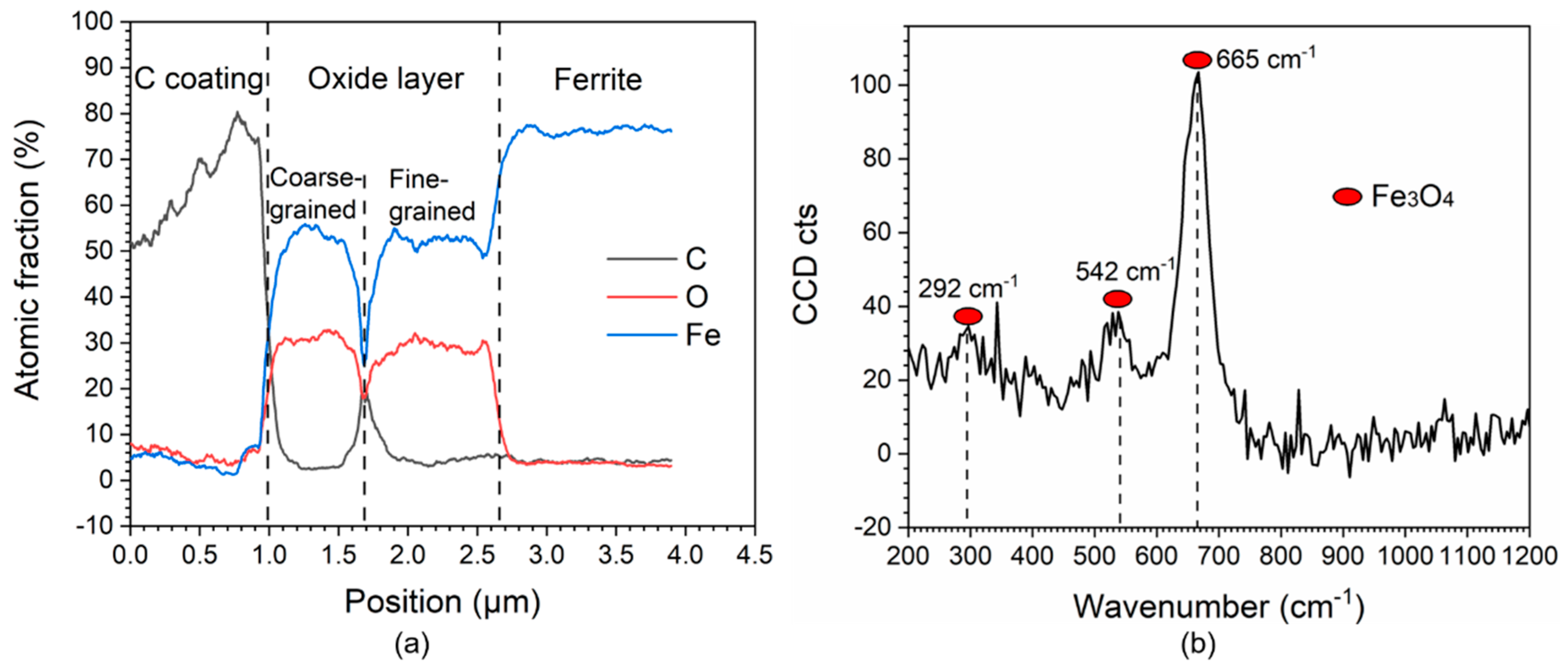
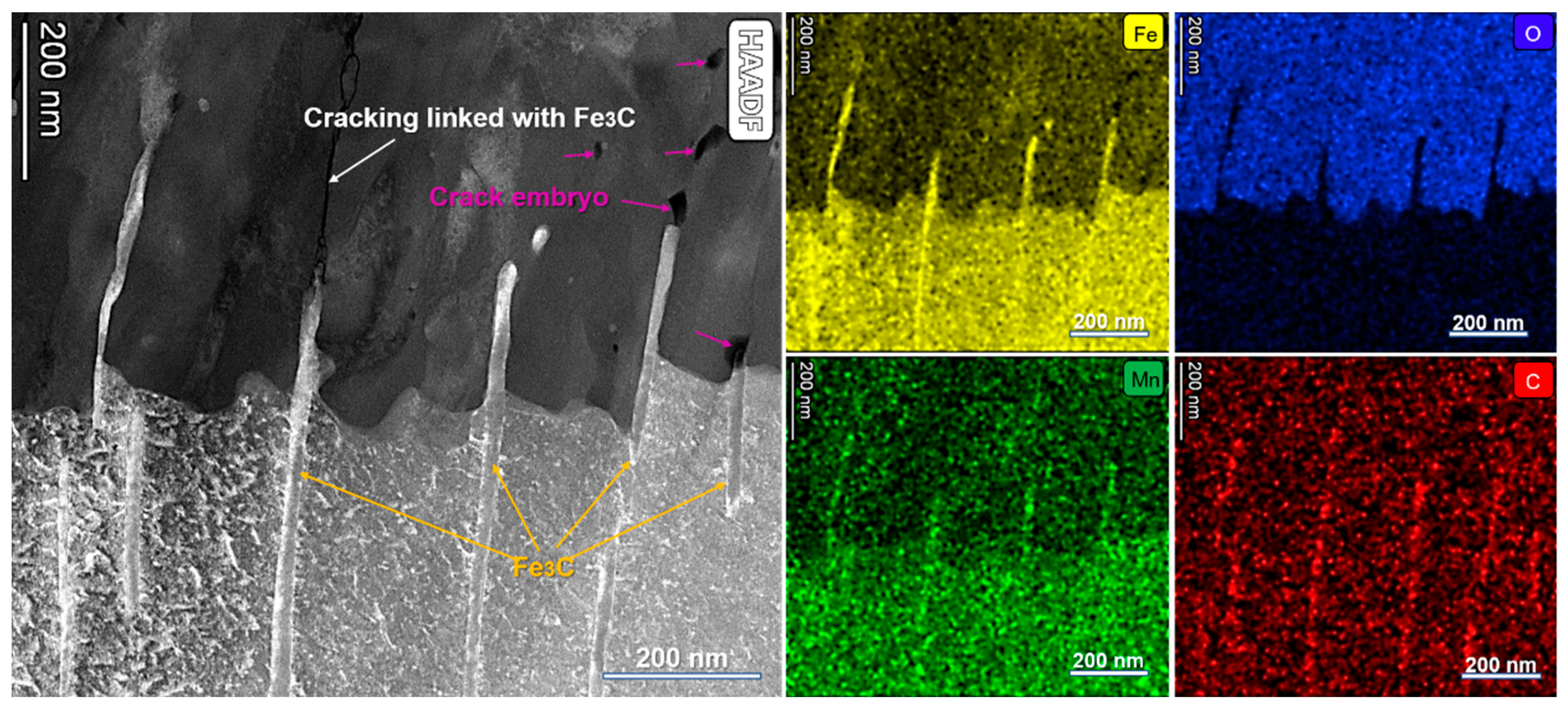
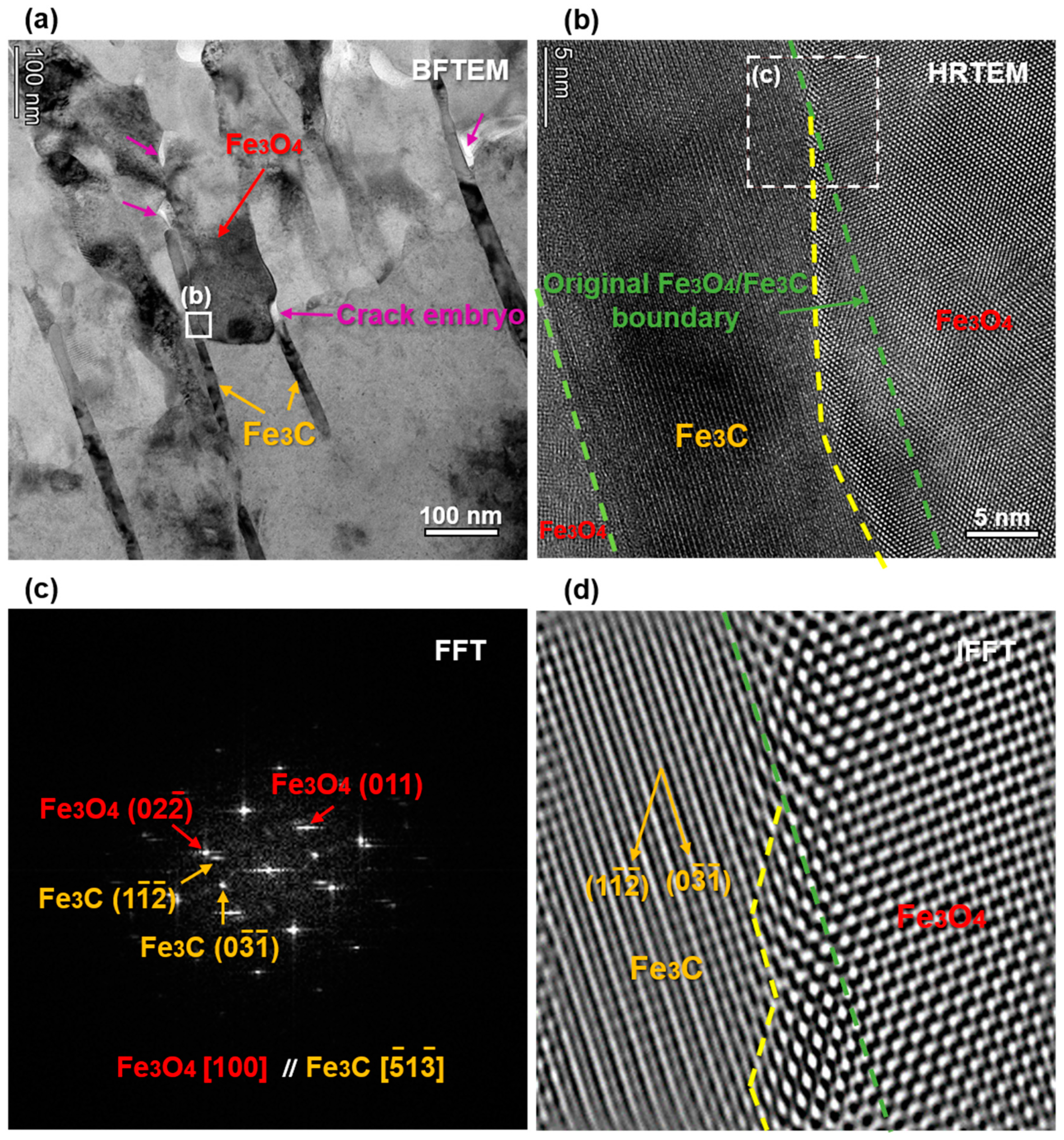
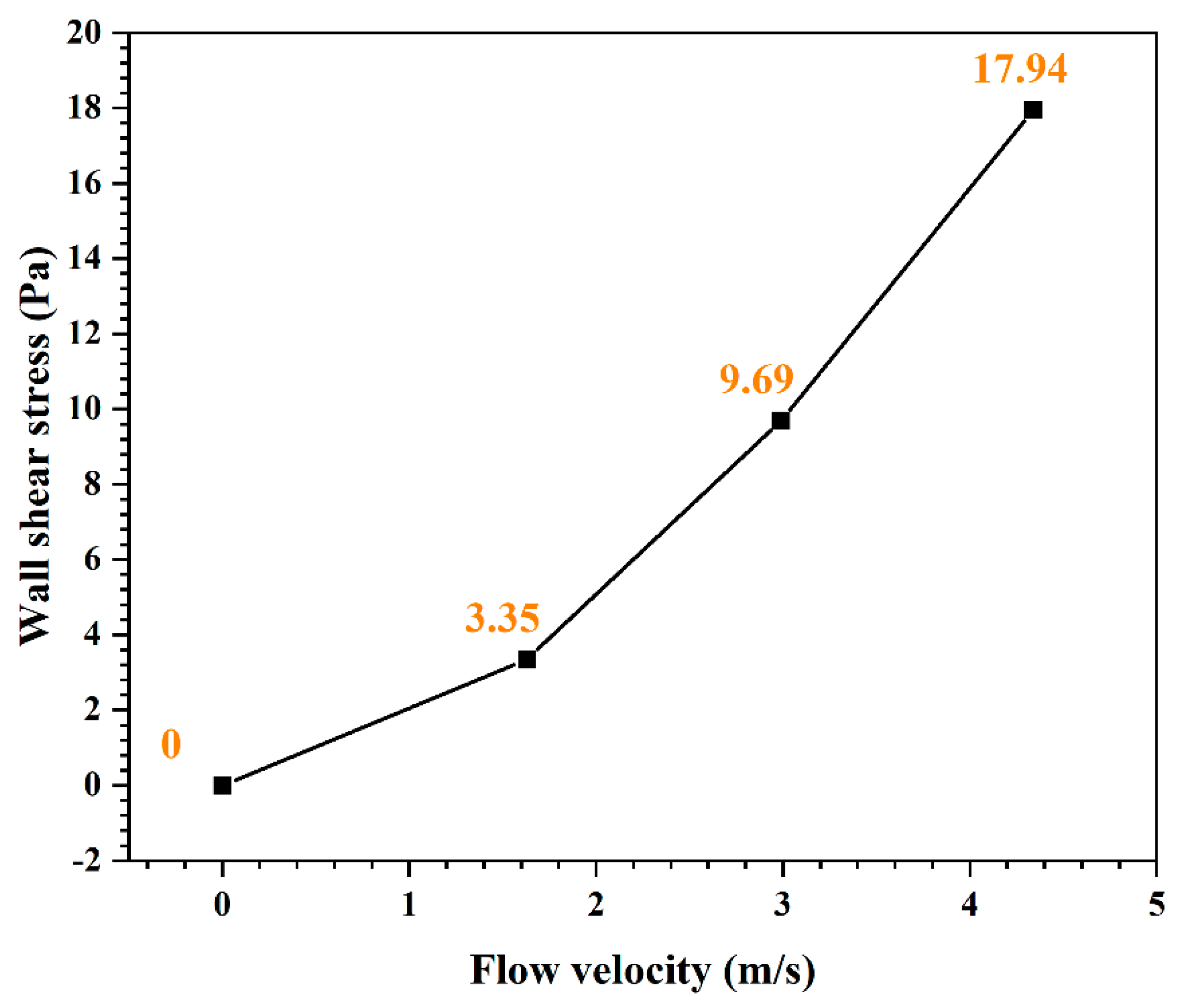
3. Results
4. Discussion
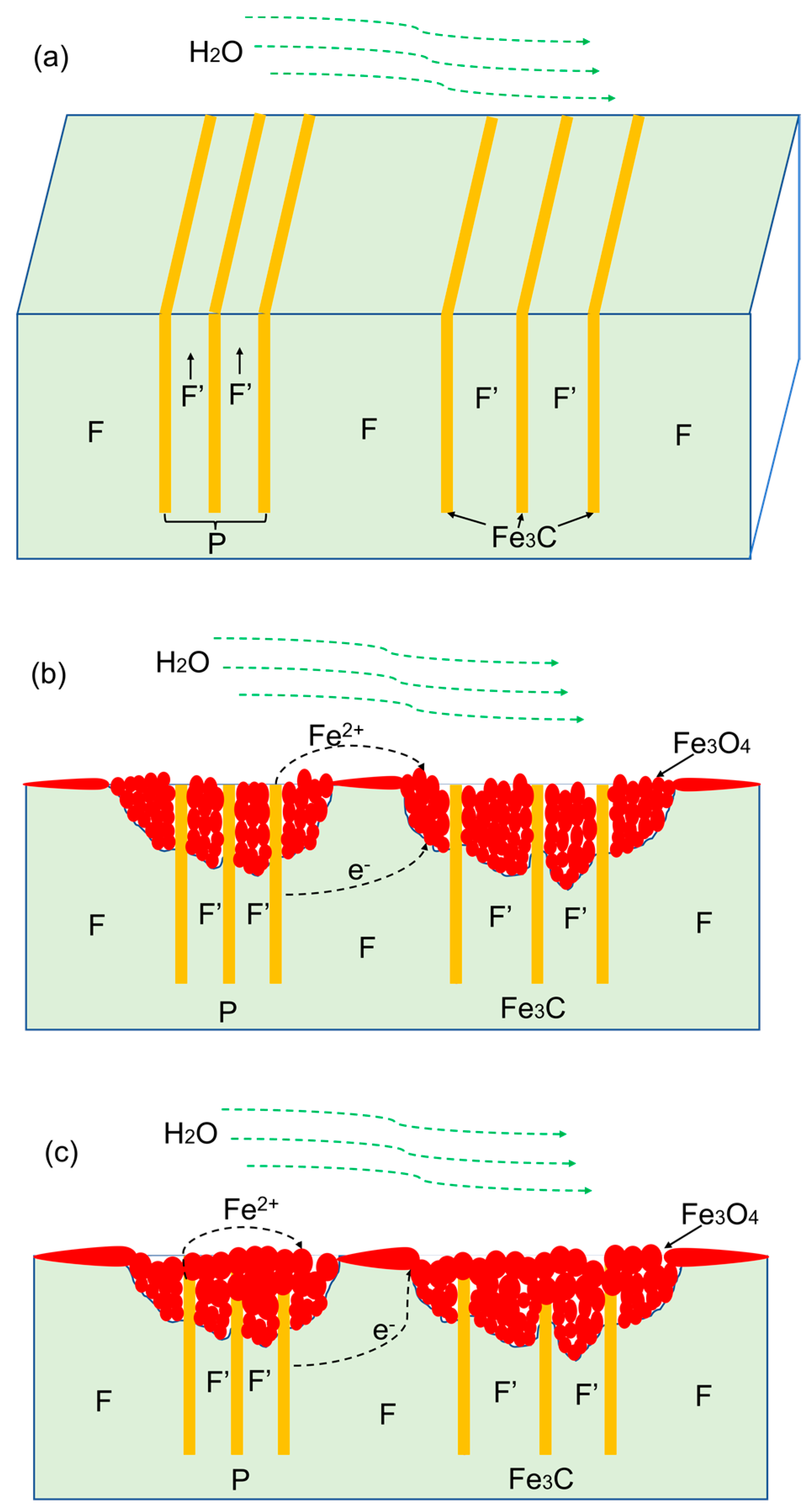
4.1. Influence of Microstructure on FAC
4.2. Influence of Flow Velocity on FAC
5. Conclusions
- ➢
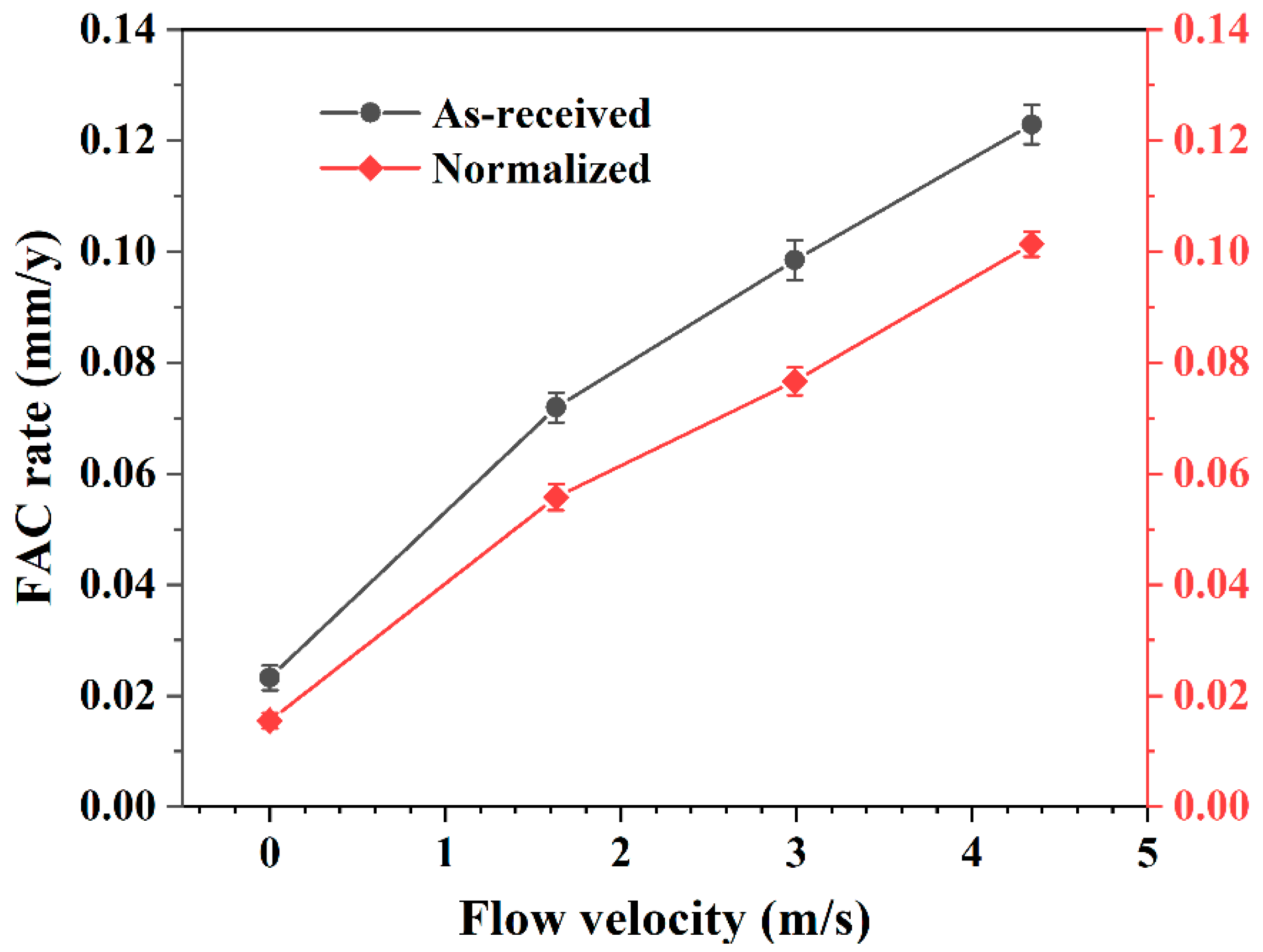
- Flow velocity converted the major corrosion type in high-temperature water. The steel prevailingly went through uniform corrosion in static high-temperature water, while localized corrosion became dominant in dynamic water. The FAC rate in dynamic high-temperature water was much larger than static high-temperature water. Furthermore, the rate increased linearly as the flow velocity increased. After normalizing, the FAC rate was reduced by 33.28% under static conditions, while and 22.47%, 22.15%, and 17.53% at flow velocity of 1.63 m/s, 2.99 m/s, and 4.34 m/s, respectively.
- ➢
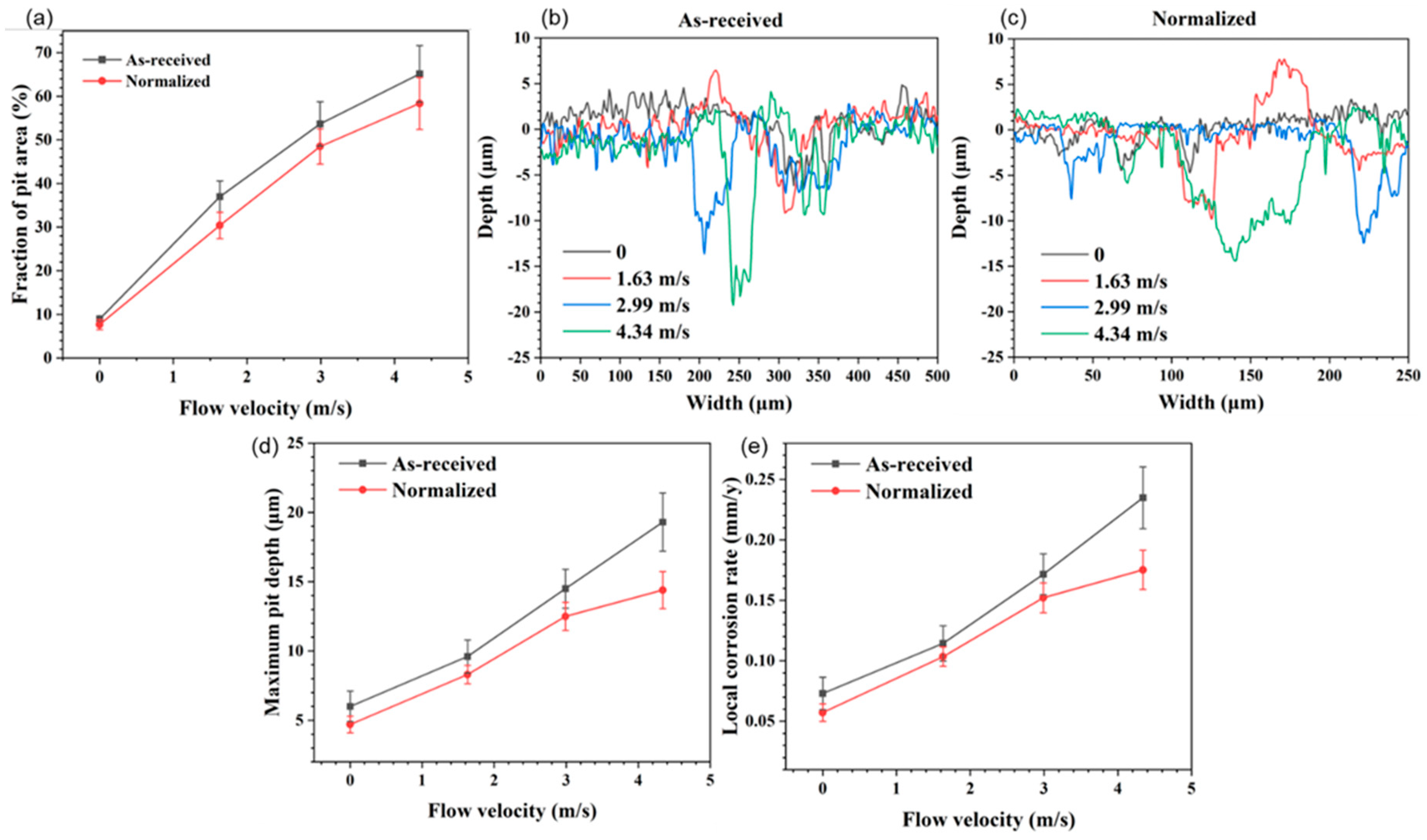
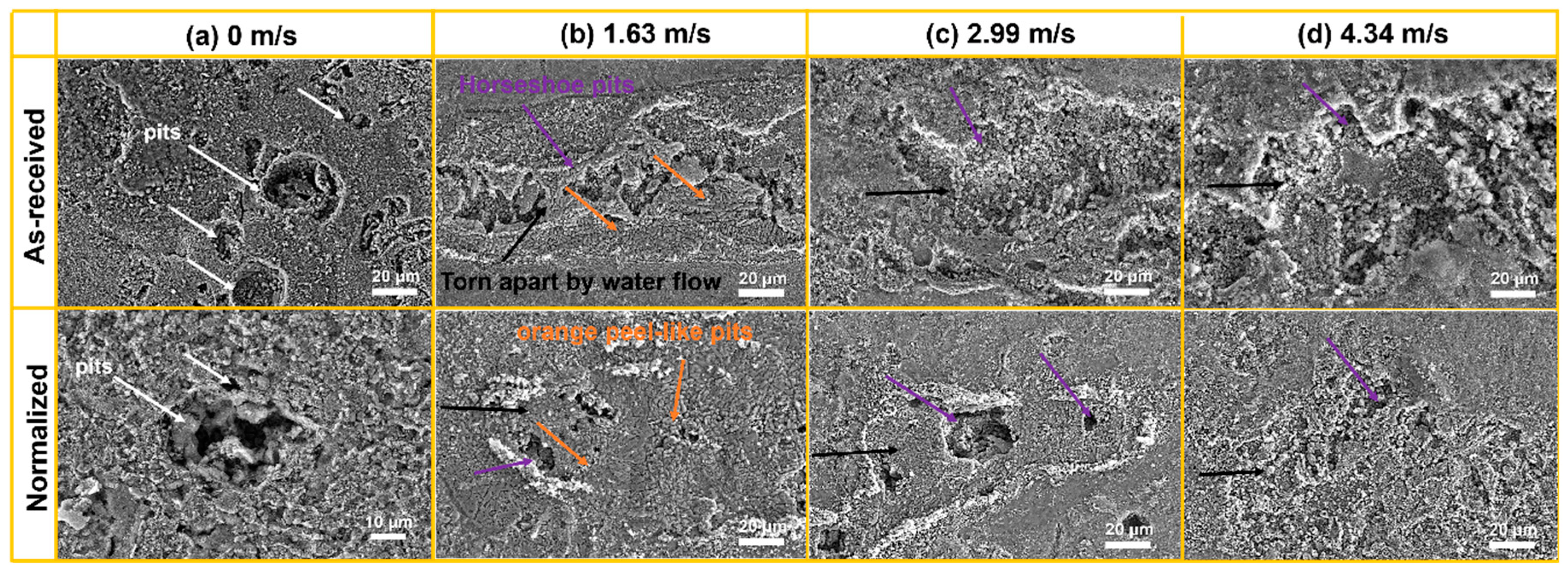
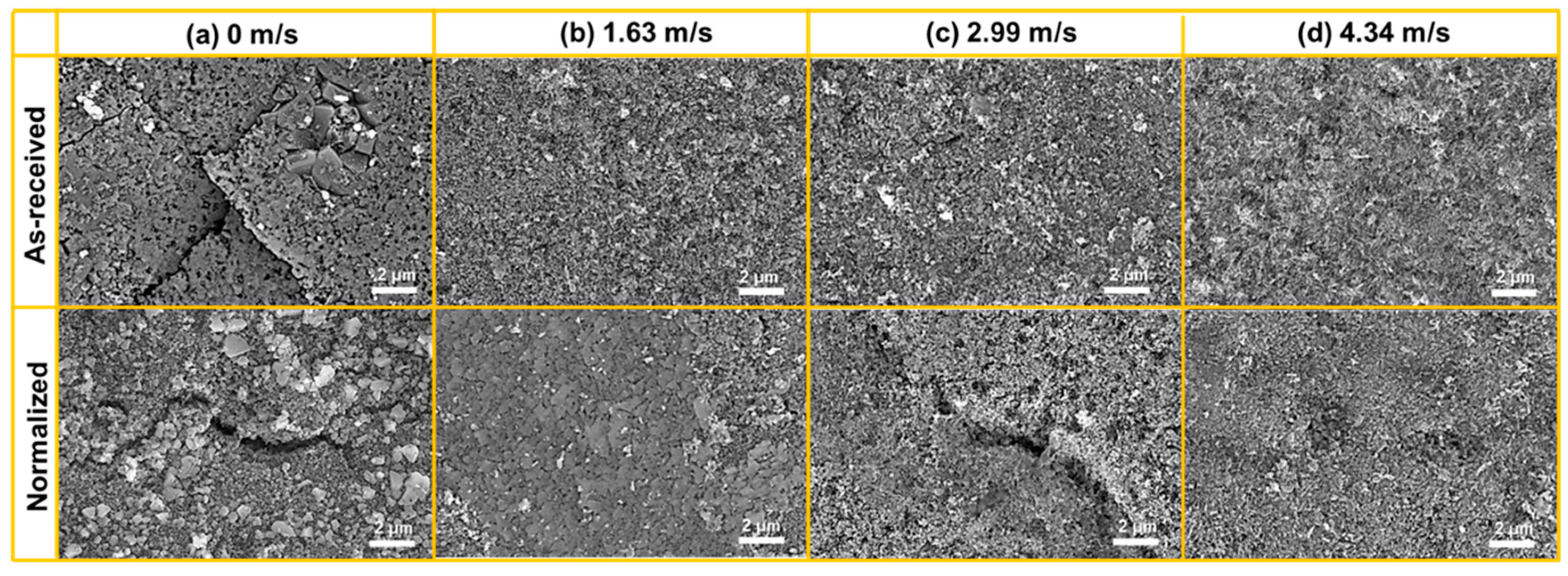
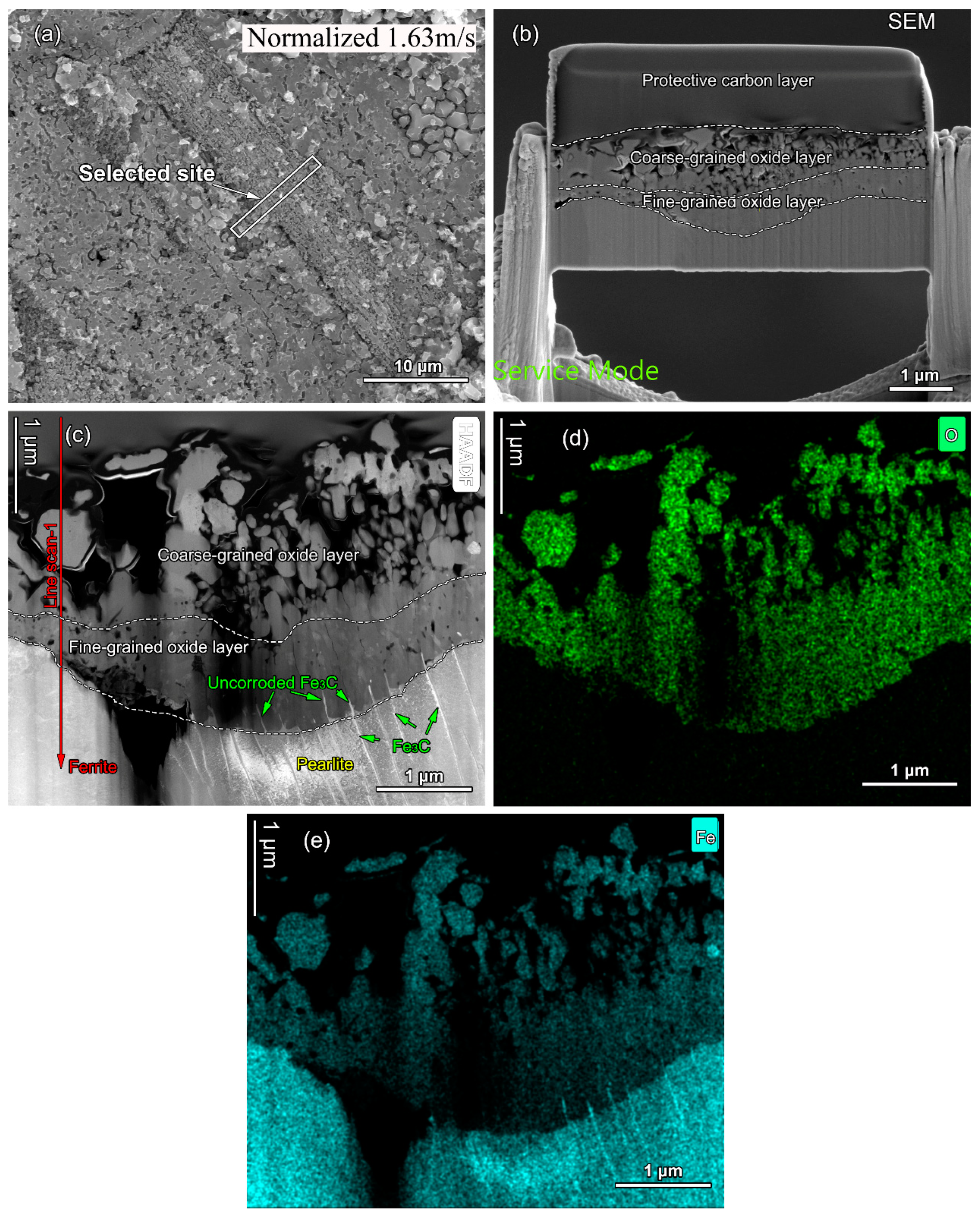
- The faster the flow velocity, the more difficult it was to form a complete oxide film inside the pits, resulting in the aggravation of localized corrosion. With increasing flow velocity, the vortex was readily created even if the pit was small in size, with the effect of accelerating the mass transfer and preventing the formation of Fe3O4 film inside the pits, resulting in the severe localized corrosion. Meanwhile, enhanced wall shear stress could intensify the spalling of oxides and influence the growth in Fe3O4 inside localized corrosion pits. After normalizing, the close of the localized corrosion pits was faster. Oxides might fully fill the pit and restrain the growth of localized corrosion, and the maximum localized corrosion rate decreased by 21.7%, 13.5%, 13.8%, and 25.4% at flow velocity of 0 m/s, 1.63 m/s, 2.99 m/s, and 4.34 m/s, respectively.
- ➢
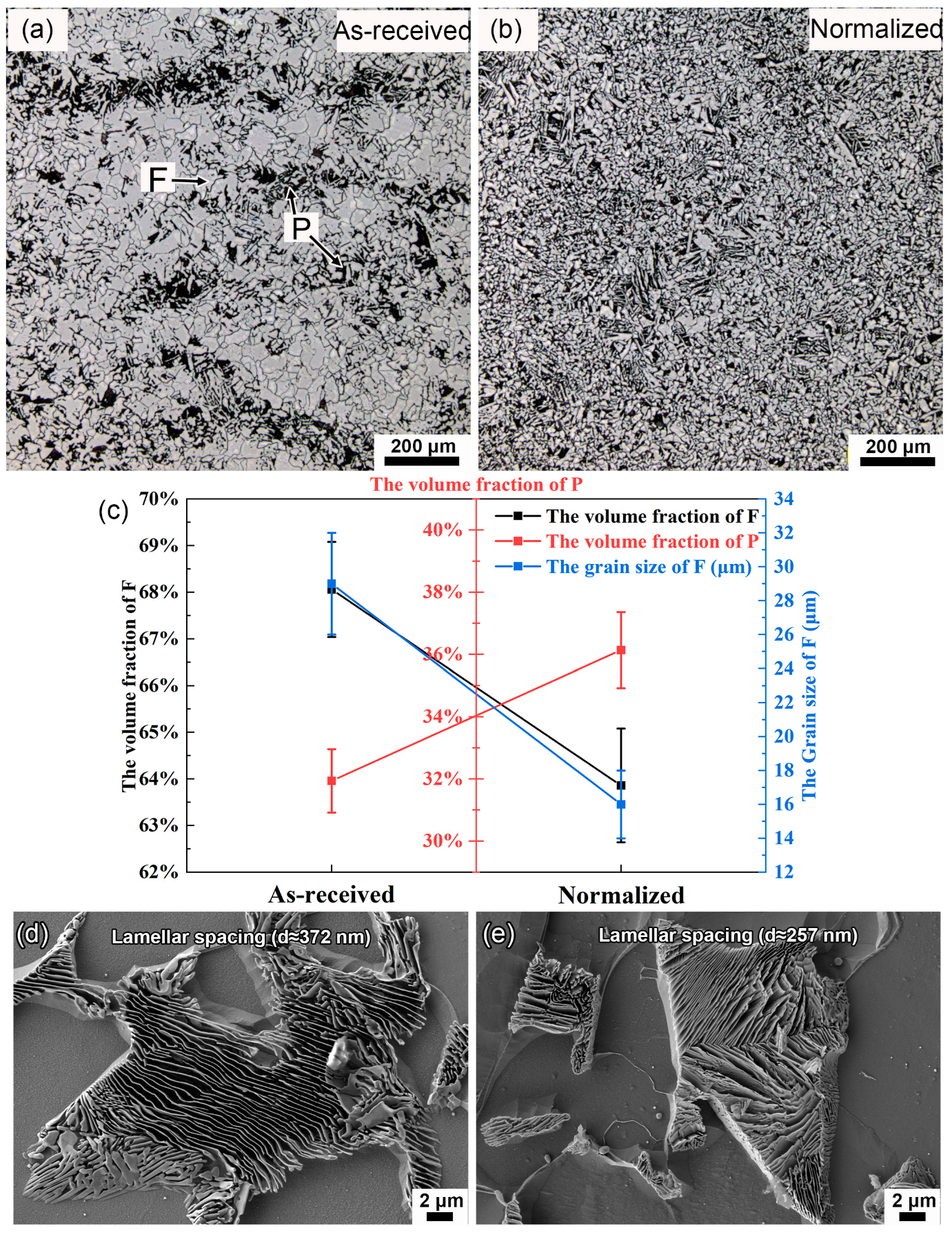
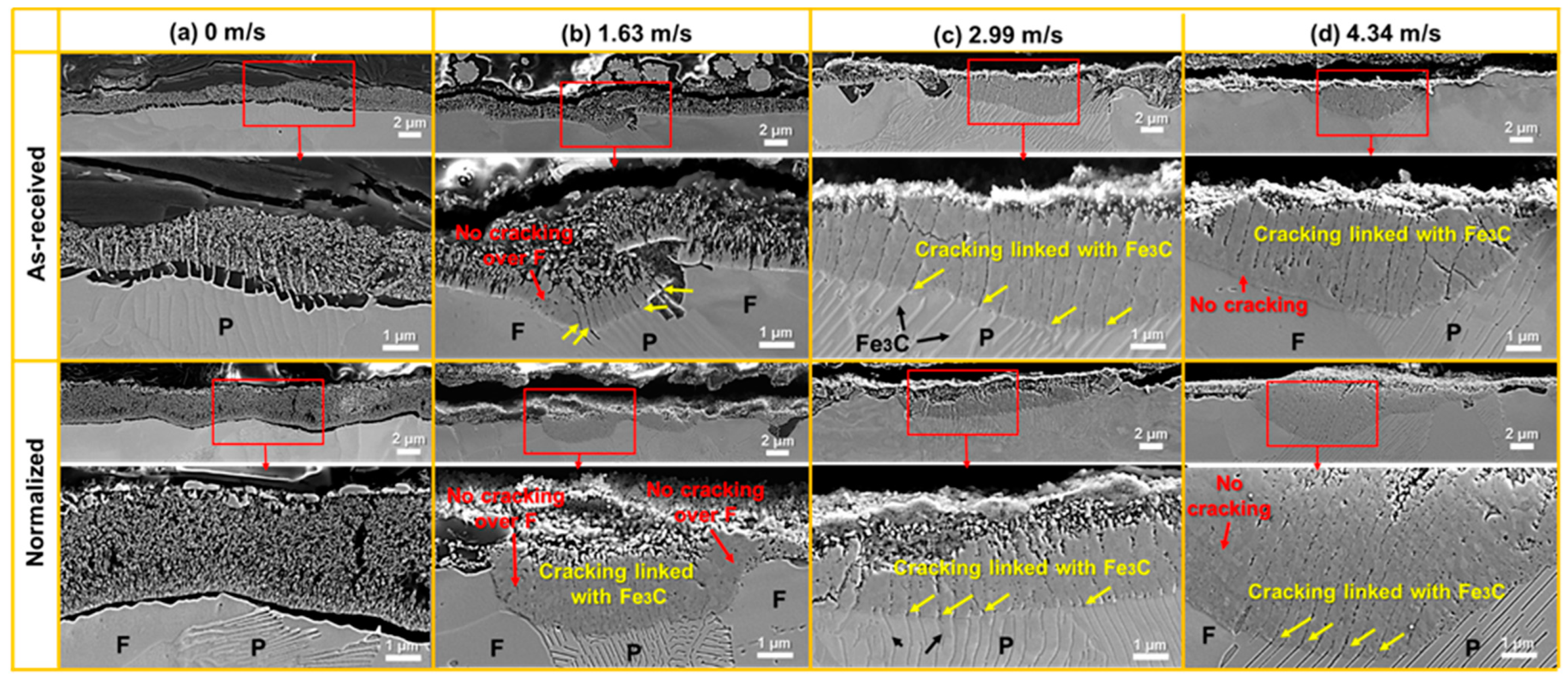
- From the as-received steel with banded structure, severe localized corrosion occurred at the location of the pearlite, which could be the prior sites for pit initiation. The cementite acting as a cathode aggregated in the pits and further reinforced the effect micro-galvanic corrosion, resulting in the acceleration of the corrosion rate. Ultimately, the cementite was reacted during the corrosion invasion along the direction of Fe3C ().
- ➢
- From the normalized steel, uniformly dispersed ferrite and pearlite were obtained, along with the disappearance of banded structure. The improvement in microstructure homogeneity relieved the localized corrosion tendency by retarding accelerated corrosion through the micro-galvanic couple. The refinement of lamellar structure reduced the volume fraction of cementite available to act as cathodic sites, resulting in a decrease in the corrosion rate. The increase in pearlite alignment weakened the tensile stresses in the oxide film and led to slower oxidation kinetics and lower cracking sensitivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, D.; Kuang, B. Study of CAP1400 secondary pipe wall thinning rate under flow accelerated corrosion. Ann. Nucl. Energy 2021, 155, 108170. [Google Scholar] [CrossRef]
- Madasamy, P.; Chandramohan, P. Flow accelerated corrosion rate on carbon steel pipe bend by thin layer activation technique and computational modeling: Under PHWR operating conditions. Eng. Fail. Anal. 2021, 121, 105125. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, S.G. On-line monitoring system development for single-phase flow accelerated corrosion. Nucl. Eng. Des. 2007, 237, 761–767. [Google Scholar] [CrossRef]
- Prasad, M.; Gopika, V. Pipe wall thickness prediction with CFD based mass transfer coefficient and degradation feedback for flow accelerated corrosion. Prog. Nucl. Energy 2008, 107, 205–214. [Google Scholar] [CrossRef]
- Bignold, G.J.; Garbett, K. Erosion-corrosion in nuclear steam generators. In Water Chemistry of Nuclear Reactor Systems 2; Thomas Telford Publishing: London, UK, 1981. [Google Scholar]
- Gipon, E.; Trevin, S. 6—Flow-accelerated corrosion in nuclear power plants. In Nuclear Corrosion; Ritter, S., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 213–250. [Google Scholar]
- Xu, Y.Z.; Tan, M.Y.J. Probing the initiation and propagation processes of flow accelerated corrosion and erosion corrosion under simulated turbulent flow conditions. Corros. Sci. 2019, 151, 163–174. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Zhou, Q.; Gao, S.; Bin Wang, B.; Wang, X.; Huang, Y. Flow accelerated corrosion and erosion−corrosion behavior of marine carbon steel in natural seawater. Mater. Degrad. 2021, 5, 56. [Google Scholar] [CrossRef]
- Potter, E.C. The First International Congress on Metallic Corrosion; Butterworths: London, UK, 1961; 712p. [Google Scholar]
- Li, L.L.; Wang, Z.B. Interaction between pitting corrosion and critical flow velocity for erosion-corrosion of 304 stainless steel under jet slurry impingement. Corros. Sci. 2019, 158, 108084. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J. Influence of the secondary phase on micro galvanic corrosion of low carbon bainitic steel in NaCl solution. Mater. Charact. 2018, 139, 401–410. [Google Scholar] [CrossRef]
- Khalid, F.A.; Farooque, M. Role of ferrite/pearlite banded structure and segregation on mechanical properties of microalloyed hot rolled steel. Mater. Sci. Technol. 1999, 15, 1209–1215. [Google Scholar] [CrossRef]
- Majka, T.F.; Matlock, D.K. Development of microstructural banding in low-alloy steel with simulated Mn segregation. Met. Mater. Trans. A 2002, 33, 1627–1637. [Google Scholar] [CrossRef]
- Neetu, P.K.; Katiyar, S. Effect of various phase fraction of bainite, intercritical ferrite, retained austenite and pearlite on the corrosion behavior of multiphase steels. Corros. Sci. 2021, 178, 109043. [Google Scholar] [CrossRef]
- Anijdan, S.M.; Sabzi, M.; Park, N.; Lee, U. Sour corrosion performance and sensitivity to hydrogen induced cracking in the X70 pipeline steel: Effect of microstructural variation and pearlite percentage. Int. J. Press. Vessel. Pip. 2022, 199, 104759. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Z. Corrosion behavior of low-alloy steel containing 1% chromium in CO2 environments. Corros. Sci. 2013, 75, 400–408. [Google Scholar] [CrossRef]
- Liu, H.; Wei, J. Influence of cementite spheroidization on relieving the micro-galvanic effect of ferrite-pearlite steel in acidic chloride environment. J. Mater. Sci. Technol. 2021, 61, 234–246. [Google Scholar] [CrossRef]
- Schmitt, G.; Mueller, M. Critical wall shear stresses in CO{sub 2} corrosion of carbon steel. In Proceedings of the CORROSION 99, San Antonio, TX, USA, 25 April 1999. [Google Scholar]
- Hwang, K.; Lee, D.; Yun, H.; Yoo, S.C.; Kim, J.H. Analysis of Material Loss Behavior According to Long-Term Experiments on LDIE-FAC Multiple Degradation of Carbon Steel Materials. World J. Nucl. Sci. Technol. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Trevin, S. 7-Flow accelerated corrosion (FAC) in nuclear power plant components. In Nuclear Corrosion Science and Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 186–229. [Google Scholar]
- Staehle, R.W.; Gorman, J.A. Quantitative assessment of submodes of stress corrosion cracking on the secondary side of steam generator tubing in pressurized water reactors: Part 2. Corrosion 2004, 60, 5–63. [Google Scholar] [CrossRef]
- Zhang, T.; Li, T.; Lu, J.; Guo, Q.; Xu, J. Microstructural Characterization of the Corrosion Product Deposit in the Flow-Accelerated Region in High-Temperature Water. Crystals 2022, 12, 749. [Google Scholar] [CrossRef]
- Ren, L.; Wang, S.C.; Xu, J.; Zhang, T.; Guo, Q.; Zhang, D.; Si, J.; Zhang, X.; Yu, H.; Shoji, T.; et al. Fouling on the secondary side of nuclear steam generator tube: Experimental and simulated study. Appl. Surf. Sci. 2022, 590, 153143. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.L.; Gao, K.W. Effect of flow rate on localized corrosion of X70 steel in supercritical CO2 environments. Corros. Sci. 2018, 136, 339–351. [Google Scholar] [CrossRef]
- Kuang, W.; Was, G.S. A high-resolution characterization of the initiation of stress corrosion crack in Alloy 690 in simulated pressurized water reactor primary water. Corros. Sci. 2020, 163, 108243. [Google Scholar] [CrossRef]
- Kadowaki, M.; Muto, I. Real-Time Microelectrochemical Observations of Very Early Stage Pitting on Ferrite-Pearlite Steel in Chloride Solutions. J. Electrochem. Soc. 2017, 164, 261–268. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X. Synergistic Effect of Al2O3 Inclusion and Pearlite on the Localized Corrosion Evolution Process of Carbon Steel in Marine Environment. Materials 2018, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pan, J. Localized corrosion behaviour of reinforcement steel in simulated concrete pore solution. Corros. Sci. 2009, 51, 2130–2138. [Google Scholar] [CrossRef]
- Liu, H.H.; Wei, J. The synergy between cementite spheroidization and Cu alloying on the corrosion resistance of ferrite-pearlite steel in acidic chloride solution. J. Mater. Sci. Technol. 2021, 84, 65–75. [Google Scholar] [CrossRef]
- Wharton, J.A.; Wood, R.J.K. Influence of flow conditions on the corrosion of AISI 304L stainless steel. Wear 2004, 256, 525–536. [Google Scholar] [CrossRef]
- Fujiwara, K.; Domae, M. Correlation of flow accelerated corrosion rate with iron solubility. Nucl. Eng. Des. 2011, 241, 4482–4486. [Google Scholar] [CrossRef]
- Kadowaki, M.; Muto, I. Anodic Polarization Characteristics and Electrochemical Properties of Fe3C in Chloride Solutions. J. Electrochem. Soc. 2019, 166, 345–351. [Google Scholar] [CrossRef]
- Mehtani, H.K.; Khan, M.I. Oxidation kinetics in pearlite: The defining role of interface crystallography. Scr. Mater. 2018, 152, 44–48. [Google Scholar] [CrossRef]
- Moss, T.; Kuang, W. Stress corrosion crack initiation in Alloy 690 in high temperature water. Curr. Opin. Solid State Mater. Sci. 2018, 22, 16–25. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, X. Comparative study on corrosion behaviors of ferrite-pearlite steel with dual-phase steel in the simulated bottom plate environment of cargo oil tanks. J. Mater. Res. Technol. 2021, 12, 399–411. [Google Scholar] [CrossRef]
- Xiang, S.; He, Y. Chloride-induced corrosion behavior of cold-drawn pearlitic steel wires. Corros. Sci. 2018, 141, 221–229. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.F.M. A direct measurement of wall shear stress in multiphase flow—Is it an important parameter in CO2 corrosion of carbon steel pipelines? Corros. Sci. 2016, 110, 35–45. [Google Scholar] [CrossRef]
- Gray, L.G.S.; Anderson, B.G. Effect of pH and Temperature on the Mechanism of Carbon Steel Corrosion by Aqueous Carbon Dioxide; CORROSION/90; NACE: Houston, TX, USA, 1990. [Google Scholar]
- Patel, V.C.; Head, M.R. Some observations on skin friction and velocity profiles in fully developed pipe and channel flows. J. Fluid Mech. 1969, 38, 181. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 31 March 2023).
- Xiong, Y.; Brown, B. Atomic Force Microscopy Study of the Adsorption of Surfactant Corrosion Inhibitor Films. Corrosion 2014, 70, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.A.; Zeng, Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Gao, K.; Fang, Y. Mechanical properties of CO2 corrosion product scales and their relationship to corrosion rates. Corros. Sci. 2008, 50, 2796–2803. [Google Scholar] [CrossRef]
- Rani, H.P.; Divya, T.; Sahaya, R.R.; Kain, V.; Barua, D. CFD study of flow accelerated corrosion in 3D elbows. Ann. Nucl. Energy 2014, 69, 344–351. [Google Scholar] [CrossRef]
- Davis, C.; Frawley, P. Modelling of erosion–corrosion in practical geometries. Corros. Sci. 2009, 51, 769–775. [Google Scholar] [CrossRef]
- Sanchez-Caldera, L.E.; Griffith, P.; Rabinowicz, E. The mechanism of corrosion–erosion in steam extraction lines of power stations. J. Eng. Gas Turbines Power 1988, 110, 180–184. [Google Scholar] [CrossRef]

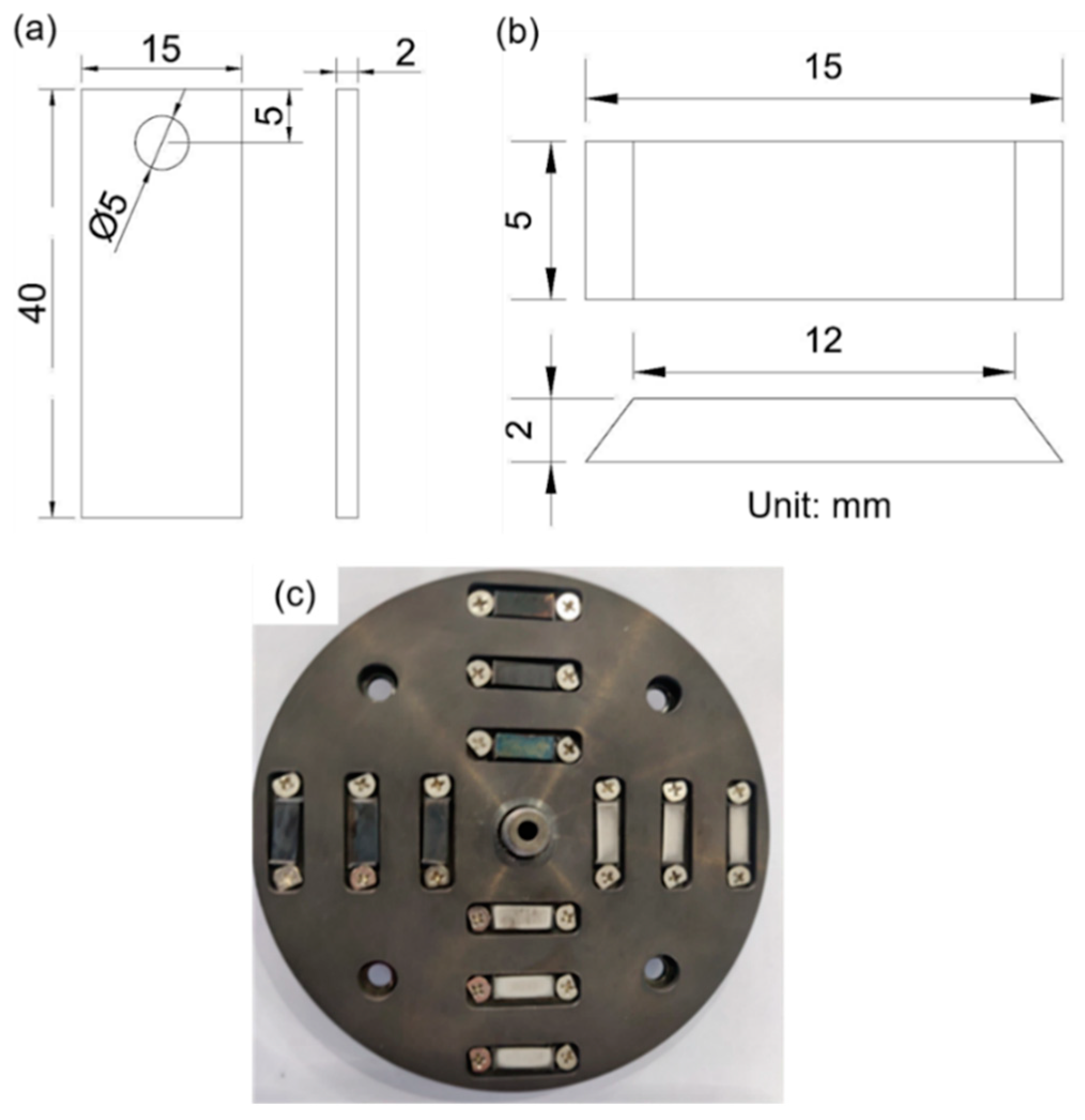


| Element | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe | C | Mn | P | S | Mo | Si | V | Cr |
| Bal. | 0.21 | 0.53 | 0.011 | 0.008 | 0.01 | 0.26 | 0.01 | 0.02 |
| Rotational Speed | Duration | Temperature | Pressure | pH | DO |
|---|---|---|---|---|---|
| 865 rpm | 720 h | 290 °C | 10 MPa | 9.7 | <10 ppb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Xin, L.; Hong, C.; Han, Y.; Lu, Y. Microstructural Understanding of Flow Accelerated Corrosion of SA106B Carbon Steel in High-Temperature Water with Different Flow Velocities. Materials 2023, 16, 3981. https://doi.org/10.3390/ma16113981
Hu Y, Xin L, Hong C, Han Y, Lu Y. Microstructural Understanding of Flow Accelerated Corrosion of SA106B Carbon Steel in High-Temperature Water with Different Flow Velocities. Materials. 2023; 16(11):3981. https://doi.org/10.3390/ma16113981
Chicago/Turabian StyleHu, Ying, Long Xin, Chang Hong, Yongming Han, and Yonghao Lu. 2023. "Microstructural Understanding of Flow Accelerated Corrosion of SA106B Carbon Steel in High-Temperature Water with Different Flow Velocities" Materials 16, no. 11: 3981. https://doi.org/10.3390/ma16113981
APA StyleHu, Y., Xin, L., Hong, C., Han, Y., & Lu, Y. (2023). Microstructural Understanding of Flow Accelerated Corrosion of SA106B Carbon Steel in High-Temperature Water with Different Flow Velocities. Materials, 16(11), 3981. https://doi.org/10.3390/ma16113981






